Abstract
Infrared spectroscopic tissue imaging is a potentially powerful adjunct tool to current histopathology techniques. By coupling the biochemical signature obtained through infrared spectroscopy to the spatial information offered by microscopy, this technique can selectively analyze the chemical composition of different features of unlabeled, unstained tissue sections. In the past, the tissue features that have received the most interest were parenchymal and epithelial cells, chiefly due to their involvement in dysplasia and progression to carcinoma; however, the field has recently turned its focus toward stroma and areas of fibrotic change. These components of tissue present an untapped source of biochemical information that can shed light on many diverse disease processes, and potentially hold useful predictive markers for these same pathologies. Here we review the recent applications of infrared spectroscopic imaging to stromal and fibrotic regions of diseased tissue, and explore the potential of this technique to advancing current capabilities for tissue analysis.
Keywords: Fibrosis, Hyper spectral imaging, Infrared Spectroscopy, Microscopy, Collagen
1. Introduction
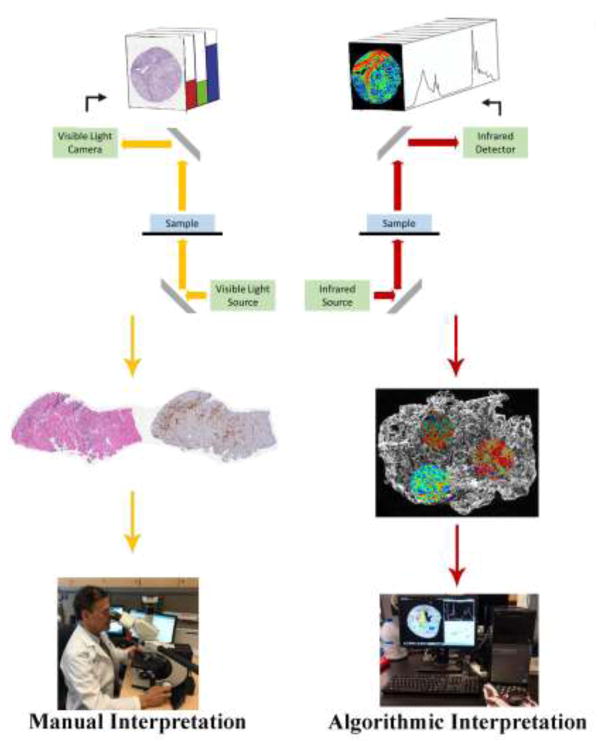
Histopathological analysis of tissue biopsy is the gold standard in disease diagnosis, relying on tissue staining to enhance contrast for light microscopy, followed by morphological pattern recognition by a trained observer (Fig. 1). While it is essential in clinical and research activities, histopathologic identification is limited by inherent operator variability and the currently available dyes and immunohistochemical techniques. Mid-infrared (IR) spectroscopic imaging (Fig. 1), an analytical modality based on the evaluation of variations in molecular vibrations, is an emerging technique that can complement current techniques for tissue analysis to offer new diagnostically-relevant information (Baker et al., 2014; Fernandez et al., 2005; Martin et al., 2010).
Fig 1.
Schematic depicting the difference between conventional histopathology and infrared spectroscopic imaging of tissue sections. Conventional pathology evaluation requires chemical staining (with one tissue section per stain), light microscopy imaging, and manual interpretation by a trained pathologist. Infrared spectroscopic imaging evaluates the biochemical characteristics of a single tissue section without the need for stains, dyes, or a trained observer.
IR imaging is a label-free chemical imaging technique that is non-destructive, and compatible with the standard clinical workflow for tissue sample preparation (Fernandez et al., 2005). Protocols have been well established for both frozen and formalin-fixed paraffin-embedded specimens. These sections must be placed onto IR-compatible slides, but may be stained following imaging and used for conventional histopathology (Baker et al., 2014).
The basis of IR spectroscopy is the measurement of varied absorption across the IR spectrum as a function of the vibrational modes of the biomolecules present within a sample. This allows for study of the molecular composition of a specimen, which will vary with tissue identity and disease state, without perturbing or altering the sample itself. In tissue, the spectral regions of interest are the fingerprint region (600–1,450 cm−1), the amide I and amide II (amide I/II) region (1,500–1,700 cm−1) and the higher-wavenumber region (2,550–3,500 cm−1). The lower-wavenumber region is associated with bending and carbon skeleton fingerprint vibrations such as C-H, C-N, N-H, O-H and P-O, while the higher-wavenumber region is associated with stretching vibrations (Baker et al., 2014).
2. Instrumentation and imaging
IR imaging combines the chemical analysis capabilities of IR spectroscopy with the appropriate optical apparatus to provide a microscopy technique that couples spatial information to the spectral data. Developing this capacity in a benchtop setting required advances in infrared sources, detectors and sampling techniques, which together revolutionized IR microspectroscopic imaging. At present, IR imaging is capable of wide-field scanning of a sample in seconds, generating tens of thousands of spectra associated with each pixel in the resulting dataset (Baker et al., 2014; Martin et al., 2010).
Multiple mid-IR imaging modalities are now available, differing in their illumination sources, detectors, and imaging modes. Traditional Fourier transform infrared (FT-IR) systems utilize a broadband source and a Michaelson interferometer to obtain data that is mathematically converted into a spectrum via the Fourier transform. Commercially-available FT-IR systems relying on a low-intensity thermal globar wire source and utilize highly sensitive infrared sensitive detectors. Liquid nitrogen-cooled focal plane array (FPA) detectors are now widely available for rapid imaging (Baker et al., 2014). Synchrotrons offer high-intensity broadband sources for FT-IR imaging, but are rendered impractical for clinical applications by size constraints (Nasse et al., 2011). Quantum cascade lasers (QCL) provide a new high-intensity source for IR imaging, and these QCL-IR systems have unique features as compared to FT-IR because of their capacity for discrete-frequency imaging (Bird and Rowlette, 2017; Pilling et al., 2017; Sreedhar et al., 2016; Tiwari et al., 2016).
Each of these systems has its own advantages and limitations, but all serve to facilitate IR imaging of tissue samples. Samples can be interrogated through transmission imaging (on expensive CaF2 or BaF2 slides), reflection imaging (on inexpensive IR-reflective low-emissivity glass slides), or attenuated total reflection (ATR) imaging (Baker et al., 2014). These modalities can acquire IR images that can approach one micron in resolution depending on wavelength and optics (Nasse et al., 2011; Reddy et al., 2013). Whatever the system and imaging modality used, the product of IR imaging of a tissue sample is a data cube, consisting of the absorption profile at every wavenumber tied to the 2-dimensional spatial information of the sample. The spatial information can be correlated to histopathology information from adjacent serial sections, or from the IR sample itself if it is stained following imaging. This dataset can then be analyzed through a diverse array of techniques, by selectively extracting spectra from features of interest within the tissue being studied (Bird and Rowlette, 2017; Martinez-Marin et al., 2016; Sreedhar et al., 2016).
3. Fibrosis as the target region
One such tissue feature that has recently attracted interest in the IR imaging community is areas of stroma or fibrosis (Bird and Rowlette, 2017; Pilling et al., 2017; Sreedhar et al., 2016; Tiwari et al., 2016). Early IR investigations of tissue samples tended to focus on cancers and other disease processes that centered on epithelia and parenchyma. However, the surrounding regions of stroma, or the reactive areas of fibrosis that accompany many disease processes have now begun to attract attention, both as pathologically significant areas in their own right and as interdependent partners in complex disease processes.
The first step in analyzing these regions of stroma and fibrosis lies in characterizing one of their chief components: collagen. This ubiquitous protein-based structural element of tissue would naturally contribute greatly to the IR absorption signatures of connective tissue regions. Differences in the collagen composition (and collagen types) would be expected to contribute to differences in spectroscopic characteristics, which would translate to pathology-specific differences between areas of stroma and fibrosis (Belbachir et al., 2009; Bird and Rowlette, 2017; Tiwari et al., 2016).
Early work in this area focused on characterizing bovine and human collagen spectra, and showed that several different collagen types could be distinguished on the basis of FT-IR data (Belbachir et al., 2009; Petibois et al., 2006). Other investigations have searched for the spectral signatures of collagen within human tissues in such diverse contexts as gliomas (Noreen et al., 2012), cardiomyopathy (Gough et al., 2003; Wang et al., 2005), myocardial infarction (Cheheltani et al., 2012), wound healing (Wiens et al., 2007), and articular cartilage (Saarakkala et al., 2010). It has also been suggested that serum can be measured using FT-IR as a surrogate for hepatic fibrosis (Scaglia et al., 2011).
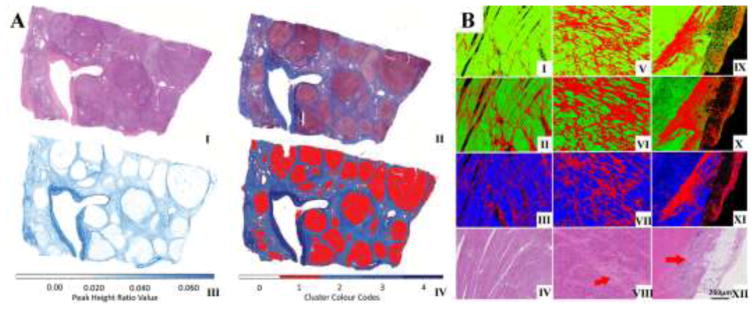
The influence of collagen in these tissue components means that regions of stroma and fibrosis have very different IR signatures than other features such as epithelia. As a result, this facilitates the segmentation of such regions from other tissue components. An example is seen in the virtual reproduction of the Masson trichrome stain (Fig. 2A) to highlight collagen-rich regions on the basis of FT-IR data alone. In a similar capacity, applications of IR imaging toward assessing cardiac transplant rejection (Fig. 2B) have also used the segmentation of fibrosis as part of their approach (Tiwari et al., 2016). The distribution of collagen deposition could also be visualized in cardiac tissue using the 1338cm−1 wavenumber (Cheheltani et al., 2012). Recent advances in high resolution IR imaging have also allowed access to stromal compartments that could previously not be visualized using IR imaging such as intralobular stroma in breast tissues (Nasse et al., 2011; Walsh et al., 2012).
Fig 2.
A) Infrared and corresponding histology images of liver (Bird and Rowlette, 2017). (I) Bright field images of H&E stained and (II) Masson’s trichrome stained liver biopsy tissue sections show the fibrotic area in the tissue. The same fibrotic regions are identified with QCL-IR discrete frequency imaging by using (III) an image of peak height ratio of 1232 cm−1 to 1336 cm−1 and (IV) a k-Means clustering image. B) Heart transplant (Tiwari et al., 2016) tissue sections with abundant fibrosis showing classification of cardiac tissue into myocardium (green or blue) or fibrosis (red) on the basis of FT-IR spectral data (I, V, IX), QCL-IR discrete frequency spectral data (II, VI, X), and only the peak height ratio of 1236 cm−1 to 1542 cm−1 from QCL IR (III, VII, XI). Corresponding H&E stained images with red arrows identifying prominent areas of fibrosis (IV, VIII, XII).
Identifying and isolating these regions allows for their spectral characteristics to be individually analyzed in tissue samples, leading to the next step, of using these data to find fibrotic changes that relate to disease processes of interest. One such connection between alterations in IR signatures and disease processes is found in diabetic changes in regions of fibrous scarring in the liver. Fibrosis is commonly seen in the progression of liver disease following multiple repeated injuries to the tissue, and QCL-IR imaging has demonstrated a robust capacity to isolate and analyze such regions on the basis of a few wavenumbers, as illustrated in Fig. 2A (Bird and Rowlette, 2017). As diabetes mellitus is a disease with metabolic implications that lead to changes in multiple organ systems, it is not surprising that some of these alterations are found in liver fibrosis. QCL-IR data has demonstrated differences between fibrotic spectra in liver samples from diabetic and nondiabetic patients spanning the range of hepatic pathology from cirrhosis to hepatocellular carcinoma (Sreedhar et al., 2016). In the context of renal pathology, FT-IR has been able to accurately detect and classify fibrosis, which is a hallmark of kidney damage (Vuiblet et al., 2015).
In addition to offering indications of systemic disease processes, the IR signatures of fibrotic regions can also offer information about what is occurring in other components of the same tissue sample, such as epithelium. These relationships arise from the fact that stromal or abnormal fibrotic regions are often the surrounding environment in which pathologic processes of the epithelium take place. For example, FT-IR investigations of co-culture of fibroblasts with breast cancer cell lines highlight the interaction between the former and the latter (Holton, S. et al., 2011; Holton et al., 2014; Holton, S.E. et al., 2011). QCL investigation of liver biopsies suggested that the spectral signatures from fibrotic regions varied between patients with no dysplasia or low grade dysplasia, and those with high-grade dysplasia or hepatocellular carcinoma (Sreedhar et al., 2016). The chemistry of the stroma in breast tissue was shown to be altered in the presence of cancer using both FT-IR imaging (Kumar et al., 2013) and more recently with QCL imaging (Pilling et al., 2017).
Ultimately, the goal of exploring areas of fibrosis with IR imaging is to obtain new information which was unavailable through current histopathology methods. For example, stromal IR features, rather than epithelium, may offer improved prediction of prostate cancer recurrence over clinical tools such as the CAPRA-S score or Kattan nomograms (Kwak et al., 2015). This finding represents novel information, with potential clinical impact, derived through stromal FT-IR analysis which is not known to be accessible to any other modality (Kwak et al., 2015).
4. Challenges and future directions
IR imaging is an attractive technique that can give additional information about the biochemical status of tissues without the need for stains or labels. There is a wealth of literature on using these techniques for extracting biomarkers from cell types directly implicated in diseases processes, principally epithelial cells. Emerging evidence demonstrates the crucial role of the adjacent stromal and fibrotic regions in cancer development (Hanahan and Weinberg, 2011). These areas represent a novel target for IR imaging which could provide new insight for patient diagnosis and prognosis.
It has been shown that IR imaging can rapidly map areas of fibrosis (Cheheltani et al., 2012; Sreedhar et al., 2016; Vuiblet et al., 2015) but new studies demonstrate that additional information also resides within these regions (Kwak et al., 2015). Stromal regions may also permit detection of changes distal to the epithelium (Kumar et al., 2013; Pilling et al., 2017) that remain unaffected by the heterogeneity in the diseased cells; this is useful as that heterogeneity can often interfere with precise diagnosis (Sreedhar et al., 2016). The exploration of the biochemistry of stromal regions remains largely untapped with future research required to determine whether these areas hold useful and clinically actionable information. Recently, it was demonstrated that interrogating regions of fibrosis in the setting of thyroid carcinoma was not able to discriminate between two types of cancer whereas the epithelial cells could (Martinez-Marin et al., 2016), demonstrating that these regions do not always hold diagnostic value. Future work must focus on adapting IR imaging technology for clinical implementation to provide useful answers to clinicians, and delve deeper into the nature of the precise stromal components contributing to diseased IR signatures.
Highlights.
Infrared (IR) spectroscopy provides chemical information about a sample based on how the chemical bonds absorb infrared radiation at different wavelengths – an infrared absorption spectrum.
Infrared spectroscopic tissue imaging combines IR spectroscopy with microscopy to yield an image of a tissue sample that is coupled to a biochemical signature (the IR spectrum) for each of its pixels.
Areas of stroma and fibrosis in tissue samples provide new targets for acquiring information relevant to disease processes.
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases [grant number R21DK103066].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker MJ, Trevisan J, Bassan P, Bhargava R, Butler HJ, Dorling KM, Fielden PR, Fogarty SW, Fullwood NJ, Heys KA. Using Fourier transform IR spectroscopy to analyze biological materials. Nature protocols. 2014;9(8):1771–1791. doi: 10.1038/nprot.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belbachir K, Noreen R, Gouspillou G, Petibois C. Collagen types analysis and differentiation by FTIR spectroscopy. Analytical and bioanalytical chemistry. 2009;395(3):829–837. doi: 10.1007/s00216-009-3019-y. [DOI] [PubMed] [Google Scholar]
- Bird B, Rowlette J. A protocol for rapid, label-free histochemical imaging of fibrotic liver. Analyst. 2017;142(8):1179–1184. doi: 10.1039/c6an02080a. [DOI] [PubMed] [Google Scholar]
- Cheheltani R, Rosano JM, Wang B, Sabri AK, Pleshko N, Kiani MF. Fourier transform infrared spectroscopic imaging of cardiac tissue to detect collagen deposition after myocardial infarction. Journal of biomedical optics. 2012;17(5):0560141–0560149. doi: 10.1117/1.JBO.17.5.056014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DC, Bhargava R, Hewitt SM, Levin IW. Infrared spectroscopic imaging for histopathologic recognition. Nature biotechnology. 2005;23(4):469–474. doi: 10.1038/nbt1080. [DOI] [PubMed] [Google Scholar]
- Gough KM, Zelinski D, Wiens R, Rak M, Dixon IM. Fourier transform infrared evaluation of microscopic scarring in the cardiomyopathic heart: effect of chronic AT 1 suppression. Analytical biochemistry. 2003;316(2):232–242. doi: 10.1016/s0003-2697(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Holton S, Walsh M, Kajdacsy-Balla A, Bhargava R. Label-free characterization of cancer-activated fibroblasts using infrared spectroscopic imaging. Biophysical journal. 2011;101(6):1513–1521. doi: 10.1016/j.bpj.2011.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton SE, Bergamaschi A, Katzenellenbogen BS, Bhargava R. Integration of molecular profiling and chemical imaging to elucidate fibroblast-microenvironment impact on cancer cell phenotype and endocrine resistance in breast cancer. PLoS One. 2014;9(5):e96878. doi: 10.1371/journal.pone.0096878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton SE, Walsh MJ, Bhargava R. Subcellular localization of early biochemical transformations in cancer-activated fibroblasts using infrared spectroscopic imaging. Analyst. 2011;136(14):2953–2958. doi: 10.1039/c1an15112f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Desmedt C, Larsimont D, Sotiriou C, Goormaghtigh E. Change in the microenvironment of breast cancer studied by FTIR imaging. Analyst. 2013;138(14):4058–4065. doi: 10.1039/c3an00241a. [DOI] [PubMed] [Google Scholar]
- Kwak JT, Kajdacsy-Balla A, Macias V, Walsh M, Sinha S, Bhargava R. Improving prediction of prostate cancer recurrence using chemical imaging. Scientific reports. 2015:5. doi: 10.1038/srep08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin FL, Kelly JG, Llabjani V, Martin-Hirsch PL, Patel II, Trevisan J, Fullwood NJ, Walsh MJ. Distinguishing cell types or populations based on the computational analysis of their infrared spectra. Nature Protocols. 2010;5(11):1748–1760. doi: 10.1038/nprot.2010.133. [DOI] [PubMed] [Google Scholar]
- Martinez-Marin D, Sreedhar H, Varma VK, Eloy C, Sobrinho-Simões M, Kajdacsy-Balla A, Walsh MJ. Accounting for tissue heterogeneity in infrared spectroscopic imaging for accurate diagnosis of thyroid carcinoma subtypes. Vibrational Spectroscopy. 2016 doi: 10.1016/j.vibspec.2016.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasse MJ, Walsh MJ, Mattson EC, Reininger R, Kajdacsy-Balla A, Macias V, Bhargava R, Hirschmugl CJ. High-resolution Fourier-transform infrared chemical imaging with multiple synchrotron beams. Nature methods. 2011;8(5):413–416. doi: 10.1038/nmeth.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreen R, Moenner M, Hwu Y, Petibois C. FTIR spectro-imaging of collagens for characterization and grading of gliomas. Biotechnology advances. 2012;30(6):1432–1446. doi: 10.1016/j.biotechadv.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Petibois C, Gouspillou G, Wehbe K, Delage JP, Déléris G. Analysis of type I and IV collagens by FT-IR spectroscopy and imaging for a molecular investigation of skeletal muscle connective tissue. Analytical and bioanalytical chemistry. 2006;386(7–8):1961–1966. doi: 10.1007/s00216-006-0828-0. [DOI] [PubMed] [Google Scholar]
- Pilling MJ, Henderson A, Gardner P. Quantum Cascade Laser Spectral Histopathology: Breast Cancer Diagnostics Using High Throughput Chemical Imaging. Analytical Chemistry. 2017 doi: 10.1021/acs.analchem.7b00426. [DOI] [PubMed] [Google Scholar]
- Reddy RK, Walsh MJ, Schulmerich MV, Carney PS, Bhargava R. High-Definition Infrared Spectroscopic Imaging. Appl Spectrosc. 2013;67(1):93–105. doi: 10.1366/11-06568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarakkala S, Rieppo L, Rieppo J, Jurvelin J. Fourier transform infrared (FTIR) microspectroscopy of immature, mature and degenerated articular cartilage. Microscopy: science, technology, applications and education. 2010;1:403–414. [Google Scholar]
- Scaglia E, Sockalingum GD, Schmitt J, Gobinet C, Schneider N, Manfait M, Thiéfin G. Noninvasive assessment of hepatic fibrosis in patients with chronic hepatitis C using serum Fourier transform infrared spectroscopy. Analytical and bioanalytical chemistry. 2011;401(9):2919–2925. doi: 10.1007/s00216-011-5402-8. [DOI] [PubMed] [Google Scholar]
- Sreedhar H, Varma VK, Gambacorta FV, Guzman G, Walsh MJ. Infrared spectroscopic imaging detects chemical modifications in liver fibrosis due to diabetes and disease. Biomedical optics express. 2016;7(6):2419–2424. doi: 10.1364/BOE.7.002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari S, Raman J, Reddy V, Ghetler A, Tella RP, Han Y, Moon CR, Hoke CD, Bhargava R. Towards Translation of Discrete Frequency Infrared Spectroscopic Imaging for Digital Histopathology of Clinical Biopsy Samples. Analytical chemistry. 2016;88(20):10183–10190. doi: 10.1021/acs.analchem.6b02754. [DOI] [PubMed] [Google Scholar]
- Vuiblet V, Fere M, Gobinet C, Birembaut P, Piot O, Rieu P. Renal Graft Fibrosis and Inflammation Quantification by an Automated Fourier–Transform Infrared Imaging Technique. Journal of the American Society of Nephrology, ASN. 2015 doi: 10.1681/ASN.2015050601. 2015050601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MJ, Holton SE, Kajdacsy-Balla A, Bhargava R. Attenuated total reflectance Fourier-transform infrared spectroscopic imaging for breast histopathology. Vibrational Spectroscopy. 2012;60:23–28. doi: 10.1016/j.vibspec.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sanad W, Miller LM, Voigt A, Klingel K, Kandolf R, Stangl K, Baumann G. Infrared imaging of compositional changes in inflammatory cardiomyopathy. Vibrational spectroscopy. 2005;38(1):217–222. [Google Scholar]
- Wiens R, Rak M, Cox N, Abraham S, Juurlink BH, Kulyk WM, Gough KM. Synchrotron FTIR microspectroscopic analysis of the effects of anti-inflammatory therapeutics on wound healing in laminectomized rats. Analytical and bioanalytical chemistry. 2007;387(5):1679–1689. doi: 10.1007/s00216-006-1095-9. [DOI] [PubMed] [Google Scholar]