Abstract
Objective
We used complete-linkage cluster analysis to identify healthy subpopulations with distinct responses to continuous theta-burst stimulation (cTBS).
Methods
21 healthy adults (age±SD, 36.9±15.2 years) underwent cTBS of left motor cortex. Natural log-transformed motor evoked potentials (LnMEPs) at 5–50 minutes post-cTBS (T5–T50) were calculated.
Results
Two clusters were found; Group 1 (n=12) that showed significant MEP facilitation at T15, T20, and T50 (p’s<.006), and Group 2 (n=9) that showed significant suppression at T5–T15 (p’s<.022). LnMEPs at T10 and T40 were best predictors of, and together accounted for 80% of, cluster assignment.
In an exploratory analysis, we examined the roles of brain-derived neurotrophic factor (BDNF) and apolipoprotein E (APOE) polymorphisms in the cTBS response. Val66Met participants showed greater facilitation at T10 than Val66Val participants (p=.025). BDNF and cTBS intensity predicted 59% of interindividual variability in LnMEP at T10. APOE did not significantly affect LnMEPs at any time point (p’s>.32).
Conclusions
Data-driven cluster analysis can identify healthy subpopulations with distinct cTBS responses. T10 and T40 LnMEPs were best predictors of cluster assignment. T10 LnMEP was influenced by BDNF polymorphism and cTBS intensity.
Significance
Healthy adults can be sorted into subpopulations with distinct cTBS responses that are influenced by genetics.
Keywords: transcranial magnetic stimulation, continuous theta-burst stimulation, plasticity, interindividual variability, cluster analysis, BDNF
1. Introduction
Transcranial magnetic stimulation (TMS) is a form of noninvasive brain stimulation through electromagnetic induction. TMS was originally developed as a neurophysiological tool to investigate the integrity of corticospinal pathways in humans (Barker et al., 1985). When applied within the recommended guidelines (Rossi et al., 2009; Rossini et al., 2015), TMS provides a safe means to trigger or modulate neural activity. A single TMS pulse applied to the primary motor cortex (M1) can generate a compound muscle action potential in a target muscle, referred to as the motor evoked potential (MEP). Various TMS protocols have been designed to study neural processes, including plasticity, in the motor and non-motor systems by applying single, paired, or repetitive TMS pulses at specific intensities and frequencies to one or more cortical areas.
Theta burst stimulation (TBS) is a form of repetitive TMS developed more than ten years ago (Huang et al., 2005). TBS was originally conceived based on the 4–7 Hz burst discharge (the theta range in electroencephalography) recorded from the hippocampus of rats during exploratory behavior (Diamond et al., 1988) and used to study synaptic plasticity in animal brain slices (Larson and Lynch, 1986, 1989; Capocchi et al., 1992). TBS consists of 50Hz bursts of three TMS pulses repeated every 200ms (at 5 Hz), for a total of 600 pulses, in one of two protocols: (1) a 2-sec on, 8-sec off intermittent TBS (iTBS) pattern for 190 sec, which in most individuals increases MEP amplitude by approximately 35% for up to 60 min, or (2) a continuous TBS (cTBS) pattern for 40 sec, which can reduce MEP amplitude by approximately 25% for up to 50 min (Wischnewski and Schutter, 2015). Suppression of MEPs by cTBS and their enhancement by iTBS are considered indices of long-term depression- (LTD-) and long-term potentiation- (LTP-) like mechanisms, respectively (Huang et al., 2005; Huerta and Volpe, 2009). Once MEP amplitudes have been altered by cTBS, the time it takes for MEP amplitudes to return to their baseline levels is considered a neurophysiologic index of the mechanisms of cortical plasticity (Oberman et al., 2010; Pascual-Leone et al., 2011; Wischnewski and Schutter, 2015; Suppa et al., 2016).
Application of cTBS to M1 and other brain areas has been used to measure abnormalities in cortical plasticity and to assess therapeutic responses to interventions aimed at restoring normal cortical plasticity in several neurological and psychiatric disorders, including Alzheimer’s disease (Freitas et al., 2011a), autism spectrum disorders and fragile X syndrome (Oberman et al., 2010, 2012, 2014, 2016), dementia (Cantone et al., 2014), epilepsy (Carrette et al., 2016), essential tremor (Chuang et al., 2014), hemispatial neglect (Cazzoli et al., 2012; Koch et al., 2012), major depression (Li et al., 2014), multiple sclerosis (Mori et al., 2013), obsessive-compulsive disorders (Wu et al., 2010; Suppa et al., 2014), Parkinson’s disease (Koch et al., 2009), schizophrenia (Poulet et al., 2009; Eberle et al., 2010; McClintock et al., 2011), stroke (Ackerley et al., 2010; Hsu et al., 2012; Di Lazzaro et al., 2013, 2016), tinnitus (Forogh et al., 2014), and Tourette syndrome (Suppa et al., 2014).
Despite the numerous TBS studies conducted among clinical populations, there is large interindividual variability in TBS response among healthy individuals that remains largely unexplained (Hamada et al., 2013; Hinder et al., 2014; López-Alonso et al., 2014). Given such high interindividual variability, it has been estimated that in order to reliably detect a 20% difference in M1 TBS response between two groups, each group may need to have at least 30 participants (Suppa et al., 2016), which is a larger sample size than used in most previous TBS studies (Wischnewski and Schutter, 2015). The large interindividual variability in TBS response among healthy individuals and, consequently, the relatively large sample sizes required to detect a meaningful difference, can limit the utility of TBS in the assessment of mechanisms of plasticity in healthy individuals and patients with neuropsychiatric disorders.
Several factors have been suggested as potential contributors to the interindividual variability in response to TBS, including the activated intracortical networks (Hamada et al., 2013), functional connectivity in the motor system (Nettekoven et al., 2014, 2015), state-dependent factors (Suppa et al., 2016), and single-nucleotide polymorphisms (SNPs) that can influence neuroplasticity.
Brain-derived neurotrophic factor (BDNF) is the most abundantly available protein of the neurotrophine family (Allen and Dawbarn, 2006) and critically involved in N-methyl-D-aspartate (NMDA)-type glutamate receptor-dependent LTP (Figurov et al., 1996) and LTD (Woo et al., 2005). A frequent BDNF polymorphism (Val66Met) influences the intracellular trafficking and packaging of the precursor peptide (pro-BDNF), which is associated with LTD, and the regulated secretion of the mature (m)BDNF, involved in LTP (Egan et al., 2003; Bramham and Messaoudi, 2005). Several studies have shown effects of BDNF polymorphism on neuroplasticity in humans, including reduced hippocampal plasticity and activity-dependent secretion of BDNF (Egan et al., 2003), reduced training-dependent facilitation of MEPs (Kleim et al., 2006; Lee et al., 2013), reduced cTBS-induced suppression (Cheeran et al., 2008) and iTBS-induced facilitation of MEPs (Cheeran et al., 2008; Antal et al., 2010; Lee et al., 2013; Di Lazzaro et al., 2015), reduced plasticity induced by paired associative stimulation (Cirillo et al., 2012), and reduced rTMS-induced motor recovery after stroke (Chang et al., 2014).
Apolipoprotein E (APOE) codes for a protein component of triglyceride-rich lipoproteins and is an important factor in cholesterol metabolism (Mahley, 1988). APOE has three major alleles (ε2, ε3, and ε4), and the presence of its ε4 allele is a major risk factor for Alzheimer’s disease (AD; Poirier et al., 1993; Saunders et al., 1993). Functional consequences of the presence of APOE ε4 in the central nervous system include poor clinical outcome after acute head trauma and stroke (Mahley and Rall Jr, 2000), reduced neuronal and hippocampal plasticity (White et al., 2001; Nichol et al., 2009), greater impairment in episodic memory among AD patients (Wolk et al., 2010), and differential patterns of rTMS-induced activation (Peña-Gomez et al., 2012).
To investigate the contributors to interindividual variability in TBS response without unfeasibly large sample sizes, one option may be to use statistical approaches such as cluster analyses (Kaufman and Rousseeuw, 2009; Rencher and Christensen, 2012). Due to their data-driven nature, cluster analyses can identify subpopulations of individuals with distinct patterns of response to TBS in a manner that is minimally biased by a priori hypotheses. The resulting subpopulations can then be compared against each other in terms of potentially important predictors. Identifying subpopulations that are more similar in their TBS response can increase the power of TBS studies that investigate differences between healthy individuals and clinical populations. In the present study, we examined the utility of cluster analysis, in the form of complete-linkage cluster analysis, for identification of subpopulations of healthy individuals with distinct patterns of response to cTBS.
As an exploratory analysis, we also assessed the effects of BDNF and APOE polymorphisms on interindividual variability in cTBS-induced plasticity. We did not set out to determine which genetic variants (from among numerous plausible genes) are associated with a particular trait, disease, or outcome measure. Rather, we aimed to test the specific hypothesis that these two well-characterized genetic polymorphisms described in signalling pathways that mediated cortical plasticity (Kleim et al., 2006; Cheeran et al., 2008; Antal et al., 2010; Li Voti et al., 2011; Cirillo et al., 2012; Peña-Gomez et al., 2012; Witte et al., 2012; Lee et al., 2013; Chang et al., 2014; Di Lazzaro et al., 2015) also contributed to the interindividual variability in response to cTBS. Since certain clinical populations, including individuals with Alzheimer’s disease, autism spectrum disorders, schizophrenia, and type-2 diabetes show TBS-induced hyper- or hypoplasticity (Freitas et al., 2011a; McClintock et al., 2011; Oberman et al., 2012; Fried et al., 2017), examining the effects of these polymorphisms on cTBS-induced plasticity would allow for comparing them between healthy individuals and clinical populations in the future.
2. Methods
2.1. Participants
21 healthy adults participated in the study that was conducted in accordance with the Declaration of Helsinki and was approved by the local Institutional Review Board. All participants provided written informed consent prior to enrollment and received monetary compensation upon completion. None of the participants had a history of medical disease or contraindication to TMS, and all of them had normal physical and neurological examinations.
Participants were predominantly male (19 out of 21) because they had been recruited as neurotypical controls in a larger ongoing study that involved individuals with ASD who were predominantly male. To maintain comparability in gender ratio between the two groups, it was necessary to have predominantly male participants in the neurotypical group as well.
2.2. Neuropsychological testing
Mini-Mental State Examination (MMSE) (Folstein et al., 1975; Crum et al., 1993) and the Abbreviated Battery of Stanford-Binet IV intelligence scale, consisting of Verbal Knowledge and Nonverbal Fluid Reasoning subscores (Thorndike et al., 1986) were conducted.
2.3. Genetic analyses
Saliva samples from 18 out of the 21 participants were used to assess brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and the presence of apolipoprotein-E (APOE) ε4 allele. Two participants did not consent to providing DNA samples and one sample was deemed unusable.
Aliquot (700μL) extraction of genomic DNA was performed on saliva samples collected using the Oragene Discover OGR-250 Kit (DNA Genotek Inc., Ottawa, ON, Canada). DNA was extracted from samples using standard methodology and the prepIT•L2P reagent (DNA Genotek Inc., 2015). The following quality control metrics were performed on each sample: PicoGreen fluorometry for double stranded DNA quantification, Nanodrop spectrophotometry as an estimate of sample purity using A260/A280 ratios and agarose gel electrophoresis for visualization of DNA integrity. The rs6265 SNP of the BDNF gene, the rs429358 and the rs7412 SNPs of the APOE gene were analyzed using a TaqMan single tube genotyping assay, which uses polymerase chain reaction (PCR) amplification and a pair of fluorescent dye detectors that target the SNP. One fluorescent dye is attached to the detector that is a perfect match to the first allele and a different fluorescent dye is attached to the detector that is a perfect match to the second allele. During PCR, the polymerase releases the fluorescent probe into solution where it is detected using endpoint analysis in an Applied Biosystems, Inc. (Foster City, CA, USA) 7900HT Real-Time instrument. Primers and probes were also obtained through Applied Biosystems.
Exact tests for deviations of BDNF and APOE SNPs from Hardy-Weinberg equilibrium (HWE) proportions (Guo and Thompson, 1992; Wigginton et al., 2005) were conducted in Python for Population Genomics (PyPop) software version 0.7.0 (Lancaster et al., 2003, 2007), implementing a Markov chain Monte-Carlo (MCMC) with 2000 dememorization steps, 1000 chain samples, and a chain sample size of 1000 (for a total of 1,000,000 steps).
The Ewens–Watterson homozygosity tests of neutrality (Ewens, 1972; Watterson, 1978) were conducted in PyPop using the Slatkin’s implementation (Slatkin, 1994). Fisher’s exact tests were used to compare the minor allele frequencies of rs6265 (BDNF), rs429358 (APOE), and rs7412 (APOE) SNPs against the corresponding frequencies in the admixed American (AMR) population in the 1000 Genomes Project (1KGP) (Auton et al., 2015), i.e., 0.1527, 0.1037, and 0.0476, respectively.
2.4. Transcranial magnetic stimulation
Participants were seated in a comfortable chair with the right arm and hand in a pronated position on a pillow. They were instructed to keep their right hand as still and relaxed as possible throughout the experiment.
All study parameters followed the current guidelines for the safe application of TMS recommended by the International Federation of Clinical Neurophysiology (Rossi et al., 2009; Rossini et al., 2015). Single TMS pulses and cTBS were applied to the left primary motor cortex as biphasic pulses with the current flowing in the brain with antero-posterior and then postero-anterior (AP-PA) direction by a MagPro X100 stimulator (with the current direction set to ‘Normal’) and a MC-B70 Butterfly Coil (outer diameter: 97mm; MagPro, MagVenture A/S, Farum, Denmark). Note that this direction is opposite to most commercial rTMS stimulators. The coil was held tangentially to the participant’s head surface, with the handle pointing occipitally and positioned at 45° relative to the mid-sagittal axis of the participant’s head. With this orientation, the induced electric current flows perpendicular to the central sulcus and results in achieving the lowest motor threshold. The optimal spot for the maximal responses of the right first dorsal interosseous muscle (FDI) was localized. A Polaris infrared-optical tracking system (Northern Digital Inc., Waterloo, ON, Canada) and a Brainsight TMS neuronavigation system (Rogue Research Inc., Montreal, QC, Canada) with a brain MRI template was used to ensure consistent targeting throughout the experiment.
To collect electromyogram (EMG) signal, two surface electrodes were placed on the belly and on the tendon of the right FDI and connected to a PowerLab 4/25T data acquisition device (ADInstruments, Colorado Springs, CO, USA). The TMS system delivered triggered pulses that synchronized the TMS and EMG systems. EMG data were digitized at 1 kHz for 500 ms following each stimulus trigger and 100 ms pre-trigger, amplified with a range of ± 10 mV (band-pass filter 0.3–1000 Hz). Peak-to-peak MEP amplitude of the non-rectified signal was calculated on individual waveforms using LabChart 8 software (ADInstruments, Colorado Springs, CO, USA). Live EMG was monitored in order to maintain hand relaxation throughout the experiment. The participants were also monitored for drowsiness and asked to keep their eyes open throughout the experiment.
Resting motor threshold (RMT) and active motor threshold (AMT) were measured individually and used to set the intensity of subsequent stimulation. RMT was defined as the lowest intensity of stimulation that elicited motor evoked potentials (MEPs) ≥ 50 μV in at least five of ten pulses in the relaxed right FDI, and AMT was defined as the lowest intensity that elicited MEPs ≥ 200 μV in at least five of ten pulses with the FDI slightly contracted.
Single TMS pulses were applied at 120% of individual RMT and were separated by a random 4–6 s interval. The cTBS protocol consisted of bursts of three pulses of 50Hz stimulation at 80% of individual AMT, repeated at 200ms inter-burst intervals for 40 seconds (for a total of 600 pulses). This protocol has been shown to inhibit cortico-motor reactivity for up to 50 minutes in healthy individuals (Huang et al., 2005; Oberman et al., 2010, 2012, 2016; Wischnewski and Schutter, 2015). Prior to cTBS, participants received three blocks of 30 single TMS pulses. There was a 5–min interval between the onsets of successive blocks. The peak-to-peak amplitude of each MEP was measured and averaged for each participant as a measure of baseline cortico-motor reactivity. To control the effects of voluntary hand movements on cTBS aftereffects (Iezzi et al., 2008), there was a 5-min break between the AMT measurement and the onset of cTBS, during which participants were instructed to maintain hand relaxation.
Following cTBS, cortico-motor reactivity was reassessed in blocks of 30 single TMS pulses at 5, 10, 15, 20, 30, 40, and 50 minutes (T5, T10, etc.). The timing of each block was centered on the time point of interest. For each block, individual MEPs > 2.5 SD from the mean were excluded.
2.5 Statistical analyses
Stata software version 13.1 (StataCorp, College Station, TX, USA) was used for statistical analyses. TMS data included two motor thresholds (RMT and AMT), assessed in terms of the percent of maximum stimulator output, one measure of baseline cortico-motor reactivity (average baseline MEP amplitude), and seven post-cTBS time-points (T5–T50), with the average amplitude of MEPs at each time-point normalized by forming a ratio of MEP amplitudes after TBS relative to the average baseline MEP amplitude for each participant. The Shapiro–Wilk test found significant deviations from normal distribution; thus, natural log-transformed data were used for statistical analyses. Log-transformed, baseline-corrected MEP amplitudes at each of the seven post-cTBS time points (LnMEP) were averaged over all participants. Additional analyses were conducted on LnMEP values at T5 and T10 as they commonly exhibit the maximal effect of cTBS (Wischnewski and Schutter, 2015). Additional analyses were also conducted on LnMEP values at T40 and T50 considering the results of some of the analyses. All comparisons of proportions were conducted with Fisher’s exact test. All analyses were two-tailed, and the α level was set to 0.05.
To identify potential subpopulations of healthy adults with distinct patterns of response to cTBS, cluster analyses were performed on the MEP data. In hierarchical agglomerative clustering methods, observations are clustered into larger and larger groups by progressively lowering the threshold for determining whether two or more observations are similar enough to belong to the same group. As more and more observations are grouped together, the dissimilarity among members of each cluster is increased (Rencher and Christensen, 2012).
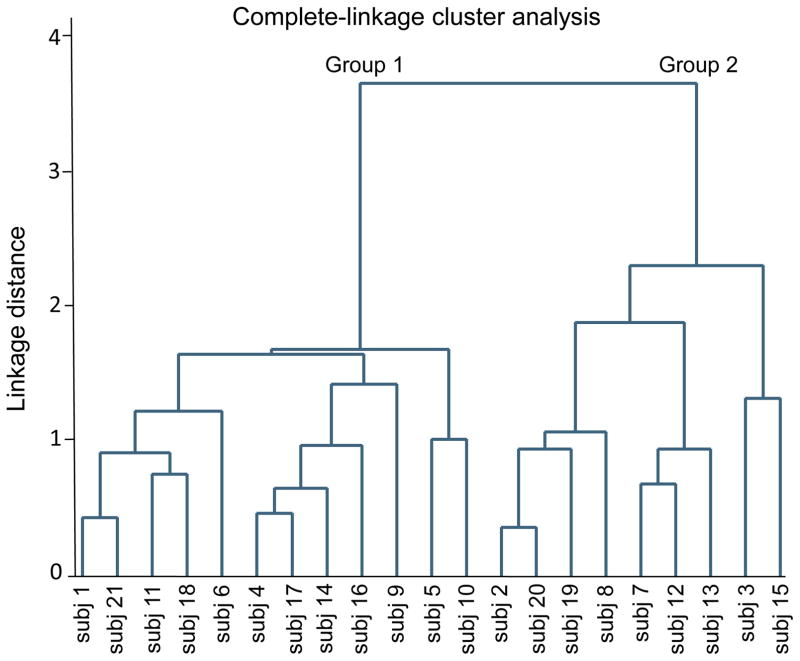
The clustering algorithm results in a dendrogram (or cluster tree) that illustrates which observations are clustered at different levels of dissimilarity (Figure 1). Each observation belongs to its own cluster at the bottom of the hierarchy. Moving up in the dendrogram, observations are linked via horizontal lines to form progressively larger clusters until they are all grouped together at the top of the cluster tree. The length of the vertical lines extending from the observations, as well as the range of the dissimilarity axis, indicate the dissimilarity between the groups (StataCorp, 2013).
Figure 1.
Dendrogram (cluster tree) summarizing the results of complete-linkage cluster analysis of natural log-transformed, baseline-corrected amplitudes of motor evoked potentials at 5 to 50 minutes following continuous theta-burst stimulation (cTBS) of the left primary motor cortex in healthy individuals. The numbers on the abscissa indicate the subject numbers. Linkage distance is defined as the Euclidean distance between the farthest observations in each of the two linked subgroups. The tree structure illustrates two distinct patterns of response to cTBS by 12 individuals in Group 1 and nine individuals in Group 2.
Complete-linkage clustering method, previously used in an rTMS study (Gangitano et al., 2002), calculates the (dis)similarity between every pair of clusters at each step, as measured by the Euclidean distance between the farthest pair of observations in each pair of clusters, and joins the two clusters with the smallest distance from each other (Kaufman and Rousseeuw, 2009; Rencher and Christensen, 2012).
To avoid bias in selecting the time points for the cluster analysis, all time points from T5 through T50 were included in the analysis. A recent meta-analysis found that cTBS aftereffects on average last approximately 50 min among healthy individuals (Wischnewski and Schutter, 2015). LnMEPs at each of the seven post-cTBS time points were averaged separately for each of the two clusters, were compared against zero using one-sample t tests, and were compared against each other using two-sample t-tests. False discovery rate (FDR) was used to adjust p values for multiple testing (Simes, 1986; Benjamini and Hochberg, 1995; Benjamini and Yekutieli, 2001).
To determine the time point(s) at which the LnMEPs had the most discriminatory power, i.e., would best predict the participants’ assignment to one of the clusters, we conducted multiple logistic regression analyses of clusters with LnMEP at each of the seven time points as a predictor and then calculated the pseudo-R2 for each model (McFadden, 1973).
To reduce the probability of failure in identifying important confounding variables (Bendel and Afifi, 1977; Mickey and Greenland, 1989), selecting covariates for potential inclusion in the linear and logistic regression models was based on a p-value cutoff of 0.25 for univariate regression (Bursac et al., 2008). Neurophysiologically important measures including RMT, AMT, and baseline MEP amplitude were always considered for potential inclusion in the model as covariates. RMT and AMT were highly correlated (R21 = 0.67, p < .001) and were never included in the same model to avoid multicollinearity. Given the sample size, up to three predictors were considered for simultaneous inclusion in any regression model.
3. Results
Demographics, single-nucleotide polymorphisms, and neuropsychological measures for individual participants are presented in Table 1. Descriptive statistics for those measures and baseline neurophysiological measures are presented in Table 2.
Table 1.
Participants’ demographics, single-nucleotide polymorphisms, and neuropsychological measures.
| Age (yr) | Gender | Race | Ethnicity | Education* (yr) | BDNF † | APOE † | MMSE | Abbreviated Stanford-Binet IQ | Verbal KN | Nonverbal FR | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participant 01 | 24 | M | Asian | non-Hispanic | 16 | Val/Met | ε3/ε3 | 30 | 100 | 10 | 10 |
| Participant 02 | 26 | M | Other | non-Hispanic | 19 | Val/Val | ε3/ε4 | 30 | 121 | 17 | 10 |
| Participant 03 | 23 | M | Multiracial | Hispanic | 16 | Val/Val | ε3/ε3 | 30 | 106 | 11 | 11 |
| Participant 04 | 56 | F | African-American | non-Hispanic | 14 | - | - | 30 | 97 | 9 | 10 |
| Participant 05 | 47 | M | White | non-Hispanic | 20+ | Val/Val | ε3/ε3 | 30 | 133 | 17 | 14 |
| Participant 06 | 21 | M | Asian | non-Hispanic | 16 | - | - | 30 | 94 | 8 | 10 |
| Participant 07 | 53 | M | White | non-Hispanic | 16 | Val/Val | ε3/ε4 | 30 | 106 | 13 | 9 |
| Participant 08 | 51 | M | White | non-Hispanic | 16 | Val/Val | ε3/ε3 | 30 | 115 | 14 | 11 |
| Participant 09 | 62 | M | White | non-Hispanic | 16 | Val/Met | ε3/ε4 | 30 | 118 | 12 | 14 |
| Participant 10 | 21 | M | White | Hispanic | 15 | Val/Val | ε3/ε4 | 30 | 109 | 14 | 9 |
| Participant 11 | 22 | M | White | Hispanic | 17 | Val/Val | ε2/ε3 | 30 | 112 | 10 | 14 |
| Participant 12 | 32 | M | White | non-Hispanic | 19 | Val/Val | ε3/ε3 | 29 | 100 | 9 | 11 |
| Participant 13 | 28 | M | White | Hispanic | - | Val/Val | ε3/ε3 | 30 | 115 | 13 | 12 |
| Participant 14 | 50 | F | African-American | non-Hispanic | 12 | - | - | 30 | 97 | 10 | 9 |
| Participant 15 | 23 | M | White | non-Hispanic | 17 | Val/Val | ε2/ε3 | 30 | 127 | 16 | 13 |
| Participant 16 | 24 | M | Multiracial | Hispanic | 17 | Val/Met | ε2/ε3 | 28 | 115 | 12 | 13 |
| Participant 17 | 22 | M | Asian | non-Hispanic | 17 | Val/Met | ε3/ε4 | 30 | 103 | 9 | 12 |
| Participant 18 | 47 | M | African-American | non-Hispanic | 18 | Val/Met | ε3/ε3 | 30 | 88 | 9 | 7 |
| Participant 19 | 32 | M | Asian | non-Hispanic | 20+ | Val/Met | ε3/ε4 | 30 | 103 | 8 | 13 |
| Participant 20 | 65 | M | Asian | non-Hispanic | 20+ | Val/Val | ε3/ε4 | 30 | 124 | 15 | 13 |
| Participant 21 | 46 | M | White | non-Hispanic | 13 | Val/Val | ε3/ε4 | 30 | 88 | 9 | 7 |
All participants were right-handed. APOE, apolipoprotein E; BDNF, brain-derived neurotrophic factor; FR, fluid reasoning; IQ, intelligence quotient; KN, knowledge; MMSE, Mini-Mental State Examination. Racial and ethnic categories were defined based on the National Institutes of Health policy and guidelines on the inclusion of women and minorities as subjects in clinical research (NIH Office of Extramural Research, 2001).
Education data were available for 20 participants.
BDNF and APOE data were available for 18 participants.
Table 2.
Participants’ demographics, single-nucleotide polymorphisms, neuropsychological tests, and baseline neurophysiological measures.
| All (N = 21) | Group 1 (n = 12) | Group 2 (n = 9) | p | |
|---|---|---|---|---|
| Age (yr, mean ± SD) | 36.9 ± 15.2 | 36.8 ± 15.8 | 37.0 ± 15.3 | 0.98 |
| Sex (M : F) | 19 : 2 | 10 : 2 | 9 : 0 | 0.49 |
| Race (White : non-White) | 10 : 11 | 5 : 7 | 5 : 4 | 0.67 |
| Ethnicity (Hispanic : non-Hispanic) | 5 : 16 | 3 : 9 | 2 : 7 | 1.00 |
| Education (yr, mean ± SD)* | 16.8 ± 2.5 | 16.0 ± 2.4 | 18.1 ± 2.2 | – |
| BDNF (Met− : Met+)† | 12 : 6 | 4 : 5 | 8 : 1 | – |
| APOE (ε4− : ε4+)† | 10 : 8 | 5 : 4 | 5 : 4 | – |
| Handedness (Right : Left) | 21 : 0 | 12 : 0 | 9 : 0 | 1.00 |
| MMSE score (mean ± SD) | 29.9 ± 0.5 | 29.8 ± 0.6 | 29.9 ± 0.3 | 0.80 |
| Abbreviated Stanford-Binet IQ (mean ± SD) | 108.1 ± 12.4 | 104.5 ± 13.4 | 113.0 ± 9.7 | 0.12 |
| Verbal KN score (mean ± SD) | 11.7 ± 2.9 | 10.8 ± 2.6 | 12.9 ± 3.1 | 0.10 |
| Nonverbal FR score (mean ± SD) | 11.0 ± 2.2 | 10.8 ± 2.6 | 11.4 ± 1.4 | 0.47 |
| RMT (% MSO, mean ± SD) | 36.1 ± 7.7 | 36.8 ± 6.6 | 35.1 ± 9.4 | 0.63 |
| AMT (% MSO, mean ± SD) | 26.1 ± 5.2 | 25.8 ± 5.0 | 26.4 ± 5.8 | 0.80 |
| Baseline MEP amplitude (mV, mean ± SD) | 1.4 ± 1.2 | 1.2 ± 1.0 | 1.6 ± 1.3 | 0.50 |
AMT, active motor threshold; APOE, apolipoprotein E; APOE ε4+, ε2/ε4 or ε3/ε4 genotype; APOE ε4−, ε2/ε3 or ε3/ε3; BDNF, brain-derived neurotrophic factor; BDNF Met−, Val66Val; BDNF Met+, Val66Met; FR, fluid reasoning; IQ, intelligence quotient; KN, knowledge; MEP, motor evoked potential; MMSE, Mini-Mental State Examination; MSO, maximum stimulator output; RMT, resting motor threshold; SD, standard deviation. Racial and ethnic categories were defined based on the National Institutes of Health policy and guidelines on the inclusion of women and minorities as subjects in clinical research (NIH Office of Extramural Research, 2001). Groups 1 and 2 were identified by complete-linkage cluster analysis of natural log-transformed, baseline-corrected MEP amplitudes at 5 to 50 minutes post-cTBS. Comparisons of proportions were conducted with Fisher’s exact test. p values were not adjusted for multiple comparisons. Education and single-nucleotide polymorphisms were not statistically compared between the two groups because the data were not available for the total sample.
Education data were available for 12 participants in Group 1 and eight participants in Group 2.
BDNF and APOE results were available for 18 participants.
3.1. Genetic analyses
Among 18 participants with available BDNF and APOE results, the frequencies of BDNF Val/Val and Val/Met genotypes were 0.67 and 0.33, respectively, while the frequencies of APOE ε3/ε4, ε3/ε3, and ε2/ε3 genotypes were 0.44, 0.39, and 0.17, respectively.
None of the three SNPs (rs6265, rs429358 and rs7412) significantly deviated from HWE proportions (all p’s > .52) or from neutrality (all p’s > .31). Similarly, Fisher’s exact tests did not find a significant difference between the ratio of major: minor alleles of any of the SNPs in our participants and the expected ratio of those alleles based on the AMR population in the 1KGP (all p’s > .33).
3.2. cTBS-induced plasticity results
Grand-average cTBS-induced plasticity results are shown in Figure 2. The overall results did not show a statistically significant change in cortico-motor reactivity at any of the seven time points following cTBS (all p’s > .18). There was no significant association between any of the demographic, neuropsychological or baseline neurophysiological measures listed in Table 2 and LnMEP at T5 or T10 (all p’s > .10).
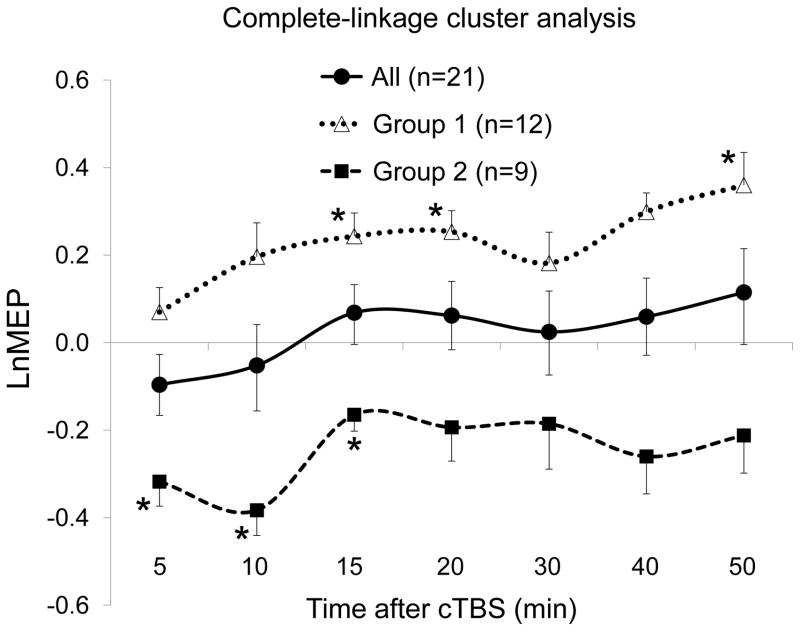
Figure 2.
Effects of continuous theta-burst stimulation (cTBS) of the left primary motor cortex in all participants and in two subgroups (Groups 1 and 2) identified by complete-linkage cluster analysis of natural log-transformed, baseline-corrected amplitudes of motor evoked potentials (LnMEP) at 5 to 50 minutes following cTBS. The overall results (‘All’) did not show a significant MEP modulation at any of the seven time points. Groups 1 and 2 were significantly different from each other at all time points except at T30. Values that were significantly different from zero are marked by *. All p-values were adjusted for multiple testing using false discovery rate. Error bars represent standard error of the mean.
Multiple regression analyses of LnMEP at T5 or T10 with AMT and baseline MEP amplitude as predictors did not result in a significant model (both p’s > .09).
3.3. Cluster analyses of cTBS-induced plasticity results
Complete-linkage cluster analysis of LnMEP values at T5 through T50 indicated the presence of two subgroups consisting of 12 and 9 individuals in Groups 1 and 2, respectively, with distinct patterns of response to cTBS (Figures 1 and 2). Comparisons of demographics, neuropsychological and baseline neurophysiological measures between the two groups are presented in Table 2.
Group 1, by itself, showed significant facilitation of cortico-motor reactivity at T15 [t(11) = 3.48, p = .005], T20 [t(11) = 3.98, p = .002], T50 [t(11) = 3.65, p = .004], and nonsignificant changes at other time points (all p’s > .07). In contrast, Group 2 showed significant suppression of cortico-motor reactivity at T5 [t(8) = 3.73, p = .006], T10 [t(8) = 4.36, p = .002], T15 [t(8) = 2.87, p = .021], and nonsignificant changes at other time points (all p’s > .08).
The difference between LnMEPs in Groups 1 and 2 was statistically significant at T5 [t(19) = 3.43, p = .003], T10 [t(19) = 4.11, p < .001], T15 [t(19) = 4.29, p < .001], T20 [t(19) = 3.57, p = .002], T30 [t(19) = 2.11, p = .048], T40 [t(19) = 4.29, p < .001], and T50 [t(19) = 3.56, p = .002].
Pooling the 21 p values in these three analyses (comparing Group 1’s LnMEPs against zero, Group 2’s LnMEPs against zero, and Groups 1 and 2’s LnMEPs against each other), all significant p values survived FDR adjustment for multiple testing (all adjusted p’s < .034), except the p value for comparing the LnMEPs at T30 between the two groups (adjusted p = .072).
Multiple logistic regression analyses of Group found significant models with LnMEP at T5 (pseudo-R2 = 0.35, p = .002), T10 (pseudo-R2 = 0.49, p < .001), T15 (pseudo-R2 = 0.45, p < .001), T20 (pseudo-R2 = 0.35, p = .002), T30 (pseudo-R2 = 0.16, p = .03), T40 (pseudo-R2 = 0.53, p < .001), or T50 (pseudo-R2 = 0.40, p < .001) as predictor. The two models with LnMEP at either T10 or T40 resulted in the largest pseudo-R2 values.
A multiple logistic regression analysis of Group with both the LnMEP at T10 and the LnMEP at T40 as predictors found a significant model (pseudo-R2 = 0.80, p < .001) but nonsignificant effects for both predictors (both p’s > 0.11). Adding either BDNF status, RMT, AMT, or baseline MEP amplitude as a predictor to the logistic regression analysis of Group with LnMEP at either T10 or T40 as the predictor did not find a significant effect of any of those additional predictors (all p’s > 0.13). None of the other predictors listed in Table 2 contributed significantly to any of the linear or logistic regression models.
3.4. Exploratory analysis of the effect of BDNF polymorphism on cTBS-induced plasticity
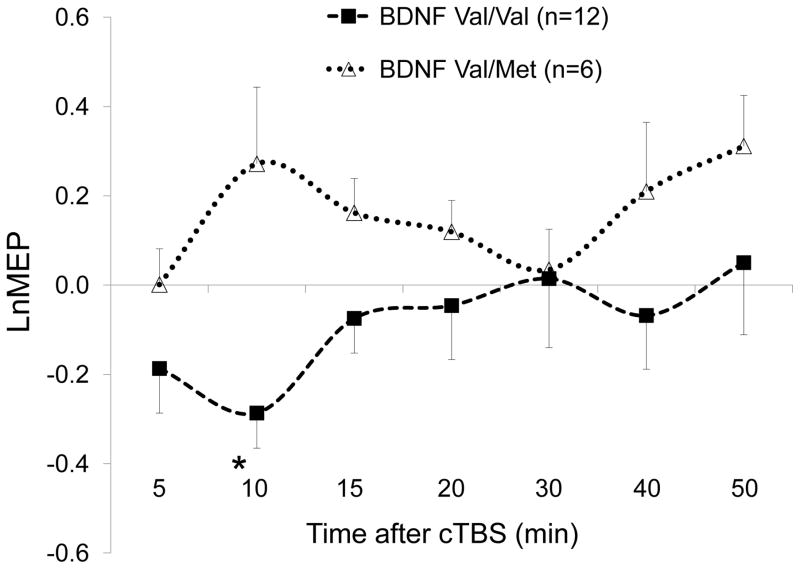
Among 18 participants, the LnMEP at T10 was significantly more positive in BDNF Val66Met (Met+) participants (mean ± SD, 0.27 ± 0.42) than in BDNF Val66Val (Met−) participants (−0.29 ± 0.27), t (16) = 3.42, p = .004 (FDR-adjusted p = .025). LnMEPs at other time points were not significantly different between BDNF Met+ and Met− participants (all p’s > .07). cTBS results in BDNF Met+ and Met− participants are illustrated in Figure 3.
Figure 3.
Effects of continuous theta-burst stimulation (cTBS) of the left primary motor cortex at 5 to 50 minutes following cTBS in BDNF Val66Val (Val/Val) and BDNF Val66Met (Val/Met) participants. * LnMEPs were significantly different between the two groups at 10 minutes post-cTBS. All p-values were adjusted for multiple testing using false discovery rate. Error bars represent standard error of the mean. BDNF, brain-derived neurotrophic factor; LnMEP, natural log-transformed, baseline-corrected amplitudes of motor evoked potentials.
Multiple regression analysis of LnMEP at T10 with BDNF status (0 for Met−,1 for Met+) and AMT as predictors resulted in a significant model (adjusted R2 = 0.59, p < .001) and significant effects for both BDNF status (B̂ = 0.73, t = 5.02, p < .001) and AMT (B̂ = −0.04, t = 3.00, p = .009).
Among variables listed in Table 2, BDNF status was found to have significant associations with Race (lower Met+ frequency in Whites), Verbal IQ subscore (lower in Met+ participants) and RMT (higher in Met+ participants), all p’s < .05. None of these variables, however, were found to have a significant effect on LnMEPs at any time point (all p’s > .20). Moreover, when added (one at a time) to the multiple regression analysis of LnMEP at T10, Race, Verbal IQ, and RMT were nonsignificant predictors (all p’s > .14), while BDNF status (all p’s < .003) and AMT (all p’s < .019) remained significant predictors in all models.
3.5. Effect of APOE polymorphisms on cTBS-induced plasticity
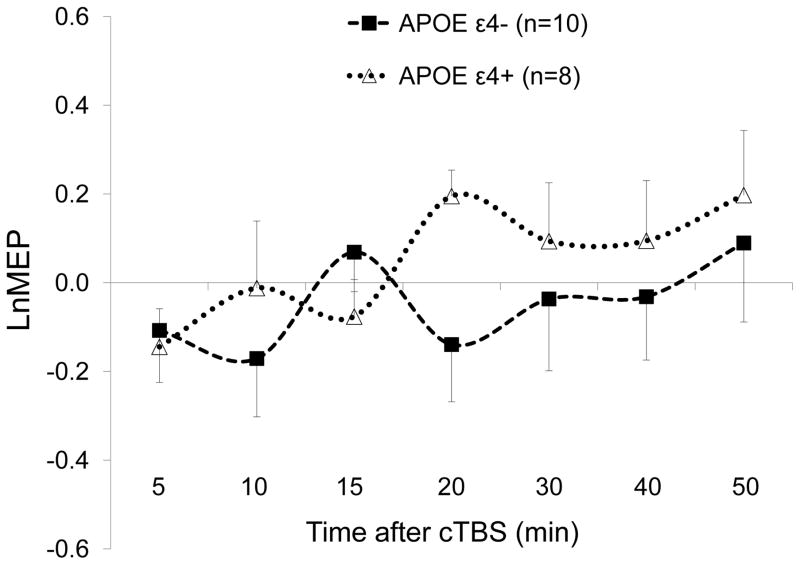
Among 18 participants, the LnMEP at T20 was significantly more positive in APOE ε4+ (ε3/ε4) participants (mean ± SD, 0.20 ± 0.17) than in APOE ε4− (ε2/ε3 or ε3/ε3) participants (−0.14 ± 0.40), t (16) = 2.17, p = .046, but the p-value did not survive adjustment for multiple comparisons (FDR-adjusted p = .32). cTBS results in APOE ε4+ and APOE ε4− participants are illustrated in Figure 4.
Figure 4.
Effects of continuous theta-burst stimulation (cTBS) of the left primary motor cortex at 5 to 50 minutes following cTBS in APOE ε4+ (ε3/ε4) and APOE ε4− (ε2/ε3 or ε3/ε3) participants. LnMEP at T20 was significantly more positive in in APOE ε4+ participants than in in APOE ε4− participants (p = .046), but the p-value did not survive adjustment for multiple comparisons (FDR-adjusted p = .32). LnMEPs were not significantly different between the two groups at other time points. Error bars represent standard error of the mean. APOE, apolipoprotein E; LnMEP, natural log-transformed, baseline-corrected amplitudes of motor evoked potentials.
Multiple regression analysis of LnMEP at T20 with BDNF and APOE statuses as predictors found a nonsignificant effect of APOE status (p = .056) and a nonsignificant effect of BDNF status (p = .42). Adding APOE status as a predictor to the regression analyses of LnMEP at T10, T40, or T50 with BDNF status as the other predictor found a significant effect of BDNF status at T10 (p = .005), but no other significant effect of either BDNF or APOE status (all p’s > .21).
4. Discussion
Interindividual variability in TBS response can limit the utility of TBS aftereffects as indices of neuroplasticity. The present work examined the interindividual variability in response to cTBS among healthy individuals applying a data-driven statistical approach, i.e., cluster analysis, to identify subgroups of healthy individuals with distinct patterns of response to cTBS applied to M1. A further, exploratory objective was to assess the effect of BDNF and APOE polymorphisms on cTBS-induced plasticity.
4.1. Overall cTBS-induced plasticity results
The grand-average cTBS aftereffects showed no significant MEP modulation at any time point in the first 50 minutes. The interindividual variability in cTBS response was indicated by the large variability in MEP modulation around zero at T10, which typically shows the maximal effect of cTBS. While these results are inconsistent with the average cTBS response among healthy individuals reported in recent reviews (Wischnewski and Schutter, 2015; Suppa et al., 2016), they are consistent with the large interindividual variability in cTBS responses reported previously (Hamada et al., 2013).
Three possible factors could be suggested to account for the finding that the grand-average cTBS results were not consistent with the results of recent systematic reviews of cTBS studies (Wischnewski and Schutter, 2015; Suppa et al., 2016). First, the less-common AP-PA current direction induced in the brain (by using the ‘Normal’ current-direction setting on the MagPro X100 stimulator and the MC-B70 Butterfly Coil) may have influenced the cTBS results compared to those obtained with other rTMS stimulators. Second, individual participants in some of the previous cTBS studies that had not shown the expected suppression of MEPs during the first few post-cTBS time points may have been excluded as “non-responders”, even though some of them might have shown significant facilitation of MEPs at certain time points (similar to many of the participants in Group 1). Third, there might have been some publication bias in reporting the effects of cTBS; i.e., studies that did not find the conventional suppressive effects of cTBS in their grand-average MEP results might have been less likely to be published.
4.2. Cluster analysis of cTBS-induced plasticity results
To examine the interindividual variability in the overall response to cTBS, we conducted a complete-linkage cluster analysis (Kaufman and Rousseeuw, 2009; Rencher and Christensen, 2012) on LnMEPs at all T5–T50 data points and found two subgroups with distinct patterns of cTBS response. The LnMEPs in the two subgroups were significantly different from each other at all time points except at T30. Group 1 showed significant facilitation of MEPs at T15, T20, and T50 (i.e., no clear return to baseline), whereas Group 2 showed significant suppression of MEPs at T5, T10, and T15. LnMEPs at T10 and at T40 had the most discriminatory power between inhibitory and facilitatory responses to cTBS. When considered together, LnMEPs at T10 and T40 predicted 80% of interindividual variability in cluster assignment.
The proportion of participants with facilitatory response to cTBS (~ 57%) was comparable with the results reported by Hamada and colleagues who found approximately 58% of their participants showed facilitatory responses to cTBS (Hamada et al., 2013). This pattern of results indicates: (1) the large interindividual variability in cTBS response among healthy individuals, and (2) the utility of cluster analysis for identification of subpopulations with distinct patterns of cTBS response in a data-driven and minimally biased manner.
Approximately 43% of participants (Group 2) showed suppression of cTBS aftereffects that, on average, returned to baseline by T20. This duration of cTBS aftereffects was shorter than expected considering the results of a recent meta-analysis of cTBS aftereffects in healthy individuals (Wischnewski and Schutter, 2015). The reduced duration of the TBS aftereffects might be due to unmeasured characteristic(s) of our participants or to the relatively small number of participants.
12 participants in Group 1 showed facilitatory cTBS aftereffects that did not return to baseline by T50. This long-lasting facilitation following cTBS could have stemmed from a combination of BDNF polymorphism and cumulative facilitatory effects of single TMS pulses: (1) if a majority of participants in Group 1 were BDNF Met+, they would likely not show the conventional suppressive effects of cTBS due to impaired GABAergic synaptic transmission (Abidin et al., 2008) presumed to be involved in cTBS aftereffects (Stagg et al., 2009; Trippe et al., 2009); (2) receiving blocks of single TMS pulses, even with a jittered 4–5s inter-pulse interval, has been shown to have cumulative, within- and between-block facilitatory effects on corticospinal excitability (Pellicciari et al., 2016).
Applying numerous single TMS pulses may gradually skew the overall MEP amplitudes towards facilitation (Pellicciari et al., 2016) and perhaps even more so among BDNF Met+ individuals following cTBS (present results). This concern, however, needs to be balanced against the findings that at least 20 pulses are required to obtain reliable estimates of MEP amplitude at a given time point (Chang et al., 2016; Goldsworthy et al., 2016). Further studies are thus needed to determine the optimal number, stimulation intensity, and inter-pulse interval of single TMS pulses for minimizing their cumulative facilitatory effects while still allowing for reliable MEP estimates at given time points following TBS and other TMS protocols.
4.3. BDNF and APOE polymorphisms and cTBS-induced plasticity
In an exploratory analysis, we evaluated the potential association between BDNF Met carrier status and cTBS aftereffects, by comparing the cTBS responses between BDNF Met+ and Met− participants, regardless of which clusters they were assigned to. The two groups differed significantly in LnMEP at T10, with Met+ and Met− participants showing facilitatory and inhibitory cTBS responses, respectively (Figure 3). Moreover, the BDNF status and the AMT (that determined the intensity of cTBS) together accounted for 59% of variability in LnMEP at T10. The BDNF status was found to have significant associations with race, verbal IQ, and RMT. Additional analyses of those covariates, however, failed to find significant evidence for confounding of the significant association between BDNF status and cTBS response at T10 by race, verbal IQ or RMT.
The results of the present study and previous studies that found associations between BDNF polymorphism and measures of cortical plasticity (Cheeran et al., 2008, 2009; Antal et al., 2010; Cirillo et al., 2012; Lee et al., 2013; Chang et al., 2014; Di Lazzaro et al., 2015) but see (Li Voti et al., 2011; Mastroeni et al., 2013), suggest the importance of controlling for BDNF polymorphism when comparing M1 cTBS responses between healthy and clinical populations.
The significant association between BDNF status and cTBS-induced plasticity may be due to the important BDNF role in synaptic plasticity and intracellular signaling (Bramham and Messaoudi, 2005; Numakawa et al., 2010), reduced activity-dependent BDNF secretion (Egan et al., 2003), impaired NMDA-dependent LTD (Woo et al., 2005) and/or aberrant GABAergic inhibition in BDNF Met carriers (Abidin et al., 2008). However, the relatively small sample size of the present study limits the strength of the conclusions and warrants further replication in larger cohorts. It may also be important to obtain in those cohorts the results for other SNPs that have been found to influence cortical plasticity as measured by TMS, including catechol-O-methyltransferase (COMT) polymorphism that has been found to influence M1 cTBS responses (Lee et al., 2014) and to interact with the effect of BDNF polymorphism (Witte et al., 2012).
After controlling for BDNF status, AMT (or cTBS intensity) was found to have a significant effect on cTBS response at T10; higher AMT values were associated with more negative values, i.e., more suppression or less facilitation of MEPs, at T10. The significant effect of AMT (or cTBS intensity), when considered together with BDNF status, on the interindividual variability in cTBS aftereffect at T10 indicates the importance of controlling for AMT or stimulation intensity in studies comparing M1 cTBS responses between healthy and clinical populations.
While there was a hint of more facilitation at T20 in APOE ε4+ participants than in APOE ε4− participants (Figure 4), the difference did not survive adjustment for multiple comparisons. This result could be due to the fact that, while both the influences of APOE ε4 on synaptic plasticity and TBS aftereffects have been found to involve NMDA receptors (Huang et al., 2007; Chen et al., 2010), cTBS-induced plasticity may also involve GABAergic mechanisms (Stagg et al., 2009; Trippe et al., 2009) that are not necessarily affected by the presence of APOE ε4 (Andrews-Zwilling et al., 2010).
4.4. Additional considerations
Several factors may limit the generalizability of the present findings. The range of baseline MEP amplitudes was relatively wide and a few participants had average baseline MEP amplitudes smaller than 0.5mV. This was perhaps in part due to the fact that the intensity of single TMS pulses was fixed at 120% of RMT and was not adjusted to obtain any particular MEP amplitude. Since the slope of increase in MEP amplitude with increase in stimulation intensity of single TMS pulses can vary among individuals, it may be beneficial to utilize input-output curves, rather than a fixed proportion of RMT, to determine the stimulation intensity of single pulses. It is also possible that higher intensity of single TMS pulses and/or larger baseline MEP amplitudes would have resulted in different patterns of cTBS aftereffects.
The present work tested the utility of cluster analysis with the complete-linkage method, previously used in an rTMS study (Gangitano et al., 2002), for identifying subpopulations of healthy participants with distinct patterns of cTBS response. There are other methods of hierarchical and nonhierarchical cluster analysis, including single-linkage, average-linkage, centroid, median, Ward’s method, k-means, etc., each with their own advantages and disadvantages (Kaufman and Rousseeuw, 2009; Rencher and Christensen, 2012). It remains to be investigated which methods of cluster analysis are the most sensitive and robust in identifying natural clusters within post-TBS MEP data.
Besides the method of clustering, choosing which time points to include in the cluster analysis of post-TBS data may also influence the composition of the resultant clusters. Because of the exploratory nature of the present work, and to avoid any bias in the selection of the time points, we included all available time points from T5 through T50 in the cluster analysis. It may be the case that choosing certain time points instead of the whole dataset can improve the sensitivity or robustness of the cluster analysis. One option may be to choose T5, previously found to be the most reliable time point for measuring MEP changes following cTBS (Vernet et al., 2014) and/or T10 to capture the peak effect of TBS, including for participants whose MEPs return rapidly to baseline levels, and perhaps one of the later time points, e.g., T40 and/or T50, to use their discriminatory power for classifying the cTBS aftereffects (present results) and for capturing the differential time of return of post-TBS MEP amplitudes to baseline levels between healthy and clinical populations (Oberman et al., 2010, 2012, 2014, 2016; Freitas et al., 2011b; McClintock et al., 2011).
Finally, the type of post-TBS MEP data may also influence the results of cluster analysis. In the present work, we chose LnMEP as a measure of TBS-induced MEP modulation that is normalized to each participant’s baseline MEP amplitude and log-transformed to achieve a near-normal distribution. It is possible that conducting the cluster analysis with other types of post-TBS MEP data, e.g., area-under-the-curve, absolute deviation from the baseline amplitude, or time of return to the baseline, can improve the results of cluster analysis.
5. Conclusions
The large interindividual variability in cTBS response among healthy individuals should be considered when utilizing cTBS as an index of the mechanisms of cortical plasticity. Relying only on grand-average results can obscure important interindividual differences in cTBS response within each group of participants. Data-driven cluster analyses can identify subpopulations of individuals with distinct patterns of cTBS response. BDNF polymorphism had a significant effect on MEP changes at 10 minutes after cTBS. Moreover, BDNF status and AMT (or cTBS intensity) accounted for a large portion of interindividual variability in cTBS responses at T10. Changes in MEPs at T10 and T40 had the most discriminatory power for categorizing the cTBS responses. When considered together, MEP changes at T10 and at T40 accounted for a large portion of interindividual variability in cluster assignment. Considering these factors can improve the utility of cTBS as index of cortical plasticity and can increase the power of studies that examine differences in cTBS response between healthy and clinical populations.
Highlights.
Cluster analysis can identify subpopulations in healthy adults with distinct cTBS responses.
MEP changes at 10 and 40 minutes post-cTBS best predicted the results of the cluster analysis.
Variability in cTBS response after 10 min was influenced by BDNF polymorphism and cTBS intensity.
Acknowledgments
We thank Stephanie Changeau, Aaron Boes, Simon Laganiere, and Ann Connor (Beth Israel Deaconess Medical Center) for assistance with evaluation of participants’ health/medical history and physical/neurological examination.
This study was primarily funded by the National Institutes of Health (NIH R01 MH100186). A.P.-L. was further supported by the Sidney R. Baer Jr. Foundation, the NIH (R01 HD069776, R01 NS073601, R21 MH099196, R21 NS085491, R21 HD07616), and Harvard Catalyst | The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). A.R. was further supported by the NIH (R01 NS088583), The Boston Children’s Hospital Translational Research Program, Autism Speaks, Massachusetts Life Sciences, The Assimon Family, Brainsway, CRE Medical, Eisai, Neuroelectrics, Roche, Sage Therapeutics and Takeda Medical. A.J. was further supported by a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada (NSERC PDF 454617). L.M.O. was further supported by the Simons Foundation Autism Research Initiative (SFARI) and the Nancy Lurie Marks Family Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, or any of the listed granting agencies.
Footnotes
Conflict of Interest Statement
A.P.-L. serves on the scientific advisory boards for Magstim, Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Constant Therapy, and Neosync; and is listed as inventor on several issued and pending patents on real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging. A.R. is a founder and advisor for Neuromotion, serves on the medical advisory board for NeuroRex, and is listed as inventor on a patent related to integration of TMS and EEG. The remaining authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abidin I, Eysel UT, Lessmann V, Mittmann T. Impaired GABAergic inhibition in the visual cortex of brain-derived neurotrophic factor heterozygous knockout mice: GABA release is impaired in visual cortex of BDNF heterozygous KO mice. J Physiol. 2008;586:1885–1901. doi: 10.1113/jphysiol.2007.148627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerley SJ, Stinear CM, Barber PA, Byblow WD. Combining Theta Burst Stimulation With Training After Subcortical Stroke. Stroke. 2010;41:1568–1572. doi: 10.1161/STROKEAHA.110.583278. [DOI] [PubMed] [Google Scholar]
- Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci. 2006;110:175–191. doi: 10.1042/CS20050161. [DOI] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, et al. Apolipoprotein E4 Causes Age- and Tau-Dependent Impairment of GABAergic Interneurons, Leading to Learning and Memory Deficits in Mice. J Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010;3:230–237. doi: 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Auton A, Abecasis GR, Altshuler DM, Durbin RM, Abecasis GR, Bentley DR, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;325:1106–1107. doi: 10.1016/S0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Bendel RB, Afifi AA. Comparison of Stopping Rules in Forward “Stepwise” Regression. J Am Stat Assoc. 1977;72:46–53. doi: 10.1080/01621459.1977.10479905. [DOI] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc Ser B Methodol. 1995;57:289–300. [Google Scholar]
- Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantone M, Di Pino G, Capone F, Piombo M, Chiarello D, Cheeran B, et al. The contribution of transcranial magnetic stimulation in the diagnosis and in the management of dementia. Clin Neurophysiol. 2014;125:1509–1532. doi: 10.1016/j.clinph.2014.04.010. [DOI] [PubMed] [Google Scholar]
- Capocchi G, Zampolini M, Larson J. Theta burst stimulation is optimal for induction of LTP at both apical and basal dendritic synapses on hippocampal CA1 neurons. Brain Res. 1992;591:332–336. doi: 10.1016/0006-8993(92)91715-q. [DOI] [PubMed] [Google Scholar]
- Carrette S, Boon P, Dekeyser C, Klooster DCW, Carrette E, Meurs A, et al. Repetitive transcranial magnetic stimulation for the treatment of refractory epilepsy. Expert Rev Neurother. 2016;16:1093–1110. doi: 10.1080/14737175.2016.1197119. [DOI] [PubMed] [Google Scholar]
- Cazzoli D, Muri RM, Schumacher R, von Arx S, Chaves S, Gutbrod K, et al. Theta burst stimulation reduces disability during the activities of daily living in spatial neglect. Brain. 2012;135:3426–3439. doi: 10.1093/brain/aws182. [DOI] [PubMed] [Google Scholar]
- Chang WH, Bang OY, Shin Y-I, Lee A, Pascual-Leone A, Kim Y-H. BDNF Polymorphism and Differential rTMS Effects on Motor Recovery of Stroke Patients. Brain Stimul. 2014;7:553–558. doi: 10.1111/j.1460-9568.2012.08177.x. [DOI] [PubMed] [Google Scholar]
- Chang WH, Fried PJ, Saxena S, Jannati A, Gomes-Osman J, Kim Y-H, et al. Optimal number of pulses as outcome measures of neuronavigated transcranial magnetic stimulation. Clin Neurophysiol. 2016;127:2892–2897. doi: 10.1016/j.clinph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeran BJ, Ritter C, Rothwell JC, Siebner HR. Mapping genetic influences on the corticospinal motor system in humans. Neuroscience. 2009;164:156–163. doi: 10.1016/j.neuroscience.2009.01.054. [DOI] [PubMed] [Google Scholar]
- Cheeran BJ, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS: BNDF polymorphism modulates response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Durakoglugil MS, Xian X, Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc Natl Acad Sci. 2010;107:12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang WL, Huang YZ, Lu CS, Chen RS. Reduced cortical plasticity and GABAergic modulation in essential tremor. Mov Disord. 2014;29:501–507. doi: 10.1002/mds.25809. [DOI] [PubMed] [Google Scholar]
- Cirillo J, Hughes J, Ridding M, Thomas PQ, Semmler JG. Differential modulation of motor cortex excitability in BDNF Met allele carriers following experimentally induced and use-dependent plasticity: BDNF polymorphisms and motor cortex plasticity. Eur J Neurosci. 2012;36:2640–2649. doi: 10.1111/j.1460-9568.2012.08177.x. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-Based Norms for the Mini-Mental State Examination by Age and Educational Level. JAMA J Am Med Assoc. 1993;269:2386. doi: 10.1001/jama.1993.03500180078038. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Capone F, Di Pino G, Pellegrino G, Florio L, Zollo L, et al. Combining Robotic Training and Non-Invasive Brain Stimulation in Severe Upper Limb-Impaired Chronic Stroke Patients. Front Neurosci. 2016:10. doi: 10.3389/fnins.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pellegrino G, Di Pino G, Corbetto M, Ranieri F, Brunelli N, et al. Val66Met BDNF Gene Polymorphism Influences Human Motor Cortex Plasticity in Acute Stroke. Brain Stimul. 2015;8:92–96. doi: 10.1016/j.brs.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Rothwell JC, Talelli P, Capone F, Ranieri F, Wallace AC, et al. Inhibitory theta burst stimulation of affected hemisphere in chronic stroke: A proof of principle, sham-controlled study. Neurosci Lett. 2013;553:148–152. doi: 10.1016/j.neulet.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Dunwiddie TV, Rose GM. Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J Neurosci. 1988;8:4079–4088. doi: 10.1523/JNEUROSCI.08-11-04079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DNA Genotek Inc. Laboratory protocol for manual purification of DNA from 0.5 mL of sample. 2015 Available at: http://www.dnagenotek.com/US/pdf/PD-PR-006.pdf.
- Eberle M-C, Wildgruber D, Wasserka B, Fallgatter AJ, Plewnia C. Relief From Chronic Intractable Auditory Hallucinations After Long-Term Bilateral Theta Burst Stimulation. Am J Psychiatry. 2010;167:1410–1410. doi: 10.1176/appi.ajp.2010.10070988. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell. 2003;112:257–269. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ewens WJ. The sampling theory of selectively neutral alleles. Theor Popul Biol. 1972;3:87–112. doi: 10.1016/0040-5809(72)90035-4. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forogh B, Yazdi-Bahri S-M, Ahadi T, Fereshtehnejad S-M, Raissi GR. Comparison of two protocols of transcranial magnetic stimulation for treatment of chronic tinnitus: a randomized controlled clinical trial of burst repetitive versus high-frequency repetitive transcranial magnetic stimulation. Neurol Sci. 2014;35:227–232. doi: 10.1007/s10072-013-1487-5. [DOI] [PubMed] [Google Scholar]
- Freitas C, Mondragón-Llorca H, Pascual-Leone A. Noninvasive brain stimulation in Alzheimer’s disease: systematic review and perspectives for the future. Exp Gerontol. 2011a;46:611–627. doi: 10.1016/j.exger.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas C, Perez J, Knobel M, Tormos JM, Oberman L, Eldaief M, et al. Changes in Cortical Plasticity Across the Lifespan. Front Aging Neurosci. 2011b:3. doi: 10.3389/fnagi.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PJ, Schilberg L, Brem AK, Saxena S, Wong B, Cypess AM, et al. Humans with type-2 diabetes show abnormal long-term potentiation-like cortical plasticity associated with verbal learning deficits. J Alzheimers Dis. 2017;55:89–100. doi: 10.3233/JAD-160505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangitano M, Valero-Cabré A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone A. Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol. 2002;113:1249–1257. doi: 10.1016/s1388-2457(02)00109-8. [DOI] [PubMed] [Google Scholar]
- Goldsworthy MR, Hordacre B, Ridding MC. Minimum number of trials required for within- and between-session reliability of TMS measures of corticospinal excitability. Neuroscience. 2016;320:205–209. doi: 10.1016/j.neuroscience.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Guo SW, Thompson EA. Performing the Exact Test of Hardy-Weinberg Proportion for Multiple Alleles. Biometrics. 1992;48:361. doi: 10.2307/2532296. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The Role of Interneuron Networks in Driving Human Motor Cortical Plasticity. Cereb Cortex. 2013;23:1593–1605. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Hinder MR, Goss EL, Fujiyama H, Canty AJ, Garry MI. Inter-and Intra-individual variability following intermittent theta burst stimulation: implications for rehabilitation and recovery. Brain Stimul. 2014;7:365–371. doi: 10.1016/j.brs.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Hsu W-Y, Cheng C-H, Liao K-K, Lee I-H, Lin Y-Y. Effects of Repetitive Transcranial Magnetic Stimulation on Motor Functions in Patients With Stroke: A Meta-Analysis. Stroke. 2012;43:1849–1857. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Chen R-S, Rothwell JC, Wen H-Y. The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin Neurophysiol. 2007;118:1028–1032. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta Burst Stimulation of the Human Motor Cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J NeuroEngineering Rehabil. 2009;6:7. doi: 10.1186/1743-0003-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iezzi E, Conte A, Suppa A, Agostino R, Dinapoli L, Scontrini A, et al. Phasic voluntary movements reverse the aftereffects of subsequent theta-burst stimulation in humans. J Neurophysiol. 2008;100:2070–2076. doi: 10.1152/jn.90521.2008. [DOI] [PubMed] [Google Scholar]
- Kaufman L, Rousseeuw PJ. Finding groups in data: an introduction to cluster analysis. John Wiley & Sons, Inc; 2009. [Google Scholar]
- Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Koch G, Bonni S, Giacobbe V, Bucchi G, Basile B, Lupo F, et al. Theta-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology. 2012;78:24–30. doi: 10.1212/WNL.0b013e31823ed08f. [DOI] [PubMed] [Google Scholar]
- Koch G, Brusa L, Carrillo F, Lo Gerfo E, Torriero S, Oliveri M, et al. Cerebellar magnetic stimulation decreases levodopa-induced dyskinesias in Parkinson disease. Neurology. 2009;73:113–119. doi: 10.1212/WNL.0b013e3181ad5387. [DOI] [PubMed] [Google Scholar]
- Lancaster A, Nelson MP, Meyer D, Single RM, Thomson G. PyPop: a software framework for population genomics: analyzing large-scale multi-locus genotype data. Pac Symp Biocomput. 2003:514–25. [PMC free article] [PubMed] [Google Scholar]
- Lancaster AK, Single RM, Solberg OD, Nelson MP, Thomson G. PyPop update - a software pipeline for large-scale multilocus population genomics. Tissue Antigens. 2007;69:192–197. doi: 10.1111/j.1399-0039.2006.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Theta pattern stimulation and the induction of LTP: the sequence in which synapses are stimulated determines the degree to which they potentiate. Brain Res. 1989;489:49–58. doi: 10.1016/0006-8993(89)90007-3. [DOI] [PubMed] [Google Scholar]
- Lee M, Kim SE, Kim WS, Lee J, Yoo HK, Park KD, et al. Interaction of Motor Training and Intermittent Theta Burst Stimulation in Modulating Motor Cortical Plasticity: Influence of BDNF Val66Met Polymorphism. PLoS One. 2013;8:e57690. doi: 10.1371/journal.pone.0057690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NJ, Ahn HJ, Jung K-I, Ohn SH, Hong J, Kim YJ, et al. Reduction of Continuous Theta Burst Stimulation-Induced Motor Plasticity in Healthy Elderly With COMT Val158Met Polymorphism. Ann Rehabil Med. 2014;38:658. doi: 10.5535/arm.2014.38.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-T, Chen M-H, Juan C-H, Huang H-H, Chen L-F, Hsieh J-C, et al. Efficacy of prefrontal theta-burst stimulation in refractory depression: a randomized sham-controlled study. Brain. 2014;137:2088–2098. doi: 10.1093/brain/awu109. [DOI] [PubMed] [Google Scholar]
- Li Voti P, Conte A, Suppa A, Iezzi E, Bologna M, Aniello MS, et al. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp Brain Res. 2011;212:91–99. doi: 10.1007/s00221-011-2700-5. [DOI] [PubMed] [Google Scholar]
- López-Alonso V, Cheeran B, Río-Rodríguez D, Fernández-del-Olmo M. Inter-individual Variability in Response to Non-invasive Brain Stimulation Paradigms. Brain Stimul. 2014;7:372–80. doi: 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Rall SC., Jr Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. doi: 10.1146/annurev.genom.1.1.507. [DOI] [PubMed] [Google Scholar]
- Mastroeni C, Bergmann TO, Rizzo V, Ritter C, Klein C, Pohlmann I, et al. Brain-Derived Neurotrophic Factor – A Major Player in Stimulation-Induced Homeostatic Metaplasticity of Human Motor Cortex? PLoS One. 2013;8:e57957. doi: 10.1371/journal.pone.0057957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock SM, Freitas C, Oberman LM, Lisanby SH, Pascual-Leone A. Transcranial Magnetic Stimulation: A Neuroscientific Probe of Cortical Function in Schizophrenia. Biol Psychiat. 2011;70:19–27. doi: 10.1016/j.biopsych.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden D. Conditional logit analysis of qualitative choice behavior. In: Zarembka P, editor. Frontiers in Econometrics. Academic Press; 1973. [Google Scholar]
- Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- Mori F, Rossi S, Piccinin S, Motta C, Mango D, Kusayanagi H, et al. Synaptic Plasticity and PDGF Signaling Defects Underlie Clinical Progression in Multiple Sclerosis. J Neurosci. 2013;33:19112–19119. doi: 10.1523/JNEUROSCI.2536-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Kutscha M, Pool E-M, Rehme AK, Eickhoff SB, et al. Dose-Dependent Effects of Theta Burst rTMS on Cortical Excitability and Resting-State Connectivity of the Human Motor System. J Neurosci. 2014;34:6849–6859. doi: 10.1523/JNEUROSCI.4993-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettekoven C, Volz LJ, Leimbach M, Pool E-M, Rehme AK, Eickhoff SB, et al. Inter-individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. NeuroImage. 2015;118:209–218. doi: 10.1016/j.neuroimage.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol K, Deeny SP, Seif J, Camaclang K, Cotman CW. Exercise improves cognition and hippocampal plasticity in APOE ε4 mice. Alzheimers Dement. 2009;5:287–294. doi: 10.1111/j.1460-9568.2012.08177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Office of Extramural Research. [Accessed February 1, 2016];NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research. 2001 Available at: https://grants.nih.gov/grants/funding/women_min/guidelines_amended_10_2001.htm.
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- Oberman LM, Eldaief M, Fecteau S, Ifert-Miller F, Tormos JM, Pascual-Leone A. Abnormal modulation of corticospinal excitability in adults with Asperger’s syndrome: Modulation of excitability in Asperger’s. Eur J Neurosci. 2012;36:2782–2788. doi: 10.1111/j.1460-9568.2012.08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Ifert-Miller F, Najib U, Bashir S, Gonzalez-Heydrich J, Picker J, et al. Abnormal Mechanisms of Plasticity and Metaplasticity in Autism Spectrum Disorders and Fragile X Syndrome. J Child Adolesc Psychopharmacol. 2016;26:617–624. doi: 10.1089/cap.2015.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Ifert-Miller F, Najib U, Bashir S, Woollacott I, Gonzalez-Heydrich J, et al. Transcranial magnetic stimulation provides means to assess cortical plasticity and excitability in humans with fragile X syndrome and autism spectrum disorder. Front Synaptic Neurosci. 2010;2:26. doi: 10.3389/fnsyn.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Pascual-Leone A, Rotenberg A. Modulation of corticospinal excitability by transcranial magnetic stimulation in children and adolescents with autism spectrum disorder. Front Hum Neurosci. 2014;8:627. doi: 10.3389/fnhum.2014.00627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, et al. Characterizing Brain Cortical Plasticity and Network Dynamics Across the Age-Span in Health and Disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011;24:302–315. doi: 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicciari MC, Miniussi C, Ferrari C, Koch G, Bortoletto M. Ongoing cumulative effects of single TMS pulses on corticospinal excitability: An intra- and inter-block investigation. Clin Neurophysiol. 2016;127:621–628. doi: 10.1016/j.clinph.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Peña-Gomez C, Solé-Padullés C, Clemente IC, Junqué C, Bargalló N, Bosch B, et al. APOE Status Modulates the Changes in Network Connectivity Induced by Brain Stimulation in Non-Demented Elders. PLoS One. 2012;7:e51833. doi: 10.1371/journal.pone.0051833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier J, Bertrand P, Kogan S, Gauthier S, Davignon J, Bouthillier D. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- Poulet E, Brunelin J, Ben Makhlouf W, D’Amato T, Saoud M. A case report of cTBS for the treatment of auditory hallucinations in a patient with schizophrenia. Brain Stimul. 2009;2:118–119. doi: 10.1016/j.brs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Rencher AC, Christensen WF. Methods of Multivariate Analysis. 3. John Wiley & Sons, Inc; 2012. Cluster analysis; pp. 501–554. [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PS, Pericak-Vance MA, Joo S, et al. Association of apolipoprotein E allele ε 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1467. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Slatkin M. An exact test for neutrality based on the Ewens sampling distribution. Genet Res. 1994;64:71–74. doi: 10.1017/s0016672300032560. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Wylezinska M, Matthews PM, Johansen-Berg H, Jezzard P, Rothwell JC, et al. Neurochemical Effects of Theta Burst Stimulation as Assessed by Magnetic Resonance Spectroscopy. J Neurophysiol. 2009;101:2872–2877. doi: 10.1152/jn.91060.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata 13 Base Reference Manual. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- Suppa A, Huang Y-Z, Funke K, Ridding MC, Cheeran B, Di Lazzaro V, et al. Ten Years of Theta Burst Stimulation in Humans: Established Knowledge, Unknowns and Prospects. Brain Stimul. 2016;9:323–335. doi: 10.1016/j.brs.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Suppa A, Marsili L, Di Stasio F, Berardelli I, Roselli V, Pasquini M, et al. Cortical and brainstem plasticity in Tourette syndrome and obsessive-compulsive disorder. Mov Disord. 2014;29:1523–1531. doi: 10.1002/mds.25960. [DOI] [PubMed] [Google Scholar]
- Thorndike RL, Hagen EP, Sattler JM. The Stanford-Binet intelligence scale: Guide for administering and scoring. Riverside Publishing Company; 1986. [Google Scholar]
- Trippe J, Mix A, Aydin-Abidin S, Funke K, Benali A. Theta burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp Brain Res. 2009;199:411–421. doi: 10.1007/s00221-009-1961-8. [DOI] [PubMed] [Google Scholar]
- Vernet M, Bashir S, Yoo W-K, Oberman LM, Mizrahi I, Ifert-Miller F, et al. Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin Neurophysiol. 2014;125:320–326. doi: 10.1016/j.clinph.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson G. The homozygosity test of neutrality. Genetics. 1978;88:405–417. doi: 10.1093/genetics/88.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F, Nicoll JAR, Roses AD, Horsburgh K. Impaired Neuronal Plasticity in Transgenic Mice Expressing Human Apolipoprotein E4 Compared to E3 in a Model of Entorhinal Cortex Lesion. Neurobiol Dis. 2001;8:611–625. doi: 10.1006/nbdi.2001.0401. [DOI] [PubMed] [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR. A Note on Exact Tests of Hardy-Weinberg Equilibrium. Am J Hum Genet. 2005;76:887–893. doi: 10.1086/429864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischnewski M, Schutter DJLG. Efficacy and Time Course of Theta Burst Stimulation in Healthy Humans. Brain Stimul. 2015;8:685–692. doi: 10.1016/j.brs.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Witte AV, Kurten J, Jansen S, Schirmacher A, Brand E, Sommer J, et al. Interaction of BDNF and COMT Polymorphisms on Paired-Associative Stimulation-Induced Cortical Plasticity. J Neurosci. 2012;32:4553–4561. doi: 10.1523/JNEUROSCI.6010-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolk DA, et al. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao C-J, Chiaruttini C, Pang PT, Milner TA, et al. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nat Neurosci. 2005;8:1069–1077. doi: 10.1038/nn1510. [DOI] [PubMed] [Google Scholar]
- Wu C-C, Tsai C-H, Lu M-K, Chen C-M, Shen W-C, Su K-P. Theta-Burst Repetitive Transcranial Magnetic Stimulation for Treatment-Resistant Obsessive-Compulsive Disorder With Concomitant Depression. J Clin Psychiatry. 2010;71:504–506. doi: 10.4088/JCP.09l05426blu. [DOI] [PubMed] [Google Scholar]