Abstract
Background
Resource-limited nations must consider their response to potential contractions in international support for HIV programs.
Objective
To evaluate the clinical, epidemiological, and budgetary consequences of alternative HIV program scale-back strategies in two recipient nations: the Republic of South Africa (RSA) and Côte d’Ivoire (CI).
Design
Model-based comparison of Current Standard (presenting CD4 0.260*109 cells/L; universal ART eligibility; 84% 5y-retention) and scale-back alternatives including: reduced HIV detection; No ART or delayed (CD4<0.350*109 cells/L) ART initiation; reduced investment in retention; and no viral load monitoring or 2nd-line ART.
Data Sources
Published RSA- and CI-specific estimates of the HIV care continuum, ART efficacy, and HIV-related costs.
Target Population
HIV-infected persons, including future incident cases.
Perspective
Modified societal perspective, excluding time and productivity costs.
Time Horizon
Five and ten years.
Outcome Measures
HIV transmissions and deaths, years of life, and budgetary outlays (2015USD).
Base Case Analysis
At 10 years, scale-back strategies increase projected HIV transmissions by 0.5–19.4% and deaths by 0.6–39.1%. Strategies can produce budgetary savings up to 30% but no more. Compared to the Current Standard, nearly every scale-back strategy produces proportionally greater HIV deaths (and transmissions, in RSA) than savings. When applying the least harmful and most efficient alternatives for achieving budget cuts of 10–20%, every year of life lost will save roughly $900 in HIV-related outlays in RSA and $600–900 in CI.
Results of Sensitivity Analysis
Scale-back programs, when combined, may result in clinical and budgetary synergies and offsets.
Limitations
The magnitude and details of budget cuts are not yet known, nor is the degree to which other international partners might step in to restore budget shortfalls.
Conclusions
Scaling back international aid to HIV programs will have severely adverse clinical consequences; for similar economic savings, certain programmatic scale-back choices result in less harm than others.
Keywords: HIV, treatment cascade, PEPfAR, survival, deaths, transmissions, budget
INTRODUCTION
For over a decade, the international HIV research and implementation communities have concentrated on scaling up HIV treatment and prevention activities. More recently, they have focused on optimizing investments in meeting the Joint United Nations Program on HIV/AIDS (UNAIDS) 90-90-90 targets by 2020 – to diagnose 90% of people living with HIV; to link 90% of persons diagnosed with HIV to antiretroviral treatment (ART); and to achieve 90% virologic suppression among those in treatment – and end the AIDS epidemic (1). The clinical benefit and economic attractiveness of such a scale-up, if not the feasibility of such an endeavor in the wake of a global economic crisis, are well established (1–4).
During the late 1990s and early 2000s, global HIV programs enjoyed robust support and success in providing ART and HIV prevention activities to resource-limited settings (5). In the last decade, however, funding has plateaued, suggesting both donor fatigue and mounting global political resistance (6). Opposition to increasing investment in the President’s Emergency Plan for AIDS Relief (PEPFAR) surfaced in 2008, when it was openly questioned by the Obama administration and some health and development experts (7–12). Most recently, the White House proposed cutting the US foreign aid budget by one-third, affecting more than $6.7 billion currently earmarked for HIV/AIDS prevention, care, and research under PEPfAR; The Global Fund to Fight HIV, Tuberculosis and Malaria; the National Institutes of Health (NIH); the Centers for Disease Control and Prevention (CDC), and the United States Agency for International Development (USAID) (13, 14). While claiming that US aid will provide “sufficient resources to maintain current commitments,” the proposal jeopardizes both the current pace of HIV treatment scale-up and the accelerated pace toward 90-90-90 (13, 15). Finally, in 2014, the Global Fund began to exclude middle-income countries from future HIV/AIDS support; PEPFAR made similar plans for some Caribbean countries, suggesting that both multilateral and bilateral agencies have already begun to triage support for HIV/AIDS (16, 17).
To date, research on global financing for HIV prevention and care has focused on questions of scale-up and budget expansions. Little is known, however, about the impact of HIV program contraction. The history of global health shows that budgetary shifts and changes in priorities occur more commonly than is generally acknowledged; we seek now to anticipate and proactively address them (18–20). For donor countries, better information regarding the clinical, epidemiological, and economic impact of cutbacks might promote a reasoned conversation about the appropriate level of development aid. Among recipient nations, analyses might assist health authorities to identify the least harmful approaches to managing program trade-offs in the context of tighter budgets and the degree to which such approaches must be tailored from one setting to the next.
METHODS
Analytic Overview
We used the Cost-Effectiveness of Preventing AIDS Complications-International (CEPAC-I) model, a first-order Monte Carlo simulation of HIV disease progression and treatment in resource-limited settings to compare the performance of alternative approaches to scaling back HIV testing, linkage, treatment, monitoring, and retention activities as a means of budgetary contraction (2). The analysis follows 10 annual cohorts of HIV-infected persons, beginning in 2016, accounting for new infections, new diagnoses, ART initiations, loss-to-follow-up (LTFU), and ART-associated virologic outcomes. Performance measures include: new HIV transmissions, deaths, years of life accrued, and overall HIV-related financial outlays (international or domestic), measured in undiscounted and discounted (for incremental cost-effectiveness analysis) 2015 US dollars. Each alternative scale-back approach is compared to a “Current Standard” benchmark, representing the present-day state of treatment. We conducted the analysis, over 5- and 10-year horizons, in the Republic of South Africa (RSA) and in Côte d’Ivoire (CI) to highlight important differences between the countries including: HIV disease burden (RSA: 6.7 million HIV-infected, prevalence 19.2%; CI: 440,000 HIV-infected, prevalence 3.2%) (21); HIV treatment cascades (proportion undiagnosed: RSA 43.3%, CI 57.0%) and standards of care (22–25); ratio of HIV care costs compared to ART costs (RSA high, CI low); per capita Gross Domestic Product (GDP: RSA $5,700, CI $1,400) (26); and relative domestic contribution to their overall HIV/AIDS program budget (in 2011: RSA 78%, CI 7%) (23, 27).
The Current Standard and Scale-back Strategies
The Current Standard emulates the status quo in RSA and CI, calibrated and validated to current data and guidelines (Table 1, Appendix Table 1) (2, 22, 25): HIV detection leads to ART initiation (mean CD4 0.260*109 cells/L) (28, 29); everyone is ART-eligible, regardless of CD4 count; 5-year retention in care is 84% (30–32). Although routine viral load monitoring is largely available in RSA, access is limited in CI (~7% in 2016); 2nd-line ART is available in both settings (22, 23, 25).
Table 1.
Selected model input values for an analysis of potential HIV program budget cuts in the Republic of South Africa and Côte d’Ivoire.
| Republic of South Africa | Côte d’Ivoire | ||||
|---|---|---|---|---|---|
|
| |||||
| Cohort | Mean CD4 (SD), 109 cells/L* | Weighted Distribution, % | Mean CD4 (SD), 109 cells/L* | Weighted Distribution, % | Reference |
| Incident | 0.667 (0.134) | N/A | 0.667 (0.134) | N/A | (28, 41, 42) |
| Prevalent undiagnosed | 0.434 (0.255) | 43.3 | 0.390 (0.191) | 57.0 | (24, 28, 34, 37, 50) |
| Prevalent, on first-line ART | 0.454 (0.246) | 33.7 | 0.395 (0.133) | 30.7 | (24, 28, 38, 50) |
| Prevalent, on second-line ART | 0.454 (0.246) | 1.8 | 0.334 (0.142) | 1.3 | (24, 28, 38, 39, 50) |
| Prevalent diagnosed, not in care | 0.257 (0.080) | 21.2 | 0.259 (0.198) | 11.0 | (24, 28, 29, 34, 37, 50) |
|
| |||||
| Cohort Characteristics | Republic of South Africa Value | Côte d’Ivoire Value | Reference | ||
|
| |||||
| Gender distribution, % female/male | 58/42 | 75/25 | (28, 50) | ||
| HIV RNA distribution after acute infection, % | (43, 46) | ||||
| >100,000 copies/ml | 42 | 34 | |||
| 30,001–100,000 copies/ml | 28 | 25 | |||
| 10,001–30,000 copies/ml | 18 | 15 | |||
| 3,001–10,000 copies/ml | 8 | 20 | |||
| ≤3,000 copies/ml | 4 | 6 | |||
|
| |||||
| Cohort Characteristics | Both Settings | Reference | |||
|
| |||||
| Mean ART efficacy, % virologic suppression at 48 weeks | 78 | (44) | |||
| Resuppression, % suppressed at 16 weeks | 54 | (45) | |||
| Loss to follow-up, probability in care at 5 (1) years, % | 84 (96) | (30–32) | |||
| Return to care | |||||
| Return to care probability after one year, monthly % | 1 | Assumption | |||
| Return to care probability upon WHO stage 3/4 OD, % | 50 | Assumption | |||
| Transmission rates (per 100PY), by disease stage and viral load | (48, 49) | ||||
| Incident infection (6 months post infection) | 65.5 | ||||
| Late stage disease (CD4 <0.200 *109 cells/L) | 9.03 | ||||
| >100,000 copies/ml | 9.03 | ||||
| 10,001–100,000 copies/ml | 8.12 | ||||
| 3,001–10,000 copies/ml | 4.17 | ||||
| 501–3,000 copies/ml | 2.06 | ||||
| 21–500 copies/ml | 0.16 | ||||
| ≤20 copies/ml | 0.16 | ||||
|
| |||||
| Cohort Characteristics | Republic of South Africa Value | Côte d’Ivoire Value | Reference | ||
|
| |||||
| Condom transmission reduction, % | 29.0 | 28.8 | (50–52) | ||
|
| |||||
| Costs, 2015 USD | |||||
|
| |||||
| ART Costs | (25) | ||||
| First-line ART, monthly (annually) | 10 (114) | 10 (114) | |||
| Second-line ART, monthly (annually) | 28 (331) | 28 (331) | |||
| Monitoring costs | (56) | ||||
| HIV viral load, cost per test | 32 | -- | |||
| CD4 count, cost per test | 6 | 9 | |||
| Routine care cost, monthly (ranges by CD4 count) | 18–140 | 26–36 | (54–56) | ||
| OD treatment costs (ranges by OD) | 217–728 | 58–402 | (55, 59) | ||
Abbreviations: SD, standard deviation; ART, antiretroviral therapy; PY, person years; OD, opportunistic disease; WHO, World Health Organization; USD, United States Dollars, N/A, not applicable
When reported as medians and interquartile ranges, CD4 cell counts were converted to means and standard deviations for reporting purposes.
We defined scale-back strategies encompassing a selection of programmatic alternatives (i.e. ‘policy levers’) available to national health authorities if funds are decreased (Appendix Table 1). Strategies 1–3 below relate to entry into care and ART initiation criteria; strategies 4–6 apply to people already receiving treatment, as well as to anyone newly initiated on ART.
No New ART. ART continues for patients already in care, but HIV screening activities are suspended; no new offers of treatment initiation are made. We assume that commitments to provide treatment to anyone in whom ART has already been initiated are maintained (13, 33).
Late Presentation. Reduced HIV testing and linkage rates result in a lower mean CD4 count at case identification (RSA 0.160*109 cells/L, CI 0.194*109 cells/L). This strategy maintains the World Health Organization (WHO) guideline of ART initiation regardless of CD4 count (29, 34, 35).
ART 0.350*109 cells/L. The CD4 treatment threshold is reduced to <0.350*109 cells/L. Patients above the threshold receive bi-annual CD4 monitoring until ART eligibility, consistent with WHO recommendations prior to 2010 (36).
Reduced Retention. This strategy simulates fewer clinics and providers, longer wait times, and limited investment in retention/adherence. Under this strategy, the fraction of people on ART and retained in care at 1 and 5 years falls from 96%/84% (Current Standard) to 92%/70%.
No Viral Load (VL) Testing. All viral load testing (routine and confirmatory) is eliminated and replaced with twice-annual CD4 counts to monitor ART success. This strategy applies only to RSA.
No 2nd-line. Patients failing 1st-line ART are provided adherence counseling and an opportunity for 1st-line resuppression (as under Current Standard) but do not have the opportunity to switch to a 2nd-line regimen.
To examine potential offsets or synergies of clinical outcomes and cost-savings, we also examined combinations of the strategies outlined above: 7) Late Presentation+ART 0.350*109 cells/L; 8) Late Presentation+Reduced Retention; 9) ART 0.350*109 cells/L+Reduced Retention; 10) Late Presentation+ART 0.350*109 cells/L+Reduced Retention; and 11) No VL+No 2nd-line (only for RSA).
To help stakeholders understand the clinical and fiscal consequences of alternative choices, we begin by reporting the absolute and proportional changes in transmissions, deaths, years of life lost, and budget for each of the scale-back strategies, compared to the Current Standard, on an undiscounted basis. Then, adopting the perspective of the in-country decision maker, we report discounted outcomes, seeking to identify the least harmful way of achieving a given level of budget reduction (discounted).
Cohort Definitions
At model outset, we define the current population – the “2016 prevalent cohort” – of HIV-infected adults (≥15 years) using country-specific data on the proportion of people currently in each stage of care (Table 1) (24, 28, 37–39). In each subsequent year, we introduce a new incident cohort and combine outcomes from all prior incident cohorts and the 2016 prevalent cohort.
The CEPAC-I Model
CEPAC-I is populated with natural history, treatment and cost data from RSA and CI (38, 40). Random draws from distributions generate individual patient characteristics (e.g., age), some of which (e.g., CD4) are specific to HIV detection and treatment stage (Table 1, top). CEPAC-I simulates HIV detection, linkage to care, ART initiation, virologic suppression, and care retention. CEPAC-I also simulates the natural history of untreated HIV disease, including immunologic/CD4 decline and increased risk of opportunistic diseases (OD). HIV is detected through the development of an OD, or via intermittent HIV screening. Patients virologically suppressed on ART experience a CD4 increase; those who are not suppressed achieve no CD4 increase and accrue ART costs (40–43).
For both countries, the 48-week virologic suppression rate is 78% for those initiating 1st-line ART, with an opportunity for resuppression after 1st-line failure (54% suppression at 16 weeks) (44, 45). In RSA, HIV RNA monitoring occurs according to country-specific guidelines; HIV RNA is not routinely monitored in CI (22, 23, 25). Patients in whom 1st-line failure is again detected after an opportunity at resuppression may be switched to a 2nd-line regimen, if patients meet national criteria for switching (22, 25). In the Current Standard, patients in care face a monthly risk of LTFU ranging from 0.2–1.1%, depending on adherence; this results in an 84% 5-year probability of remaining in care (30–32).
Transmissions
Total monthly transmissions are computed by multiplying published estimates of viral-load-specific transmission rates (Table 1) (48, 49) by model-generated estimates of patients in each viral load category. These categories depends on both baseline HIV RNA (43, 46) and clinical status: acutely infected (47); virologically suppressed; either becoming suppressed or rebounding; or advanced disease (CD4 <0.200*109 cells/L). During the 6 months of acute infection, we amplify transmissions 7.25-fold (49). We also reduce transmissions to reflect population-specific rates of condom use (RSA 29.0%, CI 28.8%) (50–52).
Costs
In both RSA and CI, 1st- and 2nd-line ART costs are $114 and $331/year (53). HIV-related care costs include OD treatment, laboratory monitoring, and CD4-stratified routine care costs; these differ between countries (Table 1). For example, CD4-stratified routine care costs (excluding ART and laboratory costs) range in RSA from $18–140/month and in CI from $26–36/month (54–56). Per patient annual costs (in HIV care) from model output were validated to published data in RSA and other similar settings (2, 57, 58); while derived similarly from CI-specific cohorts, CI aggregate costs were not externally validated due to a lack of published, CI-specific micro-costing reports (54–56, 59).
RESULTS
Clinical Outcomes
Under the Current Standard, 3.240 million new HIV transmissions will occur in the next 10 years in RSA. In the same 10-year period, HIV-infected South Africans will experience 4.258 million deaths and a total of 63.957 million years of life. In CI, 225,000 new HIV transmissions are projected in the next decade, with HIV-infected persons experiencing an estimated 270,000 deaths and 4.234 million years of life (Table 2; Appendix Tables 2–6 present these results at 2, 5, and 10 years and as a percentage change from Current Standard). Compared to these benchmarks, every scale-back strategy would result in a greater number of HIV transmissions, a greater number of deaths, and fewer years of life over a 10-year horizon. In RSA, the increase in transmissions would range from 38,000 (1.2%, No 2nd-line) to 630,000 (19.4%, No New ART), and the increase in deaths would range from 31,000 (0.7%, No 2nd-line) to 1.664 million (39.1%, No New ART). Similar results would be observed in CI, with the largest increase in transmissions (24,000, 10.5%) and deaths (93,000, 34.6%) occurring under the No New ART strategy.
Table 2.
Clinical and economic outcomes of programmatic cuts to HIV programs in the Republic of South Africa and Côte d’Ivoire at 10 years.*
| Strategy | Republic of South Africa* | Côte d’Ivoire* | ||||||
|---|---|---|---|---|---|---|---|---|
| Transmissions | Deaths | Years of Life | Budget, $M | Transmissions | Deaths | Years of Life | Budget, $M | |
| Current Standard | 3,240,000 | 4,258,000 | 63,957,000 | 32,180 | 225,000 | 270,000 | 4,234,000 | 1,500 |
|
| ||||||||
| Individual Cost-Containment Strategies Considered† | Additional Transmissions | Additional Deaths | Years of Life Lost | Budget Savings, $M‡ | Additional Transmissions | Additional Deaths | Years of Life Lost | Budget Savings, $M‡ |
|
| ||||||||
| No New ART | 630,000 | 1,664,000 | 8,518,000 | 7,740 | 24,000 | 93,000 | 479,000 | 420 |
| Late Presentation | 470,000 | 945,000 | 4,765,000 | 4,290 | 12,000 | 40,000 | 210,000 | 200 |
| ART 0.350*109 cells/L | 213,000 | 91,000 | 364,000 | 50 | 3,000 | 2,000 | 8,000 | 10 |
| Reduced Retention | 188,000 | 308,000 | 1,289,000 | 1,040 | 8,000 | 16,000 | 66,000 | 110 |
| No VL | 206,000 | 46,000 | 132,000 | −320 | - | - | - | - |
| No 2nd-line ART | 38,000 | 31,000 | 104,000 | 50 | 1,000 | 2,000 | 7,000 | 30 |
|
| ||||||||
| Combination Cost-Containments Strategies Considered† | Additional Transmissions | Additional Deaths | Years of Life Lost | Budget Savings, $M‡ | Additional Transmissions | Additional Deaths | Years of Life Lost | Budget Savings, $M‡ |
|
| ||||||||
| Late Presentation, ART 0.350*109 cells/L | 493,000 | 959,000 | 4,842,000 | 4,310 | 13,000 | 41,000 | 212,000 | 200 |
| Late Presentation, Reduced Retention | 608,000 | 1,168,000 | 5,748,000 | 5,120 | 18,000 | 53,000 | 264,000 | 290 |
| ART 0.350*109 cells/L, Reduced Retention | 390,000 | 395,000 | 1,623,000 | 1,050 | 11,000 | 17,000 | 72,000 | 110 |
| Late Presentation, ART 0.350*109 cells/L, Reduced Retention | 629,000 | 1,181,000 | 5,791,000 | 5,140 | 20,000 | 53,000 | 266,000 | 290 |
| No VL, No 2nd-line ART | 234,000 | 69,000 | 227,000 | −80 | - | - | - | - |
Abbreviations: ART: antiretroviral therapy; M: million
In 2016: RSA: 6.7 million HIV-infected (prevalence 19.2%); CI: 440,000 HIV-infected (prevalence 3.2%).
Each strategy is compared with the Current Standard, generally resulting in more transmissions and deaths and less years of life and costs accrued.
Negative numbers in the Budget Savings column denote that this strategy increases, rather than decreases, costs.
Budget Impact
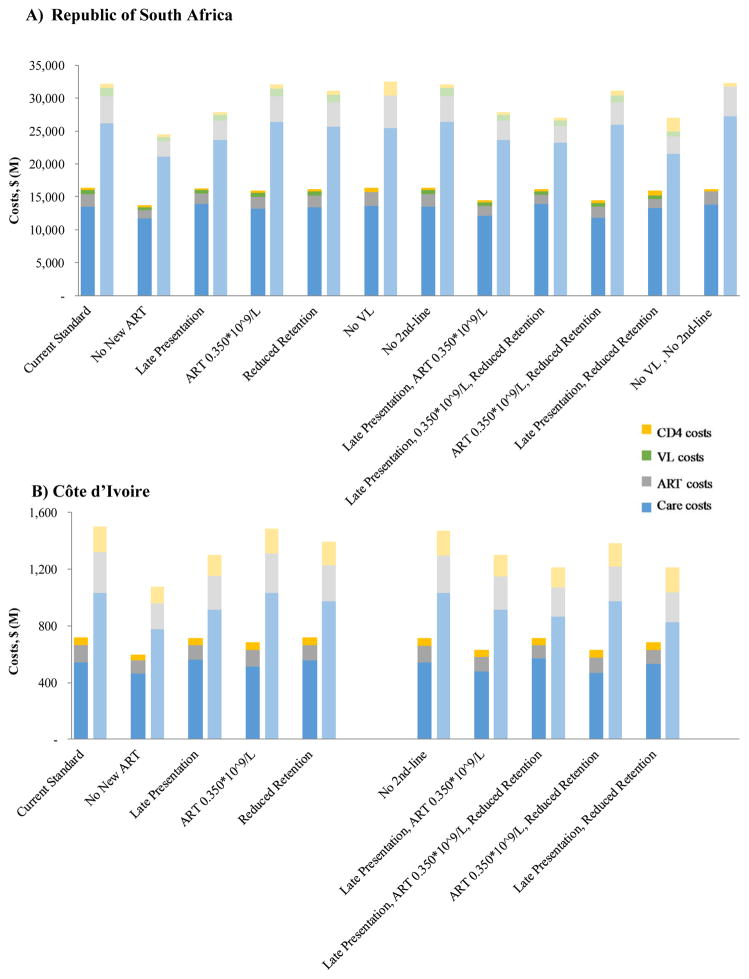
The scale-back strategies would produce 10-year reductions in financial outlays ranging from −$320 million (−1.0%, i.e., cost increases) to $7.740 billion (24.1%) in RSA and $10 million (0.7%) to $420 million (28.1%) in CI. These findings suggest that budgetary savings up to 30% but no greater can be achieved over the next decade, using the scale-back strategies we examine. Moreover, scale-back strategies would not produce constant changes in annual outlays over time (Table 2, Figure 1, Appendix Tables 3 and 5). No VL in RSA, for example, would produce a small cost savings over 2 and 5 years ($300 and $230 million) but increased cumulative outlays by year 10, due to less sensitive methods to detect ART failure and increased costs of 2nd-line treatment (Table 2 and Appendix Tables 3 and 5). By contrast, the savings from the No New ART would approximately triple in RSA (and CI) from $2.680 billion ($120 million) over 5 years (Appendix Tables 3 and 4) to $7.740 billion ($420 million) by year 10. The large savings under the No New ART Strategy reflect high mortality caused by failing to treat newly infected cases (Table 2, Appendix Table 2). In CI, the budgetary savings from any given scale-back strategy are almost always proportionally greater than in RSA, owing to the greater relative cost of drugs versus labor in CI.
Figure 1. 5- and 10-year HIV budget breakdown for alternative HIV/AIDS scale-back programs in the Republic of South Africa (A) and Côte d’Ivoire (B).
5-year outcomes are denoted by the dark bars on the left of each pair; 10-year outcomes by the light bars on the right. Alternative scale-back strategies are arrayed along the horizontal axis; the benchmark of the Current Standard is at the extreme left. Colors denote the type of expense (care [blue], ART [gray], VL monitoring [green], and CD4 monitoring [yellow]).
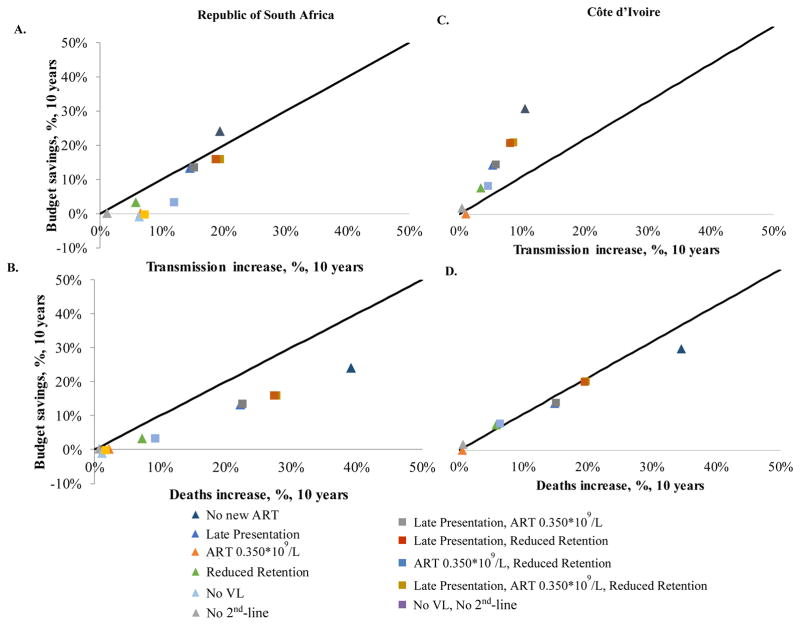
Proportional Clinical Harm and Budgetary Benefit
In RSA, nearly every scale-back program would produce a higher percent increase in adverse events (transmissions, Figure 2A; deaths, Figure 2B) than it would a percent decrease in outlays (Appendix Table 2), when compared to the Current Standard over 10 years. The No New ART program (dark blue triangle) is the only exception, where proportional savings (24.1%) would exceed the percent increase in transmissions (19.4%). In CI, the increased number of HIV transmissions resulting from any given scale-back strategy would be proportionally smaller than in RSA (Figures 2C and 2D). This reflects proportionally higher HIV transmissions in CI than RSA under the Current Standard, given higher baseline levels of undiagnosed HIV infection and lower levels of virologic suppression in the absence of viral load monitoring (Figures 2C and 2D).
Figure 2. Comparison of proportional transmission increases and death increases versus proportional budget reductions for HIV scale-back strategies in the Republic of South Africa (A and B) and Côte d’Ivoire (C and D).
The figure compares the percent increase in adverse events against the percent decrease in financial outlays for each scale-back strategy, relative to the Current Standard. The vertical axis of each panel denotes the magnitude of the budgetary savings that can be achieved; the horizontal axis denotes the concomitant proportional increase in transmissions (panels A and C) and deaths (panels B and D). The solid black line with slope equal to 1 represents the “line of identity”; this denotes instances where the percent increase in adverse events equals the percent decrease in financial outlays. Different scale-back strategies are denoted by different colored points. Programs represented by triangles (single programmatic cuts) and squares (combination programmatic cuts) that lie above the line of identity indicate scale-back strategies that deliver greater proportional budgetary savings than the offsetting proportional increase they produce in transmissions or deaths. The figure reports the proportional increase in undiscounted adverse events produced by a given proportional reduction in the budget (undiscounted). It does not represent an assessment of economic value or incremental cost-effectiveness. Because of differences in the ratio of labor compared to ART costs between the two countries, most interventions in CI save proportionally more money, exceeding the line of identity for transmissions and approaching it for deaths.
Combination Scale-back Programs
Combining scale-back programs would produce some noteworthy interactions (Table 2, bottom, Appendix Table 7). Some combinations would have roughly the same aggregate impact on transmissions, deaths, and the budget as their individual component parts (e.g., ART 0.350*109 cells/L+Reduced Retention). Other combinations would produce fewer adverse outcomes than the sum of their components (e.g., Late Presentation+ART 0.350*109 cells/L). Still other combinations would yield lower budgetary savings than the sum of their parts (e.g., No VL+No 2nd-line).
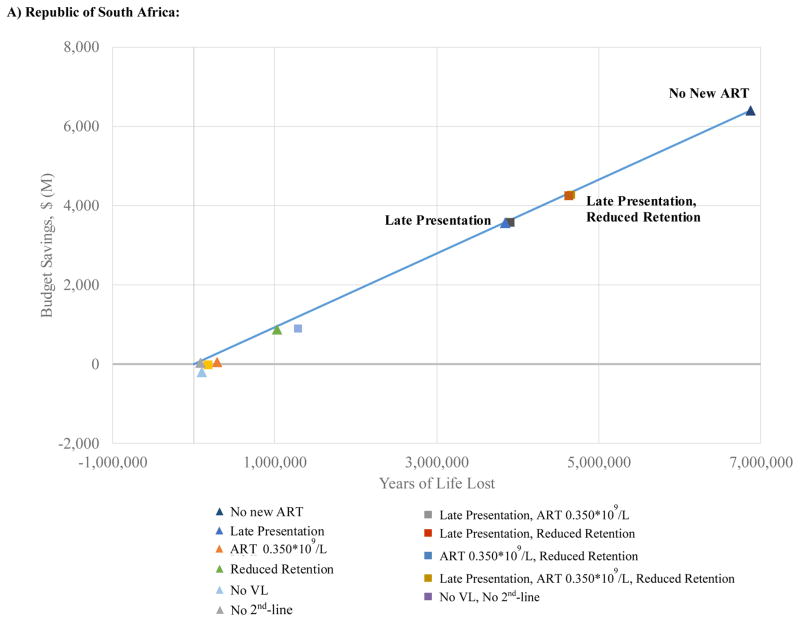
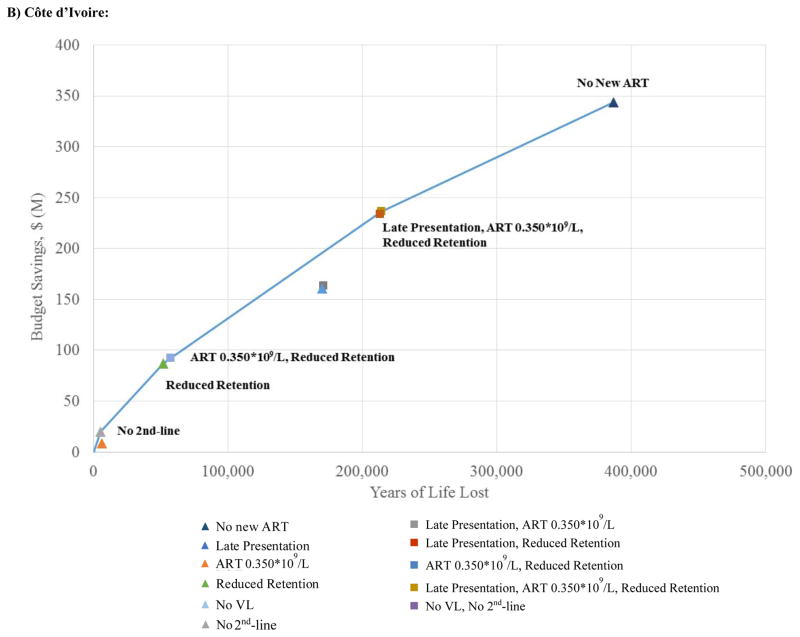
Efficiency Frontiers
Figure 3 aims to inform decision making by portraying both discounted years of life lost (horizontal axis) and discounted costs (vertical axis) for each of the scale-back strategies. The bold lines represent the “efficiency frontier,” a demarcation of the least harmful means of achieving any given reduction in the overall budget. A large number of strategies are on or close to the efficiency frontier in RSA at 10 years, including: Late Presentation (13% budgetary savings); Late Presentation+Reduced Retention (16%); and No New ART (24%). In CI, five strategies create the efficiency frontier: No 2nd-line (2% budgetary savings); Reduced Retention (7%); ART 0.350*109 cells/L+Reduced Retention (8%); Late Presentation+ART 0.350*109 cells/L+Reduced Retention (19%): and No New ART (28%). Implicit in the shape of the efficiency frontier is a tradeoff between life-expectancy and savings: for any budget cut of >10%, every year of life lost by implementing any one of the programs at or close to the efficiency frontier will save roughly $900 in HIV-related outlays in RSA and $600–$900 in CI.
Figure 3. Efficiency frontier at ten years (results discounted at 3% per year) for alternative HIV programmatic cuts in the Republic of South Africa (A) and Côte d’Ivoire (B).
The figure aims to inform decision making by portraying 10-year outcomes on a discounted basis. Colored points indicate different programmatic scale-back strategies examined. Triangles designate single programmatic cuts; squares denote combination programmatic cuts. The solid line represents the efficiency frontier. Each point represents the projected discounted budgetary savings (vertical axis) and anticipated years of life lost (horizontal axis) for a given strategy. Decision makers can maximize years of life for a given budget by opting for strategies that lie on the efficiency frontier.
DISCUSSION
Several key findings emerge from this analysis. First, the scale-back strategies we examine can accommodate budget cuts up to 30%. Commitments to patients already receiving care for their HIV infection make further budget contractions infeasible. Second, with few exceptions, any early cost savings are likely to be offset by the downstream costs of increased HIV transmissions. Third, nearly every scale-back strategy, when compared to the Current Standard, is likely to produce proportionally greater individual and population-level harm than economic savings. For example, although a strategy of decreased case identification (Late Presentation) reduces the HIV program budget (RSA 13%, CI 13%), deaths are projected to increase (RSA 22%, CI 15%). Finally, our analysis likely underestimates the number of adverse outcomes by ignoring the “youth bulge” (i.e., the demographic expansion in the number of teenagers and young adults – a sub-population at high risk for new HIV infection—in many resource-limited nations with HIV epidemics) (60).
In-country decision makers need to take note of the synergies and offsets that may exist between different alternatives for budget cutting. Shared cost structures and competing risks for harm mean that the combined effect of simultaneously implementing more than one scale-back strategy may be substantially different than the sum of the individual effects. Moreover, these differences will fluctuate from one nation to the next, reflecting variation in the relative costs of different health and medical inputs (e.g., drugs; labor) and in clinical practices and HIV epidemiology.
Finally, we note that better and worse choices exist within this set of bad alternatives. If circumstances compel in-country authorities in RSA to cut their HIV spending dramatically, the following three scale-back strategies will, for example, produce budgetary savings ranging from $4.3 billion to $7.7 billion (13–24%) over 10 years while doing the least possible harm: delaying presentation to care by limiting HIV screening activities; delaying presentation while also reducing spending on care retention; or withholding all new ART initiation. In CI, implementing either one of the following two scale-back strategies will produce budgetary savings of roughly $290 million to $420 million (19–28%) over 10 years: delaying presentation while simultaneously limiting ART eligibility to CD4 <0.350*109 cells/L and reducing spending on retention in care; or withholding all new ART initiation. These findings should be interpreted with caution; they are proposed here as the least deleterious alternatives from among a menu composed entirely of options that will cause clinical and public health harm. Indeed, for every year of life lost by implementing one of these strategies, the RSA HIV program will save roughly $900 in HIV-related outlays. In CI, with budget cuts of at least 10%, these scale-back strategies will cost a year of life for every $600–$900 saved. We leave it to readers to draw their own conclusions about whether imposing such tradeoffs on vulnerable populations accurately reflects how donor countries value life in recipient nations.
HIV funding in every resource-limited setting comes from multiple sources: domestic, multilateral, and bilateral. The mix differs from country to country, as does the type of care each funder supports. Our framework ignores domestic- and funder-specific details, as they pertain to particular line items. This reflects our view that waning support for HIV programs is a general phenomenon and our reluctance to assume that a given funder will be willing and able to jump in to fill any gaps created by other funders.
This analysis highlights the importance of considering both the proportional and the absolute cost of donor cutbacks to affected countries. Viewed on a proportional basis, the impact of cutbacks on smaller nations that depend more heavily on external sources will be devastating. In CI, for example, over 90% of HIV financing is obtained from international sources (23). A 10% cut in PEPFAR funding alone will reduce the overall HIV budget in that country by around 9%, or by $20 million. Viewed on an absolute basis, the impact of cutbacks on larger nations with more generalized epidemics will also be devastating. In RSA, for example, where the majority of HIV spending is self-financed, that same 10% cut in PEPFAR funding might only represent around 2% of the RSA HIV budget, but it will produce a much greater $40 million absolute budgetary setback (27). Both the proportional and absolute magnitude of the costs of potential scale-backs will influence the extent to which nations may be able to absorb those cuts.
Several limitations are noteworthy. First, we do not know how budget scale-backs will be imposed across recipient nations – whether all recipients would face the same, across-the-board, percent reduction; or whether cuts would be equally applied across expenditure types (e.g. among infrastructure, laboratory, and drugs); or whether prevention and treatment programs would be handled differently. Further, there are likely to be many other types of program scale-backs than those we considered here. In our view, these represent the key uncertainties of our analysis. Although we have not conducted a more traditional, parameter-driven sensitivity analysis, we have tried to manage the critical uncertainties by examining a wide variety of venues, planning horizons, and budget-cutting scenarios representing relatively recent initiatives and program expansions. Finally, our analysis did not capture cost shifts that might occur due to reduced testing programs, overall clinic closures, re-purposing of “brick and mortar” clinic sites, or how program contraction would affect the growing workforce employed to combat HIV.
We demonstrate that scale-back of international aid to HIV programs in resource-limited settings will reverse enormous strides made over the last 20 years in curbing the global HIV epidemic and in improving HIV-related survival (61). Our findings suggest that reduced HIV foreign aid will produce modest savings to donors at the expense of HIV epidemic revival and massive loss of life among recipient nations. Nonetheless, should these cutbacks materialize, in-country policy makers will be forced to confront profound ethical dilemmas in allocating remaining resources while minimizing – though not entirely avoiding – harm to individuals and communities. To be clear, we are neither endorsing any of these painful choices, nor excusing the political decisions that may make them necessary. But we demonstrate that it is possible to assess the clinical, epidemiologic, and economic effects of alternative scale-back scenarios and that some decisions made in response to the imposition of budget cuts will do less harm than others.
Supplementary Material
Acknowledgments
The authors would like to thank Alex Bulteel for his technical assistance.
This research was funded by the National Institutes of Health (R01 AI058736, R37 AI093269, K01 HL123349, R01 MH105203, R01 DA015612) and by the Steve and Deborah Gorlin MGH Research Scholars Award (Executive Committee on Research to RPW). The funding sources had no role in the design, analysis, or interpretation of the study or in the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Walensky had access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
References
- 1.90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2014. [Accessed 14 July 2017]. at http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. [Google Scholar]
- 2.Walensky RP, Borre ED, Bekker LG, Resch SC, Hyle EP, Wood R, et al. The anticipated clinical and economic effects of 90-90-90 in South Africa. Ann Intern Med. 2016;165(5):325–33. doi: 10.7326/M16-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams BG, Gupta S, Wollmers M, Granich R. The impact and cost of ending AIDS in Botswana. Lancet HIV. 2016;3(9):e409. doi: 10.1016/S2352-3018(16)30116-3. [DOI] [PubMed] [Google Scholar]
- 4.Schneider K, Garrett L. The end of the era of generosity? Global health amid economic crisis. Philos Ethics Humanit Med. 2009;4:1. doi: 10.1186/1747-5341-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daschle T, Frist B. The case for strategic health diplomacy: a study of PEPFAR. Washington, DC: Bipartisan Policy Center; 2015. [Accessed 12 July 2017]. at http://cdn.bipartisanpolicy.org/wp-content/uploads/2015/11/BPC_Strategic-Health-November-2015.pdf. [Google Scholar]
- 6.Schneider MT, Birger M, Haakenstad A, Singh L, Hamavid H, Chapin A, et al. Tracking development assistance for HIV/AIDS: the international response to a global epidemic. AIDS. 2016;30(9):1475–9. doi: 10.1097/QAD.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonsalves G. Obama’s zero-sum game in the fight against AIDS. [Accessed 12 July 2017];The Washington Post. 2011 Jan 13; at http://www.washingtonpost.com/wp-dyn/content/article/2011/01/13/AR2011011303922.html.
- 8.Nattrass N, Gonsalves G. Economics and the backlash against AIDS-specific funding. Cape Town, South Africa: Centre for Social Science Research, University of Cape Town; 2009. [Accessed 13 July 2017]. at http://open.uct.ac.za/bitstream/handle/11427/19305/Nattrass_Economicsbacklash_2009.pdf?sequence=1. [Google Scholar]
- 9.Denny CC, Emanuel EJ. US health aid beyond PEPFAR: the Mother & Child Campaign. JAMA. 2008;300(17):2048–51. doi: 10.1001/jama.2008.556. [DOI] [PubMed] [Google Scholar]
- 10.England R. Are we spending too much on HIV? BMJ. 2007;334(7589):344. doi: 10.1136/bmj.39113.402361.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halperin D. Putting a plague in perspective. [Accessed 15 July 2017];The New York Times. 2008 Jan 1; at http://www.nytimes.com/2008/01/01/opinion/01halperin.html.
- 12.The Associated Press. Experts rethinking billions spent on AIDS: with lowered infection rates, some want to shift funds to other global ills. [Accessed 17 July 2017];NBCNews.com. 2008 at http://www.nbcnews.com/id/22726852/ns/health-aids/t/experts-rethinking-billions-spent-aids/#.WS1ozOsrLct.
- 13.Office of Management and Budget, The White House. [Accessed 16 July 2017];A new foundation for American greatness: budget of the US government fiscal year 2018. at https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/budget/fy2018/budget.pdf.
- 14.U.S. President’s Emergency Plan for AIDS Relief. [Accessed 10 July 2017];Fiscal Year 2004–2017 PEPFAR Funding. at https://www.pepfar.gov/funding/budget/
- 15.Office of Management and Budget, The White House. [Accessed 12 July 2017];America first: a budget blueprint to make America great again. 2017 at https://www.whitehouse.gov/sites/whitehouse.gov/files/omb/budget/fy2018/2018_blueprint.pdf.
- 16.Burrows D, Oberth G, Parsons D, McCallum L. Transitions from donor funding to domestic reliance for HIV responses: recommendations for transitioning countries. Australia: APM Global Health; 2016. [Accessed 11 July 2017]. at http://apmglobalhealth.com/sites/apmglobalhealth.com/files/documents/transition_from_donor_funding_apmg_aidspanfinal.pdf. [Google Scholar]
- 17.Antillean Media Group. [Accessed 17 July 2017];US pulls HIV funding from hard-hit Caribbean countries. 2017 at http://www.antillean.org/us-cuts-pepfar-caribbean-139414/
- 18.Packard RM. A history of global health: interventions into the lives of other peoples. Baltimore, MD: JHU Press; 2016. [Google Scholar]
- 19.Uplekar M, Raviglione MC. The “vertical-horizontal” debates: time for the pendulum to rest (in peace)? Bull World Health Organ. 2007;85(5):413–4. doi: 10.2471/07.041756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birn AE. The stages of international (global) health: histories of success or successes of history? Glob Public Health. 2009;4(1):50–68. doi: 10.1080/17441690802017797. [DOI] [PubMed] [Google Scholar]
- 21.AIDSinfo. Geneva, Switzerland: UNAIDS; [Accessed 16 July 2017]. at http://aidsinfo.unaids.org/ [Google Scholar]
- 22.Department of Health South Africa. National consolidated guidelines: for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria, South Africa: Department of Health for the Republic of South Africa; 2015. [Google Scholar]
- 23.The United States President’s Emergency Plan for AIDS Relief. [Accessed 12 July 2017];Côte d’Ivoire Country Operational Plan (COP) 2016 Strategic Direction Summary. at https://www.pepfar.gov/documents/organization/257653.pdf.
- 24.Granich R, Gupta S, Hall I, Aberle-Grasse J, Hader S, Mermin J. Status and methodology of publicly available national HIV care continua and 90-90-90 targets: a systematic review. PLoS Med. 2017;14(4):e1002253. doi: 10.1371/journal.pmed.1002253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Départment de Prise en Charge, Programme National de Lutte contre le Sida. Directives nationales pec adultes et adolescents 2015. Abidjan, Côte d’Ivoire: Ministére de la Santé et de la Lutte contre le Sida; 2015. [Google Scholar]
- 26.World Development Indicators. Washington, D.C: World Bank; [Accessed 11 July 2017]. at http://data.worldbank.org/ [Google Scholar]
- 27.The United States President’s Emergency Plan for AIDS Relief. [Accessed 16 July 2017];South Africa Country Operational Plan (COP) 2016 Strategic Drection Summary. at https://www.pepfar.gov/documents/organization/257632.pdf.
- 28.Messou E, Anglaret X, Duvignac J, Konan-N’Dri E, Komena E, Gnokoro J, et al. Antiretroviral treatment changes in adults from Côte d’Ivoire: the roles of tuberculosis and pregnancy. AIDS. 2010;24(1):93–9. doi: 10.1097/QAD.0b013e32832ec1c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siedner MJ, Ng CK, Bassett IV, Katz IT, Bangsberg DR, Tsai AC. Trends in CD4 count at presentation to care and treatment initiation in sub-Saharan Africa, 2002–2013: a meta-analysis. Clin Infect Dis. 2015;60(7):1120–7. doi: 10.1093/cid/ciu1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyle EP, Jani IV, Lehe J, Su AE, Wood R, Quevedo J, et al. The clinical and economic impact of point-of-care CD4 testing in Mozambique and other resource-limited settings: a cost-effectiveness analysis. PLoS Med. 2014;11(9):e1001725. doi: 10.1371/journal.pmed.1001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox MP, Rosen S. Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health. 2010;15(s1):1–15. doi: 10.1111/j.1365-3156.2010.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinkhof M, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris G. Cut to AIDS treatment programs could cost a million lives. [Accessed 15 July 2017];New York Times. 2017 May 23; at https://www.nytimes.com/2017/05/23/world/africa/cuts-to-aids-treatment-programs-could-cost-a-million-lives.html?emc=eta1&_r=0.
- 34.Minga AK, Coffie P, Lewden C, et al. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2013–2016. [Accessed 11 July 2017]. Early Identification and Treatment of Early HIV Infection in Côte d’Ivoire (PRECO-CI) at https://clinicaltrials.gov/ct2/show/NCT01917175. NLM Identifier: NCT01917175. [Google Scholar]
- 35.World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 36.World Health Organization. [Accessed 15 July 2017];Antiretroviral therapy for HIV infection in adults and adoslecents: recommendations for a public health approach, 2010 revision. at http://apps.who.int/iris/bitstream/10665/44379/1/9789241599764_eng.pdf. [PubMed]
- 37.Kranzer K, Lawn SD, Johnson LF, Bekker L-G, Wood R. Community viral load and CD4 count distribution among people living with HIV in a South African township: implications for treatment as prevention. J Acquir Immune Defic Syndr. 2013;63(4):498–505. doi: 10.1097/QAI.0b013e318293ae48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouattara EN, Robine M, Eholie SP, MacLean RL, Moh R, Losina E, et al. Laboratory monitoring of antiretroviral therapy for HIV infection: cost-effectiveness and budget impact of current and novel strategies. Clin Infect Dis. 2016;62(11):1454–62. doi: 10.1093/cid/ciw117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24(6):915–9. doi: 10.1097/QAD.0b013e3283360976. [DOI] [PubMed] [Google Scholar]
- 40.Walensky RP, Ross EL, Kumarasamy N, Wood R, Noubary F, Paltiel AD, et al. Cost-effectiveness of HIV treatment as prevention in serodiscordant couples. N Engl J Med. 2013;369(18):1715–25. doi: 10.1056/NEJMsa1214720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mossong J, Grapsa E, Tanser F, Bärnighausen T, Newell M-L. Modelling HIV incidence and survival from age-specific seroprevalence after antiretroviral treatment scale-up in rural South Africa. AIDS. 2013;27(15):2471. doi: 10.1097/01.aids.0000432475.14992.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 44.Barth RE, van der Loeff MFS, Schuurman R, Hoepelman AI, Wensing AM. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10(3):155–66. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 45.Jobanputra K, Parker LA, Azih C, Okello V, Maphalala G, Kershberger B, et al. Factors associated with virological failure and suppression after enhanced adherence counselling, in children, adolescents and adults on antiretroviral therapy for HIV in Swaziland. PLoS One. 2015;10(2):e0116144. doi: 10.1371/journal.pone.0116144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19(18):2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 47.Novitsky V, Essex M. Using HIV viral load to guide treatment-for-prevention interventions. Curr Opin HIV AIDS. 2012;7(2):117–24. doi: 10.1097/COH.0b013e32834fe8ff. [DOI] [PubMed] [Google Scholar]
- 48.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 49.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 50.Shisana O, Rehle T, Simbayi LC, Zuma K, Jooste S, Zungu N, Labadarios D, Onoya D, et al. South African national HIV prevalence, incidence and behaviour survey, 2012. Cape Town, South Africa: HSRV Press; 2014. [Accessed 13 July 2017]. at http://www.hsrc.ac.za/uploads/pageContent/4565/SABSSM%20IV%20LEO%20final.pdf. [Google Scholar]
- 51.Hakim AJ, Aho J, Semde G, Diarrassouba M, Ehoussou K, Vuylsteke B, et al. The epidemiology of HIV and prevention needs of men who have sex with men in Abidjan, Cote d’Ivoire. PLoS One. 2015;10(4):e0125218. doi: 10.1371/journal.pone.0125218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes KK, Levine R, Weaver M. Effectiveness of condoms in preventing sexually transmitted infections. Bull World Health Organ. 2004;82:454–61. [PMC free article] [PubMed] [Google Scholar]
- 53.ARV market report: the state of the antiretroviral dug market in low- and middle-income countries, 2014–2019. Boston, MA: The Clinton Health Access Initiative (CHAI); 2015. [Accessed 15 July 2017]. at http://www.clintonhealthaccess.org/content/uploads/2015/11/CHAI-ARV-Market-Report-2015_FINAL.pdf. [Google Scholar]
- 54.Cleary S, Boulle A, McIntyre D, Coetzee D. Cost-effectiveness of antiretroviral treatment for HIV-positive adults in a South African township. Durban, South Africa: Health Systems Trust; 2004. [Google Scholar]
- 55.Anglaret X, Chêne G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Côte d’Ivoire: a randomised trial. Lancet. 1999;353(9163):1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 56.AIDS2031 Costs and Financing Working Group. The long run costs and financing of HIV/AIDS in South Africa. Washington DC: Results for Development Institute; 2010. [Accessed 15 July 2017]. at http://www.resultsfordevelopment.org/sites/resultsfordevelopment.org/files/aids2031%20South%20Africa%20Report_Published%202010.pdf. [Google Scholar]
- 57.Meyer-Rath G, Miners A, Santos AC, Variava E, Venter WD. Cost and resource use of patients on antiretroviral therapy in the urban and semiurban public sectors of South Africa. J Acquir Immune Defic Syndr. 2012;61(3):e25–32. doi: 10.1097/QAI.0b013e31826cc575. [DOI] [PubMed] [Google Scholar]
- 58.Menzies NA, Berruti AA, Berzon R, Filler S, Ferris R, Ellerbrock TV, et al. The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. AIDS. 2011;25(14):1753–60. doi: 10.1097/QAD.0b013e3283463eec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yazdanpanah Y, Losina E, Anglaret X, Goldie SJ, Walensky RP, Weinstein MC, et al. Clinical impact and cost-effectiveness of co-trimoxazole prophylaxis in patients with HIV/AIDS in Côte d’Ivoire: a trial-based analysis. AIDS. 2005;19(12):1299–308. doi: 10.1097/01.aids.0000180101.80888.c6. [DOI] [PubMed] [Google Scholar]
- 60.United Nations Department of Economic and Social Affairs, Population Division. [Accessed 15 July 2017];Youth population trends and sustainable development. 2015 at http://www.un.org/en/development/desa/population/publications/pdf/popfacts/PopFacts_2015-1.pdf.
- 61.The United States President’s Plan for Emergency AIDS Relief. [Accessed 17 July 2017];Annual Report to Congress. 2017 at https://www.pepfar.gov/documents/organization/267809.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.