Abstract
Background
Little research in fragile X syndrome (FXS) has prospectively examined early social communication.
Aims
To compare early social communication in infants with FXS, infant siblings of children with autism spectrum disorder (ASIBs), and typically developing (TD) infants.
Methods and Procedures
Participants were 18 infants with FXS, 21 ASIBs, and 22 TD infants between 7.5–14.5 months. Social communication was coded using the Communication Complexity Scale during the administration of Autism Observation Scale for Infants.
Outcomes and Results
Descriptively different patterns were seen across the three groups. Overall infants with FXS had lower social communication than ASIBs or TD infants when controlling for nonverbal cognitive abilities. However, infants with FXS had similar levels of social communication as ASIBs or TD infants during peek-a-boo. No differences were observed between ASIBs and TD infants. For all infants, higher social communication was related to lower ASD risk.
Conclusions and Implications
Findings provide insight into the developmental course of social communication in FXS. The dynamic nature of social games may help to stimulate communication in infants with FXS. Language interventions with a strong social component may be particularly effective for promoting language development in FXS.
Keywords: fragile X syndrome, autism spectrum disorders, social communication development, communication complexity, behavioral phenotype
1. Introduction
Communication begins during social interactions between an infant and their caregiver (Trevarthen & Aitken, 2001). Through the use of eye gaze, gestures, and vocalizations, infants communicate their needs, wants, and interests (Bates, Benigni, Bretherton, Camaioni, & Volterra, 1979; Volterra, Caselli, Capirci, & Pizzuto, 2005). As these skills are integrated and used with increasing ease, infants and young children begin to engage in reciprocal communication interactions with others (Gros-Louis, West, & King, 2014; Iverson & Thal, 1998). Impairments in early social communication may lead to subsequent deficits in language and social development, such as those often seen in children with fragile X syndrome (FXS; Abbeduto, McDuffie, Brady, & Kover, 2012). However, little research in FXS has examined the development of social communication. Deficits in social communication have also been reported in infants later diagnosed with autism spectrum disorders (ASD; Zwaigenbaum, Bryson, & Garon, 2013). In this paper, we compare the complexity of social communication in two groups who are at a high risk for being diagnosed with ASD—infants with FXS and infant siblings of children with ASD (ASIBs)—to the complexity of social communication in typically developing (TD) infants. Approximately 25–74% of individuals with FXS meet diagnostic criteria for ASD (Kaufman et al., 2004; Harris et al., 2008) and approximately 20% of ASIBs are later diagnosed with ASD (Ozonoff, et al., 2011). Our goal was to identify common and unique aspects of early social communication across these three groups.
1.1. Early Social Communication in ASD
Social communication deficits are a core feature of idiopathic ASD (APA, 2013) and are the most common developmental disruption reported in studies of ASIBs, regardless of a later diagnosis of ASD (Ozonoff et al., 2014). Prospective longitudinal studies of ASIBs indicate that individuals who receive a diagnosis of ASD show similar social communication levels as TD infants at 6 months, but then show declining trajectories with impairments emerging by 12 to 18 months (Ozonoff et al., 2010; Zwaigenbaum et al., 2013). Also, impairments in specific skills related to social communication, such as reduced social engagement, shared enjoyment, and social orienting have been detected in the first year of life (Bhat, Galloway, & Landa, 2010; Tager-Flusberg, 2010). While reduced gesture use has also been reported in the first year (Talbot, Nelson, & Tager-Flusberg, 2015), a recent study of ASIBs suggests a similar pattern of gesture use for those later diagnosed with ASD and those later diagnosed with language delays without ASD (Lebarton & Iverson, 2016). Thus, it is possible that specific aspects of early social communication deficits may represent symptoms of ASD or may signal a language disorder or other global developmental delays (Landa, Gross, Stuart, & Bauman, 2012; Lebarton & Iverson, 2016).
The subtle overlaps and variable onsets of symptoms presents challenges for early identification of ASD. Some symptoms, including social communication impairments, may not be pronounced and hence undetectable until 12 months or older (Sacrey, Bennett & Zwaigenbuam, 2015; Szatmari et al., 2016). Alternatively, screening and diagnostic measures may not be available to allow detection of these symptoms at earlier ages. Nonetheless, closer examination of early social communication in two high-risk groups for ASD may facilitate the identification of social communication deficits before 12 months of age. In the current study, we contrast early social communication impairments of infants at high-risk due to a family history of ASD—ASIBs—to another population also at high-risk for ASD—FXS.
1.2. Early Social Communication in FXS
FXS is caused by a mutation of the FMR1 gene on the X chromosome that leads to expansion of CGG repeats to greater than 200 (Santoro, Bray, & Warren, 2012). Because FXS is an X-linked disorder, more males than females are affected. This expansion results in a wide range of intellectual functioning—learning disabilities to severe cognitive impairment—that emerges in infancy (Roberts, McCary, Shinkareva, & Bailey, 2016; Van der Molen et al., 2010). In addition to being the leading inherited genetic cause of intellectual disability, FXS is the most common known single-gene cause of ASD (Auerbach, Osterweil, & Bear, 2011; Darnell et al., 2011). However, controversy exists regarding whether ASD features are common or unique in FXS contrasted to idiopathic ASD (Abbeduto, McDuffie, & Thurman, 2014).
Similar to the research on ASD, early social communication may be an early indicator of ASD in infants with FXS. In children and adults with FXS, social communication (i.e., pragmatics) is impaired, and these impairments are greater for individuals with comorbid ASD (Klusek, Martin, & Losh, 2014; Martin, Losh, Estigarribia, Sideris, & Roberts, 2013). However, the early social communication profile of infants and young children with FXS is largely unknown with few studies examining social communication at this age period (Flenthrope & Brady, 2010; Marschik et al., 2014; Roberts, Mirrett, Anderson, Burchinal, & Neebe, 2002). Marschik and colleagues (2014) investigated children with FXS 12 months and younger and found that most communication consisted of prelinguistic vocalizations with restricted gesture use. In slightly older children (21–77 months), deficits in reciprocity and gestures have also been reported (Roberts et al., 2002). Within gesture use it appears that children with FXS (15–41 months) use more contact gestures (i.e., giving, pushing away, touching an adults hand, etc.) and have yet to transition to more advanced gestures (i.e., representational gestures and distal pointing; Flenthrope & Brady, 2010). Interestingly, using eye gaze shifts—eye gaze to a person’s face that alternates between the person and an object—for the purposes of behavior requests, joint attention, and responding to communication has been reported as relatively intact in infants and young children with FXS (Marschik et al., 2014; Roberts et al., 2002). These findings contrast with research on older children, adolescents, and adults that has identified difficulties using eye gaze for initiating and maintaining social interactions (Hall et al., 2015; Williams, Porter, & Langdon, 2013) and gaze avoidance, (Cohen et al., 1988; Murphy, Abbeduto, Schroeder, & Serlin, 2007) as core aspects of the FXS phenotype. However, these studies have primarily focused on eye gaze in FXS outside of a communication context. Further study in infants during social communication is warranted.
1.3. Communication Complexity
The present study describes the pattern of social communication as measured by the Communication Complexity Scale (CCS; Brady et al., 2012), which was developed to describe levels of social communication on a developmental continuum from prelinguistic to emerging linguistic communication (Bates et al., 1979; Bates & Dick, 2002; Tomasello, 1988). Hence the CCS uniquely reflects differences in the complexity of different communication acts. It is important to use a measure that reflects differences in prelinguistic complexity because it may be this very complexity that differentially predicts outcomes. For example, children with ASD not only gesture less than children with other types of intellectual disability or TD, they point less and communicate less often for joint attention or commenting (Dawson et al., 2004; Watson, Crais, Baranek, Dykstra, & Wilson, 2013). Similarly, children with FXS without ASD use more gestures for joint attention whereas children with FXS and co-morbid ASD use relatively more giving gestures (Esplund, 2015).
1.4. Purpose of the Present Study
This study compares early social communication as measured with the CCS across three groups–infants with FXS, ASIBs, and TD infants. TD infants were included because the CCS has not yet been normed with TD infants. Characterizing social communication features in infants with FXS contrasted to ASIBs may lead to better identification of relative strengths and weaknesses in early social communication within and across these groups. The following research questions were proposed:
What is the descriptive pattern of early social communication complexity in infants with FXS contrasted to ASIBs and TD infants?
Is there a difference in early social communication complexity in infants with FXS contrasted to ASIBs and TD infants?
Are there differences in early social communication complexity across sampling contexts within and between each group?
What is the relationship between early social communication complexity and ASD risk within each group?
2. Materials and Methods
2.1. Participants
Participants were 61 infants between 7.5 and 14.5 months: 18 infants with FXS, 21 ASIBs, and 22 TD infants (see Table 1 for demographic information). There were four female infants in each group. Diagnosis of FXS was confirmed by genetic report and status as an ASIB was established by documentation of an ASD diagnosis for an older full sibling. Participants were recruited as part of a longitudinal study on the emergence and stability of ASD in FXS from across the United States. Infants with FXS and ASIBs were recruited through a research registry, networking with parent support groups, postings on a parent listservs, and postings at a national conference. TD infants were recruited from local parent groups and postings at early childhood centers.
Table 1.
Participant Characteristics
| FXS (N = 18) | ASIB (N = 21) | TD (N = 22) | p valuea | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Characteristic | M/% | SD | M/% | SD | M/% | SD | |
|
| |||||||
| Age (months) | 10.27 | 1.66 | 10.32 | 1.70 | 9.54 | 1.20 | p = .19 |
| Mental Ageb (months) | 7.81 | 2.22 | 9.94 | 1.85 | 9.51 | 1.58 | p = .002 |
| Early Learning Composite | 81.17 | 18.75 | 95.48 | 12.50 | 97.68 | 11.45 | p = .001 |
| MSEL Nonverbal | 40.92 | 14.77 | 51.81 | 9.70 | 52.02 | 7.78 | p = .003 |
| AOSI Total Score | 11.61 | 5.84 | 8.43 | 3.92 | 6.95 | 3.40 | |
| ASD Risk (%)c | 66.7 | 42.9 | 22.7 | ||||
| Family Income | 70205.88 | 56000.30 | 55309.05 | 36951.57 | 50170.26 | 38213.57 | p = .377 |
| Race/Ethnicity (%) | p = .114 | ||||||
| Caucasian | 66.7 | 81.0 | 90.9 | ||||
| African American | 16.7 | 14.3 | 9.1 | ||||
| Hispanic/Latino | -- | 4.8 | -- | ||||
| More than 1 race | 16.7 | -- | -- | ||||
| Maternal Education Level (%) | p = .575 | ||||||
| Some High School | 11.8 | -- | 4.5 | ||||
| High School Diploma | 5.6 | 14.3 | 13.6 | ||||
| Associates/Some College | 16.7 | 42.9 | 31.8 | ||||
| College Degree | 33.3 | 19.0 | 18.2 | ||||
| Some Graduate School | 5.6 | 4.8 | -- | ||||
| Graduate Degree | 22.2 | 19.0 | 31.8 | ||||
| No response | 5.6 | -- | -- | ||||
Note.
Group differences examined using ANVOA for continuous variables or Chi-Square for nominal variables.
Mental age = average age equivalence scores from the four domains of the MSEL.
ASD risk = number of infants with a Total Score of 9 or higher on the AOSI.
Participants were matched on chronological age because our focus was on early markers of social communication in FXS; therefore, we wanted the comparison group to be infants of the same age, but with different risk levels (i.e., ASIBs = high-risk; TD infants = low-risk). Mental age matching was not used because participants were very young and a chronological younger group of TD infants would not have the motor skills necessary to produce communicative gestures scored by the CCS. One-way ANOVAs indicated no significant differences between groups on chronological age, but infants with FXS had a significantly lower mental age and overall cognitive ability, measured by the Mullen Scales of Early Learning (Mullen, 1995), than the other two groups. No significant group differences were observed between family income, race, or maternal education level (see Table 1 for p values).
2.2. Procedure
Data were collected during either a home assessment around 9 months or an assessment at our lab at the [withheld for review] around 12 months. Two trained lab members conducted these assessments.
2.3. Measures
2.3.1. Mullen Scales of Early Learning
The Mullen Scales of Early Leaning (MSEL; Mullen, 1995) assesses development in the domains of expressive language, receptive language, visual reception, and fine motor skills from birth to 68 months. These domains are combined to create an estimate of overall development (i.e., Early Learning Composite). As a standardized observational assessment, the MSEL has well-established validity and reliability (Mullen, 1995). We examined nonverbal cognitive abilities by using the average of the visual reception and fine motor T-scores. Nonverbal abilities, instead of overall cognitive abilities, were used in analyses to control for the influence of language abilities on the Early Learning Composite, which may confound our examination of social communication because there is overlap in items (e.g., receptive language questions about gesture use and eye gaze). One infant with FXS had a nonverbal score that met the floor (T-score of 20 on both domains).
2.3.2. Autism Observation Scale for Infants
The Autism Observation Scale for Infants (AOSI; Bryson, Zwaigenbaum, McDermott, Rombough, & Brian, 2008) is a 15 to 20 minute semi-structured, play-based measure to identify early signs of ASD from 6 to 18 months. The 19-item version of the AOSI was used (Brian et al., 2008), which involves four main presses: free play 1, peek-a-boo, imitation, and free play 2. The examiner rates the infant’s behavior during administration from 0 to 3. A rating of 0 indicates normal behavior and ratings of 1, 2, and 3 indicate increasing levels of abnormal behavior. Ratings are summed to create a Total Score, with a score of 9 or higher indicating ASD risk (Zwaigenbaum et al., 2005). Test-retest reliability at 12 months is .61 for the Total Score (Bryson et al., 2008). Research-reliable examiners administered the AOSI. Interrater reliability was established for 20% of videos with 89% reliability at the item level.
2.3.3. Communication Complexity Scale
The CCS (Brady et al., 2012) was developed to describe levels of social communication between an individual and a communication partner. The CCS has been validated with individuals with intellectual and developmental disabilities (Brady et al., 2012), children with ASD (Brady et al. in press), and TD infants (Salley & Brady, 2015). Excellent inter-observer reliability has been reported (Brady et al., 2012; in press). Construct validity was established by showing high correlations between the CCS and the Communication Matrix (Rowland & Fried-Oken, 2010) and the expressive and receptive communication subscales of the Vineland II Adaptive Behavior Scales (Sparrow, Cicchetti, & Balla, 2005; Brady et al., in press).
2.3.3.1 Scoring and Coding of the CCS
The CCS was scored by coders from the four main presses of the AOSI: free play 1, peek-a-boo, imitation, and free play 2. These presses were selected because they provide opportunities for social communication between the infant and the examiner. Scoring is based on an ordinal scale with 12 different levels that are categorized as pre-intentional, intentional nonsymbolic, and intentional symbolic based on the behaviors—eye-gaze, gestures, vocalizations, and speech—observed during a communicative interaction (see Table 2 for definitions of CCS levels). The child’s highest communication level during each task or press of a scripted protocol was coded. For example, if a child produced a triadic eye gaze (score 7) and a triadic eye gaze with a give gesture (score 9) during peek-a-boo, only the 9 was used to compute the summary score. The CCS has been revised since this study was conducted and scanning between objects is no longer scored as communicative, but was coded in the present study. CCS/AOSI summary scores were determined by taking the average of the infant’s four CCS scores from the four presses of the AOSI. This provided an overall summary of the child’s best social communication performance consistent with the approach used in Brady and colleagues (2012).
Table 2.
Communication Complexity Scale Levels
| Code | Level | Definition | Example |
|---|---|---|---|
| 0 | No response | Not attending/orienting to objects/examiner | Toy is presented, child looks away. |
| Pre-intentional Communication | |||
| 1 | Altering Behaviors | After the onset of a communication opportunity, there is a change in behavior | Wind-up toy stops, child smiles. |
| 2 | Single Orientation | Orients to object but does not look up or orient body to the examiner | Child picks up toy and stares at it. |
| 3 | Single Orientation with one PCBa | Includes a gesture or vocalization | Child picks up toy and vocalizes. |
| 4 | Single Orientation with more than one PCB | Includes multiple gestures or vocalizations | Child reaches for toy and vocalizes. |
| 5 | Scanning with or without a PCB | Looking from one object to another object | Child looks back and forth between two toys. |
| 6 | Dual Orientation | Shift in focus/orientation between object and examiner | Child looks from toy to examiner |
| Intentional Nonsymbolic | |||
| 7 | Triadic Orientation | 3-point shift in gaze from object-examiner-object | Child looks from toy to examiner to toy. |
| 8 | Dual Orientation with one or more PCBs | Includes a gesture or vocalization | Child looks from toy to examiner and vocalizes. |
| 9 | Triadic Orientation with one PCB | Includes a gesture or vocalization | Child looks from toy to examiner to toy while vocalizing. |
| 10 | Triadic Orientation with more than one PCB | Includes multiple gestures or vocalizations | Child looks from toy to examiner to toy while reaching and vocalizing. |
| Intentional Symbolic | |||
| 11 | One Word Verbalization | Using a word/sign | Child says, “more” to request starting wind-up toy again. |
| 12 | Multiple Word Verbalization | Using multiple words | Child says, “more toy” to request starting wind-up toy again. |
Note.
PCB = Potentially communicative behavior
In this study, coders also indicated for each press the type of potential communicative behaviors (PCBs; Sigafoos et al., 2000) observed (e.g., vocalization or type of gesture) and the communicative function (i.e., behavior regulation or joint attention), if appropriate. Behavior regulation was defined as any time a child’s behavior elicited supportive action or aid from the examiner to obtain an object or event. Joint attention was defined as any time a child’s behavior directed the examiner’s attention to an object or event with the goal of sharing attention or the experience with the examiner. Social interaction functions were not coded separately because these types of communications can be assumed under either joint attention or behavior regulation (Wetherby & Prizant, 2003). For example, if a child requests more peek-a-boo, this would be coded as behavior regulation because they are asking for more of this social game. Communicative function was only coded when the social communication level the infant used involved the presence of intentional communication (i.e., triadic orientation [score 7] or higher; Table 2).
Three coders (two undergraduate research assistants and one research associate–the fourth author) who were naïve to the research questions were trained in CCS scoring and coding by the first author. Once trained, they coded two randomly selected videos with the first author, discussing each scoring/coding decision. Next, they independently coded six randomly selected videos until there was an 80% agreement between them and the first author. After training, each coder was randomly assigned videos to code as the primary coder.
2.3.3.2. Reliability
Inter-rater reliability was calculated for 20% of the videos, which were randomly assigned to the coder who had not served as the primary coder. Intraclass correlation coefficients (ICC; Shrout & Fleiss, 1979) using absolute values for single measures were: CCS score .89, type of PCB .90, and communicative function .73. Because communicative function was only coded when the CCS score included intentional communication (i.e., triadic orientation or higher) there were less opportunities for these codes, which may explain the lower ICC.
2.4. Data Analysis
Descriptive statistics were used to examine our first research question about the pattern of social communication (i.e., CCS scores, PCB use, and communicative function) on each of the four presses of the AOSI. A one-way ANCOVA was used to examine our second research question on whether there are differences in social communication between groups. We controlled for nonverbal abilities to account for effects due to cognitive deficits typically observed in FXS. CCS/AOSI summary scores were used in these analyses. The assumption of homogeneity-of-slopes was not violated (i.e., the relationship between nonverbal abilities [covariate] and the CCS/AOSI summary score did not differ significantly as a function of group). Follow-up pairwise comparisons using the LSD procedure were conducted to examine differences in the adjusted means between groups. Multilevel modeling using SAS PROC MIXED with REML estimation was used to examine our third research question on whether there was a difference in CCS scores on the four presses of the AOSI within and between groups. The random intercept model included fixed effects for group, AOSI press, and their interaction. Secondarily, a model with nonverbal abilities as a covariate was used to examine differences in CCS scores between groups. Effect sizes were calculated using the adjusted Least Squared Means (LSM) from the model and the observed standard deviations for each group. We did not include maternal education in these analyses because a significant relationship between maternal education and CCS scores was not observed (all p values greater than .25). Lastly, partial correlations controlling for nonverbal abilities were used to examine our fourth question on the relationship between the CCS/AOSI summary score, and ASD risk, as measured by the AOSI total score, within each group. Due to the low number of females in each group, we were unable to examine sex differences in our analyses. Thus, we performed secondary analyses for all research questions using males only.
3. Results
3.1. Descriptive Pattern of Social Communication
3.1.1. Social communication use
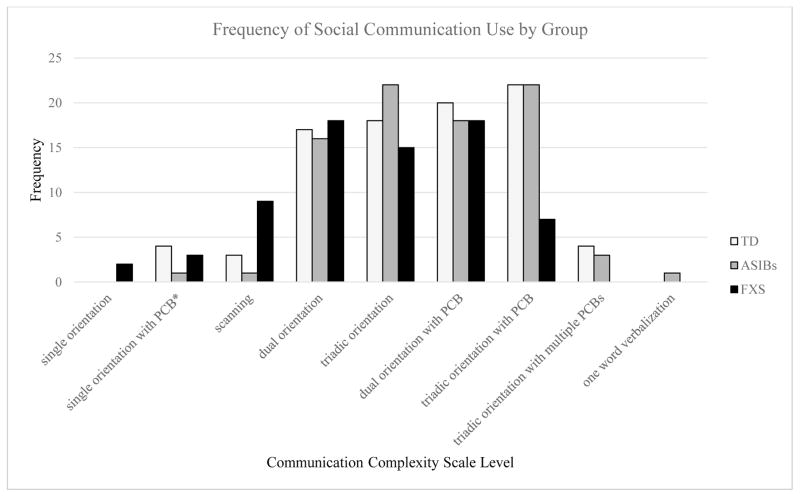
Infants used a variety of social communication during the four presses of the AOSI, but the pattern varied by group (Figure 1). Based on the four CCS scores coded during the four presses of the AOSI, the most frequently used form of social communication for infants with FXS was dual orientation with a PCB (count = 18) or without a PCB (count = 18), while TD infants most frequently used triadic orientation with a PCB (count = 22). For ASIBs, both triadic orientation with a PCB (count = 22) and without a PCB (count = 22) were the most common type of social communication. No infants with FXS used triadic orientation with multiple PCBs. Also, no infants with FXS or TD infants used any form of symbolic communication. A similar pattern was observed for males within each group. Males with FXS used dual orientation with or without a PCB most, ASIB males used triadic orientation with a PCB most, and TD males used triadic orientation most.
Figure 1.
Note. Based on each infant’s social communication during the four presses of the AOSI coded using the CCS. *Combines single orientation with one PCB and single orientation with more than one PCB.
3.1.2. Use of potentially communicative behaviors
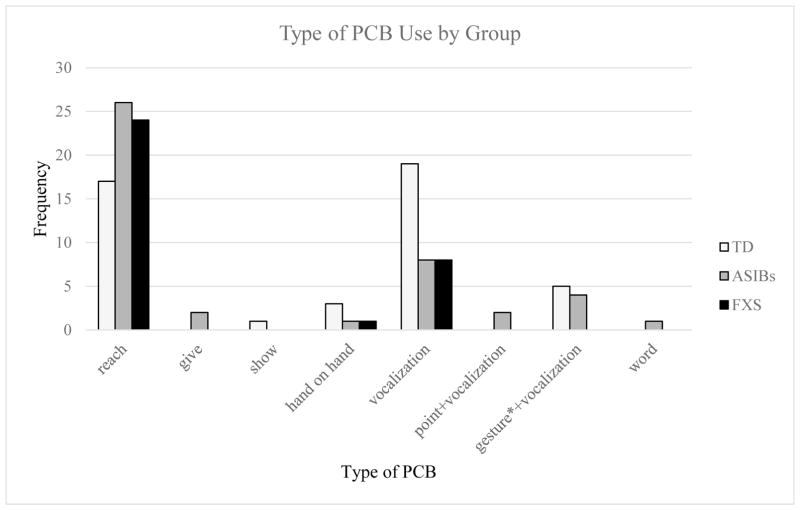
Infants with FXS used PCBs less frequently than ASIBs and TD infants based on the four CCS scores from the four presses of the AOSI (Figure 2). The most common PCB used by infants with FXS was reaching (count = 24). ASIBs used a wider variety of PCBs than the other two groups, but like infants with FXS, the most common type of PCB used was reaching (count = 26). TD infants used both vocalizing (count = 19) and reaching (count = 17) frequently. Males in all three groups used reaching most often.
Figure 2.
Note. Based on each infant’s PCB use during the four presses of the AOSI coded using the CCS. *Includes gestures other than pointing (i.e., reach, show, give, hand on hand).
3.1.3. Communicative functions
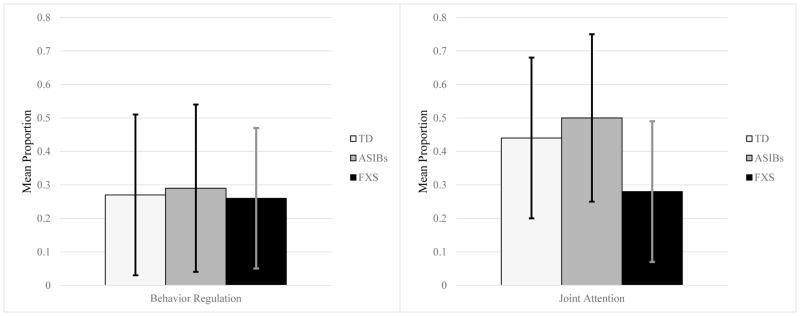
Infants with FXS had fewer opportunities for communicative functions to be coded because most of their social communication during the four presses of the AOSI was below triadic orientation (i.e., preintentional). In total, communicative functions were coded 40 times for infants with FXS during the four presses of the AOSI, while they were coded 64 times for TD infants and 66 times for ASIBs. Because of this, we examined the mean proportion of communicative functions used across the four presses of the AOSI. While infants with FXS showed similar levels of joint attention and behavior regulation, ASIBs and TD infants used joint attention more than behavior requests (see Figure 3). Infants with FXS had a lower mean proportion of joint attention than the other two groups, but behavior regulation was similar across groups. The same pattern was observed for ASIB and TD males. Males with FXS used slightly more behavior regulation (M = .30, SD = .24) than joint attention (M = .23, SD = .15).
Figure 3.
Note. Mean proportion of communicative functions during the four presses of the AOSI coded using the CCS
3.2. Differences in Social Communication between Groups
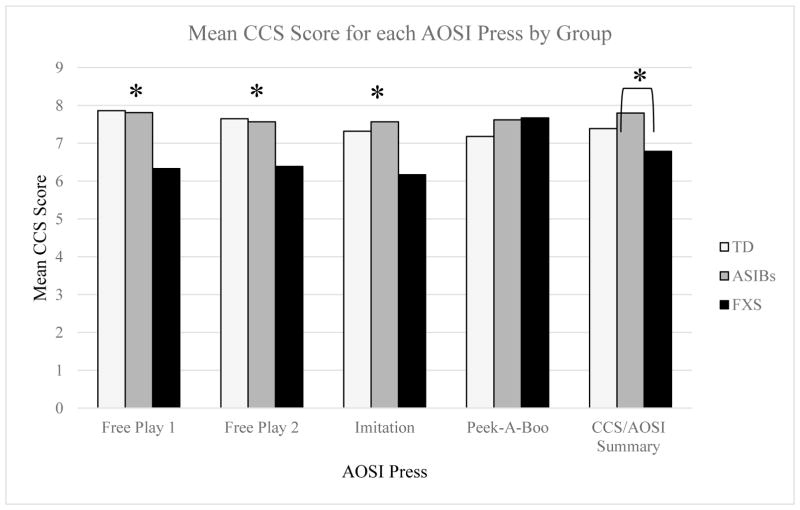
The ANCOVA for the CCS/AOSI summary score controlling for nonverbal abilities was significant, F(2, 57) = 3.25, MSE = 2.66, p = .046, η2 = .10 (Figure 4). Follow-up pairwise comparisons indicated infants with FXS had significantly lower CCS/AOSI summary scores (adjusted M = 6.79) than ASIBs (adjusted M = 7.80, p = .015). There was a trend for infants with FXS to also have lower CCS/AOSI summary scores than TD infants (adjusted M = 7.39, p = .062). There were no significant differences between ASIBs and TD infants. The male only ANCOVA model was not statistically significant (F[2, 45] = 1.35, MSE = 1.13, p = .269, η2 = .06). The follow-up comparison showed trends like the full model.
Figure 4.
Note. CCS/AOSI Summary is the average of all four AOSI presses. *Indicates signifcant differences.
3.3. Differences in Social Communication Scores Across the Sampling Context Within and Between Groups
Multilevel model indicated significant differences in intercepts across groups. The variance of the intercept was .42, SE = .16, p = .005. Residual variance was 1.725, SE = .185, p < .0001. Type 3 Tests of Fixed Effects indicated a significant group by AOSI press interaction, F(6,174) = 2.61, p =.019, and a significant main effect for group, F(2,58) = 6.37, p = .003. The main effect for press was not significant, F(3,174) = 1.33, p = .27. Follow-up tests indicated that the effect of AOSI press on CCS score was significant for infants with FXS, F(3,174) = 4.99, p = .002, but the effect of AOSI press was not significant for ASIBs (F[3,174] = 0.16, p = .925) or TD infants (F[3,174] = 1.11, p = .348). Infants with FXS used a higher level of social communication during the peek-a-boo press (LSM = 7.67, SE = .35) than during the other three presses (free play 1: LSM = 6.33, SE = .35; imitation: LSM = 6.17, SE = .35; free play 2: LSM = 6.39, SE = .35; Figure 4). Effect sizes between peek-a-boo and the other three presses were large (Cohen, 1988; free play 1: d = .88, imitation: d = .85, free play 2: d = .56).
Follow-up tests were conducted to examine of the effect of group on CCS score within each AOSI press. Results indicated a significant effect of group for free play 1 (F[2,208] = 5.28, p = .006), imitation (F[2,208] = 4.97, p = .008), and free play 2 (F[2,208] = 5.47, p = .005). However, a significant group effect was not observed for peek-a-boo, F(2,208) = .70, p = .499. Effect sizes for free play 1 were large for FXS vs. ASIB and FXS vs. TD (d = .92; d = .72) and small for ASIB vs. TD (d = .27; Cohen, 1988). For imitation, effect sizes were medium to large for FXS vs. ASIB and FXS vs. TD (d = .75; d = .56) and small for ASIB vs. TD (d = .17). For free play 2, effect sizes were medium to large for FXS vs. ASIB and FXS vs. TD (d = .58; d = .72) and small for ASIB vs. TD (d = .21). For peek-a-boo, effect sizes were medium for FXS vs. TD and ASIB vs. TD (d = .53; d = .45) and small for FXS vs. ASIB (d = .26).
When nonverbal ability was added to the model, the interaction between group and AOSI press (p = .019) and the effect of group (p = .046) remained significant. Nonverbal ability (F[1,57] = 3.35, p = .073) and AOSI press (p = .266) were not significant. The significance of the follow-up tests of the simple effects were unchanged.
Taken together, the results of the multilevel modeling indicate that infants with FXS used a significantly higher level of social communication during peek-a-boo than the other three AOSI presses. Further, while there were significant differences between the three groups on the social communicative level used during free play 1, imitation, free play 2, there were no significant differences between groups on the social communication level used during peek-a-boo.
3.3.1. Male only model
The male only multilevel model showed similar effects to the full model. The group by AOSI press and group effects on CCS score remained significant (p = .028 and .013, respectively), while the main effect of press on CCS score was not (p = .24). Infants with FXS differed in CCS score across the four presses of the AOSI (p = .009) while ASIBs (p = .40) and TD infants (p = .45) did not. One change from the full model was the effect of group on CCS score within each AOSI press. In the male only analysis, significant differences across groups were observed in free play 1 (p = .005) and free play 2 (p = .022), but not in imitation (p = .051) or peek-a-boo (p = .31). When nonverbal ability was included in the model the effect was significant, F(1,44) = 5.70, p = .02, and the group by AOSI press effect remained significant (p = .028), but the effect of group was no longer significant, F(2,44) = 1.56, p = .223. In terms of the simple effects, the difference between groups on free play 2 was no longer significant (p =.22), all other effects remained the same.
3.4. Relationship between Early Social Communication and ASD Risk within Each Group
Results indicated that higher levels of social communication were related to lower ASD risk scores on the AOSI when controlling for nonverbal abilities, but this association was only statistically significant for TD infants (r = −.74, p = < .001), and the effect size was large (Cohen, 1988). For ASIBs and infants with FXS, the direction of effects were the same, with medium to large effects for ASIBs (r = −.42, p = .067) and a small to medium effect for infants with FXS (r = −.25, p = .325; Cohen, 1988). The male only correlations showed the same pattern of associations (TD: r = −.70, p = .003; ASIBs: r = −.48, p = .059; FXS: r = −.42, p = .151).
4. Discussion
Early social communication supports language acquisition and social development. Characterizing social communication in infants with FXS may further understanding of the language and social impairments that have been noted later in development in this population. Also, describing social communication features in infants with FXS contrasted to ASIBs contributes to improved understanding of core ASD deficits. Thus, the present study sought to describe early social communication in infants with FXS compared to ASIBs and TD infants. To our knowledge this is the first prospective study of social communication in infants with FXS, and the first to contrast social communication in two etiologically distinct groups that are at high-risk for ASD.
Our results indicate that infants with FXS used lower levels of social communication than ASIBs and TD infants. Infants with FXS demonstrated emerging skills in the use of intentional communication based on their frequent use of dual orientation without and with a PCB (Bates et al., 1979, Volterra et al., 2005), while TD infants and ASIBs had already entered this stage by using triadic orientation with and without PCB’s. Considering the cognitive impairments associated with FXS (Van der Molen et al., 2010; Roberts et al., 2016), it is not surprising that infants with FXS have lower levels of social communication than ASIBs and TD infants. However, the fact that these impairments are evident even when controlling for nonverbal cognitive abilities and are observed within the first year of life highlights the importance of early identification of FXS and early intervention for this population. These findings provide additional insight into the developmental course of social communication in FXS starting in infancy.
Interestingly, infants with FXS showed higher levels of social communication during peek-a-boo than the other three presses and this was similar to the level of social communication used by TD infants and ASIBs during peek-a-boo. This is particularly interesting considering that the developmental level for infants with FXS was significantly lower. It is possible that the dynamic nature of social games, like peek-a-boo, may help to encourage communication in infants with FXS to a greater degree than a more passive or less intense interaction context such as free play. Research on language development has highlighted the importance of context for language learning, supporting that some contexts, especially social contexts, elicit greater communication both within and across groups (Bruner, 198; Hoff, 2006). Therefore, it is not surprising that a playful, social game would elicit the types of early social communication that leads to later language development. It is possible that infants with FXS “require” the level of intensity associated with dynamic social games to more fully activate their social communication skills than what is needed for the other two groups. Taken together, it is possible that using “high intensity” social games may be a good starting point for language intervention in FXS.
Like past studies on social communication in older children with FXS than those in the present study (Roberts et al., 2002), our results indicate that infants with FXS used less PCBs than their peers. Past studies have also suggested that young children with FXS have restricted gestural repertoires (Flenthrope & Brady, 2010; Marschik et al., 2014; Roberts et al., 2002), which has also been observed in other populations at risk for language delays (Luyster et al., 2011; Suttora, & Salerni, 2012). Thus, it is not surprising that infants with FXS in the present study only used one type of gesture, reaching. However, all infants in this study frequently used reaching gestures. This may be due in part to coding social communication in the context of play interactions, as the examiner may have manipulated the toys the infant liked or wanted, causing the infant to reach. It was also common for infants to reach for the blanket during peek-a-boo to “find” the examiner to share and continue in the social interaction. Furthermore, our study included infants as young as 7.5 months, which likely impacts the available repertoire of gestures due to their young age and motor ability.
We also found that infants with FXS used joint attention and behavior regulation less than ASIBs and TD infants. It has been suggested that infants with FXS have limited communicative functions (Marschik et al., 2014), but one study of older children suggested that communicative functions were used with proficiency (Roberts et al., 2002). It is possible that the use of communicative functions is slow to emerge or delayed in FXS. While ASIBs and TD infants used joint attention more than behavior regulation, infants with FXS used similar levels of joint attention and behavior regulation. This is somewhat surprising, considering many children with intellectual and developmental disabilities, including those with ASD, tend to use behavior regulation more than joint attention. Our results of higher joint attention in ASIBs and similar levels in FXS may be because the types of interactions we coded from the AOSI were designed to promote social interaction and not press for behavior regulation (e.g., activities that require the child to request or try to get the social partner to do something, such as giving the child a wind-up toy that does not work). Therefore, while it is clear from our study that infants with FXS communicate intentionally less often, more research is needed to examine differences in communication functions at these early ages.
Impairments in social communication are one of the core features of ASD (APA, 2013); consequently, it is not surprising that infants in our study who used higher levels of social communication had lower ASD risk. These findings are in line with previous studies examining the relationship between social communication and ASD (Landa et al., 2012; Ozonoff et al., 2010; 2011). The strongest relationship between ASD symptoms and social communication was evident for the TD infants, with a moderate relationship for ASIBs and a marginal to weak relationship for infants with FXS. Thus, deficits in social communication may be specific indicators of ASD risk in TD infants and ASIBs (ASD risk for ASIBs in our sample was 42.9% and 22.7% for TD infants as measured by the AOSI), but may be an indicator of more general language or social communication deficits in FXS. Given that we controlled for nonverbal cognitive abilities in these analyses, we cannot attribute the lack of a relationship solely to the lower developmental level in FXS. It may be that the relationship between social communication complexity and early ASD features differs across these etiologically distinct groups, or it could reflect a developmental issue, with the relationship between social communication complexity and ASD features strengthening over time in FXS and emerging later. Further empirical study that includes ASD diagnostic outcome data is needed to answer this question.
4.1. Limitations and Future Directions
There are several limitations and future directions for the present study. We coded the highest social communication act the infant performed during the four presses of the AOSI, which provides a snapshot of their behavior. Also, social communication was only examined at one time point in this study. Thus, longitudinal prospective studies are needed to examine the trajectory of social communication in FXS. Similarly, ASIBs in our study showed similar patterns of social communication to TD infants. Considering the wide variability that has been noted within ASIBs (Landa et al., 2012), it is possible that our cross-sectional approach was not able to detect this within group variability that may be better detected through a longitudinal study. Another possibility is that a more exhaustive examination (i.e., second by second basis) of social communication for ASIBs would identify nuanced differences in social communication behaviors that would align with different trajectories noted by Landa and colleages (2012). Future studies may also consider using standardized administrations of social communication contexts, like the Communication and Symbolic Behavior Scales (Wetherby & Prizant, 2003), that specifically press for both joint attention and behavior regulation.
While the purpose of the larger study was to examine the emergence and stability of ASD in FXS, our study materials focused on early development in neurodevelopmental to reduce bias. However, it is possible that this may have introduced some bias by leading families who were concerned about their child’s development or diagnosis to enroll in the study. Finally, there were few females in the present study, preventing comparisons of social communication between males and females within and between groups. However, some differences were observed in our analyses when only males were examined, therefore, examining early social communication in females with FXS may provide additional insight into the social profile of females with FXS. Therefore, in future studies with larger samples, it would be beneficial to stratify samples by sex, chronological age, and mental age to identify delays in social communication, especially if there is a true deficit in gesture use in FXS.
5. Conclusions
This study adds to the growing literature of identifying profiles of early communication in FXS. In addition to further characterizing the infant behavioral phenotype associated with FXS, these efforts may help to differentiate infants with FXS from infants with other intellectual and developmental disabilities, or from infants at risk for ASD. Specifically, infants with FXS in our study showed a pattern of pre-intentional communication that included high use of reaches, low vocalizations, and similar use of joint attention and behavior regulation. ASIBs showed a pattern of intentional nonsymbolic communication that included high use of reaches, moderate use of vocalizations, and high uses of joint attention. TD infants showed a pattern of intentional nonsymbolic communication with high use of reaches, vocalizations, and joint attention. Also, social communication may not serve as an early indicator of ASD in FXS and instead represent a more general area of impairment in FXS, regardless of later ASD outcome. Thus, there appears to be some early differences in social communication in these etiologically distinct groups, which has implications for early identification and intervention. Infants with FXS in our study were already showing lower levels of social communication in the first year; therefore, these skills are a prime target for early intervention, regardless of ASD diagnostic outcome. Additionally, our study suggests that language interventions that include a strong social play component may be particularly effective for promoting language development in FXS.
What this paper adds?
Early social communication supports language acquisition and social development in typically developing infants as well as infants with neurodevelopmental disorders. Characterizing social communication in infants with fragile X syndrome (FXS) may improve understanding of the language and social impairments associated with FXS and autism spectrum disorder (ASD). The present study investigated the pattern of early social communication in two groups who are at a high risk for being diagnosed with ASD—infants with FXS and infant siblings of children with ASD—as compared to typically developing infants. To our knowledge this is the first prospective study of social communication in infants with FXS, and the first to contrast social communication in two etiologically distinct groups that are at high risk for ASD.
Highlights.
Overall infants with FXS had lower social communication than ASIBs or TD infants.
Infants with FXS had similar levels of social communication during peek-a-boo.
No differences in social communication were observed between ASIBs and TD infants.
Etiologically different patterns of social communication were seen across groups.
For all infants, higher social communication was related to lower ASD risk.
Acknowledgments
This research supported by NICHD and NIMH grants T32 HD057844, R01 HD076903, P30 HD02528, P30 HD003110, R01 MH090194, L40 MH108014. This manuscript was presented at the 48th Annual Gatlinburg Conference on Research and Theory in Intellectual and Developmental Disabilities in New Orleans, LA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Laura J. Hahn, Life Span Institute, University of Kansas; Department of Psychology, University of South Carolina
Nancy C. Brady, Speech-Language-Hearing: Sciences & Disorders, University of Kansas
Lindsay McCary, Department of Psychology, University of South Carolina.
Lisa Rague, Department of Psychology, University of South Carolina.
Jane E. Roberts, Department of Psychology, University of South Carolina
References
- Abbeduto L, McGuffie A, Brady N, Kover ST. The Oxford Handbook of Intellectual Disability and Development. 2012. Language development in fragile X syndrome: Syndrome-specific features, within-syndrome variation, and contributing factors; pp. 200–216. [Google Scholar]
- Abbeduto L, McDuffie A, Thurman AJ. The fragile X syndrome-autism comorbidity: What do we really know? Frontiers in Genetics. 2014;5:1–10. doi: 10.3389/fgene.2014.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychology Association (APA) Diagnostic and statistical manual of mental disorders. American Psychiatric Pub; 2013. [Google Scholar]
- Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480(7375):63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Benigni L, Bretherton I, Camaioni L, Volterra V. The emergence of symbols: Cognition and communication in infancy. New York, NY: Academic Press; 1979. [Google Scholar]
- Bates E, Dick F. Language, gesture and the developing brain. Developmental Psychobiology. 2002;40(3):293–310. doi: 10.1002/dev.10034. [DOI] [PubMed] [Google Scholar]
- Bhat AN, Galloway JC, Landa RJ. Social and non-social visual attention patterns and associative learning in infants at risk for autism. Journal of Child Psychology and Psychiatry. 2010;51(9):989–997. doi: 10.1111/j.1469-7610.2010.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady NC, Fleming K, Thiemann-Bourque K, Olswang L, Dowden P, Saunders M, Marquis J. Development of the Communication Complexity Scale. American Journal of Speech-Language Pathology. 2012;21:16–28. doi: 10.1044/1058-0360(2011/10-0099). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady N, Fleming K, Swinburne Romine R, Holbrook A, Muller K, Kasari C. Concurrent validity and reliability for the Communication Complexity Scale. American Journal of Speech Language Pathology. doi: 10.1044/2017_AJSLP-17-0106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian J, Bryson SE, Garon N, Roberts W, Smith IM, Szatmari P, Zwaigenbaum L. Clinical assessment of autism in high-risk 18-month-olds. Autism. 2008;12(5):433–456. doi: 10.1177/1362361308094500. [DOI] [PubMed] [Google Scholar]
- Bruner J. The social context of language acquisition. Language & Communication. 1981;1(2):155–178. [Google Scholar]
- Bryson SE, Zwaigenbaum L, McDermott C, Rombough V, Brian J. The Autism Observation Scale for Infants: Scale development and reliability data. Journal of Autism and Developmental Disorders. 2008;38(4):731–738. doi: 10.1007/s10803-007-0440-y. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Mahwah, NJ: Erlbaum; 1988. [Google Scholar]
- Cohen I, Fisch G, Sudhalter V, Wolf-Scein EG, Hanson D, Hagerman R, … Brown WT. Social gaze, social avoidance, and repetitive behaviors in fragile X males: A controlled study. American Journal on Mental Retardation. 1988;92(5):436–446. [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE, … Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146(2):247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Esplund A. Spontaneous Gestural Communication as a Predictor of Autism Spectrum Diagnosis in Children with Fragile X Syndrome. master’s thesis. 2015 Retreived from KU ScholarWorks http://hdl.handle.net/1808/19188.
- Flenthrope JL, Brady NC. Relationships between early gestures and later language in children with fragile X syndrome. American Journal of Speech-Language Pathology. 2010;19(2):135–142. doi: 10.1044/1058-0360(2009/09-0018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros-Louis J, West MJ, King AP. Maternal responsiveness and the development of directed vocalizing in social interactions. Infancy. 2014;19(4):385–408. [Google Scholar]
- Hall SS, Frank MC, Pusiol GT, Farzin F, Lightbody AA, Reiss AL. Quantifying naturalistic social gaze in fragile X syndrome using a novel eye tracking paradigm. American Journal of Medical Genetic. 2015;168(7):564–572. doi: 10.1002/ajmg.b.32331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, … Hagerman RJ. Autism profiles of males with fragile X syndrome. American Journal on Mental Retardation. 2008;113(6):427–438. doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff E. How social contexts support and shape language development. Developmental Review. 2006;26(1):55–88. doi: 10.1016/j.dr.2005.11.002. [DOI] [Google Scholar]
- Iverson JM, Thal DJ. Communicative transitions: There’s more to the hand than meets the eye. In: Wetherby A, Warren SF, Reichle J, editors. Transitions in prelinguistic communication: Preintentional to intentional and presymbolic to symbolic. Baltimore, MD: Brookes; 1998. pp. 59–86. [Google Scholar]
- Kaufmann WE, Cortell R, Kau ASM, Bukelis I, Tierney E, Gray RM, … Stanard P. Autism spectrum disorder in fragile X syndrome: Communication, social interaction, and specific behaviors. American Journal of Medical Genetics. 2004;129 A(3):225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Klusek J, Martin GE, Losh M. A comparison of pragamtic language in boys with autism and fragile X syndrome. Jounral of Speech, Language, and Hearing Research. 2014;57(3):1692–1707. doi: 10.1044/2014_JSLHR-L-13-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, Bauman M. Latent class analysis of early developmental trajectory in baby siblings of children with autism. Journal of Child Psychology and Psychiatry. 2012;53(9):986–996. doi: 10.1111/j.1469-7610.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebarton ES, Iverson JM. Gesture development in toddlers with an older sibling with autism. International Journal of Language and Communication Disorders. 2016;51(1):18–30. doi: 10.1111/1460-6984.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster RJ, Seery A, Talbott MR, Tager-Flusberg H. Identifying early-risk markers and developmental trajectories for language impairment in neurodevelopmental disorders. Developmental Disabilities Research Reviews. 2011;17(2):151–159. doi: 10.1002/ddrr.1109. [DOI] [PubMed] [Google Scholar]
- Marschik PB, Bartl-Pokorny KD, Sigafoos J, Urlesberger L, Pokorny F, Didden R, … Kaufmann WE. Development of socio-communicative skills in 9- to 12-month-old individuals with fragile X syndrome. Research in Developmental Disabilities. 2014;35(3):597–602. doi: 10.1016/j.ridd.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Losh M, Estigarribia B, Sideris J, Roberts J. Longitudinal profiles of expressive vocabulary, syntax and pragmatic language in boys with fragile X syndrome or Down syndrome. International Journal of Language & Communication Disorders. 2013;48(4):432–443. doi: 10.1111/1460-6984.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Murphy M, Abbeduto L, Schroeder S, Serlin R. Contribution of social and information-processing factors to eye-gaze avoidance in fragile X syndrome. American Journal on Mental Retardation. 2007;112(5):349–360. doi: 10.1352/0895-8017(2007)112[0349:COSAIF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Iosif AM, Baguio F, Cook IC, Hill MM, Hutman T, … Sigman M. A prospective study of the emergence of early behavioral signs of autism. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(3):256–266. [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, … Steinfeld M. The broader autism phenotype in infancy: when does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry. 2014;53(4):398–407. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Hutman T. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128(3):e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, McCary LM, Shinkareva SV, Bailey DB., Jr Infant development in fragile X syndrome: Cross-syndrome comparisons. Journal of Autism & Developmental Disorders. 2016;46:2088–2099. doi: 10.1007/s10803-016-2737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Mirrett P, Anderson K, Burchinal M, Neebe E. Early communication, symbolic behavior, and social profiles of young males with fragile X syndrome. American Journal of Speech-Language Pathology. 2002;11(3):295–304. doi: 10.1044/1058-0360(2002/034). [DOI] [Google Scholar]
- Rowland C, Fried-Oken M. Communication Matrix: A clinical and research assessment tool targeting children with severe communication disorders. Journal of Pediatric Rehabilitation Medicine. 2010;3:319– 329. doi: 10.3233/PRM-2010-0144. [DOI] [PubMed] [Google Scholar]
- Sacrey LAR, Bennett JA, Zwaigenbaum L. Early infant development and intervention for autism spectrum disorder. Journal of Child Neurology. 2015;30(14):1921–1929. doi: 10.1177/0883073815601500. [DOI] [PubMed] [Google Scholar]
- Salley B, Brady N. Assessing Early Communication Behaviors in infants using the Communication Complexity Scale (CCS). Paper presented at the 48th Gatlinburg Conference on Research and Theory in Intellectual and Developmental Disabilities; New Orleans, LA. 2015. [Google Scholar]
- Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: A twenty-year perspective. Annual Review of Pathology: Mechanisms of Disease. 2012;7:219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychological Bulletin. 1979;86(2):420. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Sigafoos J, Woodyatt G, Keen D, Tait K, Tucker M, Roberts-Pennell D, Pittendreigh N. Identifying potential communicative acts in children with developmental and physical disabilities. Communication Disorders Quarterly. 2000;21(2):77–86. [Google Scholar]
- Sparrow S, Cicchetti D, Balla D. Vineland II: A revision of the vineland adaptive behavior scales: Survey/caregiver form. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Suttora C, Salerni N. Gestural development and its relation to language acquisition in very preterm children. Infant Behavior and Development. 2012;35(3):429–438. doi: 10.1016/j.infbeh.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Szatmari P, Chawarska K, Dawson G, Georgiades S, Landa R, Lord C, … Halladay A. Prospective longitudinal studies of infant siblings of children eith autism: Lessons learned and future directions. Journal of the American Academy of Child & Adolescent Psychiatry. 2016;55(3):179–187. doi: 10.1016/j.jaac.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager-Flusberg H. The origins of social impairments in autism spectrum disorder: studies of infants at risk. Neural Networks. 2010;23(8):1072–1076. doi: 10.1016/j.neunet.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott MR, Nelson CA, Tager-Flusberg H. Maternal gesture use and language development in infant siblings of children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2015;45:4–14. doi: 10.1007/s10803-013-1820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. The role of joint attentional processes in early language development. Language Sciences. 1988;10(1):69–88. [Google Scholar]
- Trevarthen C, Aitken KJ. Infant intersubjectivity: Research, theory, and clinical applications. Journal of Child Psychology and Psychiatry. 2001;42(1):3–48. doi: 10.1111/1469-7610.00701. [DOI] [PubMed] [Google Scholar]
- Van der Molen MJW, Huizinga M, Huizenga HM, Ridderinkhof KR, Van der Molen MW, Hamel BJC, … Ramakers GJA. Profiling fragile X syndrome in males: Strengths and weaknesses in cognitive abilities. Research in Developmental Disabilities. 2010;31(2):426–439. doi: 10.1016/j.ridd.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Volterra V, Caselli M, Capirci O, Pizzuto E. Gesture and the emergence and development of language. In: Tomasello M, Slobin DI, editors. Beyond nature-nurture: Essays in honor of Elizabeth Bates. Mahwah, NJ: Lawrence Erlbaum Associates; 2005. pp. 3–40. [Google Scholar]
- Watson LR, Crais ER, Baranek GT, Dykstra JR, Wilson KP. Communicative gesture use in infants with and without autism: A retrospective home video study. American Journal of Speech-Language Pathology. 2013;22(1):25–39. doi: 10.1044/1058-0360(2012/11-0145). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherby AM, Prizant BM. Communication and Symbolic Behavior Scales (CSBS) Manual. Baltimore, MD: Brookes; 2003. [Google Scholar]
- Williams TA, Porter MA, Langdon R. Viewing social scenes: a visual scan-path study comparing fragile X syndrome and Williams syndrome. Journal of Autism and Developmental Disorders. 2013;43(8):1880–1894. doi: 10.1007/s10803-012-1737-z. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Garon N. Early identification of autism spectrum disorders. Behavioural Brain Research. 2013;251:133–146. doi: 10.1016/j.bbr.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Rogers T, Roberts W, Brian J, Szatmari P. Behavioral manifestations of autism in the first year of life. International Journal of Developmental Neuroscience. 2005;23:143–152. doi: 10.1016/j.ijdevneu.2004.05.001. [DOI] [PubMed] [Google Scholar]