Abstract
Background
Although the literature on imaging of regional brain activity during sexual arousal in women and men is extensive and largely consistent, that on orgasm is relatively limited and variable, owing in part to the methodologic challenges posed by variability in latency to orgasm in participants and head movement.
Aim
To compare brain activity at orgasm (self- and partner-induced) with that at the onset of genital stimulation, immediately before the onset of orgasm, and immediately after the cessation of orgasm and to upgrade the methodology for obtaining and analyzing functional magnetic resonance imaging (fMRI) findings.
Methods
Using fMRI, we sampled equivalent time points across female participants’ variable durations of stimulation and orgasm in response to self- and partner-induced clitoral stimulation. The first 20-second epoch of orgasm was contrasted with the 20-second epochs at the beginning of stimulation and immediately before and after orgasm. Separate analyses were conducted for whole-brain and brainstem regions of interest. For a finer-grained analysis of the peri-orgasm phase, we conducted a time-course analysis on regions of interest. Head movement was minimized to a mean less than 1.3 mm using a custom-fitted thermoplastic whole-head and neck brace stabilizer.
Outcomes
Ten women experienced orgasm elicited by self- and partner-induced genital stimulation in a Siemens 3-T Trio fMRI scanner.
Results
Brain activity gradually increased leading up to orgasm, peaked at orgasm, and then decreased. We found no evidence of deactivation of brain regions leading up to or during orgasm. The activated brain regions included sensory, motor, reward, frontal cortical, and brainstem regions (eg, nucleus accumbens, insula, anterior cingulate cortex, orbitofrontal cortex, operculum, right angular gyrus, paracentral lobule, cerebellum, hippocampus, amygdala, hypothalamus, ventral tegmental area, and dorsal raphe).
Clinical Translation
Insight gained from the present findings could provide guidance toward a rational basis for treatment of orgasmic disorders, including anorgasmia.
Strengths and Limitations
This is evidently the first fMRI study of orgasm elicited by self- and partner-induced genital stimulation in women. Methodologic solutions to the technical issues posed by excessive head movement and variable latencies to orgasm were successfully applied in the present study, enabling identification of brain regions involved in orgasm. Limitations include the small sample (N = 10), which combined self- and partner-induced stimulation datasets for analysis and which qualify the generalization of our conclusions.
Conclusion
Extensive cortical, subcortical, and brainstem regions reach peak levels of activity at orgasm.
Keywords: Human Female, Orgasm, Functional Magnetic Resonance Imaging, Sexual Behavior, Sexual Arousal
INTRODUCTION
Although the literature on imaging of regional brain activity during sexual arousal in women and men is extensive and generally consistent, the literature on orgasm is relatively limited and variable. During sexual arousal, multiple subcortical and cortical regions become activated. Amygdala activation at visual stimulation-induced sexual arousal was reported in women1–3 and men,1–5 and this activation was reportedly greater in men.6 Hippocampal activity was reported to increase during sexual arousal in men.7 In women and men during sexual arousal, activation was reported in the medial prefrontal cortex,3 orbitofrontal cortex,8 and dorsolateral prefrontal cortex,9 although Huynh et al10 reported that primary and secondary visual cortices became deactivated in women watching an erotic film. Other brain regions that were reported activated during sexual arousal in response to visual erotic stimulation include the hypothalamus,1,3,5,11 anterior cingulate cortex,3,5,7,11,12 and insula.3,7,11 Ortigue et al13 reported a correlation between the degree of activation of women’s left anterior insular cortex in response to subliminal presentation of their partner’s name and the “quality” of orgasm that the women self-rated.
The relatively less extensive literature on orgasm is more variable, perhaps owing to several factors, including the technical issues of head movement and variable latencies to orgasm, the latter of which present a greater difficulty for positron-emission tomography (PET), which requires minutes for preparing the radioactive tracer immediately before orgasm, than for functional magnetic resonance imaging (fMRI), which scans continuously. During orgasm in men and women, activation was reported in the cerebellum,4,14–16 anterior cingulate,4,14–16 and dopaminergic pathway from the ventral tegmentum4 to the nucleus accumbens.14–16 In women during orgasm, activation was reported in the hippocampus1,2,14,15 and frontal cortex.14,15,17 Amygdala activity was reported to increase at orgasm in women1,2,14,15 but decrease during ejaculation in men.4 Temporal lobe activity was reported to decrease at ejaculation in men,18 although middle temporal gyrus activity increased.4 Activation was reported during ejaculation in the orbitofrontal cortex.4 Frontal cortical activation also was reported in women during orgasm,14,15,17,19 whereas deactivations of frontal cortical regions were reported based on PET studies20,21 and perfusion fMRI22 in men.
We considered that the difference (activation or deactivation) could be due to the methods used (blood oxygen-level dependent fMRI or PET, respectively) or whether the orgasm was induced in women by self-applied15–17,19 or partner-applied20,21 stimulation, respectively.
In the present study, we focused on brain activity during orgasm in relation to that before and after orgasm. We also addressed two methodologic issues raised by our previous reports: possible artifact generated by excessive head movement during orgasm and lack of correction for multiple statistical comparisons. Thus, for the present study, we developed an assembly using a custom-fitted thermoplastic whole-head mask that was molded to a neck brace and rigidly clamped to the scanner head cage; this limited head movement during orgasm to less than 1.3 mm. In addition, the fMRI data were corrected for multiple comparisons.
AIMS
The aims of the present study were (i) to compare brain activity at orgasm (self- and partner-induced) with that at the onset of genital stimulation, at an intermediate phase during the course of stimulation, immediately before the onset of orgasm, and immediately after the cessation of orgasm and (ii) to upgrade the methodology for obtaining and analyzing fMRI findings.
METHODS
Participants
Fourteen healthy women were recruited for this study by word of mouth. Data from two of the participants were excluded because they did not experience orgasm during the scanning session. Two additional datasets were discarded because of technical problems with the scans. Data from 10 participants were used (age range = 29–74 years, mean = 43.6, SD = 14.9). Each participant gave informed consent in accordance with approval by the institutional review board of Rutgers University (Newark, NJ, USA). The scanning session took place at the Rutgers University Brain Imaging Center (RUBIC) in compliance with all RUBIC MRI common practices. The participants were prescreened for MRI safety and completed screening forms in accord with RUBIC requirements. Participants were compensated $50.
Each woman was interviewed before the scanning procedure to collect information about her sexual and relational histories for purposes beyond the scope of this study. Seven participants identified themselves as exclusively heterosexual, and three reported having had “some” bisexual experience. Three participants reported being single at the time of the study, and seven described themselves as “currently in a relationship,” ranging from 2 to 20 years in duration. Four women were married to their current partners. Five women reported having had children. Only one participant reported being “postmenopausal.” All 10 participants described themselves as being “highly” orgasmic.
Each of the 10 women was accompanied by a male partner to provide the clitoral stimulation for the partner-stimulation–induced orgasm component. Each male partner was prescreened to determine suitability for study participation and gave informed consent in accordance with the Rutgers University institutional review board. In compliance with all RUBIC MRI common practices, the male participants were prescreened for MRI safety and completed screening forms in accord with RUBIC requirements. Male participants also were compensated $50.
Procedure
Each woman participated in one experimental scan consisting of clitoral stimulation-induced orgasm under two sequentially counterbalanced conditions: self-stimulation and partner stimulation.
Protocol for Self-Induced Orgasm
Cues for the self-stimulation condition were presented visually on an fMRI-compatible computer projection screen. The participant saw the instruction “rest” for the duration of 60 seconds. Then, the instruction “press when start stimulation” appeared, which cued her to press the button once she began clitoral self-stimulation. After the participant pressed the button to indicate she was self-stimulating, the instruction “press when orgasm begins” appeared on the screen to cue her to press the button when her orgasm started. Once the participant pressed the button to indicate the onset of orgasm, the instruction “press when orgasm ends” appeared to cue her to press the button when her orgasm was finished. Once the participant pressed the button to indicate that her orgasm ended, the instruction “press button when recovered” appeared to cue her to press the button when she felt physically recovered from the orgasm. Once the participant indicated by button press that she had recovered, the instruction “relax” appeared, cueing the participant to lie still for 5 minutes.
Protocol for Partner-Induced Orgasm
The women followed the same cues for the partner-stimulation condition with the exception of the stimulation procedure. For this condition, the participant’s male partner performed the clitoral stimulation. He was cued when to start and stop stimulation by prerecorded auditory instructions delivered through headphones that were prompted by the responses (button presses) of the female participants. The male partner remained in the scanning room throughout the two experimental conditions, although they participated only in this segment of the experiment.
fMRI Acquisition
The fMRI scans were performed using a 3-T Siemens Trio with a Siemens 12-channel head coil (Siemens, Malvern, PA, USA). For registration purposes, anatomic images were acquired using magnetization prepared rapid gradient echo (MPRAGE) sequences (176 slices in the sagittal plane using 1-mm-thick isotropic voxels, repetition time [TR] = 1,900 ms, echo time = 2.52 ms, field of view = 256, matrix = 256 × 256, flip angle = 9°, distance factor = 50%). For the experimental scan, gradient-echo planar sequences were acquired of the whole brain, in descending order, including the entire medulla oblongata (33 slices in the axial plane using 3-mm isotropic voxels, TR = 2,000 ms, echo time = 30 ms, inter-slice gap = 1.5 mm, flip angle = 90°, field of view = 192, matrix = 64 × 64).
Head-Immobilization Assembly
Head movement during genital self- and partner-induced stimulation and orgasm was decreased to an average of 1.3 mm through the use of a combination of two different types of commercially available head-immobilization devices: the Philadelphia Tracheotomy Collar (a two-part, light, rigid, poly-urethane foam with Velcro fasteners; all plastic; Össur, Reykjavik, Iceland) plus the Aquaplast Thermoplastic Mesh Radiology Mask (Qfix, Avaondale, PA, USA; the Aquaplast frames are too wide for the Siemens Trio head cradle, so for each participant it was necessary to cut the frames down by approximately 1 cm on all sides). The collar (three sizes, matched to each participant) was adjusted and then the first of the two thermoplastic mesh masks was softened in a 50°C hot water bath positioned under the supine participant’s head such that the lower portion of the thermoplastic mesh covered the upper part of the collar, which extends as superiorly as the occiput, and then the rigid frame of the mask was form-fitted around the back of the head and the collar to the level of the ears as the thermoplastic cooled and set rigidly into position. The cooling of the thermoplastic to rigidity takes approximately 2 minutes. The second thermoplastic frame was heated in the water bath and fitted to the participant’s face and front of the collar. The thermoplastic was gently pushed with the fingers, with the help of the participant, to form-fit to the forehead, side of the head, nose, cheekbones, mouth, chin, and front and under-chin portion of the collar, and its frame was brought to congruence with the frame of the thermoplastic mask that cradled the back of the head. For the participant’s comfort, the portion of the face mask covering the eyes, nostrils, and mouth were marked with a felt pen, and then the front (face) half of the mask was removed from the participant and an electric tool (Dremel, Mount Prospect, IL, USA) with a side-cutting bit was used to cut out the marked regions. Several strips of strong tape were applied to gently squeeze the two frames together, thus immobilizing the head and neck. When the participant was placed onto the gurney of the fMRI scanner, a non-slip plastic mesh mat was placed on the bottom of the head cradle. The participant, with noise-attenuating ear plugs and head-immobilization assembly in place, lay supine in the Siemens Trio cradle, and the 12-channel head cage cover with projection screen observation mirror was connected. Standard foam pads used to restrict head movement were pressed in around the head-immobilization assembly, and then the two Siemens adjustable clamps mounted on the head cage were locked onto the head-immobilization assembly, further preventing its movement.
Data Analysis
Self-Stimulation–Induced Orgasm and Partner-Stimulation–Induced Orgasm Groups
Fourteen experimental scanning sessions were conducted with the goal of acquiring sequentially counterbalanced within-subject sets of partner- and self-stimulation–induced orgasms. Ten participants experienced orgasm in the two conditions during the course of the study. Six of those underwent partner stimulation first and four underwent self-stimulation first. Three additional participants who underwent self-stimulation first experienced the self-stimulation–induced orgasm, but not the partner-stimulation–induced orgasm. After excluding two datasets because of technical problems, there was an uneven number of counterbalanced datasets. Significant order effects were detected in preliminary analyses. Therefore, only “first” orgasms were used for subsequent analyses: five self-stimulation–induced and five partner-stimulation–induced orgasms.
All data were preprocessed and statistically analyzed using FMRIB Software Library (FSL) 6.00 (Center for Functional Magnetic Resonance Imaging of the Brain, University of Oxford, Oxford, UK). Lower- and higher-level fMRI data processing was carried out using the FMRI Expert Analysis Tool (FEAT).23 Each participant’s functional data were split into two files using FSLUTILS (fslroi) to create two separate datasets for analysis of the sequence of stimulation leading up to, during, and after orgasm induced by self-stimulation and by partner stimulation.
The following preprocessing procedures were performed at the individual level: manual removal of skull and non-brain tissue elements from the anatomic and functional images. “FSL Motion Outliers” was used to detect and remove data at time points that were corrupted by large motion. The resulting outlier file was added as a confound variable. In addition, standard motion parameters were added to the model. For the self-stimulation–induced orgasm condition, group mean motion displacements were 1.48 mm (absolute) and 0.20 mm (relative). For the partner-stimulation–induced orgasm condition, group mean motion displacements were 1.10 mm (absolute) and 0.18 mm (relative). The data were spatially smoothed using a 5-mm isotropic full width at half-maximum Gaussian kernel with the exception of the brainstem analysis, in which the data were not spatially smoothed. For the brainstem, the analysis was carried out a second time with the exception of spatial smoothing. Spatially smoothing a dataset enables the detection of larger clusters such as those found within the forebrain; however, the application of spatial smoothing to smaller areas, such as those within the brainstem, decreases the detection of smaller clusters.24,25
Registration of the functional images to the high-resolution anatomic images was performed outside the FEAT using the FMRIB Linear Image Registration Tool (FLIRT)26 and selecting the options Mutual Information Cost Function and Sinc Interpolation (Blackman, width of Sinc window = seven voxels). Each participant’s first-level FEAT registration file was updated with the FLIRT registration conducted outside FEAT before higher-level analyses.
A challenge to the analysis of orgasm is the variability in the duration of the stimulation before orgasm, the duration of orgasm, and the duration of the recovery period. The duration of stimulation ranged from 87 to 829 seconds, and the duration of orgasm ranged from 10 to 59 seconds. The recovery period—or the time it took for participants to be “over” their orgasm—ranged from 23 to 89 seconds.
We dealt with this variability by sampling, across participants, equivalent time points reflecting comparable phases in the stimulation, orgasm, and recovery periods. For the “stimulation” phase, equivalent epochs were sampled at the beginning, middle, and end of stimulation, regardless of the individual latency to orgasm. For the “orgasm” phase, an equivalent epoch was sampled at orgasm onset. For the “recovery” phase, an equivalent epoch was sampled at its beginning. These equivalent epochs were used as the explanatory variables (EVs) in the analyses. The five EVs were early stimulation, mid-stimulation, late stimulation, orgasm, and recovery.
The durations of these equivalent data sample epochs (selected as a maximum of 20 seconds each) were indexed to each woman’s orgasm duration. Thus, seven women had orgasms longer than 20 seconds, so the first 20 seconds of their early, mid, late, and recovery epochs, respectively, was selected. Three women had orgasms 10 to 14 seconds in duration, so their corresponding epochs were matched to those durations.
First-level analyses were conducted with a high-pass filter cutoff set at 100 seconds. The FMRIB Improved Linear Model (FILM) pre-whitening option was selected to improve estimation efficiency. The data were convolved using a Gaussian hemodynamic response function with temporal derivatives. The EVs were used as regressors to determine the average activity elicited by each condition. Differential contrasts were set up to compare each condition with the adjacent conditions (eg, mid-stimulation > early stimulation; orgasm > late stimulation; and orgasm > recovery). The output files (contrast of parameter estimates, or “COPE” files) were used in the higher-level analysis to determine mean group effects and to perform contrast analyses between conditions.
Higher-level analyses were performed using the FMRIB Local Analysis of Mixed Effects (FLAME 1). A whole-brain analysis was conducted in which the FEAT files containing all contrasts from the first levels were passed up to the higher-level analysis. A two-group unpaired t-test was conducted with self-stimulation– induced orgasm inputs (EV 1) and partner-stimulation–induced orgasm inputs (EV 2). Contrasts were set up to analyze group means and group differences (ie, self-induced orgasm group > partner-induced orgasm group and partner-induced orgasm group > self-induced orgasm group). Two additional group analyses were similarly conducted, but with specific regions of interest. One analysis masked the frontal lobe and the other masked the temporal lobe using standard masks from the Montreal Neurological Institute Structural Atlas to determine whether there were any deactivations (activity significantly lower than the global baseline) in these specific regions. All group results were corrected for multiple comparisons with a cluster-forming threshold set at z equal to 2.3 (P = .001).
Combined Self- and Partner-Induced Orgasm Group
Results of first levels for the self-stimulation–induced orgasm group and partner-stimulation–induced orgasm group were combined by passing up the respective FEAT files to the higher-level group analysis using a single group average to create the combined orgasm group.
A differential contrast that was not conducted on the first levels (orgasm > mid-stimulation) was created by entering the first-level COPE files for the basic contrasts (orgasm > 0 and mid-stimulation > 0) and conducting a paired t-test between the two conditions. Two differential contrasts were created (mid-stimulation > orgasm and orgasm > mid-stimulation).
Two additional group analyses targeting the frontal and temporal lobes, respectively, were conducted for the combined orgasm group and for each group separately to determine whether there were any deactivations (activity significantly lower than the global baseline) in these regions of interest observed for the basic contrasts. A separate brainstem analysis of the combined orgasm group also was performed using a brainstem mask from the Harvard-Oxford Subcortical Structural Atlas.
All group results were corrected for multiple comparisons with a cluster-forming threshold set at z equal to 2.3 (P = .001) with one “exploratory contrast” (ie, orgasm > late stimulation) analyzed with a cluster threshold of 1.65 (P < .01).
The brainstem analyses were set at threshold of z equal to z = 1.0 or 1.5 (P < .01), depending on the specific contrast, so the brainstem nuclei could be discerned.
Time-Course Analysis
The “regions of interest” for the time-course analysis of the combined orgasm data were selected based on significantly active regions found in the following differential contrasts: mid-stimulation > early stimulation, orgasm > mid-stimulation, orgasm > late stimulation, and orgasm > early recovery. Masks were created for the brainstem, the secondary somatosensory cortex (combined bilateral regions of the operculum [OP1–4]), cerebellum, insula, paracentral lobule, frontal cortex, hypothalamus, left and right amygdala, left and right nucleus accumbens, and left and right hippocampus. The 13 masks were converted from standard space to each individual’s native space. For each participant and each region, the time series was extracted from the temporally high-pass–filtered and motion-corrected “filtered functional” data file for 11 TRs centered on the “onset” of orgasm—encompassing the 10 seconds immediately before and the 10 seconds immediately after orgasm onset. The output text files containing the time-course values were moved to Excel (Microsoft, Redmond WA, USA) for a group calculation of TR by TR (2 seconds) comparison of the percentage of change from a 60-second resting baseline.
RESULTS
Partner-Induced vs Self-Induced Orgasm
Comparison of frontal cortical activity (greater than baseline) between partner-induced orgasm and self-induced orgasm showed no significant difference between groups for any brain regions. Furthermore, there was no significant deactivation of the frontal cortex, temporal cortex, or any other brain region. The only significant difference between the two groups was found during the stimulation period: the self-stimulation group showed more activity during mid-stimulation, whereas the partner-stimulation group showed more activity during late stimulation. Because there were no significant differences between the orgasms in the two groups, we combined the data of the two groups and analyzed the sequential activity leading up to, during, and after orgasm.
Combined Self- and Partner-Induced Orgasm Group Analysis
Orgasm Compared With Early Stimulation
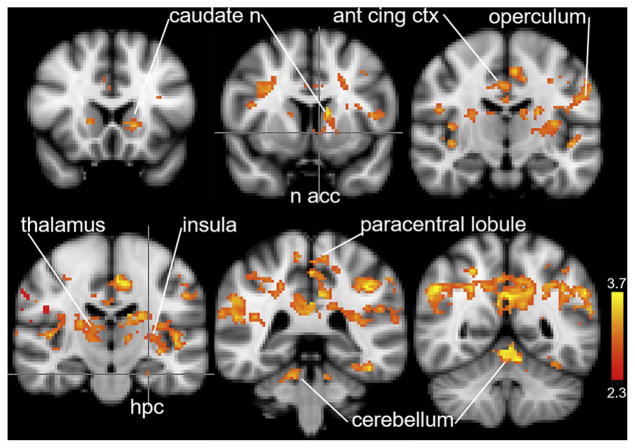
Figure 1 and Table 1 present the brain regions in which activity at orgasm was significantly higher than at early stimulation (z = 2.3, P < .001). These brain regions include the nucleus accumbens, hippocampus, operculum (SII), insula, anterior and cingulate cortices, paracentral lobule, and cerebellum. Additional regions listed in Table 1 also were significantly activated, including the hypothalamus (mammillary bodies), amygdala, and frontal cortical regions, including Broca area (BA) 44 and BA 45.
Figure 1.
Regional activation at orgasm compared with early stimulation. Regional brain activity during the 20 seconds at the initiation of stimulation was subtracted from the 20 seconds of activity immediately after the button press indicating the start of orgasm (z = 2.3, P <.001). ant cing ctx = anterior cingulate cortex; hpc = hippocampus; n = nucleus; n acc = nucleus accumbens.
Table 1.
Significant findings with coordinates of max z-score for main analyses
| z-score | x | y | z | |
|---|---|---|---|---|
| Whole brain | ||||
| Orgasm > early stimulation | ||||
| Left | ||||
| Amygdala latero-basal | 2.33 | −26 | −18 | −20 |
| Broca area 44 | 3.45 | −46 | 10 | 12 |
| Broca area 45 | 2.86 | −40 | 14 | 24 |
| Caudate | 3.47 | −10 | 12 | 6 |
| Cerebellum | 3.47 | −8 | −52 | −16 |
| Corticospinal tract | 3.43 | −32 | −26 | 36 |
| Frontal operculum cortex | 3.06 | −42 | 10 | 12 |
| Hippocampus | 3.07 | −26 | −30 | −16 |
| Inferior frontal gyrus pars opercularis | 3.45 | −46 | 10 | 12 |
| Inferior parietal lobule | 3.65 | −58 | −48 | 34 |
| Insula | 3.54 | −40 | −22 | −4 |
| Middle frontal gyrus | 3.23 | −30 | 2 | 48 |
| Nucleus accumbens | 2.92 | −14 | 20 | −2 |
| Pallidum | 2.70 | −26 | −22 | −2 |
| Parietal operculum | 3.49 | −32 | −28 | 6 |
| Pons | 2.40 | −18 | −28 | −30 |
| Putamen | 3.15 | −34 | −18 | −6 |
| Superior frontal gyrus | 2.69 | −28 | 4 | 50 |
| Superior temporal gyrus—posterior | 3.61 | −60 | −42 | 16 |
| Supramarginal gyrus—anterior | 3.76 | −40 | −32 | 38 |
| Supramarginal gyrus—posterior | 3.62 | −60 | −42 | 16 |
| Temporal pole | 2.79 | −54 | 4 | −4 |
| Thalamus | 3.39 | −14 | −8 | 16 |
| WM cingulum | 2.53 | −6 | −30 | 28 |
| Right | ||||
| Broca area 44 | 3.35 | 46 | 6 | 16 |
| Caudate | 3.51 | 14 | −2 | 16 |
| Cerebellum | 2.99 | 12 | −38 | −24 |
| Corticospinal tract | 3.06 | 30 | −26 | 44 |
| Inferior parietal lobule | 3.22 | 54 | −38 | 18 |
| Insula | 3.29 | 44 | −22 | 6 |
| Parietal operculum | 3.64 | 42 | 4 | 16 |
| Pons | 2.40 | 12 | −32 | −30 |
| Putamen | 2.55 | 26 | −14 | 14 |
| Supramarginal gyrus—posterior | 3.72 | 50 | −48 | 36 |
| Thalamus | 3.39 | 14 | −4 | 16 |
| WM cingulum | 2.47 | 6 | −48 | 26 |
| Midline | ||||
| Anterior cingulate gyrus | 3.46 | −4 | −18 | 44 |
| Cerebellum | 3.55 | −2 | −54 | −10 |
| Fornix | 3.24 | −2 | −6 | 10 |
| Mammillary body | 3.06 | 6 | −4 | 10 |
| Paracentral lobule | 3.28 | 0 | −38 | 50 |
| Posterior cingulate gyrus | 3.73 | 4 | −58 | 28 |
| Precuneus | 3.73 | 4 | −58 | 28 |
| Orgasm > early recovery | ||||
| Left | ||||
| Broca area 44 | 3.22 | −46 | 8 | 10 |
| Broca area 45 | 3.01 | −46 | 40 | −2 |
| Caudate | 3.25 | −18 | −16 | 20 |
| Central operculum cortex | 3.23 | −40 | −4 | 20 |
| Cerebellum—crus I | 3.29 | −32 | −74 | −40 |
| Cerebellum—crus II | 3.67 | −30 | −74 | −44 |
| Cerebellum—crus VI | 3.19 | −22 | −46 | −24 |
| Dorsal medial prefrontal cortex | 2.55 | −14 | 34 | 32 |
| Frontal operculum cortex | 3.11 | −36 | 26 | 2 |
| Frontal pole | 3.21 | −42 | 40 | −10 |
| Hippocampus—cornu ammonis | 3.15 | −30 | −32 | −8 |
| Hippocampus—entorhinal | 3.23 | −24 | −26 | −24 |
| Hippocampus—subiculum | 3.12 | −24 | −26 | −22 |
| Inferior frontal gyrus—pars opercularis | 3.22 | −46 | 8 | 10 |
| Insula | 3.04 | −34 | −38 | 20 |
| Orbital frontal cortex | 3.19 | −36 | 30 | −2 |
| Paracingulate gyrus | 2.84 | −10 | 30 | 32 |
| Parietal operculum—OP1 | 3.12 | −64 | −26 | 36 |
| Parietal operculum—OP2 | 2.90 | −36 | −16 | 24 |
| Parietal operculum—OP3 | 3.17 | −38 | −6 | 20 |
| Parietal operculum—OP4 | 3.01 | −40 | −8 | 14 |
| Putamen | 2.72 | −32 | −8 | 0 |
| Superior frontal gyrus | 3.39 | −16 | 14 | 50 |
| Supramarginal gyrus—posterior | 3.84 | −56 | −42 | 46 |
| Thalamus | 3.15 | −10 | −20 | 14 |
| WM corticospinal tract | 3.73 | −40 | −12 | 42 |
| Right | ||||
| Angular gyrus | 3.73 | 52 | −56 | 30 |
| Caudate | 3.42 | 14 | −2 | 16 |
| Cerebellum crus VI | 3.37 | 8 | −74 | −12 |
| Insula | 3.05 | 34 | −28 | 22 |
| Lingual gyrus | 3.56 | 6 | −78 | −12 |
| Parietal operculum—OP1 | 3.58 | 62 | −24 | 30 |
| Parietal operculum—OP2 | 3.05 | 34 | −28 | 22 |
| Parietal operculum—OP3 | 3.38 | 58 | −20 | 26 |
| Parietal operculum—OP4 | 3.50 | 54 | −22 | 26 |
| Precuneus cortex | 3.23 | 6 | −54 | 14 |
| Putamen | 2.59 | 26 | −14 | 14 |
| Superior temporal gyrus—posterior | 3.43 | 64 | −36 | 28 |
| Thalamus | 3.25 | 14 | −2 | 14 |
| WM corticospinal tract | 3.22 | 28 | −30 | 40 |
| Midline | ||||
| Cerebellum vermis VI | 3.01 | 4 | −76 | −20 |
| Cingulate gyrus—anterior | 3.26 | −6 | −18 | 44 |
| Cingulate gyrus—posterior | 2.46 | −10 | −18 | 44 |
WM = white matter.
Orgasm Compared With Immediate Post-Orgasm Phase (ie, “Recovery”)
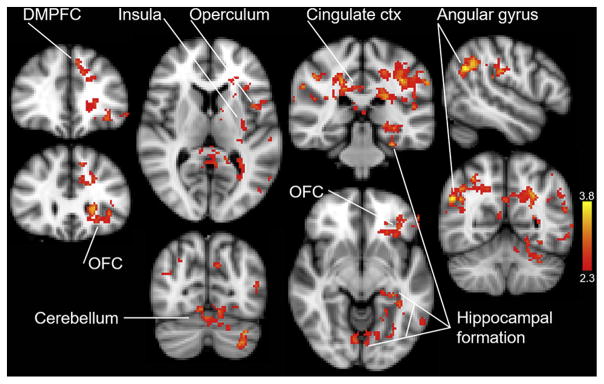
Figure 2 shows activity in brain regions at orgasm was significantly higher than at early recovery (z = 2.3, P < 0.001). The following regions were significantly activated, including the dorsomedial prefrontal cortex, orbitofrontal cortex, insula, operculum, cingulate gyrus, cerebellum including the vermis, hippocampal formation, and right angular gyrus. Additional activations included the paracingulate gyrus, caudate, thalamus, putamen, left BA 44 and 45, and posterior cingulate gyrus (Table 1).
Figure 2.
Regional activation at orgasm compared with recovery. Brain activity during the 20 seconds immediately after the button press indicating the end of orgasm was subtracted from the 20 seconds of activity starting at the onset of orgasm (orgasm > recovery; z = 2.3, P <.001). ctx = cortex; DMPFC = dorsal medial prefrontal cortex; OFC = orbital frontal cortex.
Transition to Orgasm: An Exploratory Analysis
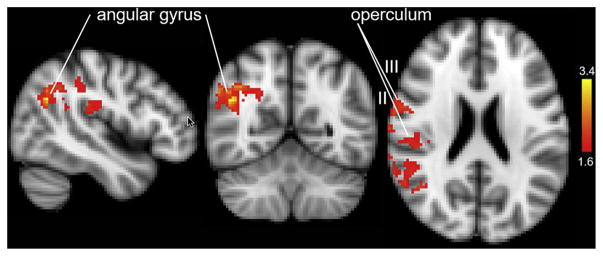
To ascertain whether there were brain regions whose activity levels were uniquely related to orgasm, we contrasted the activity during the first 20 seconds of orgasm with the activity during the 20 seconds immediately before orgasm. Thus, the data for the high level of preorgasmic arousal activity were subtracted from the data for actual orgasmic activity. Using a cluster-forming threshold of z equal to 2.3 (P < .01) yielded no significant difference between these two 20-second periods, likely because of the similarity in high activity during these two conditions and/or a limited sample size. To explore the differences between these two similar conditions, we analyzed these data using a less stringent cluster-forming threshold of z equal to 1.65 (P < .01). Based on this criterion, brain regions showed significantly higher activity during orgasm than immediately before orgasm (provisional results presented in Figure 3). These included, all on the right side, the operculum (secondary somatosensory cortex regions 1–4) and the angular gyrus and the precuneus, BA 44 and 45, pre and post central gyri, and other regions of the frontal, temporal, parietal, and occipital cortices (Table 2).
Figure 3.
Regional activation “going over” to orgasm. Brain activity during the 20 seconds immediately before the button press indicating the onset of orgasm was subtracted from the 20 seconds of activity immediately after the button press (orgasm > late stimulation; z = 1.65, P < .01).
Table 2.
Exploratory analysis and brainstem results: significant findings with coordinates of max z-score
| z-score | x | y | z | |
|---|---|---|---|---|
| Whole brain | ||||
| Orgasm > late stimulation | ||||
| Right | ||||
| Angular gyrus | 3.48 | 46 | −58 | 28 |
| Broca area 44 | 3.22 | −46 | 8 | 10 |
| Broca area 45 | 3.01 | −46 | 40 | −2 |
| Inferior frontal gyrus—pars opercularis | 2.25 | 62 | 8 | 10 |
| Inferior frontal gyrus—pars triangularis | 1.91 | 58 | 18 | 10 |
| Inferior parietal lobule | 2.18 | 52 | −22 | 28 |
| Lateral occipital cortex—superior | 3.49 | 46 | −58 | 28 |
| Middle temporal gyrus—temporooccipital | 2.37 | 62 | −48 | 16 |
| Parietal operculum—OP1 | 2.47 | 66 | −24 | 28 |
| Parietal operculum—OP2 | 1.90 | 46 | −26 | 20 |
| Parietal operculum—OP3 | 2.03 | 52 | −2 | 18 |
| Parietal operculum—OP4 | 2.55 | 66 | −6 | 18 |
| Planum temporale | 2.29 | 60 | −24 | 14 |
| Postcentral gyrus | 2.61 | 64 | −4 | 24 |
| Precentral gyrus | 2.64 | 52 | 0 | 20 |
| Precuneus cortex | 1.76 | 24 | −58 | 30 |
| Superior parietal lobule | 2.04 | 34 | −56 | 38 |
| Superior temporal gyrus—posterior | 2.43 | 66 | −38 | 18 |
| Supramarginal gyrus—anterior | 2.47 | 66 | −24 | 28 |
| Supramarginal gyrus—posterior | 3.15 | 48 | −48 | 36 |
| Brainstem | ||||
| Orgasm > early stimulation | ||||
| Left | ||||
| Nucleus cuneiformis | 1.80 | −6 | −32 | −20 |
| Pons | 2.07 | −10 | −24 | −26 |
| Substantia nigra | 1.84 | −6 | −20 | −20 |
| Right | ||||
| Pons | 2.09 | 6 | −30 | −30 |
| Substantia nigra | 2.02 | 6 | −20 | −20 |
| Midline | ||||
| Dorsal raphe | 1.88 | −2 | −28 | −16 |
| Ventral tegmental area | 2.52 | 4 | −24 | −18 |
| Orgasm > late stimulation | ||||
| Left | ||||
| Pons | 1.59 | −6 | −20 | −24 |
| Substantia nigra | 1.74 | −10 | −14 | −12 |
| Right | ||||
| Pons | 1.79 | 6 | −26 | −28 |
| Substantia nigra | 1.54 | 8 | −12 | −14 |
| Midline | ||||
| Dorsal raphe | 1.31 | 2 | −28 | −16 |
| Ventral tegmental area | 1.04 | 2 | −24 | −18 |
WM = white matter.
Brainstem Regions Activated at Orgasm
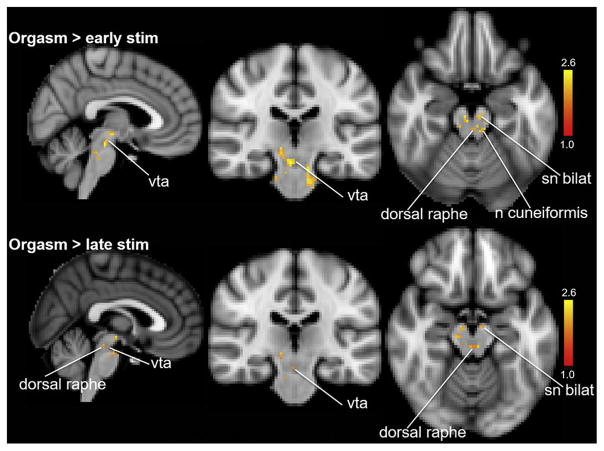
Figure 4 shows several lower brainstem regions that were activated at orgasm compared with early and late stimulation (ie, ventral tegmental area, substantia nigra, dorsal raphe, and nucleus cuneiformis). We used a lower z-score threshold for the orgasm > late stimulation because the activity levels during these two epochs were more similar to each other than those for orgasm and early stimulation (Table 2).
Figure 4.
Lower brainstem regions activated during orgasm. Top panel shows orgasm > early stimulation (z = 1.5, P <.01). Bottom panel shows orgasm > late stimulation (z = 1.0, P < .01). n = nucleus; sn bilat = bilateral substantia nigra; stim = stimulation; vta = ventral tegmental area.
Time-Course Analysis
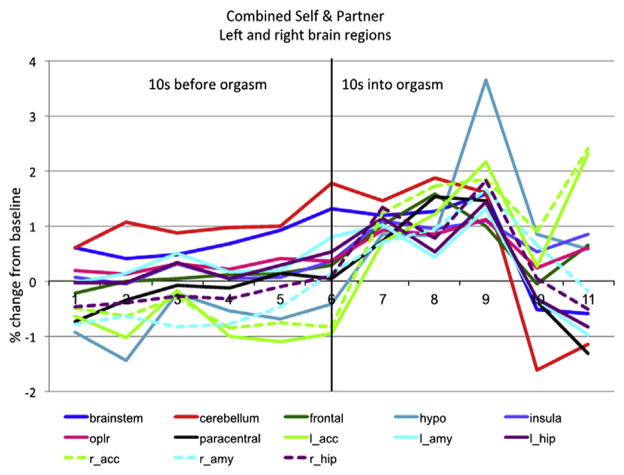
For a finer-grained analysis of this peri-orgasm phase, we plotted the activity in selected brain regions every 2 seconds during the 10-second period immediately before orgasm and during the first 10 seconds after the onset of orgasm. Figure 5 depicts this activity, showing that in most regions analyzed, there was a gradual increase that became more abrupt at the onset of orgasm and continued to increase for the next three 2-second periods. Marked activation was observed in the nucleus accumbens and hypothalamus starting at the onset of orgasm and continuing during orgasm.
Figure 5.
Composite view of the activity of selected brain regions in the 10 seconds immediately before and the 10 seconds immediately after the onset of orgasm. Note the gradual increase in overall activity leading up to orgasm, with variable patterns of increase after the onset of orgasm and some regions showing abrupt increases at orgasm onset (eg, accumbens; hypothalamus, paracentral lobule; N = 10). hypo = hypothalamus; paracentral = paracentral lobule; l_acc = left accumbens; l_amy = left amygdala; l_hip = left hippocampus; oplr = operculum (SII); r_acc = right accumbens; r_amy = right amygdala; r_hip = right hippocampus.
Activity in these brain regions increased at different rates during late stimulation. Some regions had a high level of activity overall, including the cerebellum, lower brainstem, secondary somatosensory cortex, and frontal cortex, with further increases at orgasm. Other regions, including the paracentral lobule, hippocampus, and insula, had a steadily rising level of activity toward the onset of orgasm, with a further increase at orgasm that continued throughout the 10-second orgasm epoch. Other regions, including the amygdala, had different patterns on the left and right sides, with greater activity on the left before orgasm and on the right that increased during the middle of the 10-second orgasm epoch.
DISCUSSION
The present findings provide evidence that genital stimulation activated widespread brain regions in differential temporal patterns in the approach to, during, and after orgasm involving the activation of sensory, sensory integrative, limbic, motor, frontal cortical, and other neocortical regions. We found no evidence of deactivation of frontal or temporal regions during partner-stimulation–induced or self-stimulation–induced orgasm or when these two groups were combined.
The highest level of brain activity was observed during orgasm compared with that during early stimulation and early recovery in regions that included the nucleus accumbens, insula, anterior cingulate cortex, orbitofrontal cortex, operculum, right angular gyrus, paracentral lobule, cerebellum, hippocampus, and amygdala (Figures 1 and 2 and Table 1).
We sought to ascertain whether there was activity unique to orgasm compared with the high level of arousal immediately before orgasm. The results of an analysis applying the standard cluster-forming criterion (z = 2.3) showed no significant difference between these two conditions, most likely because the activity levels were similar. However, when we used a less stringent criterion (z = 1.65, P < .01), significant activation was observed during orgasm in the angular gyrus, operculum, and other cortical regions, suggesting, based on this more exploratory approach, that they play a unique role in the experience of orgasm.
The activation in the right angular gyrus and operculum also was significant in the comparison between orgasm and recovery. The right angular gyrus has been implicated in states of altered perception (ie, “out-of-body experiences”27,28), which could be related to the “altered state of consciousness” at orgasm.29 This finding is consistent with previous studies showing right-side brain activation during orgasm30,31 and lateralization of brain injury effects on sexuality.32
The present findings obtained through the use of improved methodology, which decreased head-movement artifacts and corrected results for multiple comparisons, are consistent with our previous reports on brain activity at orgasm in women.14–19 The present findings are consistent with certain findings of Georgiadis et al20,21 who reported reliable activation in the cerebellum and pons at orgasm. However, in contrast to those reports, we did not find evidence of “deactivations” (is, activity greater during baseline) compared with orgasm for frontal or temporal regions as determined by the results of region-of-interest analyses conducted separately for the self-stimulation group, the partner-stimulation group, and combined self- and partner-stimulation group.
In addition, we found no frontal or temporal regional activity greater before orgasm than at orgasm or activity after orgasm greater than during orgasm. Rather, there were multiple regions of frontal cortical activation at orgasm, including the orbito-frontal region of the frontal cortex, an area identified as a “hedonic hot spot.”33
Contrary to what we originally believed might account for the discrepancy between our previous reports and those of Georgiadis et al,20,21 we found no significant differences at orgasm between the partner-stimulation and self-stimulation orgasm groups. This suggests that the major discrepancy between the results of the two groups arises not from the difference in type of stimulation used to induce orgasm (self- vs partner-applied), but rather from differences in the imaging methods used (fMRI vs PET). Our use of fMRI blood-oxygen-level dependent methodology in the present study allowed repetitive imaging of activity of the entire brain at 2-second intervals, rather than the single “snapshot” imaging provided by PET; this advantage enabled us to directly compare regional brain activity precisely at orgasm with the early and late stimulation and recovery phases. We note that Stoleru et al34 stated, “Results of PET studies of orgasm should be taken with caution because of their low temporal resolution. Future studies of orgasm would likely benefit from the application of fMRI” [p. 1504].
Based on results of our preliminary time-course study,15 we expected that activity of some brain regions would increase gradually with early activation, others would build over the course of self-stimulation, and others would show phasic activation just before orgasm or at orgasm; those observations were confirmed in the present study.
The present findings provide evidence of activation at orgasm of brain regions that stimulate sympathetic activity (ie, posterior hypothalamus35). There also was activation of lower brainstem regions implicated in pleasure, reward, and addiction,33 specifically the ventral tegmentum (containing the cell bodies of the mesocorticolimbic dopamine system) and the substantia nigra. Our group previously reported that pain thresholds are increased more than 100% during orgasm.36 In the present study, we observed significant activation of the dorsal raphe nucleus (which releases serotonin) and the nucleus cuneiformis,37 which are major brainstem components that mediate endogenous analgesia38 and which could account, at least in part, for the pain-attenuating effect of orgasm.
A limitation of the present study is the small number of participants in each of the two groups (N = 10). Additional significant differences between groups might be observed with a larger number of participants per group.
Another potential limitation of the study is that we combined self- and partner-stimulation datasets for analysis. Although our findings of the self- and partner-stimulation groups yielded no significant group differences at orgasm and served as a rationale for the combined orgasm group, further studies of self- and partner-induced orgasm are warranted.
CONCLUSION
Consistent with our previous reports,14–17 the present findings provide evidence of a gradual increase in the brain regions that are activated leading up to and during orgasm, followed by a decrease in these activations. We found no evidence of deactivation of brain regions leading up to or during orgasm. The activated brain regions included sensory, motor, reward, frontal cortical, and lower brainstem regions, such as the genital sensory cortex (paracentral lobule), secondary somatosensory cortex (operculum [SII] regions OP1 and OP4), precuneus, inferior parietal lobule, insula, hippocampus, amygdala, cerebellum, supplementary motor area, dorsal and ventral striatum (caudate, putamen, and nucleus accumbens), ventral tegmental area, hypothalamus, pons, anterior and posterior cingulate cortex, temporal pole, and prefrontal cortex. These brain regions showed differential patterns of activity during the post-orgasm recovery phase.
Although there has been speculation regarding the neural networks involved in processes leading up to and including orgasm,39,40 much work is yet to be done to clearly define these networks and delineate how they function. Our research group recently conducted preliminary investigations into the effective connectivity among brain components during the orgasm sequence in humans.41 Future effective connectivity42 studies should serve to elucidate how the activity of these regions develops and is integrated over the course of stimulation, orgasm, and recovery in the totality of the orgasm sequence and could provide insight into the factors contributing to anorgasmia.
Acknowledgments
We gratefully acknowledge the excellent technical assistance and support of Dr. Catherine Hanson, Dr. Stephen Jose Hanson, and Mr. Gregg Ferencz of the Rutgers University Brain Imaging Center (RUBIC), and Dr. Kachina Allen, and Ms. Wendy Birbano.
Funding: This study was supported in part by grants from the Rutgers University Brain Imaging Center (NW), the National Institute of General Medical Sciences of the NIH 2R-25-GM060826 (BRK), and by the Rutgers University Research Fund (BRK).
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
STATEMENT OF AUTHORSHIP
-
Conception and DesignNan J. Wise; Barry R. Komisaruk
-
Acquisition of DataNan J. Wise; Eleni Frangos
-
Analysis and Interpretation of DataNan J. Wise; Eleni Frangos; Barry R. Komisaruk
-
Drafting the ArticleNan J. Wise; Barry R. Komisaruk
-
Revising It for Intellectual ContentNan J. Wise; Eleni Frangos; Barry R. Komisaruk
-
Final Approval of the Completed ArticleNan J. Wise; Eleni Frangos; Barry R. Komisaruk
References
- 1.Hamann S, Herman RA, Nolan CL, et al. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7:411–416. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- 2.Rupp HA, James TW, Ketterson ED, et al. Lower sexual interest in postpartum women: relationship to amygdala activation and intranasal oxytocin. Horm Behav. 2013;63:114–121. doi: 10.1016/j.yhbeh.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karama S, Lecours AR, Leroux JM, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holstege G, Georgiadis JR, Paans AMJ, et al. Brain activation during human male ejaculation. J Neurosci. 2003;23:9185–9193. doi: 10.1523/JNEUROSCI.23-27-09185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feretti A, Caulo M, Del Gratta C, et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. Neuroimage. 2005;15:1086–1096. doi: 10.1016/j.neuroimage.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 6.Hamann S. Sex differences in the responses of the human amygdala. Neuroscientist. 2005;11:288–293. doi: 10.1177/1073858404271981. [DOI] [PubMed] [Google Scholar]
- 7.Cera N, Di Pierro ED, Sepede G, et al. The role of left superior parietal lobe in male sexual behavior: dynamics of distinct components revealed by fMRI. J Sex Med. 2012;9:1602–1612. doi: 10.1111/j.1743-6109.2012.02719.x. [DOI] [PubMed] [Google Scholar]
- 8.Spinella M. The role of prefrontal systems in sexual behavior. Int J Neurosci. 2007;117:369–385. doi: 10.1080/00207450600588980. [DOI] [PubMed] [Google Scholar]
- 9.Leon-Carrion J, Martin-Rodriguez JF, Damas-Lopez J, et al. Does dorsolateral prefrontal cortex (DLPFC) activation return to baseline when sexual stimuli cease? The role of DLPFC in visual sexual stimulation. Neurosci Lett. 2007;6:55–60. doi: 10.1016/j.neulet.2007.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Huynh HK, Beers C, Willemsen A, et al. High-intensity erotic visual stimuli de-activate the primary visual cortex in women. J Sex Med. 2012;9:1579–1587. doi: 10.1111/j.1743-6109.2012.02706.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn S, Gallinat J. A quantitative meta-analysis on cue-induced male sexual arousal. J Sex Med. 2011;8:2269–2275. doi: 10.1111/j.1743-6109.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- 12.Oei NY, Rombouts SA, Soeter RP, et al. Dopamine modulates reward system activity during subconscious processing of sexual stimuli. Neuropsychopharmacology. 2012;37:1729–1737. doi: 10.1038/npp.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortigue S, Grafton ST, Bianchi-Demicheli F. Correlation between insula activation and self-reported quality of orgasm in women. Neuroimage. 2007;37:551–560. doi: 10.1016/j.neuroimage.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Komisaruk BR, Whipple B, Crawford A, et al. Brain activation during vaginocervical self-stimulation and orgasm in women with complete spinal cord injury: fMRI evidence of mediation by the vagus nerves. Brain Res. 2004;1024:77–88. doi: 10.1016/j.brainres.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 15.Komisaruk BR, Wise N, Frangos E, et al. Neuroscience Meeting Planner. San Diego: Society for Neuroscience; 2010. An fMRI time-course analysis of brain regions activated during self-stimulation to orgasm in women. Program number 285.6. [Google Scholar]
- 16.Komisaruk BR, Wise N, Frangos E, et al. Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2011. An fMRI video animation time-course analysis of regions activated during self-stimulation to orgasm in women. Program number 495.03. [Google Scholar]
- 17.Wise NJ. Unpublished doctoral dissertation. Rutgers University; 2014. Genital stimulation, imagery, and orgasm in women: an fMRI analysis. [Google Scholar]
- 18.Holstege G, Huynh HK. Brain circuits for mating behavior in cats and brain activations and de-activations during sexual stimulation and ejaculation and orgasm in humans. Horm Behav. 2011;59:702–770. doi: 10.1016/j.yhbeh.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Komisaruk BR, Whipple B. Functional MRI of the brain during orgasm in women. Annu Rev Sex Res. 2005;16:62. [PubMed] [Google Scholar]
- 20.Georgiadis JR, Kortekaas R, Kuipers R, et al. Regional cerebral blood flow changes associated with clitorally induced orgasm in healthy women. Eur J Neurosci. 2006;24:3305–3316. doi: 10.1111/j.1460-9568.2006.05206.x. [DOI] [PubMed] [Google Scholar]
- 21.Georgiadis JR, Reinders A, Paans AM, et al. Men versus women on sexual brain function: prominent differences during tactile genital stimulation, but not during orgasm. Hum Brain Mapp. 2009;30:3089–3101. doi: 10.1002/hbm.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgiadia JR, Farrell MJ, Boessen R, et al. Dynamic subcortical blood flow during male sexual activity with ecological validity: a perfusion fMRI study. Neuroimage. 2010;50:208–216. doi: 10.1016/j.neuroimage.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;4(Suppl):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Poldrack RA, Mumford JA, Nichols TE. Handbook of functional MRI data analysis. New York: Cambridge University Press; 2011. [Google Scholar]
- 25.Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stim. 2015;8:624–636. doi: 10.1016/j.brs.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkinson M, Bannister P, Brady M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 27.Blank O, Ortigue S, Landis T, et al. Stimulating illusory own body experiences. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- 28.Tong F. Out-of-body experiences: from Penfield to present. Trends Cogn Sci. 2003;7:104–106. doi: 10.1016/s1364-6613(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 29.Meston C, Hull E, Levin R, et al. Disorders of orgasm in women. J Sex Med. 2004;1:66–68. doi: 10.1111/j.1743-6109.2004.10110.x. [DOI] [PubMed] [Google Scholar]
- 30.Tiihonen J, Kuikka J, Kupila J, et al. Increase in cerebral blood flow of right prefrontal cortex in man during orgasm. Neurosci Lett. 1994;170:241–243. doi: 10.1016/0304-3940(94)90328-x. [DOI] [PubMed] [Google Scholar]
- 31.Cohen HD, Rosen RC, Goldstein L. Electroencephalographic laterality changes during human sexual orgasm. Arch Sex Behav. 1976;5:189–199. doi: 10.1007/BF01541370. [DOI] [PubMed] [Google Scholar]
- 32.Suffren S, Braun CMJ, Guimond A, et al. Opposed hemispheric specializations for human hypersexuality and orgasm? Epilepsy Behav. 2011;21:12–19. doi: 10.1016/j.yebeh.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86:646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoleru S, Fonteille V, Cornelis C, et al. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neurosci Biobehav Rev. 2012;36:1481–1509. doi: 10.1016/j.neubiorev.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Hess WR. The functional organization of the diencephalon. New York: Grune & Stratton; 1957. [Google Scholar]
- 36.Whipple B, Komisaruk BR. Elevation of pain threshold by vaginal stimulation in women. Pain. 1985;21:357–367. doi: 10.1016/0304-3959(85)90164-2. [DOI] [PubMed] [Google Scholar]
- 37.Zelman FP, Behbehani MM. Nucleus cuneiformis and pain modulation: anatomy and behavioral pharmacology. Brain Res. 1988;2:89–102. doi: 10.1016/0006-8993(88)90146-1. [DOI] [PubMed] [Google Scholar]
- 38.Basbaum A, Fields HL. Endogenous pain control mechanisms: review and hypothesis. Ann Neurol. 1978;4:451–462. doi: 10.1002/ana.410040511. [DOI] [PubMed] [Google Scholar]
- 39.Geogiadis JR, Kringelbach LM, Pfaus JG. Sex for fun: a synthesis of human and animal neurobiology. Nat Rev Urol. 2012;9:486–498. doi: 10.1038/nrurol.2012.151. [DOI] [PubMed] [Google Scholar]
- 40.Komisaruk BR, Beyer C, Whipple B. The science of orgasm. Vol. 1. Baltimore: Johns Hopkins University Press; 2006. pp. 267–285. [Google Scholar]
- 41.Wise NJ, Allen K, Finnerty C, et al. Neuroscience Meeting Planner. New Orleans: Society for Neuroscience; 2012. Effective connectivity among brain components during the orgasm sequence in humans. Program number 676.07. [Google Scholar]
- 42.Ramsey J, Hanson S, Glymour C. Multi-subject search correctly identifies causal connections and most causal directions in the DCM models of the Smith et al. simulation study. Neuroimage. 2011;58:838–848. doi: 10.1016/j.neuroimage.2011.06.068. [DOI] [PubMed] [Google Scholar]