Abstract
Background
Aflibercept is a recombinantly-produced fusion protein that has potent anti-VEGF activity. We tested whether aflibercept has clinical activity in clear cell renal cell carcinoma (ccRCC). The recommended Phase 2 dose was 4 mg/kg but several patients treated at 1 mg/kg demonstrated prolonged progression-free survival (PFS). We therefore tested both doses in a parallel group randomized trial.
Methods
Eligible patients (pts) had histologically confirmed advanced or metastatic ccRCC and previous treatments including prior exposure to a VEGF RTKI. Patients received aflibercept (either 1 mg/kg or 4 mg/kg) day 1 of a 14-day cycle until progression. Patients randomized to 1 mg/kg could crossover to 4 mg/kg at progression. The primary endpoint was proportion alive and progression-free at 8 weeks. A Simon 2-stage design was used for each arm with 33 and 24 eligible pts/arm enrolled in stages 1 and 2.
Results
94 pts were enrolled, 59 and 35 to 4 mg and 1 mg doses, respectively. 72% had 1 prior tx most commonly sunitinib. 16 eligible pts crossed over at progression to the 4 mg dose. Most common adverse events were hypertension, proteinuria, and fatigue. Only 4 pts reported Grade 4 or higher toxicity. With 36/59 (61%) pts PFS at 8 wks, the 4-mg/kg dose met protocol specified efficacy criteria.
Conclusions
Aflibercept is active in previously treated ccRCC and may be worthy of further study.
Keywords: renal cell carcinoma, antiangiogenesis, VEGF blocker
Introduction
Renal cell carcinoma is the fourth most common genitourinary cancer with approximately 30,000 new cases diagnosed annually causing 12,000 deaths1. Approximately 45% of patients, at the time of diagnosis, have disease localized to their kidney; 30% have distant metastases; and 25% have locally advanced disease2. Surgical resection is the only potentially curative therapy. The five-year survival for stage I or II disease patients is 66%; 42% for stage III disease; and less than 5% for stage IV disease2.
Angiogenesis, an orderly and tightly controlled process through which tumor is neovascularized, is required for metastasis and tumor progression. This process is controlled by multiple factors, one of which is the vascular endothelial growth factor (VEGF) – a protein that has a critical role in initiating and sustaining tumor angiogenesis. Clear cell renal cell carcinoma (ccRCC), the most common type of kidney cancer, harbor genetic and epigenetic alteration of the von Hippel- Lindau (VHL) gene and consequently the stabilization of the transcriptional factor hypoxia inducible factors (HIFs) with down-stream upregulation of VEGF2. Over the past 10 years the use of VEGF/VEGF receptor targeting drugs has changed the treatment of ccRCC. However, despite the proven clinical benefit, eventually patients develop progressive disease due to drug resistance.
Aflibercept has been developed as a decoy receptor and blocking drug with greater binding affinity to VEGF as compared to bevacizumab3. Its ability also to bind other related proangiogenic factors such as VEGF-B and the placental growth factors, PlGF1 and PlGF2 makes this agent an appealing anti-angiogenesis drug for ccRCC. Aflibercept has been reported to have preclinical activity in several tumor models including renal cell carcinoma and has been approved for the treatment of colon carcinoma in combination with chemotherapy4–6.
In the present study we evaluated the efficacy and safety of two doses of aflibercept in the treatment of patients with metastatic ccRCC who had progressive disease after tyrosine kinase inhibitor (TKI) therapy.
Methods
Study design and participants
Eligible patients were 18 years or older with histologically confirmed metastatic or unresectable ccRCC with measurable lesions per Response Evaluation Criteria In Solid Tumors (RECIST) criteria. Patients also had evidence of progressive disease following a prior VEGF receptor TKI treatment received for at least 12 weeks; an Eastern Cooperative Oncology Group (ECOG) performance status between 0–2; adequate organ function as measured by appropriate blood chemistries; and recovered from any toxic effects of prior radiotherapy or surgical procedures within 4 weeks prior to randomization. No prior cellular therapy, vaccine, hormonal, or chemotherapy was allowed. HIV-positive patients receiving ART were also excluded due to pharmacokinetic interactions with aflibercept; patients with history of metastatic CNS, pulmonary embolism, DVT, or other thromboembolic events were excluded. Patients were randomized into two treatment arms (A and B) to determine the effect of two different doses of aflibercept. Randomization was stratified by MSKCC7 risk category and prior cytokine therapy.
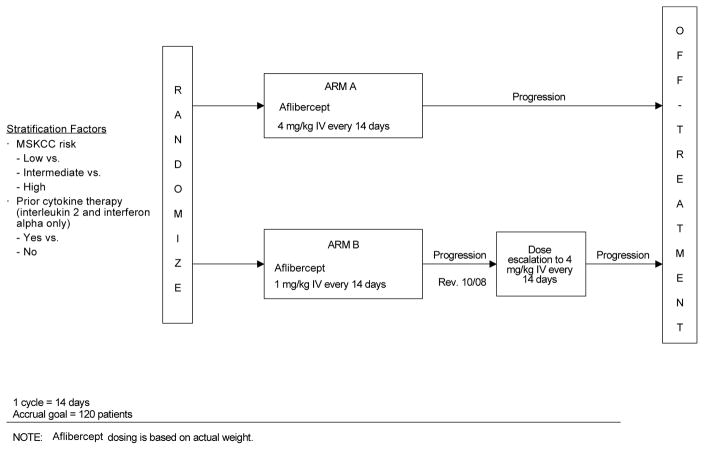
Patients randomized to Arm A received 4mg/kg of aflibercept, on Day 1 of every 14-day cycle, intravenously over 1 hour into a peripheral vein or central venous catheter using an infusion pump. Patients in Arm B received 1mg/kg using the same schedule and infusion method (Figure 1). Tumor status was assessed every 8 weeks (every 4 cycles) after starting treatment. Tumor progression was defined using RECIST criteria. At the time of progression, the dose of patients in Arm B was escalated to 4mg/kg. A patient continued receiving protocol treatment until he/she developed progressive disease or unacceptable toxicity and all patients, including those who discontinued protocol therapy early, were followed for response until progression and for survival for 3 years from the date of randomization. Aflibercept was supplied by Aventis Pharmaceuticals and Regeneron, and distributed by Cancer Trials Evaluation Program (CTEP) of the Division of Cancer Treatment and Diagnosis (DCTD), National Cancer Institute (NCI). The ClinicalTrials.gov identifier for this study is NCT00357760.
Figure 1.
Study Schema
NOTE: Aflibercept dosing is based on actual weight.
Statistical analysis
The primary endpoint was defined as the proportion of patients alive and progression-free at 8 weeks. Progression-free survival was defined as time from randomization to the earlier of documentation of progression or death. Patients alive and progression-free at the time of analysis were to be censored at the date of last disease assessment without evidence of progression. Extensive data on progression-free survival among patients who have previously been treated with TKI was not available at the time the study was designed. In this study, a progression-free proportion of 50% at 8 weeks was not of interest, while a proportion at 8 weeks of 67% signified that aflibercept was worthy of further study. Assuming an exponential distribution, this corresponds to an improvement in median progression-free survival from 8 weeks to 14 weeks.
A two-stage design was used within each arm. In the first stage, 33 eligible patients were accrued. If 16 or fewer patients were progression-free at 8 weeks, then the arm was to be closed. If at least 17 patients were progression-free at 8 weeks, then an additional 24 eligible patients would be accrued. If at least 34 of 57 eligible patients were progression-free at 8 weeks, the dose would be considered worthy of further study. Using this design, the probability on each arm of declaring a dose unworthy of further study was 91% (statistical power) if the true 8-week progression free rate was 50% and 10% (Type I error) if the true 8-week progression-free rate was 67%. To assure 33 and 24 eligible patients in the two stages of accrual, 35 and 25 patients, respectively, were to be randomized per stage and dose level for a total maximum of 60 patients per treatment arm.
Secondary endpoints were objective response rate, toxicity, response duration, and overall survival. A true objective response rate of 10% was of interest, whereas a true rate of 1% was not. Given 57 eligible patients per arm, the response rate would be considered interesting if 3 or more patients achieved an objective response. All patients receiving treatment, regardless of eligibility, were included in the assessment of toxicity. This study used Common Terminology Criteria for Adverse Events (CTCAE) Version 3 definition and only events with CTCAE attribution of possibly, probably, or definitely are reported. Response duration was defined as the time, in weeks, from onset of treatment response to disease progression. Patients without documented progression were censored at the date of last follow-up.
The efficacy analysis population included all eligible and treated patients. All treated patients, regardless of eligibility, were included in the toxicity analysis. The Kaplan-Meier (KM) method8 was used to describe overall and progression-free survival. Descriptive statistics was used to summarize patient characteristics at baseline. Two-sided 90% exact binomial CIs were computed for the assessment of toxicity and response rate.
Results
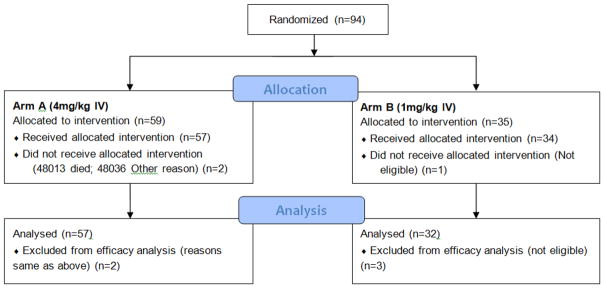
The total patient accrual was 94. The study was activated on 12/21/2007 and suspended on 03/27/2012 having reached its stage 1 accrual goal. The study was then reactivated on 06/20/2012 and only Arm A reopened to accrual – Arm B remained suspended and subsequently closed for failing to meet protocol specified efficacy criteria for continuation to stage 2. A total of 35 patients were accrued to Arm B by the end of stage 1; 3 of these 35 patients were ineligible for efficacy analysis of PFS. The number of patients alive and progression-free at 8 weeks was 16 (50%) and thus Arm B did not meet the criteria for continuing to stage 2 accrual. Fifty-nine (59) patients were accrued to Arm A by the end of stage 2 and the study was terminated on 12/06/2013. Figure 2 shows this trial’s CONSORT diagram.
Figure 2.
CONSORT diagram (with patient flow)
Patient Characteristics
Table 1 shows demographic and prognostic characteristics of patients in both treatment arms. The median age was 60 and 62 years respectively for patients in Arm A and B. More than 90% of patients in both treatment arms had an ECOG performance status of 0 or 1. The randomization process was effective in ensuring that patients in the treatment arms were comparable with respect to demographic and prognostic factors using appropriate statistical tests.
Table 1.
Patient Demographics at Baseline
| Variable | Treatment Arm N(%)
|
|
|---|---|---|
| Arm A (n=57) | Arm B (n=32) | |
|
| ||
| Age | ||
| Median | 60 | 62 |
| (Min, Max) | (35,80) | (33,73) |
| Mean ± SD | 59.7 ± 10.4 | 60.2 ± 9.0 |
|
| ||
| Sex | ||
| Male | 43 (75.4) | 22 (68.7) |
| Female | 14 (24.6) | 10 (31.3) |
|
| ||
| Race | ||
| White | 54 (94.7) | 31 (96.9) |
| Black | 3 (5.3) | 1 (3.1) |
|
| ||
| ECOG Performance Status | ||
| 0 | 28 (49.1) | 15 (46.9) |
| 1 | 26 (45.6) | 15 (46.9) |
| 2 | 3 (5.3) | 2 (6.2) |
|
| ||
| Renal Histology Type | ||
| Clear Cell Carcinoma | 53 (93.0) | 31 (96.9) |
| Mixed | 4 (7.0) | 1 (3.1) |
|
| ||
| Pre-Registration Evaluation | ||
| Progressive Disease | 57 (100) | 32 (100) |
|
| ||
| Prior Disease Treatment | ||
| Surgery | 16 (28.1) | 10 (31.3) |
| Nephrectomy | 52 (91.2) | 26 (81.3) |
| Radiation therapy | 15 (26.3) | 10 (31.3) |
| Immunotherapy | 6 (10.5) | 3 (9.4) |
| Sunitinib | 47 (82.5) | 30 (93.8) |
| Sorafenib | 14 (24.6) | 6 (18.8) |
| Interleukin 2 (IL2) | 5 (8.8) | 3 (9.4) |
| Pazopanib | 8 (14.0) | - |
| Interferon | 1 (1.8) | - |
| Axitinib | 3 (5.3) | - |
| Other TKI Anti-VEGF | 3 (5.3) | - |
| Other Prior Therapy | 8 (14.0) | 7 (21.9) |
|
| ||
| Metastatic Sites | ||
| Bone | 22 (38.6) | 9 (28.1) |
| Liver | 16 (28.1) | 7 (21.9) |
| Lung | 49 (86.0) | 19 (59.4) |
| Brain | - | - |
| Pleura | 8 (14.0) | 3 (9.4) |
| Subcutaneous | 5 (8.8) | 1 (3.1) |
| Lymph Nodes | 36 (63.2) | 20 (62.5) |
| Other | 16 (28.1) | 15 (46.9) |
Toxicity
Table 2 provides a summary of treatment-related toxicities of all grades. The denominator included patients who received protocol therapy irrespective of eligibility status. The most common treatment-related toxicities in Arm A were fatigue (63%), hypertension (54%), proteinuria (47%) and anorexia (39%). The proportion (90% CI) of severe (Grade of 3 or higher) toxicity in Arms A and B was 61.4% (49.7, 72.2) and 52.9% (37.7, 67.8) respectively. One patient in treatment Arm B experienced a grade 5 event described as intracranial hemorrhage. There were 10 grade 5 events (6 in treatment Arm A and 4 in Arm B) but were considered unrelated to treatment – 6 were due to disease progression and 4 each due to renal failure, dyspnea, infection, and upper respiratory tract related toxicity.
Table 2.
Treatment-related Toxicities (occurring in ≥10% of patients) by Treatment Arm
| Toxicity Type | Treatment Arm | |||||||
|---|---|---|---|---|---|---|---|---|
| A-4 mg/kg (n = 57) | B-1 mg/kg (n = 34) | |||||||
| Grade | Grade | |||||||
| 1,2 (%) | 3 (%) | 4 (%) | 5 (%) | 1,2 (%) | 3 (%) | 4 (%) | 5 (%) | |
| Alkaline phosphatase | 7 | - | - | - | 15 | - | - | - |
| ALT, SGPT | 11 | - | - | - | 3 | - | - | - |
| Anorexia | 37 | 2 | - | - | 47 | - | - | - |
| CNS, hemorrhage | - | - | - | - | - | - | - | 3 |
| Constipation | 19 | - | - | - | 29 | - | - | - |
| Cough | 14 | - | - | - | 18 | - | - | - |
| Creatinine | 14 | - | - | - | 18 | - | - | - |
| Diarrhea | 19 | - | - | - | 24 | - | - | - |
| Dyspnea | 12 | 5 | - | - | 32 | 3 | - | - |
| Edema limb | 7 | - | - | - | 15 | - | - | - |
| Fatigue | 60 | 4 | - | - | 56 | 21 | - | - |
| Head/headache | 30 | 4 | - | - | 24 | - | - | - |
| Hemoglobin | 19 | 4 | - | - | 26 | 9 | - | - |
| Hyperglycemia | 5 | - | - | - | 15 | - | - | - |
| Hyperkalemia | 4 | - | - | - | 12 | - | - | - |
| Hypertension | 19 | 35 | - | - | 21 | 15 | - | - |
| Hypoalbuminemia | 11 | - | - | - | 3 | 3 | - | - |
| Hyponatremia | 12 | 4 | - | - | 21 | - | - | - |
| Joint, pain | 19 | - | - | - | 24 | - | - | - |
| Muco/stomatitis (symptom) oral cavity | 9 | - | - | - | 18 | - | - | - |
| Muco/stomatitis by exam, oral cavity | - | - | - | - | 12 | - | - | - |
| Nausea | 19 | - | - | - | 50 | - | - | - |
| Neuropathy-sensory | 4 | - | - | - | 12 | - | - | - |
| Nose, hemorrhage | 5 | - | - | - | 18 | - | - | - |
| Proteinuria | 32 | 16 | - | - | 53 | 6 | - | - |
| Voice changes/dysarthria | 21 | - | - | - | 29 | - | - | - |
| Vomiting | 2 | - | - | - | 18 | - | - | - |
| Weight loss | 12 | - | - | - | 12 | - | - | - |
|
| ||||||||
| WORST DEGREE | 32 | 56 | 5 | - | 44 | 50 | - | 3 |
Efficacy
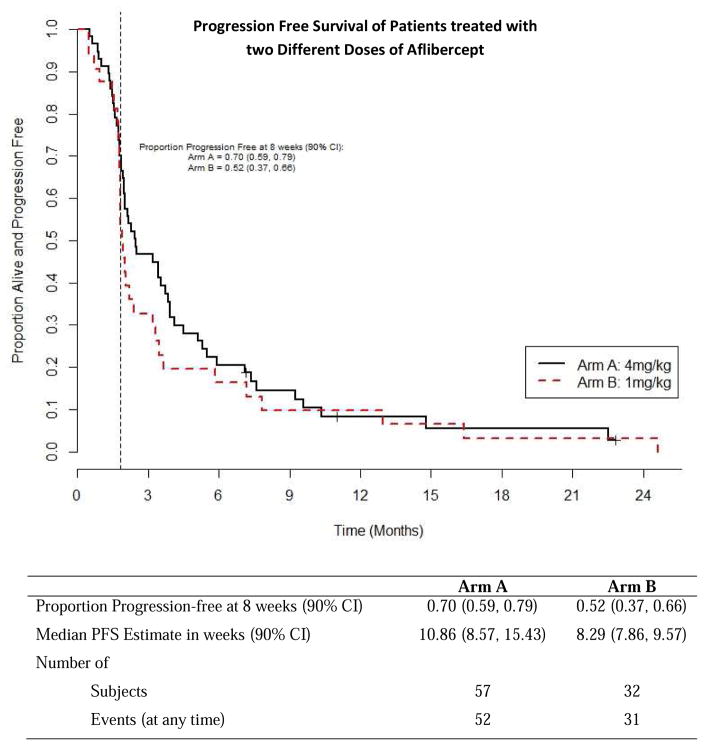
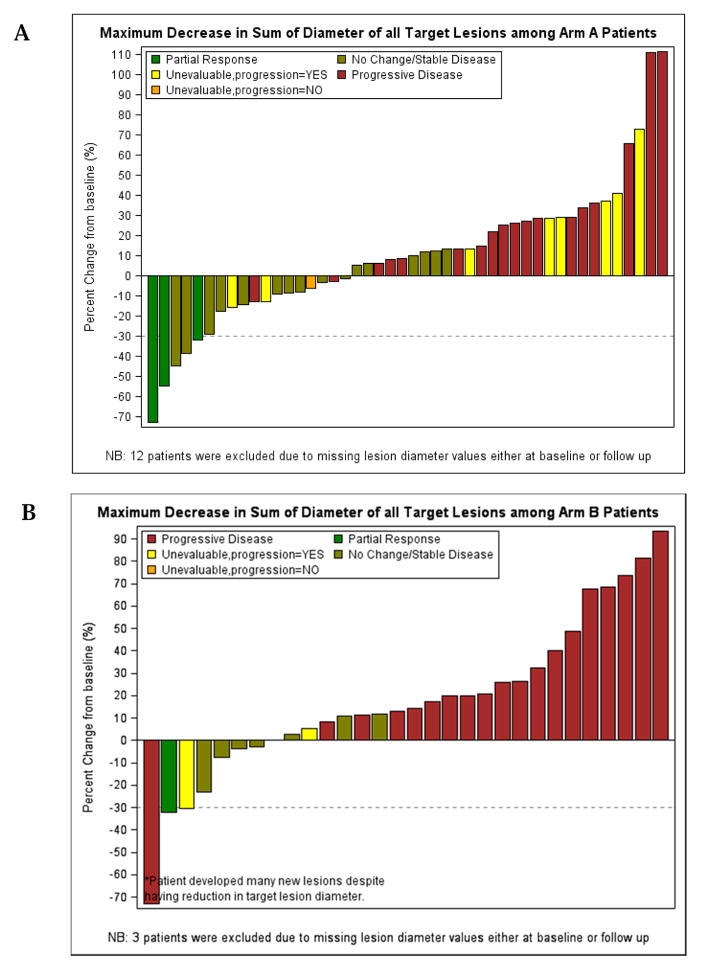
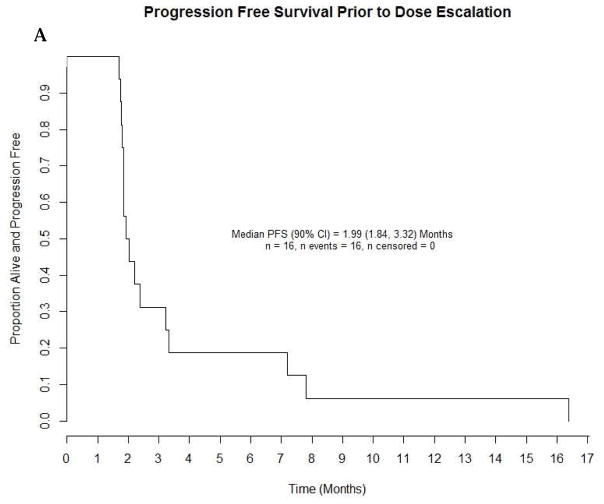
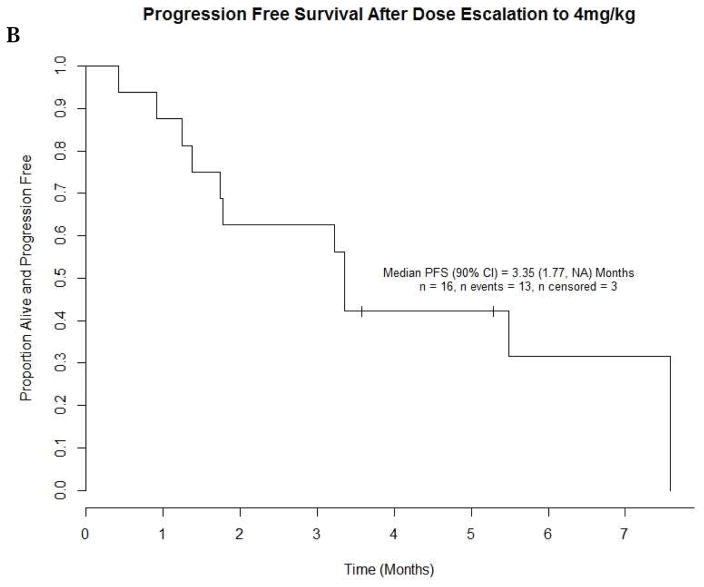
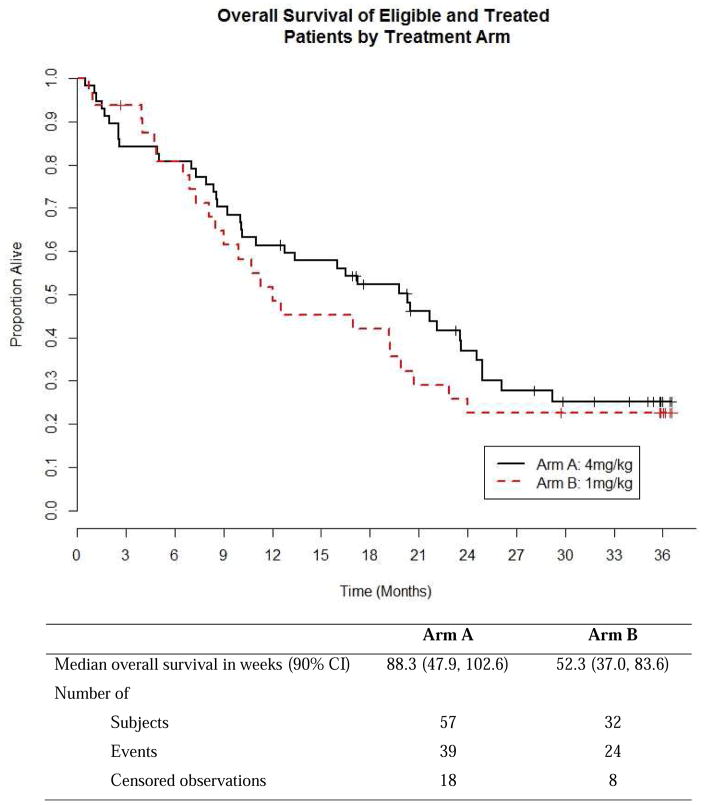
As estimated from Kaplan Meier survival estimates, the proportion of patients alive and progression-free at 8 weeks (90% CI) was 70% (59, 79%) in treatment Arm A and 52% (37, 66%) in Arm B. The proportion alive and progression free estimate of 70% for Arm A was higher than the proportion of interest value of 67% specified in the protocol. Likewise, the number of patients alive and progression free at 8 weeks was 39 which was higher than the protocol pre-specified number of 34 patients suggesting worthiness for further study. The PFS curve is shown in Figure 3. The estimated median PFS for treatment Arms A and B patients were 10.86 and 8.29 weeks respectively. The p-value of the log rank test comparing PFS between arms is 0.37; however this study was not powered to detect difference in PFS. A total of 52 (91%) events occurred among patients in treatment A and 31 (97%) in treatment B. Table 3 shows overall response by treatment arm among patients who were eligible and treated. The response rates with 90% CI for Arms A and B were 5.3% (1.4, 13.0) and 3.1% (0.2, 14.0), respectively. The pre-specified number of objective responses of interest (≥ 3 patients) was satisfied in Arm A, suggesting efficacy. Waterfall plots of maximum percent change from baseline in the sum of lesions longest diameter for both treatment arms are shown in Figure 4. A reference line at −30% represents the RECIST criteria cutoff corresponding to a partial response (PR) to treatment. A patient in Arm B was identified as having progressive disease despite having about 70% reduction in lesion size because the patient developed multiple new lesions. Four (4) patients, from both treatment arms, experienced an objective response, the KM estimated median response duration (90% CI) among these four patients was 43.9 (31.4, 56.4) weeks. The median response duration among patients (n=23) with stable disease was 13.0 (8.1, 22.6) weeks. Sixteen (16) patients in treatment Arm B (1mg/kg) had their dosage escalated to 4mg/kg after disease progression. The median PFS (90% CI) before and after escalation were 8.6 (8.0, 14.0) weeks and 14.6 (7.6, 33.0) weeks respectively (Figure 5). An overall survival curve for eligible and treated patients is shown in Figure 6. The OS and PFS curves for all randomized patients (Supplementary Fig. 1 A and B) were estimated to confirm that survival experience did not considerably differ between all randomized and only eligible, treated patients.
Figure 3.
Progression-Free Survival for Eligible and Treated Patients
Table 3.
Best Overall Response
| Best Overall Response | Treatment Arm | |
|---|---|---|
| Arm A (4 mg/kg) | Arm B (1 mg/kg) | |
| n (%) | n (%) | |
| Overall Response (OR) | 3 (5.3) | 1 (3.1) |
| Complete response (CR) | - | - |
| Partial response (PR) | 3 (5.3) | 1(3.1) |
| No change/Stable | 16 (28.1) | 7 (21.9) |
| Progression (PD) | 26 (45.6) | 19 (59.4) |
| Unevaluable | 12(21.0) | 5 (15.6) |
|
| ||
| Total | 57 | 32 |
Figure 4.
Waterfall Plots of Maximum Decrease in Sum of Lesions’ Longest Diameter by Treatment Arm
Figure 5.
Progression-Free Survival before and after Dose Escalation (1mg/kg to 4mg/kg)
Figure 6.
Overall Survival of Eligible and Treated Patients
Discussion
The results from this study confirm the hypothesis that continuous VEGF blockade following disease progression VEGF RTKIs may have clinical benefit in ccRCC patients. This study met the protocol specified criteria for significant improvement in progression-free survival among Arm A patients only (PFS of ≥ 0.67 and ≥ 34 patients alive and progression-free at 8 weeks). Also, the protocol specified criterion for objective response was met among Arm A patients only (≥3 patients with response). Overall, the treatment was well tolerated, though there was a greater percentage of patients who experienced treatment-related, grade 3 or higher, toxicities in Arms A as compared to Arm B.
The treatment landscape for ccRCC is rapidly evolving9. The recent approval of two new multi-kinase inhibitors in the second line setting such as cabozantinib and lenvatinib suggests that sequential inhibition of additional tyrosine kinases besides VEGF RTKs may induce clinical benefit10,11. The treatment options for ccRCC are also significantly expanding due to the approval and clinical development of PD1/PD-L1 inhibitors12. Continuous VEGF inhibition remains an important tool to induce prolonged clinical response in ccRCC patients. The original approval of axitinib has validated retrospective studies suggesting that sequencing of TKIs can still induce disease control despite progression following frontline therapies with anti-VEGF therapies13. The VEGF ligand blocker bevacizumab has been approved in combination with interferon α for the treatment of renal cell carcinoma in the first line setting based on the results of two Phase III randomized studies14,15. Different combination strategies with bevacizumab have been also tested but these have been hampered by toxicities, and did not show greater efficacy as compared to single agent bevacizumab16. The assessment of the role of bevacizumab in the second and third line setting in RCC patients has been relatively limited. One report showed an ORR of 9% and a median PFS of 4.4 months in previously treated ccRCC patients17. In our study, the median PFS, ORR and toxicity profile observed in patients randomized to the either the 4 mg aflibercept arm or in those who crossed over to that dose appear similar to the historical data attributed to bevacizumab in the same setting. Thus, the results from our clinical trial are consistent with a dose-dependent benefit of aflibercept that was evident also in the crossed-over patients. Similar dose-dependent effect was observed also in the original single-agent study with bevacizumab in RCC18.
As the armamentarium for treating ccRCC is growing it becomes challenging to position new drugs in the current treatment algorithm. Aflibercept has shown preclinical more potent and broader activity than bevacizumab4. Identifying patients who may most benefit from the pharmacodynamics of aflibercept may represent a clinically relevant question. As HIF-2α antagonists are becoming available, a potential combination with an effective VEGF blocker such as aflibercept appears a rational strategy for ccRCC19,20.
Conclusions
This study confirms the potential benefit of VEGF blockade in previously treated patients with metastatic ccRCC. Aflibercept shows evidence of activity in previously treated ccRCC and may be worthy of further study in rational combination strategies where continuous VEGF blockade may be beneficial.
Supplementary Material
Clinical Practice Points.
The treatment of metastatic ccRCC is rapidly evolving but therapies targeting the VEGF axis remain the main treatment of this disease.
The use of the VEGF blocker aflibercept in pretreated ccRCC patients was safe and showed clinical benefit.
Rational combination strategies with aflibercept may be beneficial in ccRCC patients.
Acknowledgments
This study was conducted by the ECOG-ACRIN Cancer Research Group (Robert L. Comis, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA180820, CA180794, CA180802, CA180844, and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Mille KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016 Jan-Feb;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Pili R, Kauffman E, Rodriguez R. Cancer of the Kidney. In: Niederhuber JE, AJ, Doroshow JH, Kastan MD, Tepper JE, editors. Abeloff’s Clinical Oncology. 5. Elsevier; 2013. pp. 1416–1444. [Google Scholar]
- 3.MacDonald DA, Martin J, Muthusamy KK, et al. Aflibercept exhibits VEGF binding stoichiometry distinct from bevacizumab and does not support formation of immune-like complexes. Angiogenesis. 2016;19:389–406. doi: 10.1007/s10456-016-9515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verheul HH, Hammers H, van Erp K, et al. VEGF Trap is a potent inhibitor of tumor growth and spontaneous lung metastases in murine renal cell carcinoma. Clin Cancer Res. 2007;13:4201–8. doi: 10.1158/1078-0432.CCR-06-2553. [DOI] [PubMed] [Google Scholar]
- 6.Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improvessurvival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol. 2012;30:3499–3506. doi: 10.1200/JCO.2012.42.8201. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Murphy BA, Bacik J, et al. Phase III trial of interferon alfa-2a with or without 13-cis-retinoic acid for patients with advanced renal cell carcinoma. J Clin Oncol. 2000;18:2972–2980. doi: 10.1200/JCO.2000.18.16.2972. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 9.Albiges L, Choueiri T, Escudier B, et al. A systematic review of sequencing and combinations of systemic therapy in metastatic renal cancer. Eur Urol. 2015;67:100–10. doi: 10.1016/j.eururo.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373:1814–23. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–82. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, McDermott DF. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;19:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 14.Escudier B, Pluzanska A, Koralewski P, et al. AVOREN Trial investigators. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007 Dec 22;370(9605):2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 15.Rini BI, Halabi S, Rosenberg JE, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008 Nov 20;26(33):5422–8. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bukowski RM, Kabbinavar FF, Figlin RA, et al. Randomized phase II study of erlotinib combined with bevacizumab compared with bevacizumab alone in metastatic renal cell cancer. J Clin Oncol. 2007 Oct 10;25(29):4536–41. doi: 10.1200/JCO.2007.11.5154. [DOI] [PubMed] [Google Scholar]
- 17.Turnbull JD, Cobert J, Jaffe T, et al. Activity of single-agent bevacizumab in patients with metastatic renal cell carcinoma previously treated with vascular endothelial growth factor tyrosine kinase inhibitors. Clin Genitourin Cancer. 2013;11:45–50. doi: 10.1016/j.clgc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Yang JC, Haworth L, Sherry RM, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Hill H, Christie A, et al. Targeting Renal Cell Carcinoma with a HIF-2 antagonist. Nature. 2016 Sep 5; doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cho H, Du X, Rizzi JP, et al. On-Target Efficacy of a HIF2α Antagonist in Preclinical Kidney Cancer Models. Nature. 2016 Sep 5; doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.