Abstract
It is increasingly apparent that ligand structure influences both the efficiency with which G protein-coupled receptors (GPCRs) engage their downstream effectors and the manner in which they are activated. Thus, ‘biased’ agonists, synthetic ligands whose intrinsic efficacy differs from the native ligand, afford a strategy for manipulating GPCR signaling in ways that promote beneficial signals while blocking potentially deleterious ones. Still, there are significant challenges in relating in vitro ligand efficacy, which is typically measured in heterologous expression systems, to the biological response in vivo, where the ligand is acting on natively expressed receptors and in the presence of the endogenous ligand. This is particularly true of arrestin pathway-selective ‘biased’ agonists. The type 1 parathyroid hormone receptor (PTH1R) is a case in point. Parathyroid hormone (PTH) is the principal physiological regulator of calcium homeostasis, and PTH1R expressed on cells of the osteoblast lineage are an established therapeutic target in osteoporosis. In vitro, PTH1R signaling is highly sensitive to ligand structure, and PTH analogs that affect the selectivity/kinetics of G protein coupling or that engage arrestin-dependent signaling mechanisms without activating heterotrimeric G proteins have been identified. In vivo, intermittent administration of conventional PTH analogs accelerates the rate of osteoblastic bone formation, largely through known cAMP-dependent mechanisms. Paradoxically, both intermittent and continuous administration of an arrestin pathway-selective PTH analog, which in vivo would be expected to antagonize endogenous PTH1R-cAMP signaling, also increases bone mass. Transcriptomic analysis of tissue from treated animals suggests that conventional and arrestin pathway-selective PTH1R ligands act in largely different ways, with the latter principally affecting pathways involved in the regulation of cell cycle, survival, and migration/cytoskeletal dynamics. Such multi-dimensional in vitro and in vivo analyses of ligand bias may provide insights into the physiological roles of non-canonical arrestin-mediated signaling pathways in vivo, and provide a conceptual framework for translating arrestin pathway-selective ligands into viable therapeutics.
Keywords: Arrestin, G protein-coupled receptor, Parathyroid hormone, Pharmacology, Osteoblast, Osteoporosis
1. Introduction
The individual cells comprising the body's tissues and organs respond to extracellular cues sent in the form of hormones and neurotransmitters that provide for coordinated regulation of multiorgan physiological processes. Conventional receptor theory envisions GPCRs as existing in spontaneous equilibrium between ‘inactive’ and ‘active’ states that are stabilized by ligand binding, such that agonist ligands increase the proportion of receptors in the active state while antagonists decrease it [1, 2]. In this context, where receptors possess unitary active states that engage the full repertoire of downstream effectors, biological responses at the cellular level are determined by receptor structure, i.e. which effectors can be engaged, and cell background, i.e. which effectors are present, while the response at the organismal level is determined by receptor distribution, i.e. which tissues/organs can detect the ligand and respond to it.
Over the past two decades, however, it has become evident that ligand structure is an important determinant not only of which receptors are activated, but also of how they are activated. GPCR signaling is ‘pluridimensional’, i.e. receptors signal via multiple G protein and non-G protein effectors, and each GPCR can adopt multiple discrete active states [3]. Since the receptor conformation that optimally engages one effector cannot be assumed to couple all effectors equally, the cellular response becomes a function of the proportion of the receptor population existing in each of several potential active states at any given time. In this context, ligand structure, by virtue of its capacity to ‘bias’ the distribution of receptors across an active conformational ensemble, assumes a vital role [4–6]. The implications of ‘functional selectivity’ or ligand ‘bias’ for new drug discovery are substantial. While the biological response at the organismal level is still determined by tissue distribution of the receptor, the response at the cellular level becomes a function not only of cell background and receptor structure, but also of ligand structure. The ability of synthetic ligands to ‘bias’ GPCR signaling output suggests that it may be possible to rationally design drugs that activate beneficial downstream signals while suppressing signals that contribute to adverse side effects.
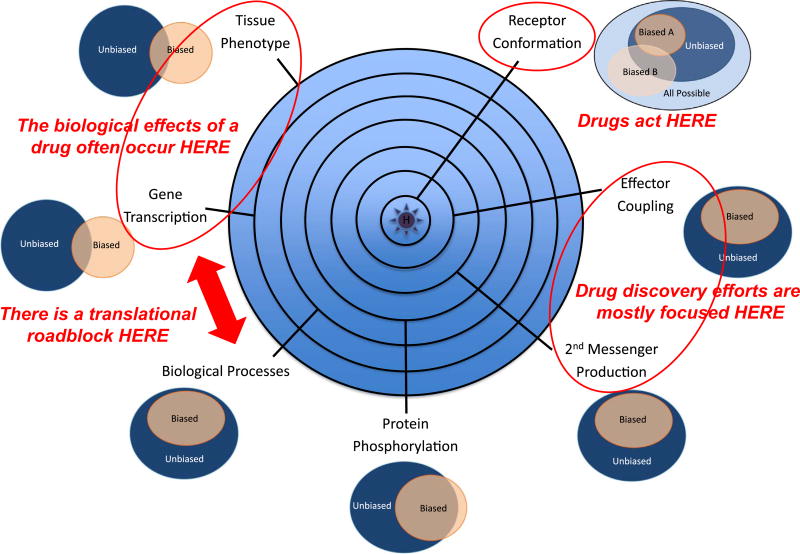
With such opportunity come challenges, however. If the ligand-receptor complex determines signal output, then a GPCR bound to a biased ligand is its own unique entity, an ‘unnatural’ receptor whose functionality is unknown [7]. This is particularly true of biased ligands that selectively activate arrestin-dependent signaling pathways whose biological roles remain incompletely understood. Fig. 1 illustrates the conceptual challenge. While many of the long term, and potentially therapeutic, actions of GPCR ligands are exerted at the level of transcriptional regulation and cell/tissue remodeling, they originate from the plasma membrane, where ligand structure, concentration, and duration of exposure determine the type, intensity, and timing of signals sent inside. Biophysical measurements of the effects of ligand binding on receptor conformation suggest that ligands stabilize a finite set of discrete receptor conformations, albeit in different proportions [8]. Compared to their native counterparts, biased ligands may favor conformations that normally represent a minor proportion of the conformational ensemble, but are unlikely to produce ‘new’ conformations that couple the receptor to novel effectors. At the level of receptor coupling to its proximal effectors, e.g. heterotrimeric G proteins and arrestins, it is likewise clear that ligand structure influences the efficiency with which different downstream effectors are engaged, but does not cause the receptor to change effectors [9]. Yet even here, subtle differences between conventional and biased ligands emerge as information within the ligand-receptor complex is transmitted allosterically to intracellular effectors. Data from intramolecular-bioluminescence resonance energy transfer (BRET) probes suggest that different ligands interacting with the same receptor can produce ligand-specific changes in the population average conformational signature of arrestins that reflect its avidity for the receptor and signaling functions [10–11]. Similarly, ligand structure can influence the rate of GTP turnover and kinetics of second messenger production by inducing subtly different conformational shifts in the G protein heterotrimer [12].
Fig. 1.
Conceptual representation of biased agonism viewed from multiple levels within a biological system. At the initial point of contact, ligand binding changes the distribution of receptor conformations leading to stabilization of one or more active states. As signals radiate outward from the ligand-receptor complex, receptors engage proximal effectors like G proteins and arrestins (seconds), second messenger and kinase cascades are activated, and global changes in downstream protein phosphorylation occur (seconds-minutes), leading to changes in cell behavior, e.g. proliferation, migration or apoptosis (hours-days). When assayed in vitro, biased ligands appear to affect a subset of the pathways activated by the native ligand (represented as Venn diagrams). But when the in vivo effects of native and biased agonists are compared, data from microarray analysis and tissue phenotyping suggest that biased agonists may have unpredictable effects arising from ‘unbalanced’ GPCR activation. This ‘disconnect’ between the in vitro and in vivo efficacy of biased ligands is a potential barrier to the rational development of biased GPCR therapeutics.
As signals propagate inwards from the plasma membrane, G proteins activate intracellular enzymes, e.g. adenylyl cyclases and phospholipase C isoforms, to generate second messengers, while arrestin recruitment promotes the assembly of GPCR based ‘signalsome’ complexes [13]. Because these steps are amenable to high throughput assay, this is the level at which most early drug discovery efforts are focused. But the existence of functional selectivity dictates that ligand classification, e.g. agonist, antagonist or inverse agonist, is assay-dependent. Thus, a single readout, e.g. cAMP generation, is insufficient to describe ligand behavior and measuring efficacy in at least two different screens is needed to detect ligand bias. While robust methods to quantify bias have been developed based on the Black-Leff operational model [14], compound screens are limited to collecting the information they seek, and assays are usually performed in ectopic expression systems at non-physiological levels of receptor expression. Indeed, most data from screens based on second messenger generation, arrestin recruitment, and cell-based proliferation/survival/migration assays suggest that biased ligands work by activating part of the native ligand response. Even global phospho-proteomic approaches to examine ligand bias in transfected cells have thus far failed to find unique biology in the short term signaling response to biased ligands [15, 16]. Whether this really means that biased ligands are only capable of initiating a subset of the conventional ligand response, or that important nuances of biased ligand efficacy are lost under high throughput assay conditions, is a question of some importance.
Yet the real gulf that presently exists is between the in vitro description of ligand efficacy and the potential unpredictability of its in vivo effects [17]. In nature, GPCRs and their ligands co-evolved to control signaling in the most physiologically adaptive manner. As a result GPCR signaling is ‘balanced’ to meet the needs of the organism. Biased agonists, on the other hand, produce a different distribution of active receptor states. The resulting signals may not be qualitatively different, but are ‘unbalanced’ compared to the native ligand, creating the potential for differences in tissue response. Additional complications arise from the fact that in vivo the biased ligand is acting in the presence of the native hormone, a factor that is rarely considered when characterizing intrinsic ligand efficacy in vitro. While conventional agonist/antagonist ligands may be thought of increasing or decreasing signal strength proportionally, biased ligands should exert mixed agonist/antagonists effects in vivo, activating some pathways directly while antagonizing the actions of the native ligand on others. At the end of the day, it may not be possible to predict the biological response to a biased agonists based either on its in vitro efficacy or prior knowledge of the physiological actions of the native hormone.
In this review, we discuss some of the issues surrounding biased agonist development using a single biomedically important GPCR, the type 1 parathyroid hormone receptor (PTH1R) as a test case. PTH1R signaling is highly susceptible to ligand structure and the biological outcomes of administration of conventional and biased PTH analogs have been studied both at the tissue and transcriptomic levels. Thus it offers one of the best opportunities to examine the complex relationships between GPCR efficacy in vitro and in vivo.
2. The biological actions of parathyroid hormone
The PTH1R is a class II GPCR that shares overall structural similarities with other peptide hormone GPCRs including those for glucagon, calcitonin and secretin [18, 19]. While widely expressed at low levels, PTH1R is most highly expressed in kidney and bone. It has two native ligands, PTH and parathyroid hormone-related protein (PTHrP). PTH is an 84-amino acid polypeptide, expressed principally in the parathyroid gland, that functions as the primary systemic regulator of calcium-phosphate homeostasis. G protein-coupled calcium-sensing receptors on parathyroid cells negatively regulate the secretion of PTH, such that a fall in serum calcium increases PTH secretion while rising calcium levels suppress it. The peripheral actions of PTH work in concert to raise serum calcium, establishing a classic hormonal negative feedback loop. In the kidney, PTH increases renal tubular calcium retention and phosphaturia. It also upregulates renal expression of the 1α-hydroxyase necessary to convert 25(OH)-vitamin D to its active form 1,25(OH)2-vitamin D, which in turn increases intestinal calcium absorption. PTH exerts complex effects in bone, its other major target organ. It directly stimulates osteoblasts to form new bone by increasing osteoblast number and activity, promoting the deposition of new matrix, and accelerating the rate of mineralization [20, 21]. At the same time, PTH increases the recruitment, differentiation, and activity of bone-resorbing osteoclasts. Osteoclasts themselves lack PTH receptors, instead being regulated by soluble factors, such as receptor activator of NFκB ligand (RANKL) and osteoprotegrin, secreted by osteoblasts in response to PTH.
On the other hand, PTHrP, which is produced as 139, 141, and 173 amino acid splice variants and subsequently processed to yield numerous biologically active fragments, is thought to function primarily in an autocrine or paracrine role in postnatal physiology [22]. Circulating PTHrP only exerts hormonal effects in the fetus, where it regulates maternal-fetal calcium transport; in the lactating breast, where it may help liberate skeletal calcium for secretion in milk; and in certain cancers, where its excessive production is a cause of hypercalcemia of malignancy. In addition, PTHrP is widely expressed in fetal tissues, where it acts as a cellular cytokine promoting cell growth and differentiation [23]. Its local roles include relaxation of vascular and intestinal smooth muscle, regulation of keratinocyte and glandular breast development, and pancreatic β-cell proliferation and insulin production. In bone, PTHrP coordinates endochondral bone formation during embryonic skeletal development and contributes to the regulation of bone remodeling in the postnatal skeleton. PTHrP is synthesized by cells early in the osteoblast lineage and acts locally rather that systemically, stimulating proliferation of preosteoblasts, increasing RANKL production and osteoclast formation, and inhibiting apoptosis of mature osteoblasts and osteocytes.
For both PTH and PTHrP, the structural features necessary to fully activate the PTH1R appear to be contained within the N-terminal 34 amino acids of each peptide [18]. In vitro, the short-term signaling responses to both ligands are similar. PTH1Rs couple most efficiently to the Gs-adenylyl cyclase pathway resulting in cAMP production and protein kinase A (PKA) activation; but can also activate the Gq/11-phospholipase Cβ pathway, leading to inositol-1,4,5-trisphosphate production, intracellular calcium mobilization, and protein kinase C (PKC) activation; and the G12/13-phospholipase D pathway, leading to RhoA activation [18, 24–26]. In renal tubular epithelium, which expresses the PDZ domain-containing scaffold protein, Na+/H+ exchanger regulatory factor 2 (NHERF2), NHERF2 binding to the PTH1R C-terminus changes its G protein coupling efficiency, enabling the receptor to engage Gi/o family G proteins and inhibit, rather than stimulate, adenylyl cyclase, while simultaneously enhancing receptor coupling to Gq/11 [27]. Activated PTH1Rs also recruit both β-arrestin1 and β-arrestin2, leading to the clathrin-dependent internalization of PTH1R-arrestin complexes, and arrestin-dependent scaffolding of the ERK1/2 cascade [28–30]. In fact, using both transfected cell lines and primary calvarial osteoblasts, it is possible to show that PTH1R activates ERK1/2 via multiple independent pathways, involving PKA, PKC and/ or arrestins [30–33]. While multiple upstream signals converge on ERK1/2 they are not redundant, in that the G protein-mediated signals produce a transient signal while activation of the arrestin pathway leads to prolonged activation of a spatially constrained ERK1/2 pool [30].
One potentially important distinction between PTH and PTHrP signaling relates to the kinetics of effector activation occasioned by a difference in ligand on-off rates. Both PTH(1–34) and a corresponding N-terminal fragment of PTHrP, PTHrP(1–36), robustly activate Gs-cAMP signaling, but the two ligands differ markedly in the kinetics of receptor association and dissociation, with PTH(1–34) exhibiting a faster on rate and very slow off rate compared to PTHrP(1–36). Consequently, PTH1R bound to PTH(1–34) remains in the activated state for a prolonged period of time and live cell imaging demonstrates that while cAMP generation in response to PTHrP(1–36) is limited to the plasma membrane, PTH(1–34) continues to stimulate cAMP production from within an endosomal compartment long after the receptor has internalized [34–36].
3. PTH1R ligand bias in vitro
The PTH1R, with its pleiotropic effector coupling profile, has long been recognized to be sensitive to ligand-induced signal bias. As noted, the C-terminal 34 amino acids of PTH, i.e. PTH(1–34), possess all of the known properties of the native hormone acting on the PTH1R, behaving as a conventional/full agonist with respect to activation of Gs and Gq/11 signaling and arrestin-dependent receptor desensitization and internalization. Yet other PTH fragments exhibit marked variations in their ability to promote PTH1R coupling to downstream G proteins. For example, shorter N-terminal fragments of the PTH peptide, e.g. PTH (1−31), activate adenylyl cyclase in ROS 17/2 rat osteosarcoma cell membranes without stimulating membrane-associated PKC [37, 38], while N-terminal truncations, e.g. PTH(3–34), activate PKC while failing to stimulate cAMP production [39, 40].
Ligand-dependent dissociation of G protein activation and arrestin recruitment is also possible. The mutated N-terminal PTHrP fragments, [Trp1]-PTHrP(1–36) and [Bpa1]-PTHrP(1–36), selectively activate Gs and generate sustained cAMP signaling while not promoting arrestin-dependent PTH1R desensitization [30, 41]. The opposite efficacy profile, engagement of arrestins without heterotrimeric G protein activation, has been described as well. The PTH(7–34) fragment, which still possesses the structural determinants necessary for relatively high affinity binding but lacks the N-terminal residues needed to stimulate guanine nucleotide exchange, antagonizes G protein signaling but still stimulates receptor phosphorylation and internalization [42]. Similarly, the bovine PTH fragment, [D-Trp12,Tyr34]-bPTH(7–34), acts as an inverse agonist for Gs coupling and fails to stimulate Gq/11, yet is capable of activating G protein-independent signaling via a β-arrestin mediated pathway [30, 33, 43].
3.1. Classification of biased PTH1R ligands
Confronted with the facts that ligand structure, receptor structure, and cell background all contribute to the observed cellular response to GPCR activation, any meaningful comparison of ligand ‘bias’ has to start from a common base. Typically this involves comparing responses generated by a panel of ligands in multiple assays of receptor activation performed in a common cell background [44]. In addition, since a ligand can only be ‘biased’ relative to the intrinsic efficacy of some other ligand acting on the same receptor, ligand bias is usually expressed in relation to a ‘reference ligand’, typically the native hormone or neurotransmitter.
Quantitative approaches have been developed to provide a framework for describing and comparing the intrinsic efficacy of GPCR ligands [45–47]. Considering any single experimental readout, the actions of a ligand can be described by two terms that together specify the relationship between receptor occupancy and cellular response, i.e. the equilibrium dissociation constant of the ligand-receptor complex [Kd] and the maximal observed change in receptor activity [Vmax]. These two measures can be combined into a single parameter according to the Black-Leff operational model [48] to determine a ‘transduction coefficient’, log(τ/KA), where τ represents the maximum observed response, itself a combination of both intrinsic efficacy and ‘system factors’ imposed by cell background, and KA reflects the level of receptor occupancy. Once the ‘transduction coefficient’ has been determined for each ligand in the same assay, the relative coupling efficiency compared to a reference agonist can be expressed as a normalized transduction coefficient, Δlog(τ/KA). And once normalized transduction coefficients have been determined in two or more assays, a ‘bias factor’, ΔΔlog(τ/KA), can be calculated as the difference in relative coupling efficiency between any two measurable downstream responses [49, 50]. An alternative approach, based on the method of Ehlert [51], that is valid for assays in which ligand concentration-response curves have Hill slopes near 1.0, is to determine ‘intrinsic relative activity’ (RAi) from EC50 and EMAX data, and calculate the bias factor from ΔΔlog (RAi).
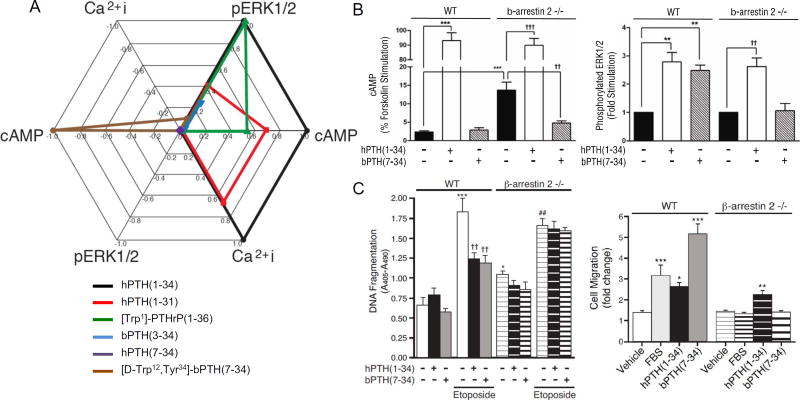
Fig. 2A illustrates PTH1R ligand bias using a simple multiaxial graphic representation of RAi data obtained from the human PTH1R ectopically expressed in a common HEK293 cell background [52]. RAi values were determined for six PTH peptide analogs in three assays: cAMP production reflecting Gs-adenylyl cyclase activation, stimulation of Ca2+ influx reflecting Gq/11-Phospholipase C activation, and ERK1/2 phosphorylation reflecting a composite of Gs, Gq/11 and arrestin-mediated signals. In this background, human hPTH(7–34) (purple) was devoid of measurable activity in all assays and is represented as a single point at the origin. Indeed, in this HEK cell system the presence of hPTH (7–34) can only detected by its ability to competitively antagonize an agonist response [53], and thus appears as a ‘neutral’ antagonist. The reference agonist, human PTH(1–34) (black) is by definition assigned an RAi of 1.0 in all assays, since its EC50 to EMAX ratio is normalized to itself. Since it was the most efficacious ligand tested in all assays, there were no RAi values > 1.0 observed within this ligand set. Compared to hPTH(1–34), the human hPTH(1–31) peptide (red) appears as a relatively ‘balanced’ partial agonist, with RAi values of 0.4 to 0.7 in all assays. The other three ligands exhibit signal bias. [Trp1]-PTHrP(1–36) (green) appears to Gs-cAMP selective, since it fails to elicit a threshold calcium signal at concentrations that robustly activate cAMP production and ERK1/2, whereas hPTH(1–34) and hPTH(1–31) exhibit proportional relative activity in all three assays. A bovine PTH derivative, bPTH(3–34) (blue), exhibits more drastic bias, retaining substantial activity in the ERK1/2 assay in the absence of a detectable cAMP or calcium signal. The most divergent ligand, however, is the modified bovine PTH derivative, [D-Trp12,Tyr34]-bPTH(7–34). While ‘reversal of potency’ where two ligands exhibit the opposite rank order of potency in two different assays of receptor activation, is one of the hallmarks of ligand bias, [D-Trp12,Tyr34]-bPTH(7–34) exhibits frank reversal of efficacy, appearing as an inverse agonist for cAMP production, neutral for calcium signaling, and a partial agonist for ERK1/2 activation. Such ‘perfect bias’ is uncommon, but has been described for some GPCRs, e.g. the β2-adrenergic [54].
Fig. 2.
PTH1R ligand bias in vitro. A. Multiaxial plot of PTH analog efficacy in cAMP, calcium and ERK1/2 assays performed using HEK293 cells overexpressing human PTH1R. Estimated RAi values for each ligand are plotted to represent the magnitude and direction of effect in each signaling response. Figure adapted from Appleton et al. (2013) Methods Enzymol 522: 229–262 [52]. B. Effects of hPTH(1–34) and [D-Trp12,Tyr34]-bPTH(7–34) [bPTH(7–34)] on cAMP production (left) and ERK1/2 activation (right) in 10 day-old cultures of differentiating primary calvarial osteoblasts isolated from wild type (WT) and β-arrestin2−/− mice. Figure adapted from Gesty-Palmer et al. (2009) Science – Transl Med 1ra1 [33]. C. Effects of hPTH (1–34) and [D-Trp12,Tyr34]-bPTH(7–34) on the survival and random migration of primary calvarial osteoblasts isolated from wild type and β-arrestin2−/− mice. Anti-apoptotic effects (left) were measured in 10 day-old differentiating osteoblast cultures subjected to serum-withdrawal or exposed to etoposide in the presence or absence of hPTH(1–34) or [D-Trp12,Tyr34]-bPTH(7–34). Random cell migration (right) was measured by scratch assay performed on 3-day old confluent monolayers of primary preosteoblasts. Figure adapted from Gesty-Palmer et al. Mol Endocrinol 27:296–314 [55].
3.2. Ligand bias in ‘real’ cells
Of course PTH does not exert its biological effects in vivo by interacting with overexpressed PTH1R in transfected HEK cells engineered to provide a fluorescent or luminescent readout. As useful as ‘transduction coefficients’ and ‘bias factors’ are for describing differences in the intrinsic efficacy of ligands, they provide only limited insight into the behavior of biased ligands interacting with natively expressed GPCRs in a relevant cell background. Differences in receptor density and level of expression of intracellular effectors or signal modifying scaffolds like NHERF2 will not change the intrinsic efficacy of a ligand, which is an innate property embodied in its structure, but can change signal strength, i.e. the observed Vmax in different downstream pathways [44]. Moreover, biological responses, e.g. cell proliferation, growth, apoptosis, differentiation or migration, represent a higher order integration of proximal signals. Any understanding, therefore, of how ligand efficacy will affect the biology of therapeutically relevant target cells has to be based on assays performed in the proper cell background.
Fig. 2B compares the in vitro efficacy of the two most divergent PTH analog peptides described in Fig. 2A, in cAMP and ERK1/2 activation assays performed using 10-day cultures of primary calvarial osteoblasts isolated from wild type and β-arrestin2−/− C57BL/6 mice [33]. Consistent with the HEK cell data, both wild type and β-arrestin2−/− osteoblasts generated robust increases in cAMP when exposed to with hPTH(1–34) for 5 min. Treatment of wild type osteoblasts with [D-Trp12,Tyr34]-bPTH(7–34), as expected did not increase intracellular cAMP levels. In the β-arrestin2 −/− background, where basal cAMP levels were modestly higher due to loss of arrestin-dependent dampening of basal signaling, [D-Trp12,Tyr34]-bPTH(7–34) significantly lowered the elevated basal cAMP levels, a pattern consistent with its reported inverse agonist efficacy for PTH1R-Gs coupling [43]. Also consistent with the HEK cell data, hPTH(1–34) increased phosphatidylinositol hydrolysis, while [D-Trp12,Tyr34]-bPTH(7–34) did not [33]. Unlike HEK cells, where hPTH(1–34) provked much larger increases in ERK1/2 phosphorylation, in wild type primary osteoblasts hPTH(1–34) and [D-Trp12,Tyr34]-bPTH(7–34) demonstrated roughly equal efficacy, presumably reflecting cell background differences related to lower receptor density and/or efficiency of Gs pathway coupling to ERK1/2 activation. Consistent with its putative β-arrestin-dependent mechanism of action [30], [D-Trp12,Tyr34]-bPTH(7–34) failed to activate ERK1/2 in the β-arrestin2−/− background, while the ERK1/2 response to the Gs-competent ligand persisted. Despite differences in signal strength, the efficacy of hPTH(1–34) and [D-Trp12,Tyr34]-bPTH (7–34) in these three readouts of receptor activation are consistent between HEK cells and primary osteoblasts.
Further insights into the possible physiological effects of ligand bias in vivo can be garnered from cell based assays of higher order processes like cell proliferation, migration and survival. Undifferentiated β-arrestin−/− calvarial preosteoblasts proliferate faster than wild type cells [55]. When grown in the presence of either hPTH(1–34) or [D-Trp12,Tyr34]-bPTH(7–34) the proliferation rate of wild type preosteoblasts is modestly slowed. This PTH1R mediated effect is absent in β-arrestin2−/− preosteoblasts, whose higher growth rate is not affected by agonist exposure. These data suggest that β-arrestin2 may act as a brake on preosteoblast proliferation and that engagement of arrestin signaling pathways by PTH1R facilitates this negative regulatory effect. β-Arrestin2 also appears to contribute to anti-apoptotic and pro-migratory signaling by the PTH1R in osteoblasts. Fig. 2C shows the effects of hPTH(1–34) and [D-Trp12,Tyr34]-bPTH(7–34) on osteoblast survival in an etoposide challenge assay of DNA damage-induced apoptosis and in a scratch assay of random cell migration [55]. When exposed to etoposide, both hPTH(1–34) and [D-Trp12,Tyr34]-bPTH(7–34) significantly improved the survival of wild type osteoblasts. The effect was absent in β-Arrestin2−/− osteoblasts, which themselves showed a higher basal apoptosis rate suggesting that a PTH1R-mediated survival pathway activated by either ligand is β-arrestin2 mediated and independent of G protein signaling. Similar results were observed in the migration assay (Fig. 2C). Here, [D-Trp12,Tyr34]-bPTH(7–34) was more effective than either serum or hPTH(1–34) at stimulating wild type preosteoblast migration. Consistent with the reported roles of arrestin scaffolds in GPCR-regulated actin cytoskeletal dynamics and chemotaxis [56], [D-Trp12,Tyr34]-bPTH(7–34) had no effect in β-arrestin2−/− preosteoblasts. Thus, in vitro assays performed in primary cells expressing endogenous PTH1R suggest that arrestins contribute to the regulation of cell proliferation, cell survival signaling, and cell migration. Since these assay are performed in the absence of the native ligand, they further suggest that these arrestin-dependent responses do not require coincident activation of heterotrimeric G proteins and do not result from arrestin-dependent desensitization of Gs- or Gq/11 signaling.
4. PTH1R ligand bias in vivo
While the intrinsic efficacy of a ligand can be described in vitro, and bias quantified, translating ligand bias into a potential therapeutic requires the demonstration of efficacy in vivo, and the leap from in vitro to in vivo efficacy can be challenging, especially for arrestin pathway-selective ligands that activate poorly understood non-canonical GPCR pathways of undetermined physiological relevance (Fig. 1). Several factors must be taken into consideration. The first relates to the downstream consequences of ‘unbalanced’ GPCR activation by a biased agonist. Natural ligand-GPCR pairs co-evolved to produce the most physiologically adaptive responses to receptor activation. Biased agonists, because they specify a different set of downstream signals than the native ligand, in effect create ‘new’ receptors whose physiological properties may be unpredictable. While the tissue distribution of the receptor will dictate where responses will occur, the structure of the ligand-receptor complex will determine the nature of the response, and as these ‘unbalanced’ responses propagate, unexpected consequences may arise [17].
A second source of uncertainty is that administration of any GPCR ligand in vivo is occurring in the presence of the native hormone or neurotransmitter. For conventional agonist/antagonist ligands, a reasonable prediction of its actions in vivo can be derived from understanding the physiological roles of the endogenous ligand. Administration of pharmacologic concentrations of a conventional agonist, e.g. hPTH(1–34), would be expected to override physiological homeostasis and produce systemic responses that to some extent mimic those observed when the native ligand is pathologically overexpressed, e.g. primary hyperparathyroidism. Conversely, a neutral antagonist, e.g. hPTH(7–34), might be expected to mimic the natural absence or deficiency of the hormone, e.g. primary hypoparathyroidism. But a ‘biased’ agonist is not merely a different entity than a conventional agonist/antagonist, it is a different entity in the presence of endogenous ligand than it is in vitro where only its intrinsic efficacy is typically measured. In vivo a biased agonist would be expected to produce mixed agonist-antagonist effects, activating some pathways directly while preventing the activation of other pathways by the native ligand. Observed physiological responses to a biased agonist could arise either from pathway-selective agonism, antagonism of the native ligand, or some combination of the two effects.
4.1. The role of G protein signaling in bone metabolism
As predicted from clinical observation of humans with primary hyperparathyroidism, mice given daily injections of hPTH(1–34) show increased indices of bone turnover [57]. Markers of accelerated bone formation, including osteoblast number, osteoid surface, serum osteocalcin level and mineral apposition rates all increase. At the same time, indices of osteoclastic bone resorption rise, including osteoclast number and urinary deoxypyrodiniline (DPD). Serum and urine calcium levels also rise, reflecting the net effect of hPTH(1–34) on bone resorption, intestinal calcium absorption and renal tubular calcium retention. Because the anabolic and catabolic effects of PTH are coupled, the net effect of hPTH(1–34) on bone mass depends on the timing of exposure, such that intermittent exposure through daily subcutaneous injection produces an increase in bone formation over resorption and an increase in trabecular bone volume and cortical thickness. Continuous exposure, on the other hand, leads to bone loss, hypercalcemia and hypercalcuria [57–61].
Data from rodent models treated with various analogs of PTH or PTHrP support the conclusion that activation of Gs-adenylyl cyclase-cAMP signaling accounts for most of the biological consequences of PTH1R activation in bone. PTH(1–34), which elicits the full range of PTH1R signaling in vitro, also reproduces the full spectrum of PTH action in vivo. PTH(1–31), which has been reported to be selective for Gs over Gq/11 signaling in rodent renal tubular cells [62], increases markers of bone formation in vivo as effectively as PTH(1–34), although the net increases in bone volume with prolonged treatment are smaller [63]. PTHrP(1–36), which like [Trp1]-PTHrP(1–36) is reportedly Gs pathway-selective, is also anabolic in vivo. Both PTH(1–34) and PTHrP (1–36) increase indices of bone formation, bone mass and bone strength in ovariectomized rats [64]. On the other hand, PTH1R-Gq/11 signaling, at least in isolation, appears insufficient to produce anabolic effects in bone. In mice, the N-terminal truncated fragment, PTH(2–34), which is dramatically impaired in Gs coupling is far less efficacious than either PTH(1–34) or PTH(1–31) [63]. Comparison of the ligand series, PTH(1–38), PTH(2–38) and PTH(3–38) in rats also supports the conclusion that Gs signaling is critical. Despite the capacity to activate PKC and simulate mitogenesis in rat osteoblastic cells in vitro, PTH(3–38) produces no detectable anabolic or catabolic effects on bone in vivo [65]. Likewise, a mutant PTH1R capable of activating Gs, but not Gq/11, is able to rescue PTH(1–34) responses in PTH1R−/− mice [66]. Consistent with the critical role of Gs signaling in bone, mice subjected to post-natal deletion of Gsα in the osteoblast lineage exhibit a dramatic reduction in trabebcular and cortical bone and fail to increase bone mass in response to PTH(1–34) [66]. Gs-cAMP signaling may not be the full story, however, since PTH(1–34) still increases osteoblast number and bone formation rate in the absence of Gsα, suggesting that alternative signaling pathways beyond Gs and Gq/11 act downstream of PTH1R on osteoblast differentiation.
4.2. The paradoxial effects of arrestin-selective bias in vivo
What about arrestins in bone? While simultaneous germline deletion of both non-visual arrestin isoforms results in embryonic lethality, β-arrestin1 or β-arrestin2 can be deleted individually so isoform-specific effects of arrestin deletion can be studied using global knockout mice [67]. Still, in the presence of circulating endogenous PTH, any skeletal phenotype observed in β-arrestin null mice could result either from exaggerated G protein signaling due to impairment of arrestin-dependent PTH1R desensitization, or from loss of non-canonical GPCR signaling due to the absence of arrestin scaffolds. Skeletal phenotyping of adult male and female β-arrestin2−/− mice reveals higher basal rates of bone turnover and an impaired anabolic response to PTH(1–34), with blunted increases in trabecular bone volume and no change in cortical thickness compared to controls [68, 69]. The attenuated anabolic response to PTH(1–34) correlates with smaller changes in osteoblast number and osteoid deposition, but preserved or exaggerated increases in osteoclast number and urine deoxypyridinoline (DPD). While this supports the conclusion that PTH1R-mediated osteoblast-osteoclast coupling and bone resorption are Gs-dependent, it does not address whether the diminished anabolic response to PTH(1–34) in β-arrestin2−/− mice reflects exaggerated cAMP signaling in the setting of impaired arrestin-mediated desensitization or loss of arrestin-mediated signaling.
One approach to dissociating the desensitizing and signaling functions of arrestins in vivo would be to compare the actions of an arrestin pathway-selective PTH1R agonist in wild type and arrestin null backgrounds. In the wild type background, such a ligand should competitively antagonize endogenous PTH while simultaneously activating arrestin-dependent pathways, thus providing a glimpse of the consequences of β-arrestin engagement in the absence of G protein activation. Exposing β-arrestin2−/− mice to the same ligand would reveal skeletal effects arising from competitive inhibition of endogenous PTH signaling alone, such that changes in bone metabolism elicited by an arrestin-biased ligand that were observed in wild type, but not β-arrestin2−/−, mice would likely be consequences of arrestin-mediated signaling, not antagonism of endogenous PTH. Conversely, administering PTH(1–34) to β-arrestin2−/− animals would allow separation of the effects of G protein-dependent signaling from β-arrestin-dependent signaling because PTH(1–34) would activate both pathways in wild type animals, but only G protein signaling in the knockout.
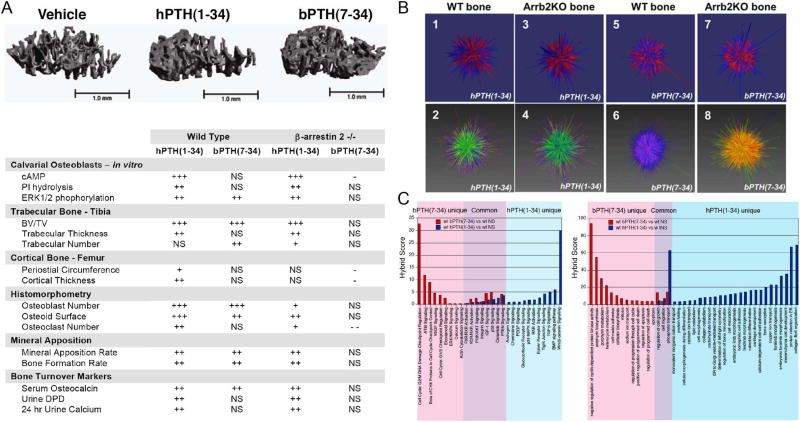
The results of such an experiment, using the conventional PTH1R agonist hPTH(1–34) and the arrestin pathway-selective agonist [D-Trp12,Tyr34]-bPTH(7–34) are summarized in Fig. 3A [33]. Contrary to predictions, in adult male wild type C57BL/6 mice, intermittent daily dosing of [D-Trp12,Tyr34]-bPTH(7–34) provokes an increase in trabecular bone formation with increased osteoblast number, osteocalcin mRNA expression and serum osteocalcin, increased trabecular number and thickness, increased rates of mineral apposition, and greater bone volume despite its antagonism of endogenous PTH. In congenic β-arrestin2−/− mice, the skeletal effects of [D-Trp12,Tyr34]-bPTH(7–34) are either absent or opposite those seen in the wild type background, suggesting that they do not result from inhibition of G protein signaling mediated by endogenous PTH. [D-Trp12,Tyr34]-bPTH(7–34) does not replicate the full response to hPTH(1–34), however. In wild type mice, hPTH(1–34) provokes the expected increases RANKL mRNA, osteoclast number, and bone turnover markers that accompany PTH1R-mediated activation of bone resorption. [D-Trp12,Tyr34]-bPTH(7–34), on the other hand, does not significantly increase these indices in wild type mice, and in the β-arrestin−/− background significantly reduces osteoclast number. In contrast, β-arrestin2−/− animals treated with hPTH(1–34) show increases in osteoclast number and urine DPD, supporting the hypothesis that PTH1R-induced bone resorption is principally mediated via Gs-cAMP dependent signaling pathways that are antagonized by the arrestin pathway-selective PTH analog in vivo.
Fig. 3.
Comparison of the in vivo efficacy of hPTH(1–34) and [D-Trp12,Tyr34]-bPTH(7–34) at the tissue and transcriptome levels. A. Representative quantitative computed tomography (qCT) images of the trabecular compartment of proximal tibia from wild type male C57BL/6 mice treated for 8 weeks with daily injection of vehicle, hPTH(1–34) or [D-Trp12,Tyr34]-bPTH (7–34) [bPTH(7–34)] (top). The table below summarizes the actions of hPTH(1–34) and [D-Trp12,Tyr34]-bPTH(7–34) in vitro and in vivo. qCT of trabecular and cortical bone, histomorphometric analysis of osteoblast and osteoclast numbers, serum osteoid surface and mineral apposition rate, and assays of bone turnover markers were performed on serum, urine and tissue samples from wild type and β-arrestin2−/− mice after 8 weeks treatment with vehicle, hPTH(1–34) or [D-Trp12,Tyr34]-bPTH(7–34). ‘+’ to ‘+++’ denotes magnitude of increase relative to vehicle-treated controls. ‘-’ denotes decrease relative to vehicle-treated. ‘NS’ denotes no significant change. The table summarizes data originally published in Gesty-Palmer et al. (2009) Science – Transl Med 1ra1 [33]. B. Transcriptomic signatures from calvarial bone of mice treated with hPTH(1–34) and [D-Trp12,Tyr34]-bPTH(7–34). Global changes in mRNA abundance in wild type and male mice treated with vehicle, hPTH(1–34) or [D-Trp12,Tyr34]-bPTH(7–34) were depicted using the vector based graphic program Omnimorph [71]. C. Parametric geneset enrichment analysis of signaling pathways and biological processes affected by hPTH(1–34) and [D-Trp12,Tyr34]-bPTH(7–34) in vivo. Analyses were performed using microarray genesets consisting of calvarial transcripts with significantly different expression between wild type (WT) mice treated with vehicle (NS), hPTH(1–34) or [D-Trp12,Tyr34]-bPTH (7–34). The left panel compares signaling pathway gene clusters identified using the WT bPTH(7–34) vs WT NS (red bars) and WT hPTH(1–34) vs WT NS (blue bars) genesets. Signaling pathways corresponding to signal transduction, growth factor signaling, nuclear receptor signaling and cell cycle control are shown. The right panel depicts an identical comparison derived by querying the Gene Ontology biological processes (GObp) database. In each panel, hybrid scores (−log10(p) × pathway enrichment ratio) reflect the statistical probability that the observed differences did not occur by chance. All signaling pathway and GObp terms shown exceed a minimal threshold significance of p ≤0.05. Figure adapted from Gesty-Palmer et al. (2013) Mol Endocrinol 27:296–314 [55].
4.3. Systems level comparison of conventional and biased agonism
How can one account for the paradoxical response to [D-Trp12,Tyr34]-bPTH(7–34) in vivo if the effects of PTH on both bone formation and resorption are solely dependent upon activation of Gs-cAMP signaling? The neutral PTH1R antagonist PTH(7–34) has no anabolic activity in a renal failure model of rats subjected to prior parathyroidectomy [70], raising the question of whether endogenous PTH is necessary for a PTH antagonist to stimulate bone formation. With intact parathyroid glands, intermittent exposure to a PTH1R antagonist might produce periodic ‘dips’ or even rebound ‘spikes’ in endogenous PTH action that might stimulate bone formation in a manner analogous to a conventional ligand, i.e. by producing cyclic modulation of PTH1R-Gs signaling.
Unlike in vitro measures of intrinsic efficacy, the in vivo effects of a biased agonist should reflect the mixed agonist-antagonist efficacy expected in the presence of the endogenous ligand. There seems little doubt that antagonism of endogenous PTH1R-Gs signaling accounts for the failure of [D-Trp12,Tyr34]-bPTH(7–34), which is an inverse agonist for PTH1R-Gs coupling in vitro [52, 53], to activate osteoclastic bone resorption and to reduce osteoclast number in β-arrestin2−/− mice [33]. However this antagonism of endogenous PTH persists in the β-arrestin2−/− background, while its anabolic effects are completely lost, suggesting that the presence of β-arrestin2 is required for its effects on osteoblast number and activity. Moreover, [D-Trp12,Tyr34]-bPTH(7–34) retains anabolic activity when administered to wild type male C57BL/6 mice by continuous infusion [71], a mode of administration that blunts or abolishes the anabolic response to PTH(1–34) [61]. While continuous infusion of a PTH1R antagonist might provoke a sustained rise in circulating endogenous PTH, persistent elevations in the native ligand are associated with accelerated bone loss, not increase. Thus the evidence, circumstantial though it may be, suggests that the ability of [D-Trp12,Tyr34]-bPTH(7–34) to dissociate the anabolic effects of PTH1R activation in the osteoblast compartment from its catabolic effects exerted through osteoblast-osteoclast coupling results both from antagonism of PTH1R-Gs signaling in vivo and some intrinsic efficacy that requires expression of β-arrestin2.
What then, are the biological processes regulated by [D-Trp12,Tyr34]-bPTH(7–34) in vivo that are associated with its action? One way of gaining insight is to apply systems level informatic approaches based on analysis of differential mRNA expression from cDNA microarrays of bone tissue combined with parametric geneset enrichment analysis [72–74]. Fig. 3B shows a holistic representation of the genomic ‘fingerprint’ left on calvarial bone by prolonged treatment of wild type and congenic β-arrestin2−/− mice with hPTH(1–34) or [D-Trp12,Tyr34]-bPTH(7–34), generated using the 3-dimensional visualization application Omnimorph. This application assigns each cDNA probe to a sector of a gridded sphere and displays differences in relative abundance between two datasets, e.g. vehicle versus drug treated, as a vector, with length denoting magnitude and color direction of change [71, 75]. Even at this gestalt level, it is apparent that both ligand structure and genetic background affect the tissue response. In the case of the conventional ligand, hPTH(1–34), which would be expected to robustly activate cAMP-mediated signaling pathways in both backgrounds, the general magnitude and direction of change is similar in the presence of absence of β-arrestin2. By contrast, [D-Trp12,Tyr34]-bPTH (7–34), which would antagonize Gs-cAMP mediated by endogenous PTH while activating β-arrestin-mediated pathways, looks dramatically different from the conventional ligand in both backgrounds. Moreover, the transcriptomic fingerprint of [D-Trp12,Tyr34]-bPTH(7–34) is highly sensitive to β-arrestin2 expression, suggesting that its actions are not merely reflective of PTH1R antagonism [71].
Applying geneset enrichment analysis to differentially expressed transcripts offers a means to probe the biological processes impacted by each ligand in vivo [72, 73]. As shown in Fig. 3C, eight weeks treatment of wild type male mice C57BL/6 mice with hPTH(1–34) leads to predictable changes in pathways associated with embryologic skeletal patterning and PTH actions in bone, including Wnt/β-catenin signaling, BMP signaling, TGF-β signaling, PI3K/AKT signaling, and ERK/MAPK signaling, and processes associated with bone formation and turnover, mineralization and resorption, and skeletal patterning and development [76–78]. Strikingly, the transcriptomic effects of [D-Trp12,Tyr34]-bPTH(7–34) show little overlap with those of the conventional agonist whose receptor it shares. Instead of affecting classical pathways of bone remodeling, the biased ligand prominently impacts basic cellular processes related to cell cycle progression, apoptosis/cell survival, and cellular adhesion and migration. Importantly, these tissue level transcriptomic effects correlate with short terms effects of [D-Trp12,Tyr34]-bPTH(7–34) on cell proliferation rate, anti-apoptosis, and cell migration observed in vitro in cell-based assays performed on primary calvarial osteoblasts (Fig. 2C), and are disrupted in vivo by deletion of β-arrestin2, strongly suggesting that they represent β-arrestin-mediated actions that are independent of its antagonism of PTH1R-Gs signaling [71]. Indeed, the transcriptomic data support the hypothesis that PTH(1–34) and [D-Trp12,Tyr34]-bPTH(7–34) affect bone mass in vivo through predominantly separate mechanisms created by largely distinct molecular signaling systems.
Data such as these clearly demonstrate that functional selectivity can be exploited to change the quality of GPCR efficacy in vivo, one of the touted benefits of biased ligands. They also highlight the challenge inherent in translating ligand bias, in that the in vivo effects of [D-Trp12,Tyr34]-bPTH(7–34) on bone could not have been readily predicted from its measured in vitro efficacy. Still, there are only two non-visual arrestin isoforms and they interact with the vast majority of GPCRs, suggesting that there may be a relatively restricted arrestin-dependent signaling repertoire. One approach to identifying such a conserved set of arrestin-dependent biological processes in vivo might be to compare the transcriptomic effects of a biased ligand across multiple different tissues, looking not at what makes each tissue unique, but at what responses appear to be conserved across different cell/tissue backgrounds. When the transcriptomic fingerprints of [D-Trp12,Tyr34]-bPTH (7–34) and hPTH(1–34) are compared in six different murine tissues after chronic drug exposure, some interesting observations emerge. First is that the arrestin pathway-selective ligand, with its limited effector coupling profile, generates a more conserved response than the conventional ligand, whose pleotropic signaling profile is more likely to be affected by differences in effector expression between tissues [71]. The second, perhaps not surprising, observation is that the response to [D-Trp12,Tyr34]-bPTH(7–34) is highly sensitive to deletion of β-arrestin2, whereas the response to hPTH(1–34) is less so. Since [D-Trp12,Tyr34]-bPTH(7–34) is capable of antagonizing endogenous PTH signaling in both wild and β-arrestin2−/− backgrounds, this suggests arrestin-dependent signaling accounts for a large part of its actions in vivo. Finally, informatic analyses indicate that the most cross-tissue conserved effects of [D-Trp12,Tyr34]-bPTH(7–34) are involved with regulation of processes related to cell growth, development, and survival [71, 79]. To the extent that arrestin-dependent signaling engenders a transcriptomic signature that might be conserved between different tissues or even different GPCRs, it may be possible uncover a conceptual framework in which the in vivo outcomes of arrestin-biased signaling can be generalized. Indeed, arrestins have been reported to play roles in cancer cell proliferation, anti-apoptotic survival signaling, and tumor invasiveness/metastasis that remarkably parallel the results of transcriptomic analysis of the actions of this arrestin pathway-selective agonist in vivo [80–84]
5. Conclusions
The preceding discussion, presented in the context of PTH1R signaling in vitro and in vivo, highlights the opportunities and challenges surrounding the development of biased GPCR ligands as therapeutics. While GPCRs have historically proven themselves to be particularly tractable as drug targets [85], within the context of conventional agonist-antagonist theory the principal avenues to GPCR drug discovery are to find ‘new’ targets through de-orphanizing novel GPCRs or to focus on sub-type selectivity for known GPCR drug targets in hope of eliciting more targeted or nuanced effects. The capacity to bias GPCR signaling, i.e. to differentially manipulate signal strength in downstream effector pathways compared to the native ligand, adds novel dimensions to GPCR pharmacology that might be exploited for therapeutic benefit.
Work to date on ligand bias in vitro has convincingly demonstrated that it is possible to tailor ligands to elicit specific efficacy profiles, favoring for example G protein activation over arrestin-dependent desensitization and signaling or vice versa, and to develop robust mathematical tools to quantitatively compare ligands across multiple dimensions of efficacy. Work in vivo has just as clearly shown that biased ligands, through their capacity to engender mixed agonist-antagonist effects in the presence of endogenous ligand, are capable of eliciting biological effects that cannot be obtained using conventional agonist or antagonist ligands. The main challenge at present appears to be the unpredictability of biased ligand effects in vivo that may arise from ‘unbalanced’ activation of GPCRs. This is especially true of so-called arrestin pathway-selective ligands given our as-yet incomplete understanding of the physiological role(s) of arrestin-dependent signaling. While some data suggest that arrestins, due to their limited signaling repertoire, may mediate a restricted and somewhat conserved set of biological responses, much remains to be learned. In effect, we are well versed in methods to detect and quantify ligand bias, but still hampered in terms of knowing what efficacy profile is needed to produce the optimal therapeutic response in any given setting.
Acknowledgments
The contents of this article do not represent the views of the Department of Veterans Affairs or the United States Government.
Funding
This work was supported by the National Institutes of Health [grant numbers R01 DK055524; R01 GM095497]; the U.S. Department of Veterans Affairs Merit Review [grant number I01 BX003188].
Abbreviations
- BRET
bioluminescence resonance energy transfer
- DPD
deoxypyridinoline
- GPCR
G protein-coupled receptor
- RAi
intrinsic relative activity
- NHERF2
Na+/H+ exchanger regulatory factor 2
- PKA
protein kinase A
- PKC
protein kinase C
- PTH
parathyroid hormone
- PTH1R
type 1 parathyroid hormone receptor
- PTHrP
parathyroid hormone-related protein
- RANKL
receptor activator of NFκB ligand
Footnotes
Conflict of interest
The authors declare no conflicts of interest related to the content of this article.
References
- 1.De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J. Biol. Chem. 1980;255:7108–7117. [PubMed] [Google Scholar]
- 2.Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- 3.Galandrin S, Oligny-Longpré G, Bouvier M. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol. Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–374. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 5.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenakin TP. Biased signalling and allosteric machines: new vistas and challenges for drug discovery. Br. J. Pharmacol. 2012;165:1659–1669. doi: 10.1111/j.1476-5381.2011.01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol. Rev. 2010;62:305–330. doi: 10.1124/pr.109.002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu JJ, Horst R, Katritch V, Stevens RC, Wüthrich K. Biased signaling pathways in β2-adrenergic receptor characterized by 19F-NMR. Science. 2012;335:1106–1110. doi: 10.1126/science.1215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saulière A, Bellot M, Paris H, Denis C, Finana F, Hansen JT, Altié MF, Seguelas MH, Pathak A, Hansen JL, Sénard JM, Galés C. Deciphering biased-agonism complexity reveals a new active AT1 receptor entity. Nat. Chem. Biol. 2012;8:622–630. doi: 10.1038/nchembio.961. [DOI] [PubMed] [Google Scholar]
- 10.Lee M-H, Appleton KM, Strungs EG, Kwon JY, Morinelli TA, Peterson YK, Laporte SA, Luttrell LM. The conformational signature of activated arrestin3 predicts its trafficking and signaling functions. Nature. 2016;531:665–668. doi: 10.1038/nature17154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuber S, Zabel U, Loreza K, Nuber A, Milligan G, Tobin AB, Lohse MJ, Hoffmann C. FRET-based β-arrestin biosensors reveal a rapid, receptor-dependent activation/deactivation cycle in living cells. Nature. 2016;531:661–664. doi: 10.1038/nature17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furness SGB, Liang Y-L, Nowell CJ, Halls ML, Wookey PJ, Dal Maso E, Inoue A, Christopoulos A, Wootten D, Sexton PM. Ligand-dependent modulation of G 1 protein conformation alters drug efficacy. Cell. 2016;167:739–749. doi: 10.1016/j.cell.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Peterson YK, Luttrell LM. The diverse roles of arrestins scaffolds in G protein-coupled receptor signaling. Pharm. Rev. 2017 doi: 10.1124/pr.116.013367. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenakin TP. 7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol. Sci. 2009;30:460–469. doi: 10.1016/j.tips.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Christensen GL, Kelstrup CD, Lyngsø C, Sarwar U, Bøgebo R, Sheikh SP, Gammeltoft S, Olsen JV, Hansen JL. Quantitative phosphoproteomics dissection of seven-transmembrane receptor signaling using full and biased agonists. Mol. Cell. Proteomics. 2011;9:1540–1553. doi: 10.1074/mcp.M900550-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kendall RT, Strungs EG, Rachidi SM, Lee M-H, El-Shewy HM, Janech MG, Luttrell DK, Luttrell LM. The β-arrestin pathway-selective angiotensin AT1A receptor agonist, Sar1Ile4Ile8-AngII, regulates a robust G protein-independent signaling network. J. Biol. Chem. 2011;286:19880–19891. doi: 10.1074/jbc.M111.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Appleton KM, Luttrell LM. Emergent biological properties of arrestin pathway-selective biased agonism. J. Recept. Signal Transduct. Res. 2013;33:153–161. doi: 10.3109/10799893.2013.769004. [DOI] [PubMed] [Google Scholar]
- 18.Jüppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Jr, Hock J, Potts JT, Jr, Kronenberg HM. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 19.Kolakowski LF., Jr GCRDb: a G-protein-coupled receptor database. Recept. Channels. 1994;2:1–7. [PubMed] [Google Scholar]
- 20.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–3638. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt I, Dobnig H, Turner R. Intermittent parathyroid hormone treatment increases osteoblast number, steady state messenger ribonucleic acid levels for osteocalcin, and bone formation in tibial metaphysis of hypophysectomized female rats. Endocrinology. 1995;136:5127–5134. doi: 10.1210/endo.136.11.7588250. [DOI] [PubMed] [Google Scholar]
- 22.Martin TJ. Parathyroid hormone-related protein, its regulation of cartilage and bone development, and role in treating bone diseases. Physiol. Rev. 2016;96:831–871. doi: 10.1152/physrev.00031.2015. [DOI] [PubMed] [Google Scholar]
- 23.Philbrick WM, Wysolmerski JJ, Galbraith S, Holt E, Orloff JJ, Yang KH, Vasavada RC, Weir EC, Broadus AE, Stewart AF. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol. Rev. 1996;76:127–173. doi: 10.1152/physrev.1996.76.1.127. [DOI] [PubMed] [Google Scholar]
- 24.Abou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT., Jr Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc. Natl. Acad. Sci. U. S. A. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bringhurst FR, Juppner H, Guo J, Urena P, Potts JT, Jr, Kronenberg HM, Abou-Samra AB, Segre GV. Cloned, stably expressed parathyroid hormone (PTH)/PTH-related peptide receptors activate multiple messenger signals and biological responses in LLC-PK1 kidney cells. Endocrinology. 1993;132:2090–2098. doi: 10.1210/endo.132.5.8386606. [DOI] [PubMed] [Google Scholar]
- 26.Singh AT, Gilchrist A, Voyno-Yasenetskaya T, Radeff-Huang JM, Stern PH. G alpha12/G alpha13 subunits of heterotrimeric G proteins mediate parathyroid hormone activation of phospholipase D in UMR-106 osteoblastic cells. Endocrinology. 2005;146:2171–2175. doi: 10.1210/en.2004-1283. [DOI] [PubMed] [Google Scholar]
- 27.Mahon MJ, Donowitz M, Yun CC, Segre GV. Na(+)/H(+) exchanger regulatory factor 2 directs parathyroid hormone 1 receptor signalling. Nature. 2002;417:858–861. doi: 10.1038/nature00816. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari SL, Behar V, Chorev M, Rosenblatt M, Bisello A. Endocytosis of ligand-human parathyroid hormone receptor 1 complexes is protein kinase C-dependent and involves beta-arrestin2. Real-time monitoring by fluorescence microscopy. J. Biol. Chem. 1999;274:29968–29975. doi: 10.1074/jbc.274.42.29968. [DOI] [PubMed] [Google Scholar]
- 29.Vilardaga JP, Krasel C, Chauvin S, Bambino T, Lohse MJ, Nissenson RA. Internalization determinants of the parathyroid hormone receptor differentially regulate beta-arrestin/receptor association. J. Biol. Chem. 2002;277:8121–8129. doi: 10.1074/jbc.M110433200. [DOI] [PubMed] [Google Scholar]
- 30.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct conformations of the parathyroid hormone receptor mediate G protein and beta-arrestin dependent activation of ERK1/2. J. Biol. Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 31.Verheijen MH, Defize LH. Parathyroid hormone activates mitogen-activated protein kinase via a cAMP-mediated pathway independent of Ras. J. Biol. Chem. 1997;272:3423–3429. doi: 10.1074/jbc.272.6.3423. [DOI] [PubMed] [Google Scholar]
- 32.Lederer ED, Sohi SS, McLeish KR. Parathyroid hormone stimulates extracellular signal-regulated kinase (ERK) activity through two independent signal transduction pathways: role of ERK in sodium-phosphate cotransport. J. Am. Soc. Nephrol. 2000;11:222–231. doi: 10.1681/ASN.V112222. [DOI] [PubMed] [Google Scholar]
- 33.Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, Luttrell LM. A beta-Arrestin biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci. Transl. Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dean T, Vilardaga JP, Potts JT, Jr, Gardella TJ. Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol. Endocrinol. 2008;22:156–166. doi: 10.1210/me.2007-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okazaki M, Ferrandon S, Vilardaga JP, Bouxsein ML, Potts JT, Jr, Gardella TJ. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:16525–16530. doi: 10.1073/pnas.0808750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, Gardella TJ, Vilardaga JP. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jouishomme H, Whitfield JF, Gagnon L, MacLean S, Isaacs R, Chakravarthy B, Durkin J, Neugebauer W, Willick G, Rixon RH. Further definition of the protein kinase C activation domain of the parathyroid hormone. J. Bone Miner. Res. 1994;9:943–949. doi: 10.1002/jbmr.5650090620. [DOI] [PubMed] [Google Scholar]
- 38.Whitfield JF, Morley P. Small bone-building fragments of parathyroid hormone: new therapeutic agents for osteoporosis. Trends Pharmacol. Sci. 1995;16:382–386. doi: 10.1016/s0165-6147(00)89079-3. [DOI] [PubMed] [Google Scholar]
- 39.Jouishomme H, Whitfield JF, Chakravarthy B, Durkin JP, Gagnon L, Isaacs RJ, MacLean S, Neugebauer W, Willick G, Rixon RH. The protein kinase-C activation domain of the parathyroid hormone. Endocrinology. 1992;130:53–60. doi: 10.1210/endo.130.1.1727720. [DOI] [PubMed] [Google Scholar]
- 40.Takasu H, Gardella TJ, Luck MD, Potts JT, Jr, Bringhurst FR. Amino-terminal modifications of human parathyroid hormone (PTH) selectively alter phospholipase C signaling via the type 1 PTH receptor: implications for design of signal-specific PTH ligands. Biochemistry. 1999;38:13453–13460. doi: 10.1021/bi990437n. [DOI] [PubMed] [Google Scholar]
- 41.Bisello A, Chorev M, Rosenblatt M, Monticelli L, Mierke DF, Ferrari SL. Selective ligand-induced stabilization of active and desensitized parathyroid hormone type 1 receptor conformations. J. Biol. Chem. 2002;277:38524–38530. doi: 10.1074/jbc.M202544200. [DOI] [PubMed] [Google Scholar]
- 42.Sneddon WB, Bisello A, Magyar CE, Willick GE, Syme CA, Galbiati F, Bisello A, Friedman PA. Ligand-selective dissociation of activation and internalization of the parathyroid hormone receptor. Conditional efficacy of PTH peptide fragments. Endocrinology. 2004;145:2815–2823. doi: 10.1210/en.2003-1185. [DOI] [PubMed] [Google Scholar]
- 43.Gardella TJ, Luck MD, Jensen GS, Schipani E, Potts JT, Jr, Juppner H. Inverse agonism of amino-terminally truncated parathyroid hormone (PTH) and PTH-related peptide (PTHrP) analogs revealed with constitutively active mutant PTH/PTHrP receptors. Endocrinology. 1996;137:3936–3941. doi: 10.1210/endo.137.9.8756569. [DOI] [PubMed] [Google Scholar]
- 44.Luttrell LM, Maudsley S, Bohn LM. Fulfilling the promise of “biased” G protein-coupled receptor agonism. Mol. Pharmacol. 2015;88:579–588. doi: 10.1124/mol.115.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenakin TP. 7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol. Sci. 2009;30:460–469. doi: 10.1016/j.tips.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Kenakin T, Watson C, Muniz-Medina V, Christopoulos A, Novick S. A simple method for quantifying functional selectivity and agonist bias. ACS Chem. Neurosci. 2012;3:193–203. doi: 10.1021/cn200111m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stahl EL, Zhou L, Ehlert FJ, Bohn LM. A novel method for analyzing extremely biased agonism at G protein-coupled receptors. Mol. Pharmacol. 2015;87:866–877. doi: 10.1124/mol.114.096503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Black JW, Leff P. Operational models of pharmacological agonist. Proc. R. Soc. Lond. B. 1983;220:141–162. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 49.Kenakin T. Quantifying biased β-arrestin signaling. Handb. Exp. Pharmacol. 2014;219:57–83. doi: 10.1007/978-3-642-41199-1_3. [DOI] [PubMed] [Google Scholar]
- 50.Namkung Y, Radresa O, Armando S, Devost D, Beautrait A, Le Gouill C, Laporte SA. Quantifying biased signaling in GPCRs using BRET-based biosensors. Methods. 2016;92:5–10. doi: 10.1016/j.ymeth.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Ehlert FJ. Analysis of allosterism in functional assays. J. Pharmacol. Exp. Ther. 2005;315:740–754. doi: 10.1124/jpet.105.090886. [DOI] [PubMed] [Google Scholar]
- 52.Appleton KM, Lee MH, Alele C, Alele C, Luttrell DK, Peterson YK, Morinelli TA, Luttrell LM. Biasing the parathyroid hormone receptor: relating in vitro ligand efficacy to in vivo biological activity. Methods Enzymol. 2013;522:229–262. doi: 10.1016/B978-0-12-407865-9.00013-3. [DOI] [PubMed] [Google Scholar]
- 53.Leonard AP, Appleton KM, Luttrell LM, Peterson YK. A high-content, live-cell, and real-time approach to the quantitation of ligand-induced β-arrestin2 and class A/class B GPCR mobilization. Microsc. Microanal. 2013;19:150–170. doi: 10.1017/S1431927612014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gesty-Palmer D, Yuan L, Martin B, Wood WH, 3rd, Lee MH, Janech MG, Tsoi LC, Zheng WJ, Luttrell LM, Maudsley S. β-arrestin-selective G protein-coupled receptor agonists engender unique biological efficacy in vivo. Mol. Endocrinol. 2013;27:296–314. doi: 10.1210/me.2012-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeFea KA. Stop that cell! Beta-arrestin-dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu. Rev. Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- 57.Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology. 1995;136:3632–3638. doi: 10.1210/endo.136.8.7628403. [DOI] [PubMed] [Google Scholar]
- 58.Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982;110:506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- 59.Hock JM, Gera I. Effects of continuous and intermittent administration and inhibition of resorption on the anabolic response of bone to parathyroid hormone. J. Bone Miner. Res. 1992;7:65–72. doi: 10.1002/jbmr.5650070110. [DOI] [PubMed] [Google Scholar]
- 60.Ishizuya T, Yokose S, Hori M, Noda T, Suda T, Yoshiki S, Yamaguchi A. Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J. Clin. Invest. 1997;99:2961–2970. doi: 10.1172/JCI119491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qin L, Raggatt LJ, Partridge NC. Parathyroid hormone: a double-edged sword for bone metabolism. Trends Endocrinol. Metab. 2004;15:60–65. doi: 10.1016/j.tem.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 62.Friedman PA, Gesek FA, Morley P, Whitfield JF, Willick GE. Cell-specific signaling and structure-activity relations of parathyroid hormone analogs in mouse kidney cells. Endocrinology. 1999;140:301–309. doi: 10.1210/endo.140.1.6462. [DOI] [PubMed] [Google Scholar]
- 63.Mohan S, Kutilek S, Zhang C, Shen HG, Kodama Y, Srivastava AK, Wergedal JE, Beamer WG, Baylink DJ. Comparison of bone formation responses to parathyroid hormone(1-34), (1-31), and (2-34) in mice. Bone. 2000;27:471–478. doi: 10.1016/s8756-3282(00)00355-0. [DOI] [PubMed] [Google Scholar]
- 64.Stewart AF, Cain RL, Burr DB, Jacob D, Turner CH, Hock JM. Six-month daily administration of parathyroid hormone and parathyroid hormone-related protein peptides to adult ovariectomized rats markedly enhances bone mass and biomechanical properties: a comparison of human parathyroid hormone 1-34, parathyroid hormone-related protein 1-36, and SDZ-parathyroid hormone 893. J. Bone Miner. Res. 2000;15:1517–1525. doi: 10.1359/jbmr.2000.15.8.1517. [DOI] [PubMed] [Google Scholar]
- 65.Hilliker S, Wergedal JE, Gruber HE, Bettica P, Baylink DJ. Truncation of the amino terminus of PTH alters its anabolic activity on bone in vivo. Bone. 1996;19:469–477. doi: 10.1016/s8756-3282(96)00230-x. [DOI] [PubMed] [Google Scholar]
- 66.Sinha P, Aarnisalo P, Chubb R, Poulton IJ, Guo J, Nachtrab G, Kimura T, Swami S, Saeed H, Chen M, Weinstein LS, Schipani E, Sims NA, Kronenberg HM, Wu JY. Loss of Gsα in the postnatal skeleton leads to low bone mass and a blunted response to anabolic parathyroid hormone therapy. J. Biol. Chem. 2016;291:1631–1642. doi: 10.1074/jbc.M115.679753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. Beta-arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrari SL, Pierroz DD, Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML. Bone response to intermittent parathyroid hormone is altered in mice null for beta-Arrestin2. Endocrinology. 2005;146:1854–1862. doi: 10.1210/en.2004-1282. [DOI] [PubMed] [Google Scholar]
- 69.Bouxsein ML, Pierroz DD, Glatt V, Goddard DS, Cavat F, Rizzoli R, Ferrari SL. Beta-Arrestin2 regulates the differential response of cortical and trabecular bone to intermittent PTH in female mice. J. Bone Miner. Res. 2005;20:635–643. doi: 10.1359/JBMR.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sebastian EM, Suva LJ, Friedman PA. Differential effects of intermittent PTH(1-34) and PTH(7-34) on bone microarchitecture and aortic calcification in experimental renal failure. Bone. 2008;43:1022–1030. doi: 10.1016/j.bone.2008.07.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maudsley S, Martin B, Gesty-Palmer D, Cheung H, Johnson C, Patel S, Becker KG, Wood WH, 3rd, Zhang Y, Lehrmann E, Luttrell LM. Delineation of a conserved arrestin-biased signaling repertoire in vivo. Mol. Pharmacol. 2015;87:706–717. doi: 10.1124/mol.114.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maudsley S, Chadwick W, Wang L, Zhou Y, Martin B, Park SS. Bioinformatic approaches to metabolic pathways analysis. Methods Mol. Biol. 2011;756:99–130. doi: 10.1007/978-1-61779-160-4_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maudsley S, Siddiqui S, Martin B. Systems analysis of arrestin pathway functions. Prog. Mol. Biol. Transl. Sci. 2013;118:431–467. doi: 10.1016/B978-0-12-394440-5.00017-6. [DOI] [PubMed] [Google Scholar]
- 74.Chen H, Martin B, Daimon CM, Siddiqui S, Luttrell LM, Maudsley S. Textrous!: extracting semantic textual meaning from gene sets. PLoS One. 2013;8:e62665. doi: 10.1371/journal.pone.0062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin B, Chen H, Daimon CM, Chadwick W, Siddiqui S, Maudsley S. Plurigon: three dimensional visualization and classification of high-dimensionality data. Front. Physiol. 2013;4:190. doi: 10.3389/fphys.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deng ZL, Sharff KA, Tang N, Song WX, Luo J, Luo X, Chen J, Bennett E, Reid R, Manning D, Xue A, Montag AG, Luu HH, Haydon RC, He TC. Regulation of osteogenic differentiation during skeletal development. Front. Biosci. 2008;13:2001–2021. doi: 10.2741/2819. [DOI] [PubMed] [Google Scholar]
- 77.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev. Endocr. Metab. Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 78.Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88. doi: 10.1038/cr.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maudsley S, Martin B, Janssens J, Etienne H, Jushaj A, van Gastel J, Willemsen A, Chen H, Gesty-Palmer D, Luttrell LM. Informatic deconvolution of biased GPCR signaling mechanisms from in vivo pharmacological experimentation. Methods. 2016;92:51–63. doi: 10.1016/j.ymeth.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, Chellappan S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J. Clin. Invest. 2006;116:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chun KS, Lao HC, Trempus CS, Okada M, Langenbach R. The prostaglandin receptor EP2 activates multiple signaling pathways and beta-arrestin1 complex formation during mouse skin papilloma development. Carcinogenesis. 2009;30:1620–1627. doi: 10.1093/carcin/bgp168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moussa O, Ashton AW, Fraig M, Garrett-Mayer E, Ghoneim MA, Halushka PV, Watson DK. Novel role of thromboxane receptors beta isoform in bladder cancer pathogenesis. Cancer Res. 2008;68:4097–4104. doi: 10.1158/0008-5472.CAN-07-6560. [DOI] [PubMed] [Google Scholar]
- 83.Li TT, Alemayehu M, Aziziyeh AI, Pape C, Pampillo M, Postovit L-M, Mills GB, Babwah AV, Bhattacharya M. Beta-arrestin/Ral signaling regulates lysophosphatidic acid-mediated migration and invasion of human breast tumor cells. Mol. Cancer Res. 2009;7:1064–1077. doi: 10.1158/1541-7786.MCR-08-0578. [DOI] [PubMed] [Google Scholar]
- 84.Rosanò L, Cianfrocca R, Masi S, Spinella F, Di Castro V, Biroccio A, Salvati E, Nicotra MR, Natali PG, Bagnato A. Beta-arrestin links endothelin a receptor to beta-catenin signaling to induce ovarian cancer cell invasion and metastasis. Proc. Natl. Acad. Sci. U. S. A. 2009;106:2806–2811. doi: 10.1073/pnas.0807158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ellis C. The state of GPCR research in 2004. Nat. Rev. Drug Discov. 2004;3:577–626. doi: 10.1038/nrd1458. [DOI] [PubMed] [Google Scholar]