Abstract
Rationale: Burn injuries represent one of the major worldwide public health problems causing more severe physiological stress than other traumas. Effective treatment of burn injuries is mandatory to prevent the numerous life-threatening complications and possible disabilities. Stem cells, a population of multipotent cells retaining the properties of self-renewal and differentiation, are the main player in tissue regeneration after major trauma. Thus, they are thought to play a key role in wound healing inducing efficient and physiological skin regeneration. Stem cell-based regeneration is quickly gaining scientific grounds. Objective: This study was designed as a comparative prospective study to evaluate and compare the regenerative effect of bone marrow derived mesenchymal stem cells (BM-MSCs) and umbilical cord blood derived mesenchymal stem cells (UC-MSCs) compared to conventional early excision and graft (EE&G) in recent thermal full thickness burned patients. Subject & Methods: Recruited burned patients were randomly divided into three groups (20 patients on each group) having recent thermal full thickness percentage ranging from 10% to 25% total body surface area (TBSA). After receiving allocated treatment, they were assessed as regards: rate of burn healing, presence of post-burn complications both early (such as loss of graft and infections) and late (as hypertrophic scars, keloid, hypo- or hyperpigmentation or contracture of the wound), hospitalization time and cost. Results: This study showed significantly improved rate of healing in both BM-MSC and UC-MSC groups as compared to EE&G group with no significant difference between bone marrow and umbilical cord groups. Comparing the incidence of early complications, partial and total loss of graft occurred in 50% patients in (EE&G) group, while infection complication appeared in 25% of patients of (BM-MSCs) group and in 70% of patients in (UC-MSCS) group. The late complications (hypertrophic scars) were observed in 40% of (EE&G) patients group, in 15% of (BM-MSCs) treated patients group and 20% of (UC-MSCS) patients group. Contractured scars were present in 15% in (EE&G) group, 10% in (BM-MSCs) group, 10% in (UC-MSCS) group. Hypopigmentation occurred in 20% of patients in (EE&G) group, 20% in (BM-MSCs) group and 10% in (UC-MSCS) group. Hyperpigmentation was present in 20% of patient in (EE&G) group, 30% in (UC-MSCS) group but no hyperpigmentation occurred in (BM-MSCs) group. There was no late complication in 5% of patient in (EE&G) group, 55% in (BM-MSCs) group and 30% in (UC-MSCS) group. The results of this study revealed that the hospitalization period was significantly reduced in both (BM-MSCs) group and (UC-MSCS) group as compared to (EE&G) group. Conclusion: this study proves that mesenchymal stem cells, both from bone marrow and cord blood origin, can effectively improve healing of burn injuries.
Keywords: Stem cells, burn, contractured scars, hypopigmentation, graft loss
Introduction
Burn injury represents one of the important causes of morbidity and mortality all over the world. Proper wound healing requires a complex process of cell migration and proliferation in addition to the extracellular matrix deposition and remodelling. The standard treatment of choice for burn wounds is autologous skin grafting with subsequent elastic bandage. The major drawback for this option is the limited donor sites in cases of major burns. Proper skin regeneration requires the formation of epidermal appendages destroyed in deep burns such as sebaceous and sweat glands and hair. Bioengineered skin equivalents do not replace these lost structures. Restoration of such skin appendages requires stem cell-based regeneration. Dermal stem cells have not been successfully cultivated. Hair follicle stem cells represent a possible source of multipotent stem cells in skin; repopulating epidermis, epidermis, hair follicles, and sebaceous glands [1,2]. However, the main drawback is their scanty number and difficult ex-vivo expansion.
Stem cell application as a treatment option in burn wounds represents a number of repair pathways through both tissue regeneration and prevention of fluid loss and bacterial [3].
Mesenchymal stromal cells (MSCs) represent a population of multipotent adult stem cells abundant in all human tissues. MSCs can be isolated from nearly all body tissues; however, the most common sites are bone marrow, umbilical cord blood and tissues and adipose tissue. MSCs have considerable ability for proliferation in vitro and differentiation into many cell types [4,5]. Bone marrow derived MSCs are reported to play an important role in accelerating wound healing [6] as well improving the quality of healing through reduction of scar formation and regeneration of normal functioning skin and appendages [3].
Bone marrow derived MSCs have been successfully induced to trans-differentiate into a number of cell lineages including skin [7,8].
Umbilical cord blood and tissue contains a population of MSCs with the ability to differentiate into bone, skin, endothelium, neural and cartilage lineages [9-11].
Using stem cells for skin regeneration in burns offers a number of advantages. It represents an efficient, high-quality approach for skin coverage especially in deep burns through regeneration of skin appendages in addition to the minimal risk of hypertrophic scarring. In addition, stem cell-mediated skin regeneration lowers morbidity through addressing systemic effects of burn injury, namely hypermetabolism and inflammation.
Stem cell application for burn wound treatment could be performed in the operating room, immediately after debridement. Cells can be used as cellular suspension sprayed into the wound with fibrin sealant followed by application of cover in the form of dermal substitute, skin graft or film, to enhance cell paracrine signaling and homing of the cells, improving wound healing [12]. Cells can also be used after seeding on a scaffold or a skin graft.
In addition to their function in wound repair, MSCs also enhance wound healing through for their mostly paracrine anti-inflammatory properties, which can be to modulate severe burns.Current applications of MSCs’ anti-inflammatory properties in acute lung injury/respiratory distress syndrome, shows promising results [13].
This study was designed as a case-control prospective study to compare the effect of (BMSCs) and (UCMSCs) with standard regimen in the form of early excision and grafting (EE&G) in the treatment of recent thermal full thickness burned patients with percentage ranging from 10% to 25% total body surface area (TBSA).
Patients and methods
This prospective comparative study was conducted in the Plastic, Reconstructive and Burn Surgery Department of Al-Azhar University, El-Helmia Military Burn Center and Ghamra Military Hospital (Obstetric Department).
Institutional Ethical committee approval was taken before starting the study. All patients signed informed consent.
The study included 60 patients with recent thermal full thickness burned patients with percentage ranging from 10% to 25% total body surface area (TBSA).
Inclusion criteria
Both sexes; Age range: from 15 to 50 years; Patients with recent thermal full thickness burns from 10% to 25% TBSA.
Exclusion criteria
Patients with co-morbidity (chronic diseases as heart diseases, liver or renal impairment, diabetes mellitus and obesity); Below the age of 15 years old and over 50 years old; Patients had inhalation injury; Patients with superficial burns and old burns (greater than two weeks); Patients with other types of burn as chemical, electric and radiation burns; Patients with associated injury in addition to burns.
All patients were fully evaluated in Emergency Department of El Helmia Military Burn Center and Plastic and Burn Department in Al-Azhar University.
Then, patients were randomly divided into the following equal groups (20 patients in each group).
Group I (control group): early excision and graft-treated group (EE&G)
They were managed with daily dressing with gentamycin ointment for three to five days to stabilize the general condition of patient, then early excision and graft was performed.
Group II: bone marrow derived mesenchymal stem cells-treated group (BM-MSC)
They were treated by injection of adult stem cells derived from bone marrow. Two days after surgical excision of deep burned tissue, autologous BM-MSCs were injected in the burned area (1 ml/cm2 in the concentration of 100,000 MSC/ml). The injection session was repeated after 10 days. Closed dressing with gentamicin ointment was used every three days.
Group III: umblical cord blood mesenchymal stem cells-treated group (UCMSC)
They were treated by topical umbilical cord-derived mesenchymal stem cells on burned area. Two days after surgical excision of deep burned tissues, UC MSCs were injected in the same manner as in Group II. Closed dressing with gentamicin ointment was used every three days.
Methods
Complete History Taking: including type of burn, onset, causes, mechanism and site of burn, special habits of medical importance, previous operations and chronic diseases.
General examination: weight of patients, vital signs (pulse, blood pressure, temperature and respiratory rate), chest examination, abdominal examination and other trauma.
Local examination: examination of the burns including surface area (percentage) by using Lund and Browder chart, depth and areas of body affected.
Investigations: routine laboratory investigations were performed including complete blood count, renal function tests, liver function tests, blood gases and serum electrolytes assay at enrollment and every 3 days to assess the general condition of the patients.
Cases were photographed before the onset of treatment, every 2 weeks from the start of treatment and when the burns healed. Post discharging, they were photographed in the follow up at 2 weeks, one month and six months.
Group I (control group)
Early excision and autograft were performed to 20 patients by the following steps on five to seven days after injury: anesthesia: general or spinal; operation: by tangential excision of burned skin with Watson knife. Split thickness skin graft was harvested from healthy skin from either the thigh or the abdomen; The graft was meshed to be porous to increase its surface area to around 1:2 or 1:3 to minimize the area of donor site needed and to allow the drainage of seroma under the graft; the skin graft was fixed using stapler; Dressing with Vaseline gause for three or five days Figures 3, 4.
Figure 3.
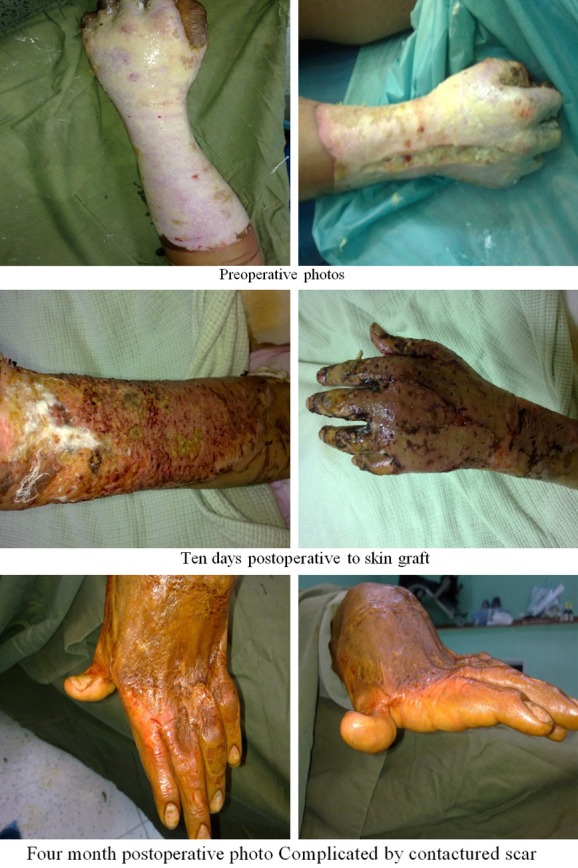
Photos of patients with early excision and graft.
Figure 4.
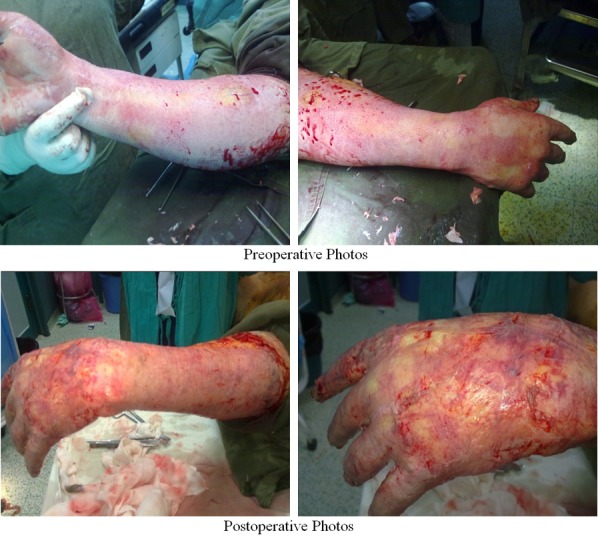
Photos of patients with early excision and graft.
Groups II and III (mesenchymal stem cells groups)
Anesthesia: general or spinal; Excision of burned skin by tangential excision with Watson knife after three or five days from injury; Group II: BM-MSCs were injected in two sessions: the first was 2 days after surgical excision of deep burned tissue. Administration of already prepared MSCs was done by its injection in the bed of the 20 cm2 burned areas (1 mL stem cells in 1 cm2 of burn). The second session was done after 10 days. Closed dressing with Gentamycin ointment was used every three days; Group III: 2 days after surgical excision of deep burned tissues, UC-SC administration was applied topically with gentamycin ointment coverage. The dressing was changed every five to seven days.
Methods of stem cells preparation
Sampling
Bone marrow stem cells
The posterior iliac spine was sterilized, and local anesthesia, 1% Xylocaine, was applied to the periosteum and the skin of that area. The bone marrow was then aspirated (100 mL), from each side, under aseptic conditions on preservative-free heparin.
Umbilical cord stem cells
Cord blood was collected ex-utero in Caesarean sections. Cord blood was collected after informed consent of mothers in Ghamra Military Hospital, and subjected to complete infectious disease investigations.
Mesenchymal stem cells (MSC) separation
Mesenchymal stem cells were separated using standard double physical separation. Briefly, mononuclear cells (MNC) separation was done using density gradient (Ficoll-Plaque 1.077 g/mL, GE Biosciences, Piscataway, NJ, USA). MNCs were washed with Dulbecco’s low glucose modified Eagle’s medium (Hyclone, Logan, UT, USA) containing 1% antibiotic-antimycotic (Gibco, Grand Island, NY, USA), resuspended in complete culture medium (Minimum Essential Media [MEM], Gibco/BRL, Gaithersburg, MD, USA); 200 ul of the patient’s own serum, 100 units/ml of penicillin/streptomycin, and 2 mM L-glutamine (Gibco/BRL). Cells were plated in T25 flasks (Falcon Plastics, Los Angeles, CA, USA) at a density of 500,000 cells and incubated at 37°C, with 5% carbon dioxide. After 24 hours, non-adherent cells were discarded, and the adherent cells were morphologically evaluated every other day with media change until 80-90% confluence is reached.
Harvest: adherent cells were then washed with phosphate-buffered saline (PBS) and harvested by incubation for 4 minutes at 37°C in 4 mL of 0.25% trypsin-EDTA. After incubation, the trypsin was inactivated by the addition of 5 mL of MEM. The cells were washed twice with PBS.
Cell viability was determined using the trypan blue exclusion test. According to the International Society for Cellular Therapy position statement in 2006, mesenchymal lineage was confirmed by plastic adherence, fibroblast-like morphology, trilineage differentiation potential and typical immunophenotyping [14].
Progress assessment
General Assessment: vital sign (pulse, temperature, blood pressure and urine output); Laboratory data (serum albumin level, hemoglobin percent, renal and liver functions) every three days.
Local Assessment: assessment of the status of the wound bed and granulation tissue.
Study groups were compared regarding: Rate of healing; Occurrence of early complications: a. Graft loss; Infections; Occurrence of late complications: a. Hypertrophic scars; b. Keloid; c. Contracture; d. Hypopigmentation or hyperpigmentation; Assessment of hospital stay and cost of treatment.
Follow up of the patients, after discharge; two weeks, one month, three months, and six months for studying the post burn wound healing.
Statistical analysis
All results in the present study were expressed as mean ± SD of the mean. Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL, USA) program, version 20.0 was used. Differences were considered significant when P ≤ 0.05. Frequency and percentage of non-numerical data were performed.
Results
Demographic data of the studied patient groups are summarized in Table 1 and Figure 1.
Table 1.
Changes in ages of patients, the extent of burn, percent of burn extent (%), hospitalization time and the cost of hospitalization in the different studied groups
| EE&G | BMSCs | UCBSCS | ||
|---|---|---|---|---|
| Ages of patients | Mean ± SD | 25.3 ± 4.38 | 23 ± 1.9 | 22.9 ± 2.73 |
| Range | 18-35 | 20-27 | 18-29 | |
| The extent of burn | Mean ± SD | 18.15 ± 2.87 | 17 ± 2.94 | 15.95 ± 2.89 |
| Range | 15-25 | 12-22 | 10-20 | |
| Percent of Burn Extent (%) | Mean ± SD | 18.15 | 17 | 15.95 |
| P1 | NS | < 0.05 | ||
| P2 | NS | |||
| Hospitalization Time | Mean ± SD | 18.35 ± 4.53 | 14.50 ± 3.5 | 15.6 ± 3.86 |
| Range | 10-25 | 10-20 | 10-22 | |
| P1 | < 0.005 | < 0.05 | ||
| P2 | NS | |||
| The Cost Of Hospitalization | Mean ± SD | 17.08 ± 2.49 | 17.25 ± 1.75 | 17.80 ± 1.93 |
| Range | 11-20.5 | 15-20 | 15-21 | |
| P1 | NS | NS | ||
| P2 | NS |
P1: Significance by LSD by ANOVA at P < 0.05 from early excision and graft group. P2: Significance by LSD by ANOVA at P < 0.05 from bone marrow stem cells group.
Figure 1.
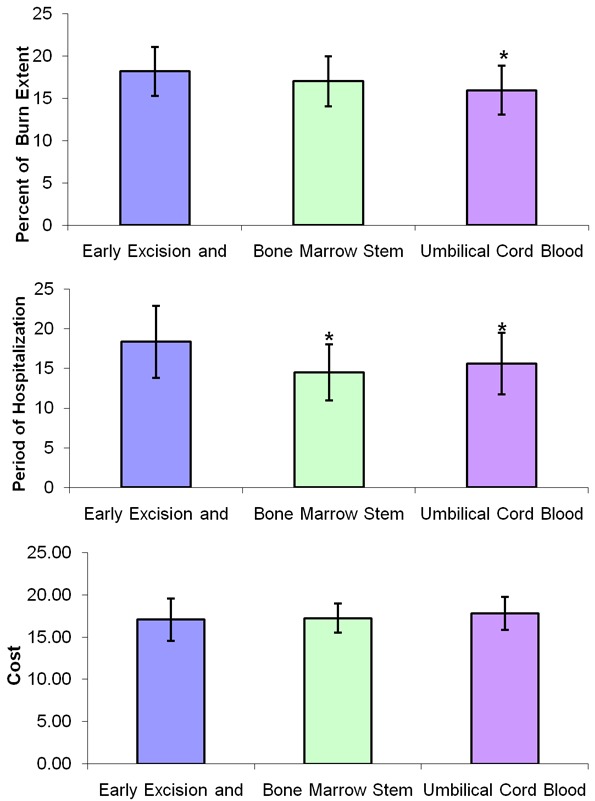
Changes in the percent of burn extent, hospitalization time and the cost of hospitalization in the different studied group. *: Significance by LSD by ANOVA at P < 0.05 from early excision and graft group.
Rate of healing
Regarding the extent of burn, initial percentage ranged between (15-25%), (12-22%) and (10-20%) in the three groups respectively. After follow-up, the percentage of burn extent was significantly reduced in both UCBSC and BMSC groups as compared to early excision and graft group; with insignificant difference between UCBSC and BMSC groups.
Hospital stay
The period of hospitalization was significantly reduced in both bone marrow stem cells group and umbilical cord blood stem cells group when each group was compared to early excision and graft group. Non-significant changes were observed when comparing bone marrow stem cell group to umbilical cord blood stem cells group as shown in Table 1 and Figure 1.
Early complications
Regarding early complications which include infection, partial and total loss of graft as shown in Tables 2, 3, 4 and 5 and Figure 2, the control (EE&G) group did not manifest any early complications in 50% of cases while the other 50% of cases in this group developed either graft loss (alone in 10% of cases, combined with infection in 15% of cases) or infection (25% cases). In BM-MSC group Figures 5, 6, 7 and 8, there was no early complication (in 75% of cases), while infection was present as an early complications (in 25% of cases). In UC-MSC group Figures 9, 10 and 11, 30% of cases did not have any manifestations of early complications while 70% of cases were complicated with infection.
Table 2.
Description of early complications in early excision and graft group
| Early Complications | Frequency | Percent % | Valid Percent | Cumulative Percent |
|---|---|---|---|---|
| No complication | 10 | 50 | 50 | 50 |
| Infection | 5 | 25 | 25 | 75 |
| Partial Loss of graft | 2 | 10 | 10 | 85 |
| Infection & Partial Loss of graft | 3 | 15 | 15 | 100 |
| Total | 20 | 100 | 100 |
Table 3.
Description of early complications in BM-MSC group
| Early Complications | Frequency | Percent % | Valid Percent | Cumulative Percent |
|---|---|---|---|---|
| No complication | 15 | 75 | 75 | 75 |
| Infection | 5 | 25 | 25 | 100 |
| Total | 20 | 100 | 100 |
Table 4.
Description of early complications in UCMSC group
| Early Complications | Frequency | Percent % | Valid Percent | Cumulative Percent |
|---|---|---|---|---|
| No complication | 6 | 30 | 30 | 30 |
| Infection | 14 | 70 | 70 | 100 |
| Total | 20 | 100 | 100 |
Table 5.
Comparison of early complications in the different studied groups
| Early Excision and Graft group | Bone Marrow Stem Cells group | Umbilical Cord Blood Stem Cells group | Chi-square test | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. | % | No. | % | No. | % | X2 | P-value | |
| No complication | 10 | 50% | 15 | 75% | 6 | 30% | 8.142 | 0.017 |
| Early complication | 10 | 50 % | 5 | 25% | 14 | 70% | ||
Figure 2.
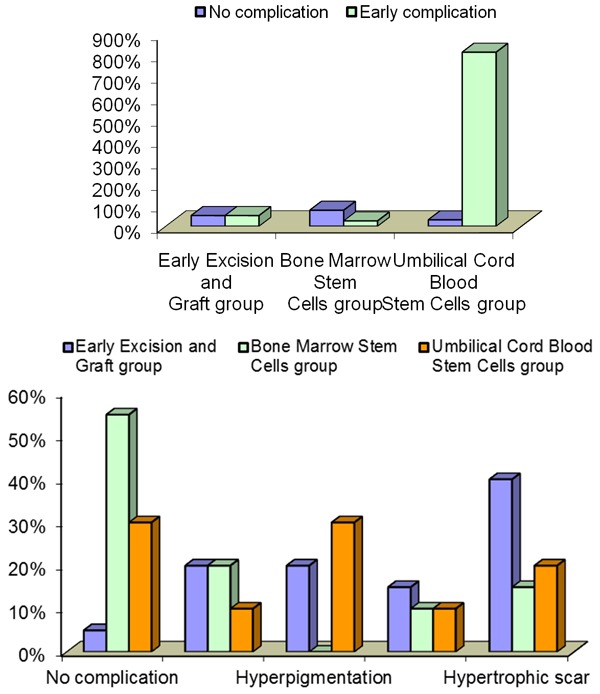
Changes in the frequency of early and late complications in the different studied group.
Figure 5.
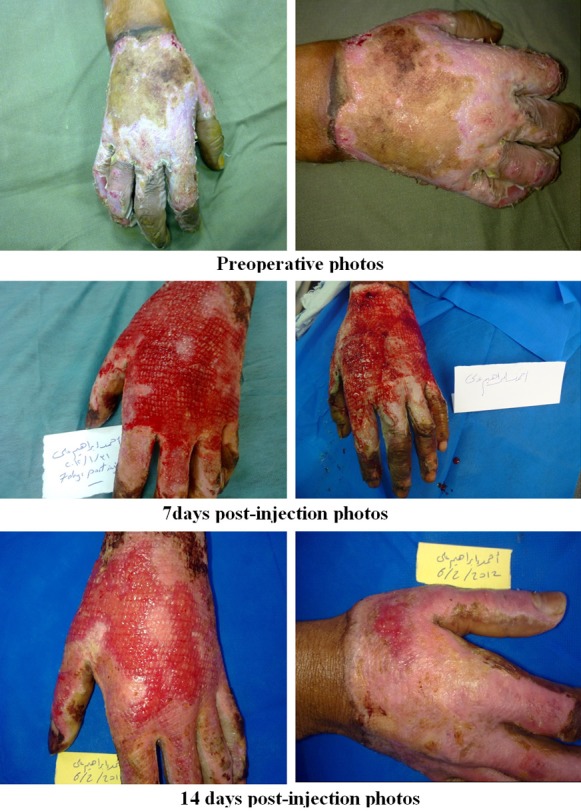
Photos of patients in the BM-MSC group.
Figure 6.
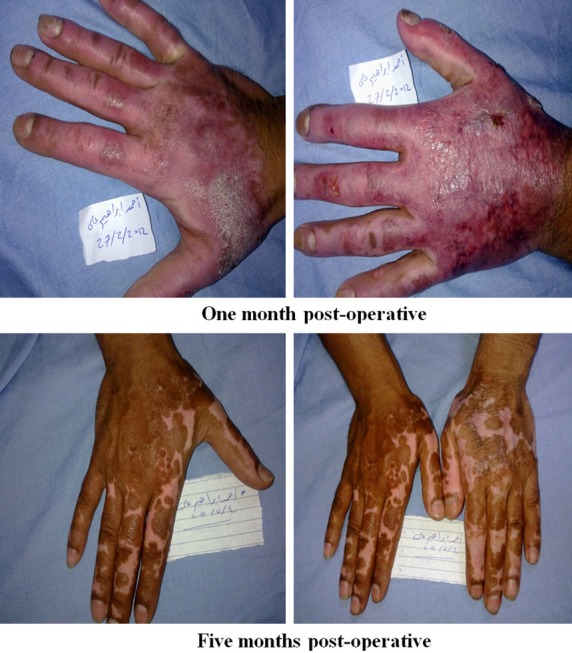
Photos of patients in the BM-MSC group.
Figure 7.
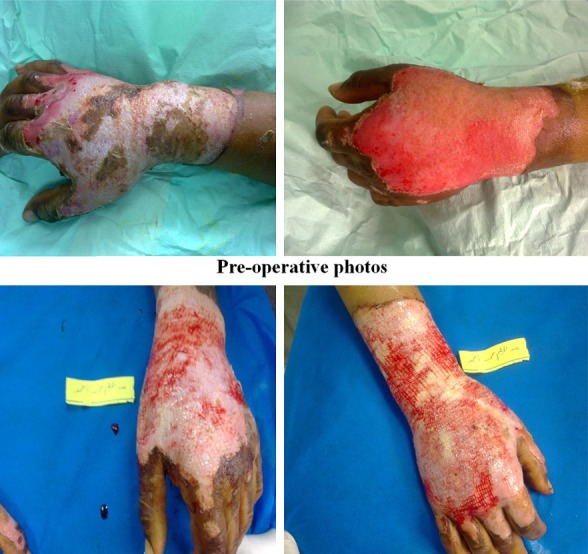
Photos of patients with bone marrow stem cells application.
Figure 8.
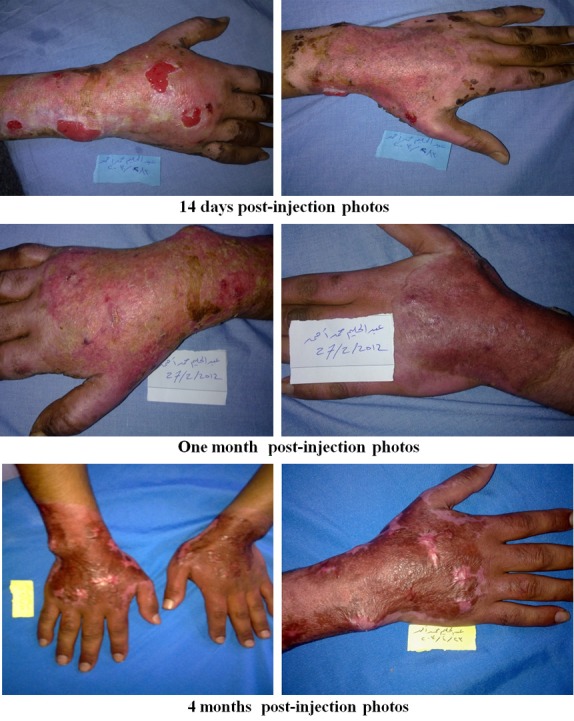
Photos of patients with bone marrow stem cells application.
Figure 9.
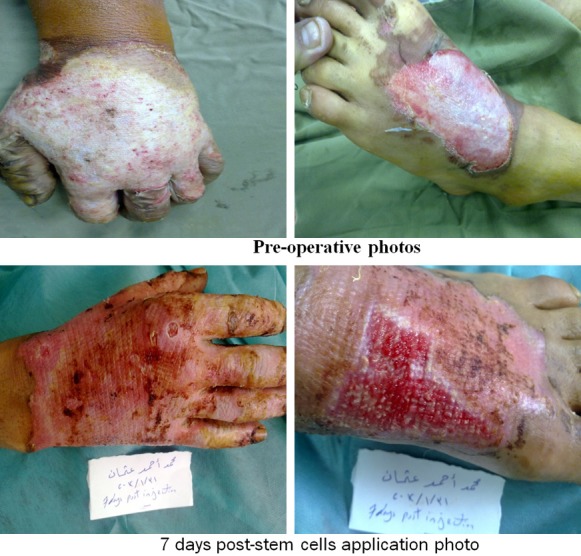
Photos of patients in the UCMSC group.
Figure 10.
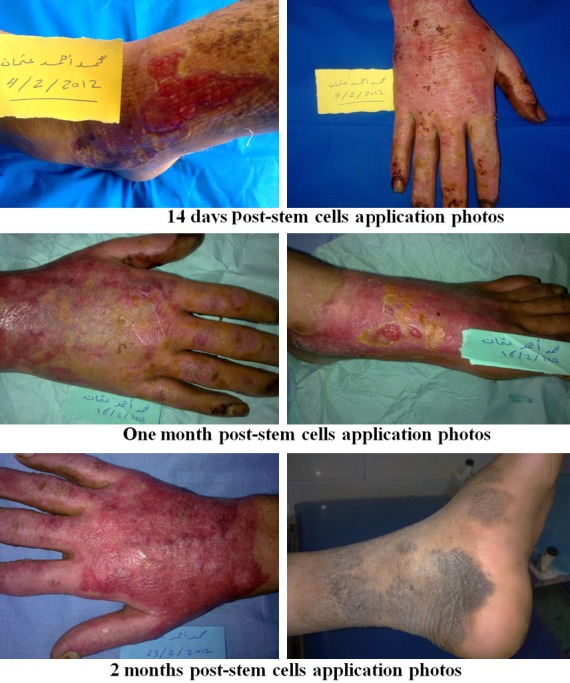
Photos of patients in the UC-MSC group.
Figure 11.
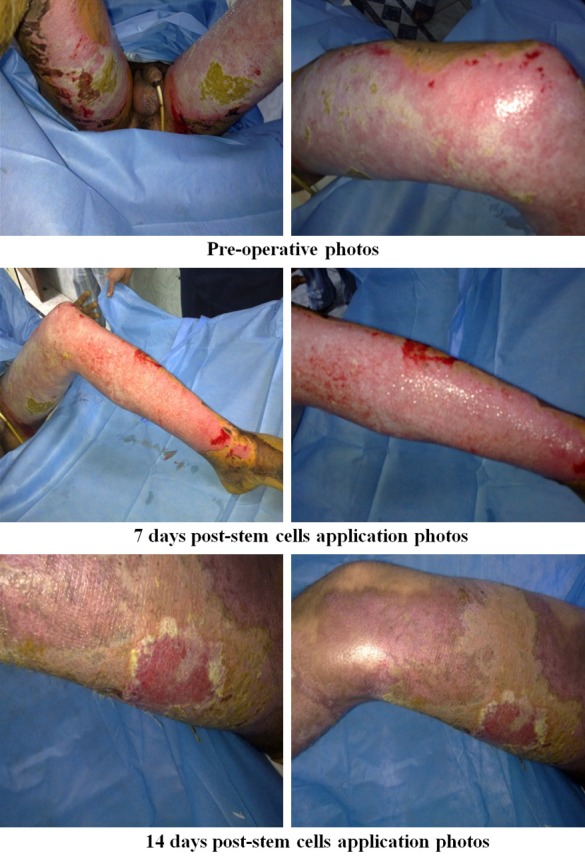
Photos of patients in the UCMSC group.
Late complications
Data represented in Table 6 showed the using of BMSCs in treatment of patients resulting no late complication 55% versus (5% and 30%) for the EE&G and UCMSCs respectively. While patients treated with UCMSCs showed hypopigmentation 10% versus (20% and 20%) for EE&G and BM-MSCs respectively. Patients treated with BMSCs exhibit no hyperpigmentation versus (20% and 30%) for the group EE&G and UCMSCs. Group treated with BM-MSCs and UCMSCs exhibits 10% of contractured scar when compared with EE&G group. However, lower values in groups treated with stem cells either BM-MSCs or UCMSCs (15% and 20%) versus 40% for EE&G group. These differences were statistically signedas shown in Table 6 and Figure 2.
Table 6.
Comparison of late complications in the different studied groups
| Early Excision and Graft group | Bone Marrow Stem Cells group | Umbilical Cord Blood Stem Cells group | Chi-square test | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. | % | No. | % | No. | % | X2 | P-value | |
| No complication | 1 | 5% | 11 | 55% | 6 | 30% | 17.819 | 0.022 |
| Hypopigmentation | 4 | 20% | 4 | 20% | 2 | 10% | ||
| Hyperpigmentation | 4 | 20% | 0 | 0% | 6 | 30% | ||
| Contractured scar | 3 | 15% | 2 | 10% | 2 | 10% | ||
| Hypertrophic scar | 8 | 40% | 3 | 15% | 4 | 20% | ||
Treatment expenses
The total cost of hospitalization of patients admitted in El Helmia Military Burn Center (admission and treatment) was estimated in Egyptian currency. The patients in excision and graft group costed maximum 20.500 thousand Egyptian pounds versus 20-21 thousand for the other groups. The cost also ranged between (11-20.5), (15-20) and (15-21) in the groups respectively as shown in Table 1 and Figure 1.
Discussion
Major burns represent a considerable health problem, with dual burden both on individuals and on the health system. Patients suffering from handicapping wounds require frequent operations, prolonged hospitalization and intense rehabilitation [15].
Standard management of major burn patients consists of autologous skin grafting followed by application of an elastic bandage. However, donor sites are limited especially in extensive deep burns Lu et al. [3]. Efficient wound healing requires full thickness regeneration of the skin and its appendages; a target which is rarely achieved in conventional management, and which the application of stem cells for burn wound healing can achieve.
The use of stem cells in burn management as an alternative to EE&G could be beneficial, not only to temporarily prevent the loss of body fluids and bacterial infection, but also to achieve satisfactory repairing effects [16,17]. Also, it was reported that stem cell application in the burn either by local spray with umbilical cord blood derived stem cells or by injection of bone marrow stem cells deep the burned area after excision were applied as a new method for regeneration of skin and decrease complication of skin graft or donor site [3,18].
This study recruited patients with recent deep burn of up to 25%; randomly divided into three groups: conventional treatment (early excision and grafting EE&G), bone marrow derived mesenchymal stem cells (BM-MSC) and umbilical cord derived mesenchymal stem cells (UCMSC). Patients and controls were followed-up to evaluate wound healing, incidence of early and late complications as well as duration and cost of hospitalization.
In this study, evaluation of the rate of healing indirectly through the duration of hospital stay showed significant decrease in both stem cell groups as compared to the control group; with no significant difference between the bone marrow and cord blood derived mesenchymal stem cell groups.
In the current study, 50% of the control group (EE&G) developed either early complications, such as infection and graft loss, or late complications, such as keloid, hypertrophic scars and limitation of movement. On the other hand, early complications occurred in less incidence, in both BM-MSC and UC-MSC groups as compared to the conventional EE&G.
The results encountered in the present study revealed that hypertrophic scar and limitation of movement were observed in early excision and graft patients, which served as a control group because it is the usual procedure in 10-25% TBSA burn.
Mohammadi et al. [19] studied the difference between early and late excision and graft in 50 patients suffering from deep thermal burn 30% TBSA. No statistically significant differences were found between both groups either in deformity, scar formation, sensation, development of disability as well as overall satisfaction. The most common site of involvement was the metacarpophalangeal (MCP) joint with frequency of 39% and 40% in early and late excision groups.
As regards duration of hospitalization, there was significant decrease in both bone marrow stem cells group and umbilical cord blood stem cells group when each group was compared to early excision and graft group. Non-significant changes were observed when comparing bone marrow stem cell group to umbilical cord blood stem cells group.
The explanation of the increase the time of hospitalization in the 1st group (ES&G) is the presence of early complication in a 50% of patients in this group as infection and partial loss of the graft.
Regarding the direct cost of hospitalization, non-significant changes were observed between the three groups regarding the direct cost of treatment. However, the overall cost of treatment may decrease as a result of decrease the time of hospitalization.
Zou et al. [20] found positive experience of using topical applications of non-processed autologous bone marrow aspirates on non-healing chronic wounds. Similarly, the applied autologous bone marrow aspirates to chronic unhealed burn wounds and slow-healing donor site wounds and there was a dramatic healing response observed in the present study.
Branski et al. [21] studied umbilical cord blood stem cells application by using a fibrin matrix after seeding stem cells, however, a Gentamycine cream was used in the present study with Vaslin guse which was effective against local bacterial infection.
Huang and Burd [22] suggested that stem cells can be used even in patients scheduled for autologous skin grafting to improve the vascularity of the wound bed before operation.
Yamaguchi et al. [23] explained that MSCs have a tremendous ability for differentiation into both hematopoietic cells including endothelial cells, osteoblasts, neural, fibroblasts and keratinocytes. MSCs transdifferentiated into endothelial progenitor cells (EPCS) play as important role in neovascularization and wound healing.
Also, Ravari et al. [24] studied the effect of bone marrow MSCs in both acute and chronic wounds including deep burns. They reported that the combined therapy using topical application and edge injection of the bone marrow aspirates together with application of culture-expanded BM-MSCs resulted in successful healing of the previously non-healing ulcers and in treating the radiation burns. Both local (topical or subcutaneous injection) and systemic applications have been used. With respect to the local application, the concomitant use of acellular matrices or scaffolds was shown to increase cell homing, differentiation, mobilization and adhesion [17,18]. In the present study, in the group of umbilical cord blood stem cells the used application of stem cells was applied either local injection or spray but without scaffold application.
Balaji et al. [25] explained stem cell-based therapy for wound healing in epithelium lined organs. Immediately after injury, there is a surge in the inflammatory response at the wound site to eliminate microbes and activate the healing process. MSCs interact with adjacent epithelial and inflammatory cells, initiating a combination of cellular and paracrine effects promoting efficient wound healing. Stem cells applied to wounds can contribute to healing by numersous effects, namely, direct transdifferentiation into the required cell types, secretion of growth factors and effector molecules integrating the regeneration processes, stimulation of proliferation of resident stem cells and lastly stimulation of angiogenesis needed for tissue regeneration.
In conclusion, the use of MSCs to enhance wound regeneration in deep burns represents a new modality offering great hope for this patient group.
Disclosure of conflict of interest
None.
References
- 1.Barret D, Rassef N, Franck Skin injury. Burn. 2002;vol. 1 ch. 3: pp. 29-42. [Google Scholar]
- 2.Hyun I. Magic eggs and the frontier of stem cell center. Hastings Cent Rep. 2006;36:16–19. doi: 10.1353/hcr.2006.0025. [DOI] [PubMed] [Google Scholar]
- 3.Lu W, Zhang YJ, Jin Y. Potential of stem cells for skin regeneration following burns. Expert Rev Dermatol. 2009;4:97–99. [Google Scholar]
- 4.Kastrinaki MC, Sidiropoulos P, Roche S, Ringe J, Lehmann S, Kritikos H, Vlahava VM, Delorme B, Eliopoulos GD, Jorgensen C, Charbord P, Häupl T, Boumpas DT, Papadaki HA. Functional, molecular and proteomic characterisation of bone marrow mesenchymal stem cells in rheumatoid arthritis. Ann Rheum Dis. 2008;67:741–9. doi: 10.1136/ard.2007.076174. [DOI] [PubMed] [Google Scholar]
- 5.Al-Nbaheen M, Vishnubalaji R, Ali D, Bouslimi A, Al-Jassir F, Megges M, Prigione A, Adjaye J, Kassem M, Aldahmash A. Human stromal (mesenchymal) stem cells from bone marrow adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential. Stem Cell Rev. 2003;9:32–43. doi: 10.1007/s12015-012-9365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–43. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 7.Korbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z. Hepatocytes and epithelial cells of donor origin in recipients of peripheral blood stem cells. N Engl J Med. 2002;346:738–46. doi: 10.1056/NEJMoa3461002. [DOI] [PubMed] [Google Scholar]
- 8.Kuehnle I, Goodell MA. The therapeutic potential of stem cells from adults. BMJ. 2002;325:372–376. doi: 10.1136/bmj.325.7360.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forraz N, McGuckin CP. The umbilical cord: a rich and ethical stem cell source to advance regenerative medicine. Cell Prolif. 2011;44:60–69. doi: 10.1111/j.1365-2184.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev. 2010;19:491–502. doi: 10.1089/scd.2009.0192. [DOI] [PubMed] [Google Scholar]
- 11.Cyranoski D. Simple switch turns cells embryonic. Nature. 2007;447:540–543. doi: 10.1038/447618a. [DOI] [PubMed] [Google Scholar]
- 12.Bannasch H, Unterberg T, Föhn M, Weyand B, Horch RE, Stark GB. Burns, cultured keratinocytes in fibrin with decellu larised dermis close porcine full-thickness wounds in a single step. Burns. 2008;34:1015–1021. doi: 10.1016/j.burns.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2006;8:315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 15.Chaby G, Senet P, Vaneau M, Martel P, Guillaume J, Meaume S, Teot L, Deure C, Dompmartin A, Bachelet H, Carsin H, Matz V, Richard J, Rochet M, Sales-Aussias N, Zagnoli A, Denis C, Guilloet B, Chosidow O. Dressing for acute and chronic wounds. Arch Dermatol. 2007;143:1297–1304. doi: 10.1001/archderm.143.10.1297. [DOI] [PubMed] [Google Scholar]
- 16.Rasulov MF, Vasilchenkov AV, Onishchenko NA, Krasheninnikov ME, Kravchenko VI, Gorshenin TL, Pidtsan RE, Potapov IV. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med. 2005;139:141–4. doi: 10.1007/s10517-005-0232-3. [DOI] [PubMed] [Google Scholar]
- 17.Ghieh F, Jurjus R, Ibrahim A, Geagea A, Daouk H, El-Baba B, Chams S, Matar M, Zein W, Jurjus A. The use of stem cells in burn wound healing: a review. Biomed Res Int. 2015;2015:684084. doi: 10.1155/2015/684084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arno A, Smith AH, Blit PH, Al Shehab M, Gauglitz GG, Jeschke MG. Stem cell therapy: a new treatment for Burns. Pharmaceuticals. 2011;4:1355–1380. doi: 10.3390/ph4101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammadi AA, Bakhshaeekia AR, Marzban S, Abbasi S, Ashraf AR, Mohammadi MK, Toulide-Ie HR, Tavakkolian AR. Early excision and skin grafting versus delayed skin grafting in deep hand burns (a randomised clinical controlled trial) Burns. 2011;37:36–41. doi: 10.1016/j.burns.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Zou Z, Zhang Y, Hao L, Wang F, Liu D, Su Y. More insight into mesenchymal stem cells and their effects inside the body. Expert Opin Biol Ther. 2010;10:215–230. doi: 10.1517/14712590903456011. [DOI] [PubMed] [Google Scholar]
- 21.Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35:171–180. doi: 10.1016/j.burns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Burd A. An update review of stem cell applications in burns and sound care. Indian J Plast Surg. 2012;45:229–236. doi: 10.4103/0970-0358.101285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamaguchi Y, Yoshida S, Sumikawa Y, Kubo T, Hosokawa K, Ozawa K, Hearing VJ, Yoshikawa K, Itami S. Rapid healing of intractable diabetic foot ulcers with exposed bones following a novel therapy of exposing bone marrow cells and then grafting epidermal sheets. Br J Dermatol. 2004;151:1019–28. doi: 10.1111/j.1365-2133.2004.06170.x. [DOI] [PubMed] [Google Scholar]
- 24.Ravari H, Hamidi-Almadari D, Salimifar M, Bonakdaran S, Parizadeh MR, Koliakos G. Treatment of non-healing wounds with autologous bone marrow cells, platelets, fibrin glue and collagen matrix. Cytotherapy. 2011;13:705–711. doi: 10.3109/14653249.2011.553594. [DOI] [PubMed] [Google Scholar]
- 25.Swathi B, Keswani SG, Crombleholme TM. Stem cell-based therapy for wound healing in epithelium lined organs. 2012;1:159–165. [Google Scholar]


