This study reports a method for removing photoreceptors from rodent whole mount retina while preserving the architecture of the inner retina. The method enables easier access to the inner retina for studies of neural processing, such as by patch clamp recording.
Keywords: retina whole mount, photoreceptor removal, patch clamp, bipolar cell
Abstract
Patch clamp recordings of neurons in the inner nuclear layer of the retina are difficult to conduct in a whole mount retina preparation because surrounding neurons block the path of the patch pipette. Vertical slice preparations or dissociated retinal cells provide access to bipolar cells at the cost of severing the lateral connection between neurons. We have developed a technique to remove photoreceptors from the rodent retina that exposes inner nuclear layer neurons, allowing access for patch clamp recording. Repeated application to and removal of filter paper from the photoreceptor side of an isolated retina effectively and efficiently removes photoreceptor cells and, in degenerate retina, hypertrophied Müller cell end feet. Live-dead assays applied to neurons remaining after photoreceptor removal demonstrated mostly viable cells. Patch clamp recordings from bipolar cells reveal responses similar to those recorded in traditional slice and dissociated cell preparations. An advantage of the photoreceptor peel technique is that it exposes inner retinal neurons in a whole mount retina preparation for investigation of signal processing. A disadvantage is that photoreceptor removal alters input to remaining retinal neurons. The technique may be useful for investigations of extracellular electrical stimulation, photoreceptor DNA analysis, and nonpharmacological removal of light input.
NEW & NOTEWORTHY This study reports a method for removing photoreceptors from rodent whole mount retina while preserving the architecture of the inner retina. The method enables easier access to the inner retina for studies of neural processing, such as by patch clamp recording.
patch clamp recordings of rodent retinal bipolar cell somas from in vitro whole mount retina preparations are difficult to obtain because bipolar cells are located between several layers of retinal neurons. In the normal mouse retina, a pipette approaching from the photoreceptor side would have to pass through 23-µm-long (approximate) densely packed outer segments, 10–14 layers of 4- to 7-µm-diameter photoreceptor somas, and the outer plexiform layer before reaching bipolar cell somas (Barhoum et al. 2008; Carter-Dawson and LaVail 1979). From the ganglion cell side, the pipette would have to traverse the inner limiting membrane, the ganglion cell layer, and a 60-µm (approximate) thick inner plexiform layer (IPL) (Fisher 1979). Each approach is technically difficult because debris may impair visualization of the neurons, and the pipette tip may become soiled, preventing the giga seal required for electrical recording. Assuming that a successful giga seal is obtained on a neuron in the inner nuclear layer (INL), there is a possibility that the neuron will be a horizontal cell, amacrine cell, or Müller cell instead of a bipolar cell, further reducing the efficiency of the intended experiment.
Vertical slice or retinal dissociation techniques are commonly used for patch clamp recording from inner retinal neurons. The vertical slice preparation provides ready access to retinal neurons and is useful for interrogating the vertical phototransduction pathway through the retina (Boos et al. 1993; Edwards et al. 1989; Van Hook and Thoreson 2013). However, the lateral processes of horizontal and amacrine cells are severed. Dissociated cell preparations are prepared by enzymatic digestion followed by mechanical trituration of the whole retina to prepare a primary culture (Grozdanov et al. 2010; Sarthy and Lam 1979; Yamashita and Wässle 1991;). This technique facilitates single cell recordings from any neuron, but the neuron is completely disconnected from the retinal networkk and exhibits altered ion channel functionality due to enzymatic digestion (Klumpp et al. 1995).
Although the slice and dissociated cell preparations have demonstrated great utility in retinal research, some studies would benefit from bipolar cell recordings conducted in whole mount retinas. Retinal prosthesis studies examining the response of INL neurons to electrical stimulation will benefit from preparation conditions that better mimic the operational conditions of the prosthesis. Prior studies of bipolar cell responses have relied on the vertical slice preparation for bipolar cell access, although each study notes that the slice preparation may not be the ideal model because there is significant current shunting around the retina, and the laterally projecting neurites have been severed (Cameron et al. 2013; Margalit and Thoreson 2006; Margalit et al. 2011).
To overcome the difficulty of obtaining patch clamp recordings from INL neurons in whole mount retina, we have developed a method to mechanically remove photoreceptors from the retina by repeated application and peeling away of filter paper adhering to tissue on the photoreceptor side of an isolated retina. The photoreceptor peel technique described exposes the INL for single-cell recording while maintaining cell viability. A subset of these results has been reported previously (Walston et al. 2015).
METHODS
Transgenic normal Tg(Gng13-EGFP)GI206Gsat (PD > 60) and rd10 Tg(Gng13-EGFP)GI206Gsat (PD > 300) mice with a C57BL/6J background were used (Gong et al. 2003; Huang et al. 2003). These transgenic mice express enhanced green fluorescent protein (EGFP) in ON-type bipolar cells. Normal mice were gifts from A. Sampath (University of Southern California, Los Angeles, CA). Normal mice were crossed with rd10 mice to produce rd10 mice containing enhanced green fluorescent protein (EGFP). Mice were anaesthetized with ketamine-xylazine (80 mg/kg, 5 mg/kg), followed by cervical dislocation. Eyes were enucleated and placed on moist filter paper. A small slit was made across the cornea with a razor blade. The eye was then placed into 30°C Ames’ media (no. A1420; Sigma-Aldrich, St. Louis, MO) buffered with sodium bicarbonate and penicillin-streptomycin (no. P4333; Sigma-Aldrich) (280 mOsm, pH 7.3). The cornea was excised above the ora serrata, followed by removal of the lens. The vitreous was removed with forceps, and four radial relief cuts were made around the eyecup. The retina was severed from the optic nerve and removed from the eyecup. A quarter of the retina was isolated such that it contained the optic disk. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Southern California.
Photoreceptor peel technique.
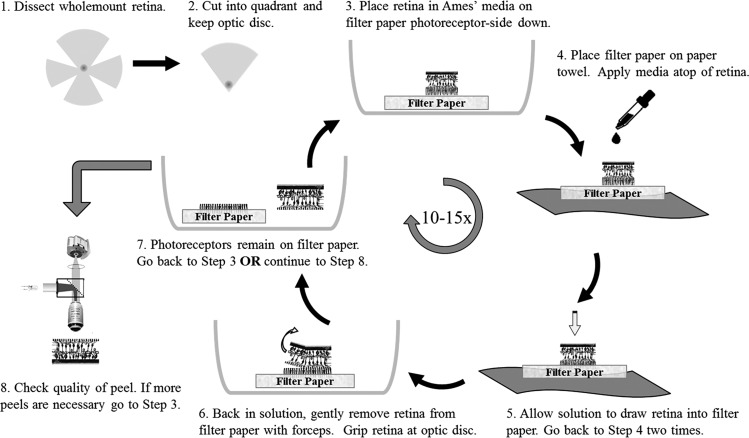
To remove photoreceptor outer segments, inner segments, and somas, the photoreceptor peel technique was performed as depicted in Fig. 1. The quarter of retina was placed photoreceptor-side down on a piece of VWR 415 Grade Qualitative Filter Paper (no. 28320; VWR International, Radnor, PA) bathed in oxygenated solution. The size of the filter paper piece was 5 × 5 mm (∼3 times larger than the quarter of retina). The coarse filter paper was made of pure cellulose with 25-µm particle retention. The filter paper-containing retina was removed from the solution and placed filter paper-side down on a paper towel. Drops of solution were applied atop the retina with a transfer pipette. The solution was allowed to move through the retina and filter paper. Care was taken to keep the retina hydrated at all times. Additionally, the retina was outside of the oxygenated solution for ≤10 s at any time. The retina was placed back into the medium and gently removed from the filter paper, using forceps to lift up the retina by grasping at the optic disk. When necessary, the sides of the retina were also lifted with forceps. This process was repeated ∼10–15 times to remove the majority of photoreceptor outer segments, inner segments, and somas. The photoreceptor peel technique was performed only one or two times on the remaining outer nuclear layer of the rd10 retina, leaving out step 5 and medium application in step 4 shown in Fig. 1. Only a quarter of the retina was used because it was easier to lay flat and because grasping at the optic disk (at the corner of the quarter) reduced retinal tears. The VWR 415 filter paper was used because its surface roughness was sufficiently coarse to remove layers of neurons yet fine enough to avoid tearing the retina in its entirety during the peeling process, but filter paper properties were not investigated systematically.
Fig. 1.
Flow diagram for the photoreceptor peel technique.
Fluorescence imaging was used to determine the thoroughness of the photoreceptor peel process. The retina was mounted ganglion cell-side down on a glass bottom petri dish, held in place with a PTFE membrane (JVWP01300; Millipore) weighted by a titanium ring, and viewed with an upright microscope. Photoreceptor removal was judged adequate by the clarity of EGFP fluorescence arising from bipolar cells and by the presence of three or fewer layers of photoreceptor somas. The retina was removed from the microscope stage, and the peeling process was resumed if necessary.
Live-dead assay.
We assessed retinal health 45 min after performing the photoreceptor peel technique using a live-dead assay. The free-floating retina was incubated in Ames’ media supplemented with 4 µM calcein-blue AM (no. C1429; Invitrogen, Grand Island, NY) and 1 µM ethidium homodimer-1 (no. E1169; Invitrogen) for the live and dead assay, respectively. The solution was continuously oxygenated and maintained at 30°C for 25 min. After incubation, the retina was mounted ganglion cell side-down on a glass bottom petri dish and perfused in Ames’ media for 30 min while being gently held down with the weighted membrane. Fluorescence imaging was performed at multiple regions across the retina under ×60 magnification. Images were manually postprocessed in ImageJ with the Cell Counter plugin to quantify the number of cells labeled with EGFP, calcein-blue, and ethidium homodimer-1 and to determine fluorescence colocalization.
Imaging of vertical slices.
Retinas with and without photoreceptors removed were prepared for imaging in vertical slices. After dissections, retinas were fixed in 4% paraformaldehyde for 2 h. They were incubated in 0.01 M phosphate-buffered saline thre times for 5 min on a shaker. The retinas were mounted flat in blocks of 4% agarose. Each agarose block was prepared on a vibratome and sectioned at 50-µm increments to obtain vertical retina slices. Retinal slices were mounted on microscope slides, covered with an ethanol-based mounting solution supplemented with 1 µM ethidium homodimer-1, covered with glass coverslips, and imaged.
Patch clamp electrophysiology.
After the photoreceptor peel technique was performed, the retina was mounted ganglion cell-side down on a glass bottom petri dish. The retina was held in place with a PTFE membrane containing manually perforated 1-mm-diameter access holes and weighted by a titanium horseshoe. The retina was perfused with buffered Ames’ media at 30°C at a flow rate of 4–6 ml/min. A silver-silver/chloride pellet (no. 64-1305; Warner Instruments, Hamden, CT) reference electrode was placed in solution. Borosilicate glass (outer diameter = 1.2 mm, inner diameter = 1.0 mm, no. B120-69-10; Sutter Instruments, Novato, CA) was pulled to obtain resistances of 7–14 MΩ (when pipette was filled with internal solution described below) using a laser pipette puller (NO. P-2000; Sutter Instruments). Pipettes were filled with internal solution containing the following components (in mM): 120 K-gluconate, 10 NaCl, 3 ATP-Mg, 0.3 GTP-Na, 10 HEPES, 0.5 EGTA, 10 phosphocreatine disodium hydrate adjusted to pH = 7.3 with 1M KOH (Sigma), and an osmolality of 270 mOsm. K-gluconate was substituted for 140 mM Cs-gluconate, and 20 mM tetraethylammonium (TEA) was added to the external solution for tests blocking potassium channels. The junction potential of 14 mV and 15.2 mV was corrected in control and blocker solutions, respectively (Neher 1992). An EPC9 amplifier (HEKA, Bellmore, NY) under control of PatchMaster software was used for data acquisition. Input resistance from recorded neurons averaged 2.78 ± 1.31 GΩ, and membrane capacitance averaged 4.59 ± 1.55 pF (means ± SD).
Whole cell voltage-clamp recordings were collected from ON-type bipolar cell somas. Cells were held at −80 mV and stepped in increments of 10 mV from −80 to 60 mV. Each step was held for 100 ms. Current-voltage (I–V) curves were obtained from the sustained current in response to each voltage step. In the current clamp, the holding current was 0 pA, and current injections of −10 to 20 pA in steps of 2 pA were delivered through the pipette for 6 or 8 s. Membrane potentials were estimated from the median membrane potential, and oscillation amplitudes were estimated from the standard deviation of the membrane potential during the current step.
RESULTS
Photoreceptor removal.
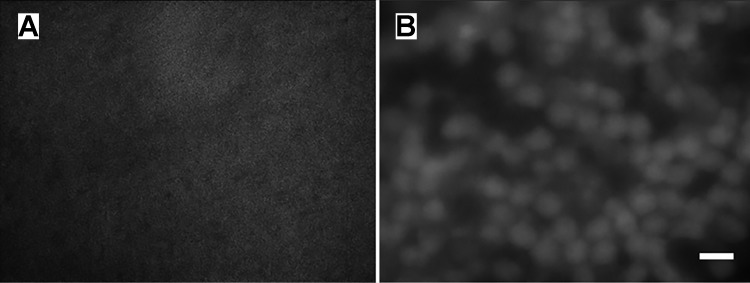
The photoreceptor peel technique effectively removes photoreceptor outer segments, inner segments, and somas from normal retina. EGFP fluorescence identifies ON-type bipolar cells in the INL, but the fluorescence is scattered by photoreceptors when imaging from the subretinal side (Fig. 2A). After photoreceptors are removed, the contours of bipolar cells are readily visualized (Fig. 2B). The majority of photoreceptor outer segments were removed from the retina after the first few peels; however, 10–15 cycles of the process were necessary to provide adequate bipolar cell visualization and access. Remaining photoreceptor soma layers ranged from 0 to 3, depending on the location. In the rd10 retina, the objective of the photoreceptor peel process is to loosen the hypertrophied glial seal covering the INL. Only one or two gentle peels were necessary. In rd10 retina, the fluorescence from the ON-type bipolar cells is visible without the photoreceptor peel technique, but we found that successful “giga seal” recordings were easier to obtain due to less soiling of the patch pipette tip from the glial cell contact.
Fig. 2.
Photoreceptor peeling allows visualization of bipolar cells.
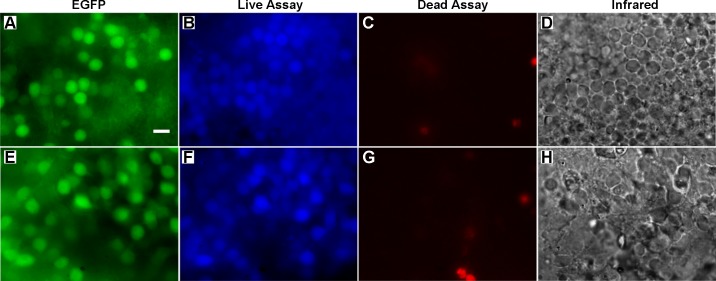
After mechanically removing photoreceptors in three retina preparations, we performed a live-dead assay to assess the health of bipolar cells. Calcein blue-AM and ethidium homodimer-1 served as the live and dead assay, respectively. Figure 3 illustrates representative images of how the assays labeled the retina. The ON-type bipolar cells are labeled with EGFP (Fig. 3A). Figure 3D illustrates the cell contours in the INL. The live assay stains cells in the INL, including the ON-type bipolar cells (Fig. 3B). The labeling of the live assay appears to be limited to the outer surface of the retina, which restricts our evaluation to the most exposed neurons. From 34 image regions, we identified 886 ON-type bipolar cells, 691 (77.9%) of which were colabeled by the live assay. In the same image regions, 151 cells were labeled by the dead assay in total. Although these cells were labeled by the dead assay, none were colocalized with the EGFP-labeled bipolar cells (Fig. 3C). This result demonstrates that the majority of labeled ON-type bipolar cells have intact cell membranes, suggesting healthy cells. The same live-dead assay procedure was applied to two rd10 retina preparations after the application of one or two peels with the filter paper. From 23 image regions, we identified 868 ON-type bipolar cells (Fig. 3E), 679 (78.2%) of which were labeled with the live assay (Fig. 3F). The contours of cell somas are illustrated in the infrared image (Fig. 3H). One-hundred three cells were labeled with the dead assay (Fig. 3G), but none were colocalized with the EGFP-labeled bipolar cells; 0.3% of cells labeled by the live assay were also labeled by the dead assay. The live-dead assay was also performed on 4 rd10 retinas without the peel technique being applied to assess potential effects of the peel technique. The staining performance of the live assay was heterogeneous and less robust, which may be due to the glial seal. In 11 image areas with discernible labeling, 236 (73%) of EGFP-labeled bipolar cells were colocalized with the live assay. None were colocalized with the dead assay.
Fig. 3.
Live-dead assay of the retina after the photoreceptor peel technique was performed.
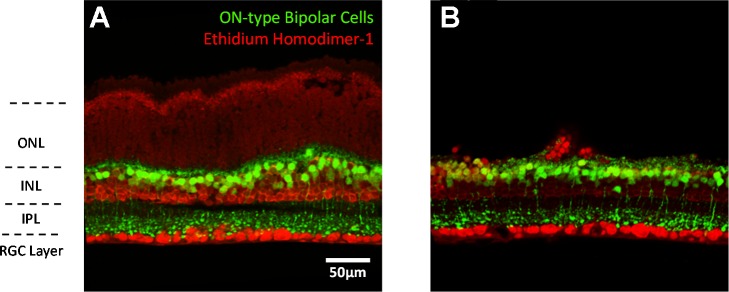
Vibratome sectioning of the retina reveals that the retina is still morphologically intact. The normal retina without photoreceptor peeling contains 10–14 layers of photoreceptors somas above the INL (Fig. 4A). ON-type bipolar cells are identified in the INL by EGFP fluorescence. Neurons not labeled with EGFP in the INL are OFF-type bipolar cells, horizontal cells, amacrine cells, and Müller cells. ON-type bipolar cell terminals are also observed in the lower strata of the IPL. Ganglion cells and displaced amacrine cells in the ganglion cell layer are also labeled by ethidium homodimer-1. Figure 4B depicts the retina after the photoreceptor peel technique has been performed. The majority of photoreceptor somas in the outer nuclear layer have been removed. The number of somas removed is determined largely by the number of peel cycles. The remaining inner retina appears intact, with a similar structural morphology to the control retina.
Fig. 4.
Vertical retina slice view of the photoreceptor peel technique. ONL, outer nuclear layer INL, inner nuclear layer; IPL, inner plexiform layer; RGC, retinal ganglion cell.
Electrophysiological measurements.
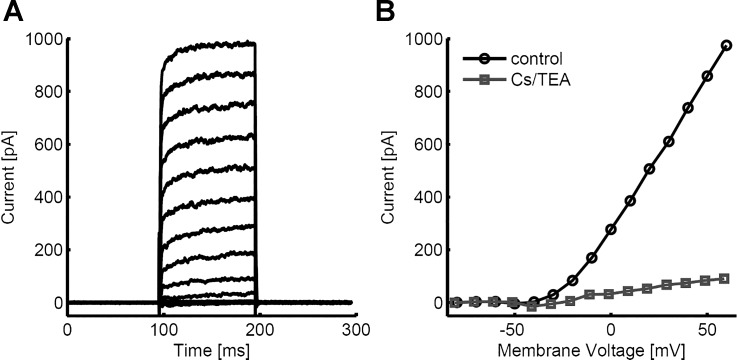
Whole cell patch clamp recordings were performed on ON-type bipolar cells in wholemount mouse retina after photoreceptor removal. The transmembrane current was recorded in response to voltage steps from a holding potential of −80 mV (Fig. 5A). Membrane voltage was stepped from −80 to +60 mV in steps of 10 mV. Outward currents are observed above approximately −40 mV and increase above −10 mV. Steady-state current responses are plotted as a function of step potential in Fig. 5B. Recordings with 140 mM Cs-gluconate internal and 20 mM TEA in the external solution significantly reduced the outward current,s indicating that they are largely dependent on potassium channels (Fig. 5B). Although reduced, the potassium current at steady state increases approximately linearly with membrane potential for step potentials greater than approximately −10 mV. The addition of potassium channel blockers also revealed small inward currents when voltage was stepped to −30 and −40 mV.
Fig. 5.
Current-voltage (I–V) curves recorded from ON-type bipolar cells in normal retina.
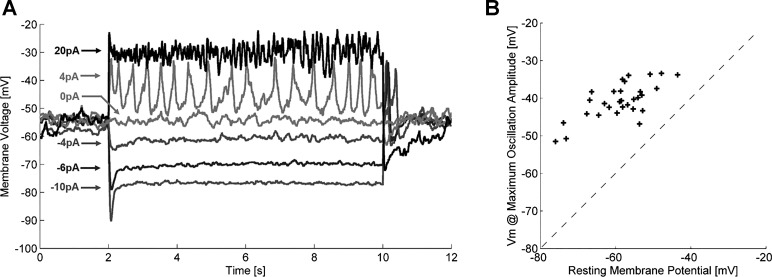
In the current clamp, we injected current into ON-type bipolar cells (n = 30) in the normal retina and measured the voltage response. Injections ranged from −10 to 20 pA in steps of 2 pA. Negative current steps resulted in hyperpolarization of the membrane potential and reduction in the amplitude of membrane oscillations (Fig. 6A). Positive current steps elevated the membrane potential, which resulted in fast depolarizing transients. Figure 6B shows the relation between resting membrane potential and the membrane potential at which oscillations were maximal for 30 bipolar cells. The average membrane potential at which the maximum oscillation amplitude occurred was −40.6 ± 4.7 mV (means ± SD). The average resting membrane potential of these cells was −58.8 ± 7.6 mV.
Fig. 6.
Bipolar cell response to current injections in current clamp in normal retina.
DISCUSSION
Mechanically peeling photoreceptors from whole mount retina using filter paper is an effective technique to expose neurons in the INL. The removal of retina layers is relatively uniform across the treated area (Fig. 4), the retina is kept in viable physiological condition, and the progress of removing photoreceptors can be monitored. Live-dead assays indicate that the majority of remaining bipolar cells are still viable after the procedure (Fig. 3). In our investigation, we used a transgenic mouse line expressing EGFP in ON-type bipolar cells to provide a priori discrimination of these neurons. This strategy can be adapted with other transgenic lines to allow visualization of other retinal cells. Adjusting the number of times the filter paper is applied and removed provides some control over the number of photoreceptor somas removed from the retina. This feature is beneficial, as it allows one to efficiently adjust which layer(s) of neurons will be removed and exposed. For example, in this study we determined that only a couple of peels were necessary to carefully remove the glial seal covering the INL of the rd10 degenerate retina in contrast to the 10–15 required to remove the majority of photoreceptor outer segments, inner segments, and somas in the normal retina. This investigation did not test the ability of filter paper to remove the inner limiting membrane.
Patch clamp electrophysiology with this preparation provided baseline bipolar cell measurements similar to those obtained in traditional slice and dissociated cell preparations (Frech and Backus 2004; Klumpp et al. 1995; Zenisek and Matthews 1998). I–V curves reveal strong outward rectifying potassium currents, initiating at membrane potentials more positive than −40 mV. Although complete blockade was not observed, the K+ channel blockers cesium and TEA significantly reduced this outward current and revealed small inward currents that are consistent with previous reports of calcium channels in bipolar cell terminals (de la Villa et al. 1998). Furthermore, application of current steps into bipolar cells revealed that the bipolar cell membrane potentials transition into and out of oscillatory behavior, peaking at approximately −40 mV. The magnitude of the responses showed dependence on membrane potential and appears to be in agreement with previously reported bipolar cell calcium spikes (Baden et al. 2011; Palmer 2006; Protti et al. 2000; Zenisek and Matthews 1998). The correspondence between the various techniques is encouraging because each preparation approach is different. However, the advantage of this preparation is that it exposes INL neurons in a whole mount retina, which may preserve certain aspects of signal processing.
Adjusting the number of peel steps makes the photoreceptor removal technique adaptable to different types of experiments. Proteomics or DNA analyses can be performed on either individual retinal layers that are removed or on the retinal tissue remaining after the peel technique is performed, and because no chemicals or enzymes are used during the processing, contamination by chemicals and enzymes is minimal. Individual bipolar cell subclass signaling pathways can be examined without contamination from broad photoreceptor input to the entire retina. Activity could be elicited, for example, by targeting optogenetic channels to single bipolar cell subtypes and then measuring the response characteristics of postsynaptic ganglion cells or amacrine cells. A few peel steps could be used to remove light input into photoreceptors without the use of pharmacological blockers. The technique is also useful for recording INL responses to electrical stimulation from microelectrode arrays. A large portion of the retina whole mount would remain intact and cover the stimulating electrode. This provides a better model of a human implant because electric current paths will be directed through the retina rather than shunting around the tissue as in the retinal slice or dissociated cell preparations.
Removing retinal layers in rodent whole mount retina has also been reported using other methodologies (Enoki et al. 2006; Feigenspan and Babai 2015; Hayashida et al. 2004; Shiosaka et al. 1984). One method uses a mounting medium to hold the retina before slicing through the retina horizontally with a fine blade (Enoki et al. 2006; Feigenspan and Babai 2015). One drawbacks to this preparation is that it is difficult to align the retina relative to the blade, which is necessary for precise removal of a specific layer. Care must also be taken to prevent exposing the retina to high temperatures when covering it in mounting medium and to maintain a healthy retinal environment throughout the entire process. Manual horizontal slicing with a blade has also been described atop filter paper without mounting media (Hayashida et al. 2004). Another method uses enzymatic trypsin digestion and two sets of filter paper attached on both retinal surfaces to remove retinal layers (Shiosaka et al. 1984). Cell exposure to digestion enzymes loosens the extracellular matrix holding neurons in place, resulting in as few as five applications of filter paper to remove the entire outer nuclear layer (ONL) and inner nuclear layer (INL) from the retina compared with the 10–15 required to remove the ONL without enzymatic digestion in our preparation. However, enzymes must be used with caution because their effect on ion channels may fundamentally change neural activity (Holt et al. 2001; Klumpp et al. 1995).
No chemicals or digestion enzymes are used during our photoreceptor peel technique. It does not require the retina to be submerged in a mounting medium, nor does it require the alignment of a fine blade to cut the retina. The limitation of the photoreceptor peel technique is that it severs the connection between photoreceptors and INL neurons. As such, it is not possible to use this technique and record light-activated responses from the remaining retina. Additionally, the removal of photoreceptors will alter the input to bipolar cells and horizontal cells, which could affect baseline retinal activity. In our study, we did not directly assess the integrity of lateral connections in the INL. Maintenance of such connections is a potential benefit of this approach compared with retinal slice, but must be proven through further experimentation.
In conclusion, we have developed and assessed a method for improving the access to bipolar cells of both normal and degenerate mammalian whole mount retina for patch clamp recording. Photoreceptor and glial membranes are removed by repeated application and peeling of commercially available filter paper to the retina. Cell viability and electrophysiological measures suggest that the bipolar cells are viable after this procedure.
GRANTS
This research has been supported by the National Eye Institute (Grants RO1-EY-022931 and EY-022931-S1), the National Science Foundation (Grant No. CBET 1343193), the University of Southern California Institute for Biomedical Therapeutics, and a departmental grant from Research to Prevent Blindness.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.T.W. and Y.-C.C. performed experiments; S.T.W. analyzed data; S.T.W., J.D.W., and R.H.C. interpreted results of experiments; S.T.W. prepared figures; S.T.W. drafted manuscript; S.T.W., J.D.W., and R.H.C. edited and revised manuscript; S.T.W., Y.-C.C., J.D.W., and R.H.C. approved final version of manuscript.
REFERENCES
- Baden T, Esposti F, Nikolaev A, Lagnado L. Spikes in retinal bipolar cells phase-lock to visual stimuli with millisecond precision. Curr Biol 21: 1859–1869, 2011. doi: 10.1016/j.cub.2011.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhoum R, Martínez-Navarrete G, Corrochano S, Germain F, Fernandez-Sanchez L, de la Rosa EJ, de la Villa P, Cuenca N. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience 155: 698–713, 2008. doi: 10.1016/j.neuroscience.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Boos R, Schneider H, Wässle H. Voltage- and transmitter-gated currents of all-amacrine cells in a slice preparation of the rat retina. J Neurosci 13: 2874–2888, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron MA, Suaning GJ, Lovell NH, Morley JW. Electrical stimulation of inner retinal neurons in wild-type and retinally degenerate (rd/rd) mice. PLoS One 8: e68882, 2013. doi: 10.1371/journal.pone.0068882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol 188: 263–272, 1979. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- de la Villa P, Vaquero CF, Kaneko A. Two types of calcium currents of the mouse bipolar cells recorded in the retinal slice preparation. Eur J Neurosci 10: 317–323, 1998. doi: 10.1046/j.1460-9568.1998.00051.x. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch 414: 600–612, 1989. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Enoki R, Jakobs TC, Koizumi A. Horizontal slice preparation of the retina. J Vis Exp 20: 108, 2006. doi: 10.3791/108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenspan A, Babai N. Functional properties of spontaneous excitatory currents and encoding of light/dark transitions in horizontal cells of the mouse retina. Eur J Neurosci 42: 2615–2632, 2015. doi: 10.1111/ejn.13016. [DOI] [PubMed] [Google Scholar]
- Fisher LJ. Development of synaptic arrays in the inner plexiform layer of neonatal mouse retina. J Comp Neurol 187: 359–372, 1979. doi: 10.1002/cne.901870207. [DOI] [PubMed] [Google Scholar]
- Frech MJ, Backus KH. Characterization of inhibitory postsynaptic currents in rod bipolar cells of the mouse retina. Vis Neurosci 21: 645–652, 2004. doi: 10.1017/S0952523804214134. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425: 917–925, 2003. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Grozdanov V, Müller A, Sengottuvel V, Leibinger M, Fischer D. A method for preparing primary retinal cell cultures for evaluating the neuroprotective and neuritogenic effect of factors on axotomized mature CNS neurons. Curr Protoc Neurosci Chapter 3: 22, 2010. doi: 10.1002/0471142301.ns0322s53. [DOI] [PubMed] [Google Scholar]
- Hayashida Y, Partida GJ, Ishida AT. Dissociation of retinal ganglion cells without enzymes. J Neurosci Methods 137: 25–35, 2004. doi: 10.1016/j.jneumeth.2004.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Lioudyno M, Athas G, Garcia MM, Perin P, Guth PS. The effect of proteolytic enzymes on the alpha9-nicotinic receptor-mediated response in isolated frog vestibular hair cells. Hear Res 152: 25–42, 2001. doi: 10.1016/S0378-5955(00)00225-2. [DOI] [PubMed] [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit Gɣ13 is coexpressed with Gαo, Gβ3, and Gβ4 in retinal ON bipolar cells. J Comp Neurol 455: 1–10, 2003. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Klumpp DJ, Song EJ, Ito S, Sheng MH, Jan LY, Pinto LH. The Shaker-like potassium channels of the mouse rod bipolar cell and their contributions to the membrane current. J Neurosci 15: 5004–5013, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit E, Babai N, Luo J, Thoreson WB. Inner and outer retinal mechanisms engaged by epiretinal stimulation in normal and rd mice. Vis Neurosci 28: 145–154, 2011. doi: 10.1017/S0952523810000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit E, Thoreson WB. Inner retinal mechanisms engaged by retinal electrical stimulation. Invest Ophthalmol Vis Sci 47: 2606–2612, 2006. doi: 10.1167/iovs.05-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods Enzymol 207: 123–131, 1992. doi: 10.1016/0076-6879(92)07008-C. [DOI] [PubMed] [Google Scholar]
- Palmer MJ. Modulation of Ca(2+)-activated K+ currents and Ca(2+)-dependent action potentials by exocytosis in goldfish bipolar cell terminals. J Physiol 572: 747–762, 2006. doi: 10.1113/jphysiol.2006.105205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti DA, Flores-Herr N, von Gersdorff H. Light evokes Ca2+ spikes in the axon terminal of a retinal bipolar cell. Neuron 25: 215–227, 2000. doi: 10.1016/S0896-6273(00)80884-3. [DOI] [PubMed] [Google Scholar]
- Sarthy PV, Lam DM. Isolated cells from a mammalian retina. Brain Res 176: 208–212, 1979. doi: 10.1016/0006-8993(79)90889-8. [DOI] [PubMed] [Google Scholar]
- Shiosaka S, Kiyama H, Tohyama M. A simple method for the separation of retinal sublayers from the entire retina with special reference to application for cell culture. J Neurosci Methods 10: 229–235, 1984. doi: 10.1016/0165-0270(84)90059-1. [DOI] [PubMed] [Google Scholar]
- Van Hook MJ, Thoreson WB. Simultaneous whole-cell recordings from photoreceptors and second-order neurons in an amphibian retinal slice preparation. J Vis Exp: 1–10, 2013. doi: 10.3791/50007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston ST, Chow RH, Weiland JD. Patch clamp recordings of retinal bipolar cells in response to extracellular electrical stimulation in wholemount mouse retina. Conf Proc IEEE Eng Med Biol Soc 2015: 3363–3366, 2015. doi: 10.1109/EMBC.2015.7319113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Wässle H. Responses of rod bipolar cells isolated from the rat retina to the glutamate agonist 2-amino-4-phosphonobutyric acid (APB). J Neurosci 11: 2372–2382, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. Calcium action potentials in retinal bipolar neurons. Vis Neurosci 15: 69–75, 1998. doi: 10.1017/S0952523898151064. [DOI] [PubMed] [Google Scholar]