We demonstrate that BDNF-induced pMF requires downstream signaling via PKCθ but not MEK/ERK or PI3K/Akt signaling. These data are essential to understand the sequence of the cellular cascade leading to BDNF-dependent phrenic motor plasticity.
Keywords: respiratory plasticity, phrenic motor facilitation, BDNF, MEK/ERK, PI3K/Akt, PLC/PKCθ, motor neuron
Abstract
Spinal brain-derived neurotrophic factor (BDNF) is necessary and sufficient for certain forms of long-lasting phrenic motor facilitation (pMF). BDNF elicits pMF by binding to its high-affinity receptor, tropomyosin receptor kinase B (TrkB), on phrenic motor neurons, potentially activating multiple downstream signaling cascades. Canonical BDNF/TrkB signaling includes the 1) Ras/RAF/MEK/ERK MAP kinase, 2) phosphatidylinositol 3‐kinase (PI3K)/Akt, and 3) PLCγ/PKC pathways. Here we demonstrate that spinal BDNF-induced pMF requires PLCγ/PKCθ in normal rats but not MEK/ERK or PI3K/Akt signaling. Cervical intrathecal injections of MEK/ERK (U0126) or PI3K/Akt (PI-828; 100 μM, 12 μl) inhibitor had no effect on BDNF-induced pMF (90 min after BDNF; U0126 + BDNF: 59 ± 14%, PI-828 + BDNF: 59 ± 8%, inhibitor vehicle + BDNF: 56 ± 7%; all P ≥ 0.05). In contrast, PKCθ inhibition with theta inhibitory peptide (TIP; 0.86 mM, 12 μl) prevented BDNF-induced pMF (90 min after BDNF; TIP + BDNF: −2 ± 2%; P ≤ 0.05 vs. other groups). Thus BDNF-induced pMF requires downstream PLCγ/PKCθ signaling, contrary to initial expectations.
NEW AND NOTEWORTHY We demonstrate that BDNF-induced pMF requires downstream signaling via PKCθ but not MEK/ERK or PI3K/Akt signaling. These data are essential to understand the sequence of the cellular cascade leading to BDNF-dependent phrenic motor plasticity.
phrenic motor facilitation (pMF) can be elicited by acute intermittent hypoxia (AIH) or by drugs injected in the cervical spinal segments near the phrenic motor nucleus. When pMF is induced by AIH, it is known as phrenic long-term facilitation (pLTF; Devinney et al. 2013; Fuller et al. 2000; Hayashi et al. 1993; Mitchell et al. 2001; Mitchell and Johnson 2003; Powell et al. 1998); pMF is a more general term that includes AIH-induced pLTF (Dale-Nagle et al. 2010a; Mitchell and Johnson 2003). Multiple, distinct cellular cascades give rise to pMF.
The Q pathway to pMF is the predominant contributor to pLTF following moderate AIH; it is termed the Q pathway since multiple Gq protein-coupled metabotropic receptors initiate a similar response (Dale-Nagle et al. 2010a; Fuller et al. 2000). The prevailing cellular model of the Q pathway involves (in sequence) 1) spinal serotonin type 2 receptor activation (5-HT2; Fuller et al. 2001; MacFarlane et al. 2011), 2) protein kinase C-theta (PKCθ) activity (Devinney et al. 2015), 3) new synthesis and release of brain-derived neurotrophic factor (BDNF; Baker-Herman et al. 2004), 4) activation of the high-affinity BDNF receptor tropomyosin receptor kinase B (TrkB; Baker-Herman et al. 2004; Dale et al. 2017), and 5) MEK-ERK activation (Hoffman et al. 2012). Although the requirement for each molecule/step is necessary for pLTF, the specific sequence has been inferred and not tested directly.
New BDNF protein synthesis is necessary for moderate AIH-induced pLTF, and BDNF/TrkB signaling alone elicits pMF (Baker-Herman et al. 2004). To establish the sequence of events downstream from BDNF/TrkB signaling, we studied intrathecal BDNF-induced pMF followed by selective inhibition of three potential TrkB signaling pathways. Canonical BDNF/TrkB signaling can involve any (or all) of the following: 1) Ras/Raf/MEK/ERK, 2) phosphatidylinositol 3-kinase (PI3K)/Akt, or 3) PLCγ/PKC signaling (Minichiello 2009). In light of several recent publications, we hypothesized that BDNF/TrkB signals via the MEK/ERK pathway with moderate AIH-induced pLTF (Devinney et al. 2013; Hoffman et al. 2012) whereas PKCθ signaling occurs upstream from new BDNF synthesis (Devinney et al. 2015). Thus we hypothesized that MEK/ERK inhibition would abolish BDNF-induced pMF, with minimal impact from PI3K/Akt or PKCθ inhibition. To our surprise, BDNF-induced pMF requires PKCθ signaling and is independent of MEK/ERK or PI3K/Akt signaling. Consequently, required MEK/ERK signaling in the Q pathway to pMF must be upstream from BDNF/TrkB activation, at least in normal rats.
MATERIALS AND METHODS
Animals
Adult male (3–4 mo) Sprague-Dawley rats (Harlan, Colonies 211 and 217) were used in all experiments. Rats were housed in a controlled environment (12:12-h light-dark cycle; daily humidity and temperature monitoring), with food and water ad libitum. The University of Wisconsin Animal Care and Use Committee approved all experimental protocols.
Experimental Series
We tested our hypothesis in three experimental series to investigate the role of three pathways associated with BDNF/TrkB signaling in other model systems. The first experimental series tested the hypothesis that spinal BDNF-induced pMF is MEK/ERK dependent. In this series, each group received either 1) intrathecal MEK/ERK inhibitor (U0126) before intrathecal BDNF (MEK/ERK inhibitor + BDNF group; n = 6) or 2) intrathecal MEK/ERK inhibitor (U0126) before intrathecal BDNF vehicle injections [artificial cerebrospinal fluid (aCSF) + 0.1% BSA] (MEK/ERK inhibitor + aCSF group; n = 6).
The second experimental series tested the hypothesis that BDNF-induced pMF is Akt/PI3K independent. In this series, each group received either 1) intrathecal Akt/PI3K inhibitor (PI-828) before intrathecal BDNF (Akt/PI3K inhibitor + BDNF group; n = 5) or 2) intrathecal Akt/PI3K inhibitor (PI-828) before intrathecal BDNF vehicle (aCSF + 0.1% BSA) (Akt/PI3K inhibitor+ aCSF group; n = 4).
In the third experimental series, we tested the hypothesis that BDNF-induced pMF is PKCθ independent. In this series, groups received either 1) intrathecal PKCθ inhibitor [theta inhibitory peptide (TIP)] before intrathecal BDNF (PKCθ inhibitor + BDNF group; n = 6) or 2) intrathecal PKCθ inhibitor (TIP) before intrathecal BDNF vehicle (aCSF + 0.1% BSA) (PKCθ inhibitor + aCSF group; n = 5).
Control Groups
Control groups included 1) intrathecal 20% DMSO-80% saline (inhibitor vehicle for U0126 and PI-282) before intrathecal BDNF (n = 5); 2) intrathecal aCSF (TIP inhibitor vehicle) before intrathecal BDNF (n = 5); 3) intrathecal 20% DMSO-80% saline (inhibitor vehicle for U0126 and PI-282) before intrathecal BDNF vehicle (aCSF + 0.1% BSA) (n = 3); and 4) intrathecal aCSF (TIP inhibitor vehicle) before intrathecal BDNF vehicle (aCSF + 0.1% BSA) (n = 5).
Since there were no statistically significant differences (2-way ANOVA, P = 0.47) between 20% DMSO-80% saline + BDNF (n = 5) and 100% aCSF + BDNF (n = 5), these groups were combined and renamed Inhibitor Vehicle + BDNF (n = 10). Since there were no significant differences (t-test, P = 0.30) between 20% DMSO-80% saline + aCSF + 0.1% BSA (n = 3) and 100% aCSF + aCSF + 0.1% BSA (n = 5), these groups were also combined and renamed Time Control (n = 8).
Surgical Protocol
Rats were anesthetized with isoflurane in a closed chamber and placed on a temperature-regulated table. A nose cone was then used to continue isoflurane administration throughout the surgery (isoflurane, 3.5% in O2 50%, balance N2). Body temperature was assessed with a digital rectal probe and maintained between 36.5 and 37.5°C. For intravenous infusions, a tail vein catheter (24 gauge × 3/4 in. iv catheter; Surflo) was placed (infusion rate: 0.5–1.2 ml·kg−1·h−1) throughout surgical preparations and the experimental protocol. Intravenous infusions were mixed to maintain fluid and acid-base balance (6:3:1, respectively): lactated Ringer solution, HesPan (6% hetastarch in 0.9% NaCl), and bicarbonate solution (8.4% solution). A tracheotomy was performed to enable artificial ventilation (Rodent Respirator, model 683; Harvard Apparatus, Holliston, MA; tidal volume 2.5 ml, frequency ~70–80). Before protocols began, the lungs were hyperinflated (2 breaths) every 1.5 h to minimize alveolar collapse.
A flow-through CO2 analyzer connected to the tracheal catheter was used to assess end-expired Pco2 levels (maintained between 40 and 45 mmHg during surgical preparation; Capnogard, Novametrix, Wallingford, CT). To prevent entrainment of respiratory neural activity to the ventilator, rats were bilaterally vagotomized in the midcervical region. A catheter was placed in the right femoral artery (polyethylene catheter PE-50; Intramedic) to monitor blood pressure and draw arterial blood samples for blood-gas and acid-base analysis (0.2- to 0.4-ml samples; ABL-800 Flex; Radiometer, Westlake, OH). Blood pressure was monitored continuously with a pressure transducer (Gould P23ID). Measurements were made on blood samples drawn during baseline and at 15, 30, 60, and 90 min after treatment.
The left phrenic nerve was isolated with a dorsal approach, cut distally, desheathed, and covered with a cotton ball soaked with saline until protocols began. A laminectomy (C2) was performed in all rats, and a small incision was made in the dura to place intrathecal catheters for drug delivery near the phrenic motor nucleus. Two soft silicone catheters (2 Fr; Access Technologies, Skokie, IL) were inserted 4 mm caudally from the C2 durotomy until the tip rested above the C4 segment. Intrathecal catheters were attached to 50-μl Hamilton syringes filled with appropriate solutions (inhibitors, BDNF, or vehicles).
After surgery, rats were converted to urethane anesthesia (1.85 g/kg iv; delivered in multiple 0.2- to 0.4-ml bolus injections over 15–20 min) while isoflurane was withdrawn. Once urethane anesthesia was established, anesthetic depth was confirmed via toe pinch with a hemostat during monitoring of changes in phrenic nerve activity, blood pressure, and/or intentional movements. After conversion, a minimum of 1 h was allowed before protocols were initiated. Rats were euthanized via urethane overdose at the end of experiments.
Neurophysiological Measurements
Pancuronium bromide (2.5 mg/kg iv) was used to paralyze rats during protocols. The phrenic nerve was covered in mineral oil and placed on bipolar silver electrodes for nerve recordings. Phrenic nerve signals were amplified (gain 10,000×), band-pass filtered (100–10,000 Hz; model 1800, A-M Systems, Carlsborg, WA), rectified and integrated (Paynter filter, time constant 50 ms; MA-821, CWE, Ardmore, PA). Integrated phrenic nerve bursts were digitized (8 kHz) and analyzed with a WINDAQ data-acquisition system (DATAQ Instruments, Akron, OH).
Before protocols were initiated, the CO2 apneic threshold was determined by lowering end-tidal CO2 until phrenic nerve activity ceased for ~1 min. The recruitment threshold was then determined by slowly increasing end-tidal CO2 until nerve activity resumed. End-tidal CO2 was raised ~2 mmHg above the recruitment threshold, and ~15–20 min was allowed to achieve a stable baseline.
Drug Treatments
Brain-derived neurotrophic factor.
Recombinant BDNF protein (Promega) was diluted in double-distilled water to make a stock solution (5 µg), aliquoted into multiple vials, and stored at −20°C. The BDNF stock solution was diluted on the day of use to 100 ng in aCSF + 0.1% BSA solution. The intrathecal BDNF dose (100 ng, 12 μl) was the same as that used in a previous study from our group (Baker-Herman et al. 2004). Control groups only received aCSF (in mM: 120 NaCl, 3 KCl, 2 CaCl, 2 MgCl, 23 NaHCO3, and 10 glucose, bubbled with 95% O2-5% CO2) with 0.1% BSA.
MEK/ERK inhibitor (U0126).
The membrane-permeant MEK/ERK inhibitor [1,4-diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto) butadiene (U0126); Promega] was dissolved in 100% DMSO and then diluted with saline to create a 100 μM solution in 20% DMSO-80% saline. The inhibitor was diluted to its injected concentration on the day of use and was administered (100 μM, 12 μl) 20 min before BDNF (or BDNF vehicle) administration.
Dose-response relationships with intrathecal U0126 had been established in prior studies from our laboratory (Dale et al. 2012; Dale-Nagle et al. 2011; Hoffman et al. 2012). We chose a dose shown to inhibit BDNF-dependent pLTF elicited by moderate AIH (Hoffman et al. 2012). Controls were 20% DMSO-80% saline injections of the same volume. To demonstrate drug efficacy, we confirmed that this drug dose blocks moderate AIH-induced pLTF in two rats (data not shown).
PI3K/Akt inhibitor (PI-828).
The PI3K/Akt inhibitor [2-(4-morpholinyl)-8-(4-aminopheny)l-4H-1-benzopyran-4-one (PI-828); Tocris] was dissolved in 100% DMSO and frozen in aliquots. On the day of use, it was diluted with saline to a final concentration of 100 μM in 20% DMSO-80% saline. The inhibitor was administered (100 μM, 12 μl) 20 min before BDNF administration. Earlier studies from our laboratory used this same PI-828 dose and confirmed efficacy (Dale et al. 2012; Dale-Nagle et al. 2011; Hoffman et al. 2012). For controls, we administered 20% DMSO-80% saline at the same volume as the inhibitor. To confirm drug efficacy, we gave the same dose and concentration of the PI3K inhibitor before severe AIH, since this form of pLTF is adenosine 2A receptor and PI3K/Akt dependent (Nichols et al. 2012); severe AIH-induced pLTF was successfully blocked in two rats (data not shown).
PKCθ inhibitor (TIP).
TIP (Calbiochem) is a myristoylated peptide mimicking the pseudosubstrate domain of PKCθ. TIP was dissolved in 100% aCSF and stored at −20°C (1 mM). On the day of use, it was diluted with aCSF to a final concentration of 0.86 mM. The inhibitor was administered (0.86 mM; 12 μl total, 1 μl/15 s) 15 min before BDNF administration. We previously demonstrated that this same dose and route of TIP administration block moderate AIH-induced pLTF (Devinney et al. 2015). For controls, we administered 100% aCSF at the same volume and rate as the inhibitor. To test the drugs’ effect, we gave the same dose and concentration of TIP before delivering inactivity phrenic motor facilitation (iPMF). iPMF is a form of plasticity dependent on protein kinase C zeta (PKCζ), and independent from PKCθ (Baertsch and Baker-Herman 2015). As expected, TIP failed to block iPMF (n = 2; data not shown).
Time Control
Time control experiments controlled for time-dependent changes in phrenic nerve activity characteristic in anesthetized animals. Our time control experiments were the inhibitor vehicle (20% DMSO-80% saline or aCSF) and the BDNF vehicle (aCSF + 0.1% BSA). Inhibitor vehicle injections were done 15–20 min before BDNF vehicle, mimicking the timing of drug injections described above.
Statistical Analysis
Integrated phrenic burst amplitude was normalized as a percent change from baseline. Respiratory frequency was also normalized as a change from baseline levels (% baseline) but was expressed as an absolute difference (bursts per minute). Statistical comparisons were made within and between treatment groups with a two-way ANOVA with a repeated-measures design (Prism 6; GraphPad Software). The same statistical comparisons were done for mean arterial pressure (MAP), arterial blood gases, pH, and rectal temperature (baseline and 90 min time). To detect significant differences between experimental groups, a two-way repeated-measures ANOVA was used (Prism 6; GraphPad Software); comparisons were made within and between treatment groups for phrenic nerve burst amplitude and frequency (baseline, 15, 30, 60, and 90 min). Individual comparisons were made with Tukey’s post hoc test. To determine significance between control groups, two different tests were used. For 20% DMSO-80% saline + BDNF vs. aCSF+ BDNF a two-way ANOVA was used, and for 20% DMSO-80% saline + aCSF + 0.1% BSA vs. 100% aCSF + aCSF + 0.1% BSA a t-test was performed. For all analyses, the significance level was set to 0.05; data are means ± SE.
RESULTS
Blood Gases and Arterial Pressure: Baseline and 90 min After Treatment
Throughout protocols, arterial Pco2 () was successfully regulated within ~2 mmHg from baseline value and arterial Po2 () was kept above 150 mmHg. and regulation during protocols was similar in all experimental groups (Table 1). MAP, temperature, and pH were similar among groups at baseline and at 90 min after hypoxia (Table 1). Thus differences in , , MAP, temperature, or pH regulation among groups cannot account for differential pMF responses observed in this study.
Table 1.
, , MAP, temperature, and pH during baseline and 90 min of experimental groups
| Experimental Groups | , mmHg | , mmHg | MAP, mmHg | Temperature, °C | pH |
|---|---|---|---|---|---|
| MEK/ERK + BDNF | |||||
| Baseline | 45.3 ± 2.1 | 310.0 ± 21.0 | 94.6 ± 7.5 | 36.8 ± 0.1 | 7.4 ± 0 |
| 90 min | 45.9 ± 2.7 | 293.1 ± 17.8 | 84.7.0 ± 7.3 | 37.1 ± 0.2 | 7.4 ± 0 |
| MEK/ERK + aCSF | |||||
| Baseline | 47.0 ± 1.7 | 317.1 ± 11.8 | 101.3 ± 0.6 | 37.1 ± 0.2 | 7.4 ± 0 |
| 90 min | 47.5 ± 1.7 | 276.5 ± 25.9 | 91.2 ± 8.0 | 37.2 ± 0.2 | 7.4 ± 0 |
| PI3K/Akt + BDNF | |||||
| Baseline | 47.3 ± 1.9 | 331.8 ± 18.6 | 89.9 ± 6.2 | 37.1 ± 0.1 | 7.4 ± 0 |
| 90 min | 47.5 ± 1.5 | 289.0 ± 42.2 | 76.9 ± 6.5 | 37.2 ± 0.2 | 7.4 ± 0 |
| PI3K/Akt + aCSF | |||||
| Baseline | 49.5 ± 1.6 | 308.5 ± 24.2 | 114.9 ± 2.1 | 37.1 ± 0.2 | 7.4 ± 0 |
| 90 min | 49.5 ± 1.7 | 283.2 ± 36.0 | 97.1 ± 1.4 | 37.2 ± 0.1 | 7.4 ± 0 |
| PKCθ + BDNF | |||||
| Baseline | 44.5 ± 0.7 | 328.5 ± 3.5 | 104.2 ± 5.0 | 36.9 ± 0.1 | 7.4 ± 0 |
| 90 min | 44.6 ± 1.4 | 317.5 ± 2.9 | 99.6 ± 7.0 | 37.1 ± 0.1 | 7.4 ± 0 |
| PKCθ + aCSF | |||||
| Baseline | 43.6 ± 0.9 | 323.25 ± 4.7 | 109.6 ± 6.4 | 37.5 ± 0.1 | 7.4 ± 0 |
| 90 min | 42.7 ± 0.9 | 303.0 ± 11.4 | 92.8 ± 5.7 | 37.1 ± 0.1 | 7.4 ± 0 |
| Vehicle + BDNF | |||||
| Baseline | 45.5 ± 0.6 | 317.5 ± 8.3 | 96.9 ± 3.5 | 37.1 ± 0.1 | 7.4 ± 0 |
| 90 min | 44.9 ± 0.8 | 293.1 ± 13.5 | 88.7 ± 10.9 | 37.3 ± 0.1 | 7.4 ± 0 |
| Vehicle + aCSF | |||||
| Baseline | 45.5 ± 3.9 | 294.7 ± 39.8 | 100.4 ± 6.1 | 37.2 ± 0.2 | 7.4 ± 0 |
| 90 min | 44.8 ± 3.6 | 295.2 ± 33.3 | 88.7 ± 10.9 | 37.1 ± 0.2 | 7.4 ± 0 |
Values are expressed as means ± SE. Animals were treated with MEK/ERK inhibitor (U0126), PI3K/Akt inhibitor (PI-828), PKCθ inhibitor (TIP), or inhibitor vehicle [20% DMSO-80% saline (U0126 and PI-828) or 100% aCSF (TIP)]. The animals were administered BDNF or aCSF (BDNF vehicle) after inhibitor or after inhibitor vehicle administration. MAP, mean arterial pressure. There were no significant differences in any group at baseline vs. 90 min.
BDNF-Induced pMF Is MEK/ERK Independent
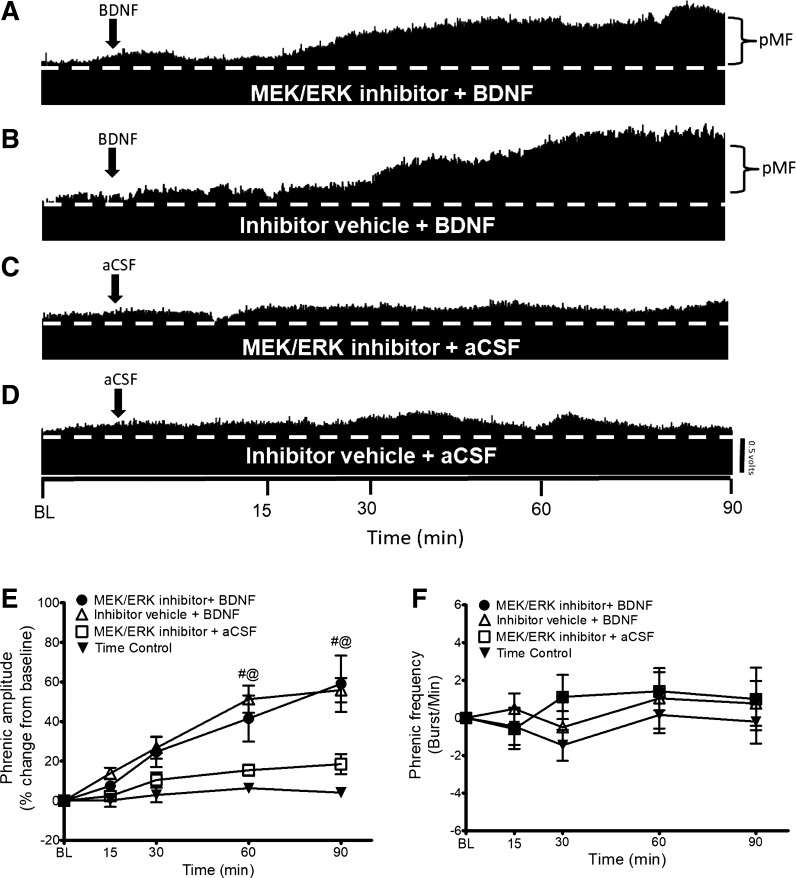
To test the hypothesis that BDNF-induced pMF requires MEK/ERK signaling, we delivered the MEK/ERK inhibitor U0126 (intrathecal delivery) 20 min before BDNF injections. Typical neurograms representing phrenic activity in Fig. 1, A–D, illustrate that MEK/ERK inhibition does not affect BDNF-induced pMF. Indeed, pMF magnitude was not significantly different in the MEK/ERK inhibitor + BDNF group (60 min after BDNF: 41 ± 11%, 90 min: 59 ± 14%; n = 6) compared with the Inhibitor Vehicle + BDNF group (60 min: 49 ± 7%, 90 min: 57 ± 6%; n = 10) (P ≥ 0.05; Fig. 1E).
Fig. 1.
A–D: representative traces of compressed integrated phrenic neurograms. BDNF-induced pMF is MEK/ERK independent since intrathecal delivery of the MEK/ERK inhibitor U0126 does not affect pMF. A: intrathecal MEK/ERK inhibitor U0126 (100 μM, 12 μl) administration 20 min before intrathecal BDNF administration had no significant effect on pMF. B: intrathecal BDNF after the inhibitor vehicle (20% DMSO-80% saline) elicits pMF. C: MEK/ERK inhibitor + BDNF vehicle (aCSF) does not exhibit pMF. D: intrathecal administration of inhibitor vehicle + aCSF does not exhibit pMF. E: group data for phrenic burst amplitude expressed as % change from baseline (BL). MEK/ERK inhibitor + BDNF (n = 6), Inhibitor Vehicle + BDNF (n = 10), MEK/ERK inhibitor + aCSF (n = 6), and Time Control (n = 8) groups were compared to determine significance between groups. There were no significant differences at any time between MEK/ERK inhibitor + BDNF- vs. inhibitor vehicle + BDNF-treated rats. There were no significant differences at any time between MEK/ERK inhibitor + aCSF and Inhibitor Vehicle + aCSF groups. There were significant differences between the MEK/ERK inhibitor + BDNF and Inhibitor Vehicle + BDNF groups vs. MEK/ERK inhibitor + aCSF and Time Control groups at 60 and 90 min after BDNF or vehicle injection. Significance is P ≤ 0.05: #significantly different from MEK/ERK inhibitor + aCSF; @significantly different from Inhibitor Vehicle + aCSF. F: group data for phrenic frequency; there were no significant differences in frequency between any groups at any time.
On the other hand, pMF was greater in both the MEK/ERK inhibitor + BDNF (60 min: 41 ± 11%, 90 min: 59 ± 14%; n = 6) and Inhibitor Vehicle + BDNF (60 min: 49 ± 7%, 90 min: 57 ± 6%; n = 10) groups vs. the MEK/ERK inhibitor + aCSF (60 min: 15 ± 2%, 90 min: 18 ± 5%; n = 6) and Time Control (60 min: 5 ± 3%, 90 min: 4 ± 3%; n = 7) groups (P ≤ 0.05; Fig. 1E). The MEK/ERK inhibitor + aCSF and Time Control groups were not significantly different from one another at any time (P ≥ 0.05; Fig. 1E). There was no significant difference in frequency at any time in any group (P ≥ 0.05; Fig. 1F).
BDNF-Induced pMF Is PI3K/Akt Independent
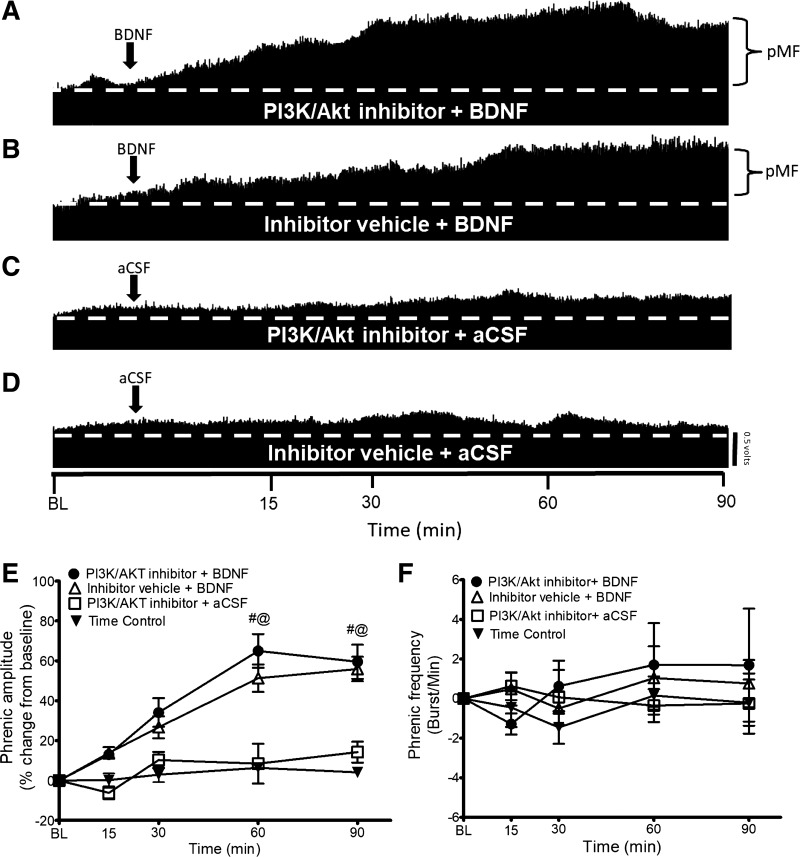
To test the hypothesis that BDNF-induced pMF requires PI3K/Akt signaling, we delivered the PI3K/Akt inhibitor PI-828 (intrathecal delivery) 20 min before BDNF. Typical neurograms representing phrenic nerve output (Fig. 2, A–D) illustrate that the PI3K/Akt inhibitor does not affect BDNF-induced pMF. Indeed, pMF magnitude was not significantly different in the PI3K/Akt inhibitor + BDNF group (60 min after BDNF: 64 ± 8%, 90 min: 59 ± 8%; n = 5) compared with the Inhibitor Vehicle + BDNF group (60 min: 49 ± 7%, 90 min: 57 ± 6%; n = 10) (P ≥ 0.05; Fig. 2E).
Fig. 2.
A–D: representative traces of compressed integrated phrenic neurograms. BDNF-induced pMF is PI3K/Akt independent since the PI3K/Akt inhibitor PI-828 has no effect. A: intrathecal PI3K/Akt inhibitor PI-828 (100 μM, 12 μl) administration 20 min before intrathecal BDNF does not affect pMF. B: intrathecal BDNF after the inhibitor vehicle (20% DMSO-80% saline) elicits pMF. C: PI3K/Akt inhibitor + BDNF vehicle (aCSF) does not exhibit pMF. D: intrathecal administration of inhibitor vehicle + aCSF does not exhibit pMF. E: group data for phrenic burst amplitude expressed as % change from baseline. PI3K/Akt inhibitor + BDNF (n = 5), Inhibitor Vehicle + BDNF (n = 10), PI3K/Akt inhibitor + aCSF (n = 5), and Time Control (n = 8) groups were compared to determine significance between groups. There were no significant differences at any time between PI3K/Akt inhibitor + BDNF- and inhibitor vehicle + BDNF-treated rats. There were no significant differences at any time between PI3K/Akt inhibitor + aCSF and Inhibitor Vehicle + aCSF groups. There were significant differences between the PI3K/Akt inhibitor + BDNF and Inhibitor Vehicle + BDNF groups vs. PI3K/Akt inhibitor + aCSF and Time Control groups at 60 and 90 min after BDNF. Significance is P ≤ 0.05: #significantly different from PI3K/Akt inhibitor PI-828 + aCSF; @significantly different from Inhibitor Vehicle + aCSF. F: group data for phrenic frequency; there were no significant differences in frequency between any groups at any time.
On the other hand, pMF was greater in both the PI3K/Akt inhibitor + BDNF (60 min: 64 ± 8%, 90 min: 59 ± 8%; n = 5) and Inhibitor Vehicle + BDNF (60 min: 49 ± 7%, 90 min: 57 ± 6%; n = 10) groups vs. the PI3K/Akt inhibitor + aCSF (60 min: 8 ± 9%, 90 min: 18 ± 5%; n = 4) and Time Control (60 min: 5 ± 3%, 90 min: 4 ± 3%; n = 7) groups (P ≤ 0.05; Fig. 2E). The PI3K/Akt inhibitor + aCSF and Time Control groups were not significantly different from one another at any time (P ≥ 0.05; Fig. 2E). There was no significant difference in frequency at any time in any group (P ≤ 0.05; Fig. 2F).
BDNF-Induced pMF Is PKCθ Dependent.
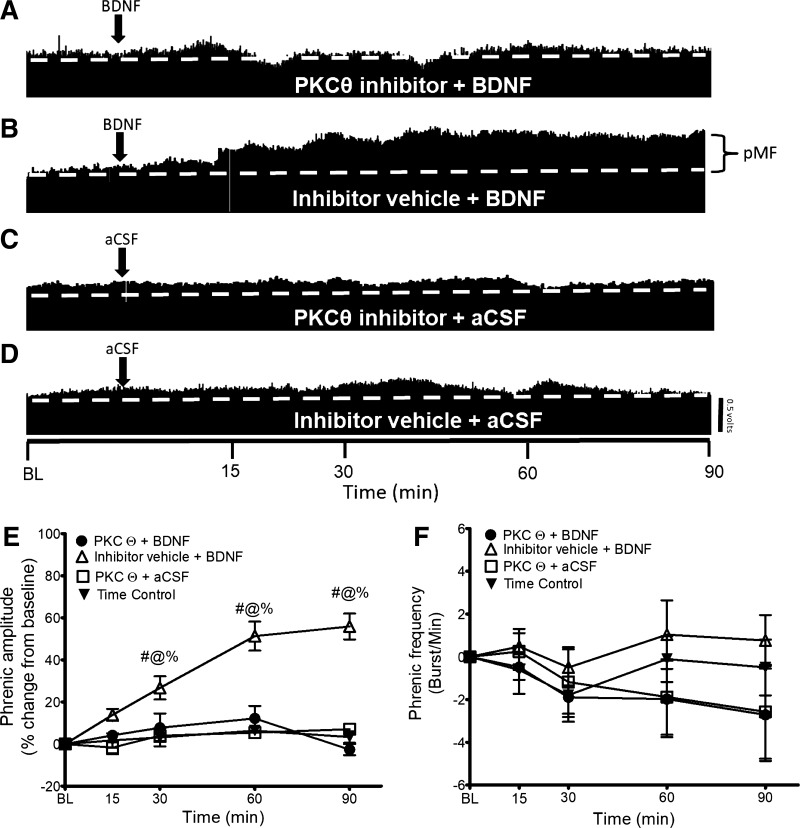
To test the hypothesis that BDNF-induced pMF is independent of PLC/PKCθ signaling, we delivered the PKCθ inhibitor TIP (intrathecal delivery) 15 min before BDNF administration. Typical neurograms representing phrenic nerve output are shown in Fig. 3, A–D, illustrating that, contrary to our hypothesis, PKCθ inhibition abolishes BDNF-induced pMF. In PKCθ inhibitor + BDNF treated rats (n = 6), there was a significant decrease in the magnitude of pMF, starting at 30 min after BDNF administration, relative to the Inhibitor Vehicle + BDNF group (n = 10) (30 min: PKCθ inhibitor+ BDNF 7 ± 6% vs. Inhibitor Vehicle + BDNF 26 ± 6%; 60 min: PKCθ inhibitor + BDNF 12 ± 5% vs. Inhibitor Vehicle + BDNF 49 ± 7%; 90 min: PKCθ inhibitor + BDNF −2 ± 2% vs. Inhibitor Vehicle + BDNF: 57 ± 6%; all P ≤ 0.05; Fig. 3E).
Fig. 3.
A–D: representative traces of compressed integrated phrenic neurograms. BDNF-induced pMF is PKCθ dependent since the PKCθ inhibitor TIP abolishes pMF. A: intrathecal PKCθ inhibitor TIP (0.86 mM, 12 μl) administration 15 min before intrathecal BDNF abolishes pMF. B: intrathecal BDNF after the inhibitor vehicle elicits pMF. C: PKCθ inhibitor + BDNF vehicle (aCSF) does not exhibit pMF. D: intrathecal administration of inhibitor vehicle + aCSF does not exhibit pMF. E: group data for phrenic burst amplitude expressed as % change from baseline. PKCθ inhibitor + BDNF (n = 6), Inhibitor Vehicle + BDNF (n = 10), PKCθ inhibitor + aCSF (n = 5), and Time Control (n = 8) groups were compared to determine significance between groups. There were significant differences at 30, 60, and 90 min after BDNF between PKCθ inhibitor + BDNF and Inhibitor Vehicle + BDNF, PKCθ inhibitor + aCSF, and Inhibitor Vehicle + aCSF groups. There was no significant difference at any time between Inhibitor Vehicle + BDNF, PKCθ inhibitor + aCSF, and Inhibitor Vehicle + aCSF groups. Significance is P ≤ 0.05: #significantly different from PKCθ inhibitor + aCSF; @significantly different from Time Control; %significantly different from Inhibitor Vehicle + BDNF. F: group data for phrenic frequency; there were no significant differences in frequency between any groups at any time.
The PKCθ inhibitor + BDNF group was not significantly different at any time from PKCθ inhibitor + aCSF and Time Control groups (P ≥ 0.05; Fig. 3E). There were no significant differences in frequency at any time in any group (P ≥ 0.05; Fig. 3F).
DISCUSSION
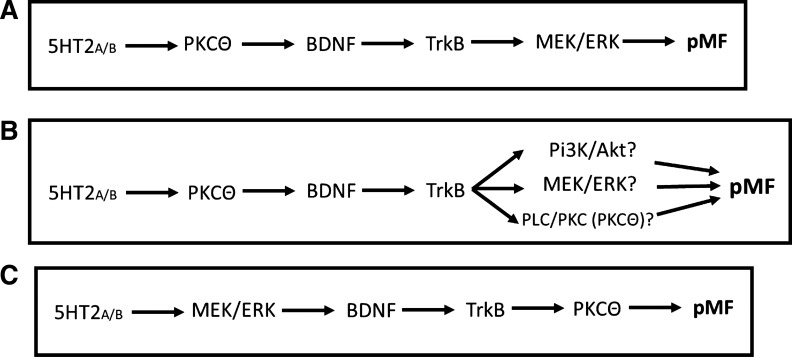
Contrary to our initial hypothesis, BDNF-induced pMF requires PKCθ activity and is independent of MEK/ERK or PI3K/Akt activity in normal rats (Fig. 4). Doses and selectivity of inhibitors were verified in other studies from our laboratory. Thus previously published working models of the Q pathway to pMF require revision (Dale et al. 2014; Dale-Nagle et al. 2010a, 2010b). We now suggest that the correct protein activation sequence in moderate AIH-induced pLTF (i.e., the Q pathway) is 1) MEK/ERK signaling upstream from new BDNF synthesis and release and 2) BDNF/TrkB signaling via PLC/PKCθ activation. These findings elaborate our model and increase understanding of mechanisms giving rise to phrenic motor plasticity.
Fig. 4.
Summary of studies and new suggested working model of the Q pathway to pMF. A: former working model of the Q pathway to pMF in which Gq-coupled receptors elicit complex downstream signaling. The initiating receptor was initially postulated to signal via PKCθ, leading to new BDNF synthesis with subsequent TrkB receptor activation. Downstream signaling was thought to be dependent on TrkB induced MEK/ERK signaling, ultimately leading to pMF (Baker-Herman et al. 2004; Devinney et al. 2013; Feldman et al. 2003; Mitchell et al. 2001). B: we activated the Q pathway by pharmacologically manipulating key proteins involved in the signaling cascade leading to pMF (BDNF/TrkB). We administered inhibitors that blocked the three canonical TrkB signaling pathways including MEK/ERK, PI3K/Akt, and PLC/PKC (PKCθ), followed by intrathecal BDNF administration to elicit pMF. C: present study demonstrates that PKCθ is the only pathway necessary for BDNF/TrkB induced pMF. Thus our working model of the Q pathway to pMF (A) requires revision; MEK/ERK must be signaling upstream from BDNF/TrkB signaling, whereas PKCθ is a downstream mediator (C).
BDNF-Induced pMF Is MEK/ERK Independent
We demonstrate that MEK/ERK signaling is not required for BDNF-induced pMF (Fig. 1). These data are surprising, since we initially hypothesized that BDNF-induced pMF required MEK/ERK signaling in light of the observation that MEK/ERK inhibition abolishes moderate AIH-induced pLTF (Hoffman et al. 2012) and MEK/ERK is a common TrkB signaling pathway (Minichiello 2009). Hoffman and colleagues (Hoffman et al. 2012) demonstrated that when the Q pathway is elicited by moderate AIH intrathecal U0126 blocks pLTF, demonstrating that MEK/ERK activity is necessary in the underlying mechanism but giving no real insight concerning where in the signaling cascade this molecule was activated. When U0126 is administered <5 min after moderate AIH, pLTF stalls but does not return to baseline values, thereby decreasing (but not reversing) pLTF (Hoffman et al. 2012). This observation is consistent with the interpretation that ERK is upstream from BDNF synthesis vs. signaling downstream from TrkB activation in BDNF-dependent pMF.
BDNF-Induced pMF Is PI3k/Akt Independent
We confirmed that PI3K/Akt signaling is not necessary for BDNF-induced pMF, just as with moderate AIH induced pLTF (Hoffman et al. 2012) (Fig. 2). On the other hand, the PI3K/Akt signaling pathway is necessary for the BDNF-independent, TrkB-dependent S pathway to pMF (Dale-Nagle et al. 2010a; Golder et al. 2008; Hoffman et al. 2012; Nichols et al. 2012).
BDNF-Induced pMF Is PKCθ Dependent
Our finding that PKCθ signaling is necessary for BDNF-induced pMF (Fig. 3) was surprising but is consistent with reports that TrkB can signal via PLC-γ/PKC in other model systems (Minichiello 2009). We did not test the hypothesis that PLC-γ is necessary for BDNF induced pMF since there are no selective PLC-γ inhibitors currently available. On the other hand, Devinney and colleagues (2015) demonstrated that the novel PKC isoform PKCθ is necessary for moderate AIH-induced pLTF, although it was not determined whether the necessary PKCθ activity is upstream vs. downstream from BDNF/TrkB signaling. Devinney et al. (2015) also demonstrated PKCθ expression within phrenic motor neurons and that PKCθ knockdown via intrapleural administration of siRNAs targeting PKCθ mRNA abolishes pLTF. Here we used the peptide PKCθ inhibitor TIP; it reliably reproduced the results of intrapleural siPKCθ injections, blocking moderate AIH-induced pLTF (Devinney et al. 2015). Since TIP blocked BDNF-induced pMF, we suggest that the necessary PKCθ activity is downstream from BDNF/TrkB activation in the Q pathway to pMF. Our findings do not rule out involvement of additional PKC isoforms upstream from BDNF/TrkB signaling; for example, a currently unknown conventional/novel PKC isoform is likely involved downstream from the Gq protein-coupled receptors initiating the Q pathway but upstream from BDNF/TrkB signaling. Investigating this possibility was beyond the scope of the present study. Although we cannot ensure that mechanisms of BDNF-induced pMF are identical to those giving rise to moderate AIH-induced pLTF, the parsimonious explanation is that PKCθ also acts downstream from BDNF/TrkB signaling after moderate AIH.
Significance and Conclusions
We conclude that BDNF-induced pMF requires PLC/PKCθ signaling but is independent of MEK/ERK or PI3K/Akt activation. On this basis, we suggest a new working model with a revised sequence of protein activation in the Q pathway to pMF (Fig. 4C).
AIH-induced pLTF is highly relevant as a model of motor neuron plasticity. In prior studies, we demonstrated that moderate AIH (or repeated AIH) elicits plasticity in other motor systems, including the hypoglossal motor nucleus (Bach and Mitchell 1996), inspiratory intercostal motor neurons (Fregosi and Mitchell 1994; Navarrete-Opazo and Mitchell 2014), recurrent laryngeal motor neurons (Xing et al. 2013), and cervical or lumbar motor neurons that innervate the limbs (Lovett-Barr et al. 2012; Trumbower et al. 2012), presumably via BDNF/TrkB-dependent mechanisms (Dale et al. 2014; Lovett-Barr et al. 2012). Thus cellular mechanisms revealed in the present study inform our understanding of nonrespiratory motor systems.
From another perspective, phrenic motor plasticity has already served as a guide to clinical translation, inspiring the use of moderate AIH or repetitive AIH as a treatment to restore breathing capacity and walking ability in neuromuscular disorders that compromise movement, including cervical spinal injury and amyotrophic lateral sclerosis (Dale et al. 2014; Gonzalez-Rothi et al. 2015; Mahamed and Mitchell 2007). For example, AIH restores breathing ability in rodent models of cervical spinal injury (Golder and Mitchell 2005; Lovett-Barr et al. 2012; Navarrete-Opazo et al. 2015, 2017) or amyotrophic lateral sclerosis (Nichols et al. 2013) and has already been demonstrated to induce functional recovery of breathing (Tester et al. 2014) and walking ability (Hayes et al. 2014; Navarrete-Opazo et al. 2017) in humans with chronic incomplete spinal cord injury. By greater understanding of cellular mechanisms giving rise to this plasticity, we can hope to harness combinatorial strategies (including drugs) to enhance the functional benefits of these promising therapeutic strategies. Alternately, specific drugs may be an excellent alternative for patients who do not tolerate repeated hypoxic exposures by inducing plasticity without systemic hypoxia (Almendros et al. 2014).
GRANTS
Support for this work was provided by National Heart, Lung, and Blood Institute (NHLBI) Grants HL-111598 and HL-69064. I. Agosto-Marlin was supported by NHLBI supplement to HL-111598.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
I.M.A.-M. and G.S.M. conceived and designed research; I.M.A.-M. performed experiments; I.M.A.-M. analyzed data; I.M.A.-M. and G.S.M. interpreted results of experiments; I.M.A.-M. prepared figures; I.M.A.-M. drafted manuscript; I.M.A.-M. and G.S.M. edited and revised manuscript; I.M.A.-M. and G.S.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Bradley Wathen for expert technical assistance.
REFERENCES
- Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol 307: L129–L140, 2014. doi: 10.1152/ajplung.00089.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baertsch NA, Baker-Herman TL. Intermittent reductions in respiratory neural activity elicit spinal TNF-α-independent, atypical PKC-dependent inactivity-induced phrenic motor facilitation. Am J Physiol Regul Integr Comp Physiol 308: R700–R707, 2015. doi: 10.1152/ajpregu.00359.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced respiratory and nonrespiratory motor function. Physiology (Bethesda) 29: 39–48, 2014. doi: 10.1152/physiol.00012.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Fields DP, Devinney MJ, Mitchell GS. Phrenic motor neuron TrkB expression is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. Exp Neurol 287: 130–136, 2017. doi: 10.1016/j.expneurol.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Satriotomo I, Mitchell GS. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J Neurosci 32: 5973–5983, 2012. doi: 10.1523/JNEUROSCI.3873-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010a. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Satriotomo I, Lovett-Barr MR, Vinit S, Mitchell GS. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann NY Acad Sci 1198: 252–259, 2010b. doi: 10.1111/j.1749-6632.2010.05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 31: 7682–7690, 2011. doi: 10.1523/JNEUROSCI.0239-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Fields DP, Huxtable AG, Peterson TJ, Dale EA, Mitchell GS. Phrenic long-term facilitation requires PKCθ activity within phrenic motor neurons. J Neurosci 35: 8107–8117, 2015. doi: 10.1523/JNEUROSCI.5086-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013. doi: 10.1111/nyas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266, 2003. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol 121: 135–146, 2000. doi: 10.1016/S0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol (1985) 90: 2001–2006, 2001. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 25: 2925–2932, 2005. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rothi EJ, Lee KZ, Dale EA, Reier PJ, Mitchell GS, Fuller DD. Intermittent hypoxia and neurorehabilitation. J Appl Physiol (1985) 119: 1455–1465, 2015. doi: 10.1152/japplphysiol.00235.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993. [DOI] [PubMed] [Google Scholar]
- Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: a randomized trial. Neurology 82: 104–113, 2014. doi: 10.1212/01.WNL.0000437416.34298.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol (1985) 113: 1184–1193, 2012. doi: 10.1152/japplphysiol.00098.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012. doi: 10.1523/JNEUROSCI.2908-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience 178: 45–55, 2011. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Minichiello L. TrkB signalling pathways in LTP and learning. Nat Rev Neurosci 10: 850–860, 2009. doi: 10.1038/nrn2738. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90: 2466–2475, 2001. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol (1985) 94: 358–374, 2003. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Dougherty BJ, Mitchell GS. Enhanced recovery of breathing capacity from combined adenosine 2A receptor inhibition and daily acute intermittent hypoxia after chronic cervical spinal injury. Exp Neurol 287: 93–101, 2017. doi: 10.1016/j.expneurol.2016.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Mitchell GS. Recruitment and plasticity in diaphragm, intercostal, and abdominal muscles in unanesthetized rats. J Appl Physiol (1985) 117: 180–188, 2014. doi: 10.1152/japplphysiol.00130.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete-Opazo A, Vinit S, Dougherty BJ, Mitchell GS. Daily acute intermittent hypoxia elicits functional recovery of diaphragm and inspiratory intercostal muscle activity after acute cervical spinal injury. Exp Neurol 266: 1–10, 2015. doi: 10.1016/j.expneurol.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol (1985) 112: 1678–1688, 2012. doi: 10.1152/japplphysiol.00060.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NL, Van Dyke J, Nashold L, Satriotomo I, Suzuki M, Mitchell GS. Ventilatory control in ALS. Respir Physiol Neurobiol 189: 429–437, 2013. doi: 10.1016/j.resp.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. doi: 10.1016/S0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term facilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med 189: 57–65, 2014. doi: 10.1164/rccm.201305-0848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 26: 163–172, 2012. doi: 10.1177/1545968311412055. [DOI] [PubMed] [Google Scholar]
- Xing T, Fong AY, Bautista TG, Pilowsky PM. Acute intermittent hypoxia induced neural plasticity in respiratory motor control. Clin Exp Pharmacol Physiol 40: 602–609, 2013. doi: 10.1111/1440-1681.12129. [DOI] [PubMed] [Google Scholar]