Abstract
Objective
The long-term use of proton pump inhibitors (PPIs) may induce adverse events in many organs, including the stomach. The chronic use of PPIs has been associated with the growth of fundic gland polyps (FGPs) and of gastric black spots. This study assessed the incidence of gastric lesions with cobblestone-like appearance in PPI users.
Methods
The clinical characteristics and endoscopic findings of patients who underwent upper gastrointestinal endoscopy after using PPIs for at least six months were analyzed. The biopsy specimens from patients with gastric cobblestone-like lesions (GCLLs) were examined histopathologically.
Patients
This study analyzed 171 patients who underwent upper gastrointestinal endoscopy after more than 6 months of PPI use in Mitsugi Public General Hospital from January 1, 2015, to March 31, 2016.
Results
Of the 171 patients, 60 (35.1%) had GCLLs and 111 (64.9%) did not. There were no significant between-group differences in age, sex, duration of PPI use, and receipt of Helicobacter pylori eradication therapy. Atrophic gastritis of the corpus was significantly less frequent in the GCLL than in the non-GCLL group (55.0% vs. 47.8%, p=0.0097). Among the GCLL group, histological examinations of 24 patients revealed cystic dilation of the fundic gland in 19 (79.2%), parietal cell hyperplasia in 18 (75.0%), and cytoplasmic vacuolation in 7 (29.2%).
Conclusion
GCLLs occurred frequently in long-term PPI users, especially in patients without atrophic gastritis. The pathological findings of GCLLs included parietal cell hyperplasia and fundic gland cysts. The clinical importance of these new lesions remains uncertain, but they should be observed carefully.
Keywords: cobblestone-like appearance, proton pump inhibitor, drug-related adverse reaction, parietal cell protrusion
Introduction
Proton pump inhibitors (PPIs) are used worldwide as maintenance therapy for patients with gastroesophageal reflux disease (GERD) and to prevent gastric complications of non-steroidal anti-inflammatory drugs (NSAIDs) and low-dose aspirin. Patients are frequently treated with these agents for long periods of time, and the long-term use of PPIs may cause various adverse events. In the stomach, the chronic use of PPIs has been reported to be associated with the growth of fundic gland polyps (FGPs) (1-4), and several case reports have described PPI-induced neuroendocrine tumors (NETs) (5). We recently described a new type of PPI-induced gastric lesion in the stomach called “black spots” (6). This report describes a second new type of gastric lesion, those with cobblestone-like appearance, in chronic PPI users.
Materials and Methods
The medical and pharmacy records of Mitsugi General Hospital were reviewed to identify patients who underwent upper gastrointestinal endoscopy (UGIE) from January 1, 2015, to March 31, 2016, after having taken PPIs for more than 6 months. Patients with previous gastrectomy, insufficient monitoring of the gastric corpus and percutaneous endoscopic gastrostomy were excluded. All endoscopy findings were checked by 3 endoscopists ― 1 each with 5, 22, and 40 years of experience.
Gastric cobblestone-like lesions (GCLLs) were defined as lesions with a cobblestone-like appearance in the gastric corpus (Fig. 1). Patients were divided into those with and without GCLLs. The factors compared in the two groups included age, sex, duration of PPI use (<1 year, 1-3 years, 3-6 years, 6-9 years, or >9 years), hemodialysis (yes/no), Helicobacter pylori eradication therapy (yes/no), reasons for PPI prescription, atrophic gastritis of the corpus (yes/no), and drug combinations. Symptoms in patients with GCLLs were ascertained based on their medical records. The percentages of patients with other endoscopic lesions, including white-and-flat elevated lesions (WFELs), black spots (BSs), and FGPs, were also compared. WFELs are observed mainly on the gastric fornix and corpus of patients not infected with H. pylori or after H. pylori eradication. WEFLs are pathologically hyperplasia of the crypt epithelium in fundic glands and have been shown to be associated with gastric secretion inhibitors, including PPIs (7,8). Black spots are new gastric findings observed as black dots endoscopically in the mucosa of the gastric corpus and as pathologically brownish pigmentations in fundic gland cysts (6).
Figure 1.
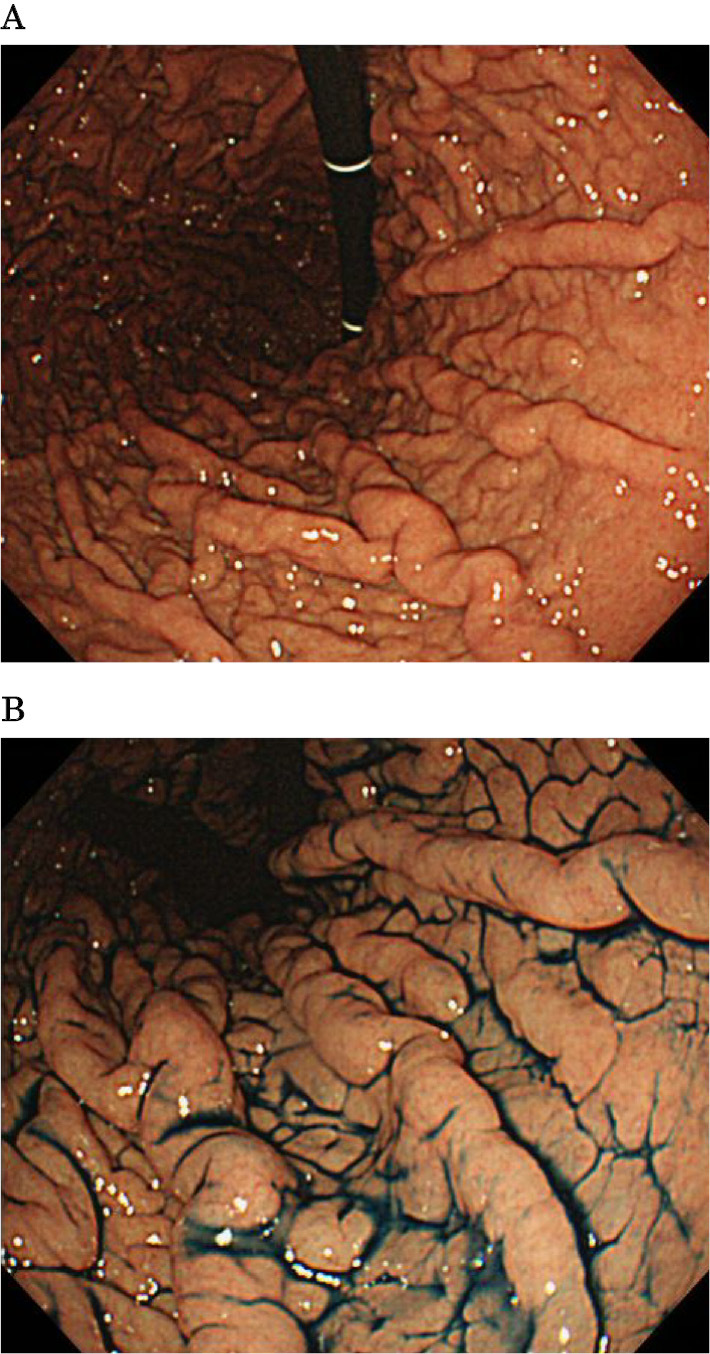
Endoscopic appearance of gastric cobblestone-like lesions (GCLLs) in the gastric corpus. A: Endoscopic examination shows GCLLs. B: Highlighted finding after dry spraying with indigo carmine solution.
One or two biopsy specimens were taken from each patient with and without GCLLs for a histopathological examination. Each specimen was embedded in paraffin wax and cut into 4-μm-thick sections. The serum gastrin concentrations were measured by enzyme-linked immunosorbent assay (ELISA).
Patient ages were compared by independent t-tests, whereas other baseline characteristics, including drug combinations and endoscopic findings, were analyzed by Fisher's exact tests. All statistical analyses were performed using the R software program, version 3.3.0 [R Development Core Team. (2016) R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria], with p values <0.05 considered statistically significant.
This retrospective study was approved by the medical ethics committees of Mitsugi General Hospital on April 8, 2016.
Results
A review of the medical and pharmacy records of our center identified 189 patients who had undergone UGIE from January 1, 2015, to March 31, 2016, after having taken PPIs for more than 6 months. Fourteen of these patients were excluded, including four post-gastrectomy, six with insufficient observation of the gastric corpus, and four who underwent UGIE for the purpose of percutaneous endoscopic gastrostomy. After excluding these 14 patients, 171 patients were included in this study. Of these 171 patients, 60 (35.1%) had GCLLs, and 111 (64.8%) did not.
Table 1 shows the baseline characteristics of the two groups. There were no significant differences in the age, sex, and percentage receiving H. pylori eradication therapy. The duration of PPI use by 28 patients (12 GCLL and 16 non-GCLL) could not be determined precisely because their date of starting PPI treatment could not be confirmed. After excluding these 28 patients, we found that PPIs were used for <1 year, 1-3 years, 3-6 years, 6-9 years, and >9 years by 12.5%, 27.1%, 33.3%, 14.6%, and 12.5%, respectively, of the patients in the GCLL group and by 7.4%, 34.7%, 32.6%, 12.6%, and 12.6%, respectively, of the patients in the non-GCLL group. The percentage of patients on hemodialysis was significantly higher in the GCLL than in the non-GCLL group (8.3% vs. 0.90%, p=0.021). The symptoms in the 60 patients with GCLL included epigastric pain (26.7%), heartburn (11.7%), nausea (6.7%), anorexia (5.0%), epigastric discomfort (5.0%), pharyngeal discomfort (3.3%), and weight loss (1.7%). Thirty-two patients (53.3%) in this group, however, had no symptoms in their medical records. Table 2 shows the percentages of patients with atrophic gastritis and the degree of gastritis in each group. The percentages of patients without atrophic gastritis (55.0% vs. 47.8%, p=0.0097) and with endoscopic evidence of WFEL (23.3% vs. 3.6%, p=0.00012) were significantly higher in the GCLL than in the non-GCLL group. Although there were no significant differences in the percentages with FGPs and BSs, the percentage of patients with BSs tended to be higher in the GCLL than in the non-GCLL group (25.0% vs. 13.5%, p=0.09). Table 3 shows the indications for PPI use, with GERD being the most frequent reason in both groups. Table 4 shows the drug combinations used to treat these patients, with antihypertensive agents being most frequently combined with PPI in both groups and no significant between-group differences in these agents.
Table 1.
Demographic and Clinical Characteristics of Long-term Users of PPIs with and without Gastric Cobblestone-like Lesions (GCLLs).
| Characteristics | GCLL group(n=60) | Non-GCLL group(n=111) | p value |
|---|---|---|---|
| Age (years) | 75.0 (38-96) | 76.2 (34-96) | 0.75 |
| Sex (male) | 33 (55.0 %) | 53 (47.8 %) | 0.42 |
| Success of H.pylori eradication | 5 (8.3 %) | 15 (13.5 %) | 0.46 |
| Hemodialysis | 5 (8.3%) | 1 (0.90 %) | 0.021 |
| PPI use within 1 year | 6 (10.0%) | 7 (6.3 %) | 0.38 |
Table 2.
Presence and Degree of Atrophic Gastritis of Long-term Users of PPIs with and without Gastric Cobblestone-like Lesions (GCLLs).
| Endoscopic findings | GCLL group(n=60) | Non-GCLL group(n=111) | p value |
|---|---|---|---|
| No atrophic gastritis | 33 (55.0 %) | 38 (34.2 %) | 0.0097 |
| Closed type atrophic gastritis | 12 (20.0 %) | 26 (23.4 %) | 0.70 |
| Open type atrophic gastritis | 15 (25.0 %) | 47 (42.3 %) | 0.030 |
Table 3.
Reasons for Long-term Use of PPIs by Patients with and without Gastric Cobblestone-like Lesions (GCLLs).
| Reasons of PPI | GCLL group(n=60) | Non-GCLL group(n=111) | p value |
|---|---|---|---|
| GERD | 29 (48.3 %) | 63 (56.8 %) | 0.34 |
| Low dose aspirin | 7 (11.7 %) | 8 (7.2 %) | 0.40 |
| Post peptic ulcer | 8 (13.3 %) | 6 (5.4 %) | 0.084 |
| NSAIDs | 4 (6.7 %) | 9 (8.11 %) | 1.0 |
| Corticosteroids | 0 | 7 (6.3%) | 0.098 |
| Functional dyspepsia | 1 (1.7 %) | 2 (1.8 %) | 1.0 |
| Unclear | 11 (18.3 %) | 8 (7.2 %) | - |
GERD: gastroesophageal reflux disease, NSAIDs: non-steroidal anti-inflammatory drugs
Table 4.
Classes of Major Medications Administered along with PPIs to Patients with and without Gastric Cobblestone-like Lesions (GCLLs).
| Co-administered medications | GCLL group(n=60) | Non-GCLL group(n=111) | p value |
|---|---|---|---|
| CCB | 25 (41.7 %) | 38 (34.2 %) | 0.41 |
| ARB/ACE-I | 24 (40.0 %) | 40 (36.0 %) | 0.62 |
| Rebamipide | 5 (8.3 %) | 9 (8.1 %) % | 1.0 |
| Mosapride | 5 (8.3 %) | 17 (15.3 %) | 0.24 |
| DPP-4 inhibitors | 6 (10.0 %) | 9 (8.1 %) | 0.78 |
| Aspirin | 13 (21.7 %) | 12 (10.8 %) | 0.070 |
| NSAIDs | 6 (10.0 %) | 14 (12.6 %) | 0.80 |
| Statins | 14 (23.3 %) | 26 (23.4 %) | 1.0 |
| Misoprostol | 2 (3.3 %) | 2 (1.8 %) | 0.61 |
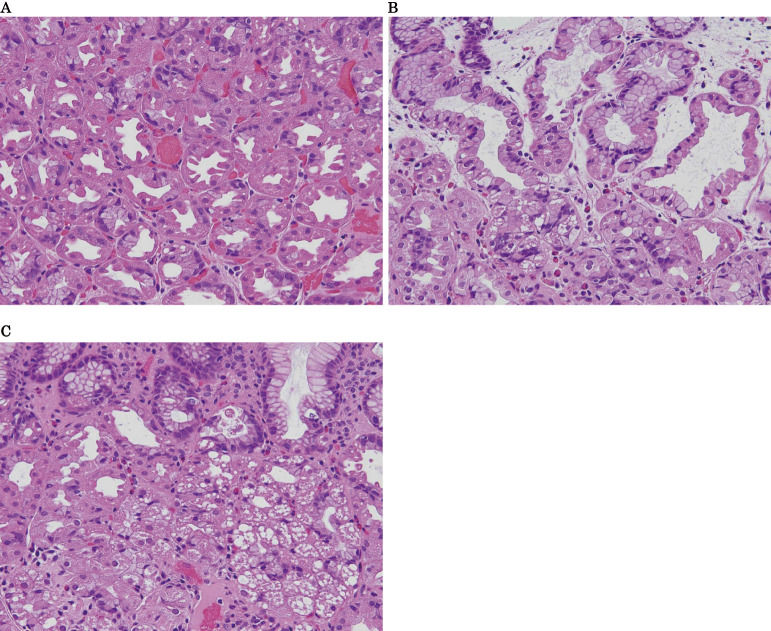
Biopsy samples were obtained from 24 patients in the GCLL group and from 18 in the non-GCLL groups. Of 24 patients with GCLL, 18 (75.0%) were positive for parietal cell hyperplasia (Fig. 2A), 19 (79.2%) for cystic dilatation of the fundic gland (Fig. 2B), and 7 (29.2%) for cytoplasmic vacuolation (Fig. 2C). In contrast, of 18 patients without GCLL, 2 (11.1%) had parietal cell hyperplasia and cystic dilatation of the fundic gland.
Figure 2.
Pathological characteristics of gastric cobblestone-like lesions (GCLLs). A: Parietal cell hyperplasia and protrusions. B: Cystic dilatation of the fundic glands. C: Cytoplasmic vacuolation of parietal cells (All, Hematoxylin and Eosin staining ×20).
Serum gastrin concentrations were measured in nine patients with and five without GCLLs. The gastrin concentrations in patients with GCLLs ranged from 110 pg/mL to more than 3,000 pg/mL, whereas these concentrations in patients without GCLLs ranged from 350 pg/mL to 2,100 pg/mL.
Discussion
This study showed that long-term PPI users experienced three important changes in their gastric mucosa. First, GCLLs were not uncommon in these patients, affecting 35% of 171 patients. Second, GCLLs were found more frequently among patients without than with atrophic gastritis. Finally, GCLLs were able to be pathologically characterized as parietal cell hyperplasia and fundic gland cysts.
Tissue with a cobblestone-like appearance has been observed in patients with pathologic conditions of the esophagogastrointestinal tract, including those with eosinophilic esophagitis, H. pylori gastritis, gastric MALT lymphoma, and Crohn's disease (9-12). The new type of gastric lesion observed in this study is in line with these previous findings, indicating that gastritis, whether due to PPI use or other conditions, can induce a cobblestone-like appearance in gastric tissues.
GCLLs were frequently observed in long-term PPI users. Although the absence of a control group consisting of patients not taking PPIs makes it difficult to conclude that PPI use results in GCLLs, these findings suggest that PPI use is strongly related to the development of GCLLs. In analyzing the clinical characteristics of these patients, we found that GCLLs were more frequent among patients without than with atrophic gastritis. The tissues of the fundic glands decrease under conditions of atrophic gastritis, suggesting that GCLLs may be induced by changes in the fundic glands.
We also found that GCLLs were more frequent among dialysis patients, although the small number of these patients included in this study precluded a precise determination of the relationship between these factors. However, the serum gastrin concentrations were reportedly higher in patients with than without chronic renal failure (13), suggesting that GCLL development may be related to serum gastrin levels. Although this correlation was not observed in this study, further study of the possible correlation between GCLLs and serum gastrin concentrations is necessary.
We originally hypothesized that GCLLs would be more frequent in patients with than without excessive gastric acid secretion. However, we did not observe any correlation between the frequency of GCLLs and the reasons for PPI treatment. Although patients with CGLLs had symptoms such as epigastric pain, we were unable to determine whether or not these symptoms were induced by GCLLs. Because 53% of patients in our GCLL group were asymptomatic, GCLLs are more likely to be asymptomatic lesions than symptomatic ones. Furthermore, because other drugs used to treat these patients were similar in the two groups, GCLLs were likely unrelated to drug-drug interactions.
GCLLs may be induced by changes in the fundic glands, including parietal cell hypertrophy and cystic dilatation of the fundic glands. Although 2 of 18 patients without GCLLs were partially positive for these pathological findings, endoscopic findings in these patients were difficult to evaluate, due to the high degree of atrophy of the gastric corpus.
Long-term use of PPIs has been reported to result in parietal cell protrusions (PCPs) and fundic gland cysts (FGCs) (14). PCPs are intraluminal protrusions of the parietal cells, with swelling and bulging. The results of this study confirmed the occurrence of these pathological changes, which, in clinical practice, can be evaluated endoscopically. Our finding that GCLLs are more frequent in patients without than with atrophic gastritis or H. pylori infection suggests that GCLLs may be due to increased numbers of parietal cells in the gastric mucosa. H. pylori eradication, however, was unrelated to the development of GCLLs in this study.
This study had two major limitations. First, not all long-term users of PPIs underwent UGIE. Second, the histological findings were not investigated in all patients.
GCLLs may become more frequent in Japan, as the rates of H. pylori infection and gastric atrophy have decreased continuously since the 1970s (15). Although the clinical importance of these lesions is currently unclear, these new lesions should be monitored carefully, as they have been histologically characterized as parietal cell hyperplasia, which may induce hypersecretion of acid.
The authors state that they have no Conflict of Interest (COI).
References
- 1. el-Zimaity HM, Jackson FW, Graham DY. Fundic gland polyps developing during omeprazole therapy. Am J Gastroenterol 92: 1858-1860, 1997. [PubMed] [Google Scholar]
- 2. Jalving M, Koornstra JJ, Wesseling J, Boezen HM, De Jong S, Kleibeuker JH. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment Pharmacol Ther 24: 1341-1348, 2006. [DOI] [PubMed] [Google Scholar]
- 3. Hongo M, Fujimoto K; Gastric Polyps Study Group.. Incidence and risk factor of fundic gland polyp and hyperplastic polyp in long-term proton pump inhibitor therapy: A prospective study in Japan. J Gastroenterol 45: 618-624, 2010. [DOI] [PubMed] [Google Scholar]
- 4. Graham JR. Gastric polyposis: Onset during long-term therapy with omeprazole. Med J Aust 157: 287-288, 1992. [DOI] [PubMed] [Google Scholar]
- 5. Jianu CS, Fossmark R, Viset T, et al. . Gastric carcinoids after long-term use of a proton pump inhibitor. Aliment Pharmacol Ther 36: 644-649, 2012. [DOI] [PubMed] [Google Scholar]
- 6. Hatano Y, Haruma K, Ayaki M, et al. . Black spot, a novel gastric finding potentially induced by proton pump inhibitors. Intern Med 55: 3079-3084, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamada T, Murao T, Osawa M, et al. . A total of 379 multiple white and flat elevated lesions in the gastric fundus are induced by acid suppressive agents and long-term proton pump inhibitor treatment. Gastroenterology 148: S-139, 2015. [Google Scholar]
- 8. Haruma K, Shiotani A, Kamada T, et al. . Adverse effects induced by long-term use of proton pump inhibitor: development of gastric polyp. Shoukaki Naika 56: 190-193, 2013. (in Japanese, Abstract in English). [Google Scholar]
- 9. Jung da H, Yun GW, Lee YJ, Jo Y, Park H. Clinicopathologic analysis of proton pump inhibitor-responsive esophageal eosinophilia in Korean patients. Gut Liver 10: 37-41, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vetro C, Romano A, Amico I, et al. . Endoscopic features of gastro-intestinal lymphomas: from diagnosis to follow-up. World J Gastroenterol 20: 12993-13005, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamada T, Hata J, Tanaka A, et al. . Nodular gastritis and gastric cancer. Dig Endosc 18: 79-83, 2006. [Google Scholar]
- 12. Stenson SF, Korzenik J. Inflammatory bowel disease. In: Textbook of Gastroenterology. 4th ed. Yamada T, Alpers DH, Kaplowitz N, et al. , Eds. Lippincott Williams & Wilkins, Philadelphia, PA, 2003: 1699-1759. [Google Scholar]
- 13. Taylor IL, Sells RA, McConnell RB, Dockray GJ. Serum gastrin in patients with chronic renal failure. Gut 21: 1062-1067, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cats A, Schenk BE, Bloemena E, et al. . Parietal cell protrusions and fundic gland cysts during omeprazole maintenance treatment. Hum Pathol 31: 684-690, 2000. [DOI] [PubMed] [Google Scholar]
- 15. Kamada T, Haruma K, Ito M, et al. . Time trends in Helicobacter pylori infection and atrophic gastritis over 40 years in Japan. Helicobacter 20: 192-198, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]