Abstract
A 70-year-old man with diabetes mellitus presented with an enlarged pituitary stalk in 2014. IgG4-related parotitis and submandibular sialoadenitis were diagnosed in 2012. He denied any symptoms related to a pituitary mass. His visual field was intact, and his hypopituitarism was subtle. The serum IgG4 level was elevated. A lip biopsy revealed strong fibrosis and hyper-infiltration of IgG4-positive plasma cells. Based on these findings, IgG4-related hypophysitis was diagnosed. The patient was carefully followed without specific intervention. His clinical condition showed no change until December 2016, suggesting a stable, natural course. Care should be taken when considering glucocorticoid therapy, especially for elderly diabetic patients, given possible side effects.
Keywords: IgG4, hypophysitis, hypopituitarism, glucocorticoid, diabetes mellitus, natural course
Introduction
IgG4-related hypophysitis is a novel clinical entity characterized by the enlargement of the pituitary stalk, an elevated serum IgG4 level, and infiltration by IgG4-positive plasma cells in the pituitary gland (1). The first case was reported in 2004, and to date, 34 cases have been documented in the English-language literature (2). However, all of these cases were associated with severe hypopituitarism or symptoms caused by pituitary mass effect and treated with glucocorticoids and/or hormonal replacement therapy immediately after the diagnosis. Therefore, the natural course of IgG4-related hypophysitis is unknown, and the indications for glucocorticoid therapy have not been explored. We herein report an unusual case of IgG4-related hypophysitis associated with diabetes mellitus in which the visual function was normal and the hypopituitarism remained subtle for over four years despite a lack of treatment.
Case Report
In November 2014, a 70-year-old Japanese man was admitted to our department with an enlarged pituitary stalk. He gradually developed akinesia and gait disturbance, which became noticeable in March 2014. After Parkinson's disease was diagnosed in October 2014 at the Department of Neurology, the patient underwent magnetic resonance imaging (MRI) of the brain.
The patient had suffered since 1992 from diabetes mellitus, hypertension, dyslipidemia, hyperuricemia, and benign prostatic hyperplasia with urinary symptoms, all of which were treated with oral medications such as glibenclamide, valsartan, tocopherol, allopurinol, and tamsulosin. His medical history also included IgG4-related parotitis and submandibular sialoadenitis, clinically diagnosed at the Department of Rhinolaryngology in April 2012 without a biopsy on the basis of an increase in the serum IgG4 level (604 mg/dL). At the time, enlargement of the pituitary stalk was visible on whole-body contrast computed tomography (CT), but no further investigations were performed.
On presentation at the hospital, the patient was awake, alert, and orientated without headache, nausea, diplopia, or stomachache. His vital signs were normal. His height was 165.8 cm, and his weight was 69.9 kg. The bilateral submandibular glands were enlarged and elastic-hard without tenderness. No signs of Cushing's syndrome, acromegaly or hyperthyroidism were noted. Pigmentation, goiter, and enlargement of the lymph nodes were absent. He did not have trouble with his eyesight or diabetic retinopathy. The abdominal findings were normal. A neurological examination revealed reduced deep tendon reflexes and decreased vibratory sensation in addition to the characteristic physical findings of Parkinson's disease, such as mask-like face, rigidity, and slurred voice.
Laboratory results revealed the following: urine albumin 4.1 mg/day, serum sodium 141 mEq/L, potassium 4.1 mEq/L, total bilirubin 0.5 mg/dL, aspartate aminotransferase 22 U/L, alanine aminotransferase 24 U/L, amylase 586 U/L, creatinine 0.96 mg/dL, C-reactive protein 0.07 mg/dL, fasting plasma glucose 108 mg/dL, HbA1c 6.6% [National Glycohemoglobin Standardization Program (NGSP)], C-peptide immunoreactivity 1.71 ng/mL, IgG 1,651 mg/dL (normal reference range: 870-1,700 mg/dL), IgG4 425 mg/dL (normal reference range: 4.8-105 mg/dL), and IgE 617.7 IU/mL (normal reference range: ≤202.3 IU/mL). We observed normal levels of soluble interleukin (IL)-2 receptor, angiotensin I-converting enzyme, tumor markers, and autoimmune antibodies including anti-glutamic acid decarboxylase (GAD), anti-thyroglobulin antibody, anti-thyroid peroxydase antibody, anti-Sjögren's syndrome (SS)-A/Ro, and anti-SS-B/La antibodies. In addition, the QuantiFERON TB-3G test was negative.
An assessment of the anterior pituitary function showed the following: urine free cortisol 26.5 μg/day [normal reference range: 11.2-80.3 μg/day, immunoradiometric assay (IRMA)], plasma adenocorticotropic hormone (ACTH) 24.9 pg/mL [(7.2-63.3 pg/mL, electro-chemiluninescence immunoassay (ECLIA)], serum cortisol 10.5 μg/dL (6.2-19.4 μg/dL, ECLIA), thyroid stimulating hormone (TSH) 2.85 μIU/mL (0.500-4.300 μIU/mL, ECLIA), Free T3 2.30 pg/mL (2.30-4.10 pg/mL, ECLIA), Free T4 0.64 ng/dL (0.70-1.70 ng/dL, ECLIA), growth hormone (GH) 0.08 ng/mL (<2.47 ng/mL, ECLIA), insulin-like growth factors (IGF)-1 112 ng/mL (63-206 ng/mL for a 70-year-old man, IRMA), luteinizing hormone (LH) 1.9 mIU/mL (0.79-5.72 mIU/mL, CLIA), follicle stimulating hormone (FSH) 2.63 mIU/mL (2.00-8.30 mIU/ml, CLIA), prolactin (PRL) 21.8 ng/mL (4.29-13.69 ng/mL, ECLIA), and testosterone 0.17 ng/mL (1.31-8.71 ng/mL, ECLIA). The ACTH, cortisol, TSH, PRL, and GH responses to subsequent provocative tests were normal, whereas the LH and FSH responses decreased (Table 1). An assessment of the posterior pituitary function revealed a low antidiuretic hormone (ADH) level but normal urine concentration capacity: ADH <0.8 pg/mL [≤3.8 pg/mL, double-antibody radioimmuno assay (RIA)], spot urine osmolality 682 mOsm/kg H2O, and urine output <1,500 mL/day. During a subsequent water deprivation test, the ADH level remained low, whereas the urine osmolality exceeded 300 mOsm/kg H2O, and urine output stopped in the middle of the test (Table 2). The urine osmolality also exceeded 300 mOsm/kg H2O in a later hypertonic saline test, whereas the ADH level showed paradoxically low values (Table 3). His pituitary insufficiency was slight and did not require hormonal replacement therapy using hydrocortisone, thyroxine, or desmopressin.
Table 1.
Responses of Pituitary and Adrenal Hormones to Intravenous Injection of CRH (100 µg), GRH (100 µg)/GHRP-2 (100 µg), TRH (500 µg), and LHRH (100 µg).
| Year | Nov 2014 | Dec 2015 | Dec 2016 | |||
|---|---|---|---|---|---|---|
| Values | Basal | Peak | Basal | Peak | Basal | Peak |
| TSH, μIU/mL | 2.17 | 21.6 | 2.13 | 21.2 | 1.82 | 9.89 |
| Cortisol, μg/dL | 4.70 | 19.0 | 10.0 | 18.1 | 6.27 | 15.8 |
| ACTH, pg/mL | 8.50 | 47.7 | 24.8 | 57.9 | 17.5 | 43.2 |
| GH, ng/mL | 0.10 | 4.05 | 0.10 | 4.37 | 0.75 | 4.55 |
| (GRH 100 µg) | (GRH 100 µg) | (GHRP-2 100 µg) | ||||
| LH, mIU/mL | 2.32 | 9.50 | 1.98 | 10.0 | 2.65 | 12.4 |
| FSH, mIU/mL | 2.89 | 5.28 | 4.24 | 8.44 | 5.87 | 10.8 |
| PRL, ng/mL | 18.2 | 46.3 | 17.9 | 37.2 | 10.8 | 26.5 |
CRH: corticotropin-releasing hormone, GRH: growth hormone (GH)-releasing hormone, GHRP-2: GH-releasing peptide-2, TRH: thyrotropin-releasing hormone, LHRH: lutenizing hormone (LH)-releasing hormone ACTH: adenocorticotropic hormone, FSH: follicle stimulating hormone, PRL: prolactin
Table 2.
Responses of ADH, Urine Osmolality, and Serum Osmolality to Water Deprivation in 2014.
| Time point | 0 min | 60 min | 120 min | 180 min | 240 min |
|---|---|---|---|---|---|
| Serum osmolality, mOsm/kg·H2O | 287 | 288 | 289 | 289 | 287 |
| Urine osmolality, mOsm/kg·H2O | 203 | 238 | No urine output | 409 | No urine output |
| ADH, pg/mL | <0.8 | <0.8 | 1.1 | <0.8 | <0.8 |
| Body weight, kg | 68.5 | 67.9 |
ADH: antidiuretic hormone
Table 3.
Responses of Serum Na, Serum Osmolality, Urine Osmolality, and ADH to Hypertonic Saline Test in 2015.
| Time point | 0 min | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|
| Serum Na, mEq/L | 139 | 143 | 145 | 149 | 151 |
| Serum osmolality, mOsm/kg·H2O | 290 | 297 | 300 | 304 | 308 |
| Urine osmolality, mOsm/kg·H2O | No urine output | No urine output | 605 | No urine output | 644 |
| ADH, pg/mL | 1.3 | <0.8 | <0.8 | <0.8 | <0.8 |
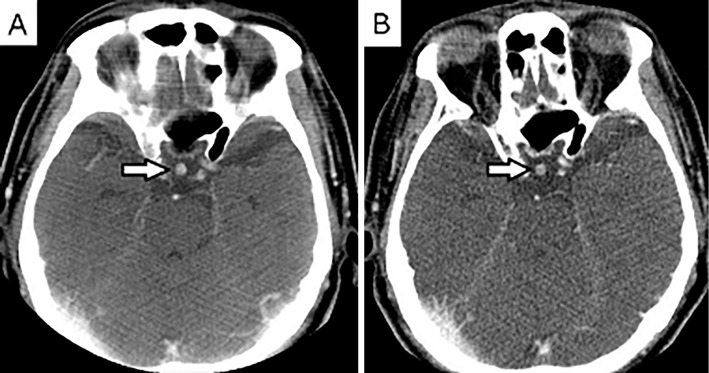
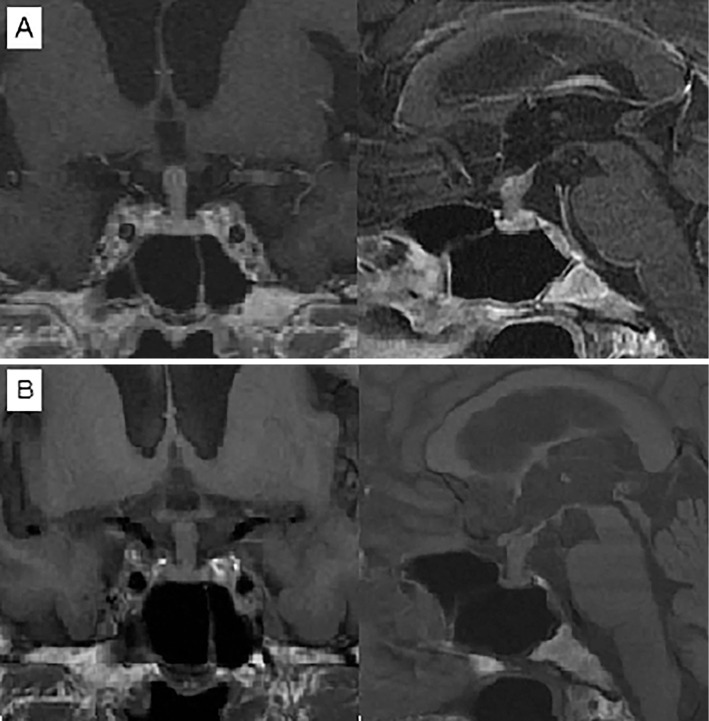
Head CT showed a thickened pituitary stalk. The same degree of thickening was previously detected in 2012 (Fig. 1). MRI of the pituitary gland corroborated these findings. T1-weighted imaging failed to visualize a physiological posterior pituitary bright spot clearly, but the thickened pituitary stalk was uniformly enhanced after gadolinium administration despite not being adjacent to the optic chiasm (Fig. 2).
Figure 1.
A: Head CT taken in 2012 showed a thickened pituitary stalk. B: Head CT taken in 2014 showed no appreciable change in the diameter of the pituitary stalk.
Figure 2.
A: The sagittal and coronal sections of the head from a gadolinium-enhanced MRI brain scan in 2014 showed enlargement of the stalk. B: Head MRI in 2016 showed no appreciable change in the diameter of the pituitary stalk. The posterior pituitary bright spot was absent.
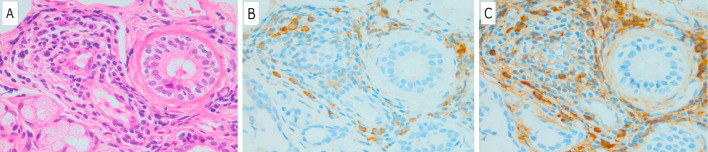
A lip biopsy revealed atrophy and fibrosis of the acinar cells in addition to lymphocyte infiltration. There were more than 10 IgG4-positive plasma cells per high-power field (HPF), and the IgG/IgG4-positive plasma cell ratio exceeded 40% (Fig. 3). The elevated serum IgG4 level and enlargement of the pituitary stalk corroborated the diagnosis of IgG4-related hypophysitis, which was later confirmed by the pathological diagnosis of IgG4-related parotitis and submandibular sialoadenitis.
Figure 3.
A biopsy specimen obtained from the lip demonstrated inflammatory cell infiltration. A: Hematoxylin and Eosin staining, ×400 magnification. B: Stained with IgG monoclonal antibody, ×400 magnification. C: Stained with IgG4 monoclonal antibody, ×400 magnification (more than 10 IgG4-positive plasma cells per HPF). HPF: high-power field
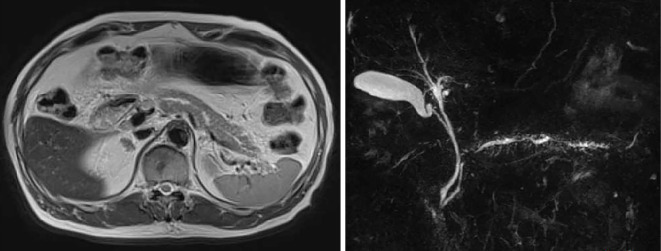
Abdominal MRI showed slight enlargement of the pancreas and a tortuous, narrowing duct (Fig. 4). The patient also had autoimmune pancreatitis, which did not require any treatment given the absence of abdominal symptoms, physical findings, and laboratory data. Considering his age as well as his diabetes mellitus, glucocorticoid therapy was not started.
Figure 4.
The T2-weighted horizontal section of the abdomen from an MRI abdominal scan and magnetic resonance cholangiopancreatography in 2014 showed enlargement of the pancreas and a tortuous duct with stenosis.
Follow-up abdominal MRI in September 2015 showed that stenosis of the tortuous duct was worsening, although the patient denied abdominal pain, vomiting, or diarrhea. The liver function test results remained normal, and amylase decreased to 392 U/L. The IgG4 level was 605 mg/dL. Enhanced abdominal CT and endoscopic ultrasonography denied sclerosing cholangitis and pancreatic cancer. Glucocorticoid therapy was offered as an option to prevent the future loss of the pancreatic function, but the patient refused it, claiming not to have had any trouble with his pancreatitis in daily life and fearing possible side effects.
At the final examination on December 2016, he denied headache, polyuria, vomiting, abdominal pain, and diarrhea, which might have been caused by the pituitary mass effect, hypopituitarism or worsening of the pancreatitis. He lost weight after stopping glibenclamide and maintained a body weight of 65 kg. His HbA1c was maintained at around 7.0% by metformin, mitiglinide, and miglitol. The IgG4 level was 567 mg/dL, while the liver function test results remained normal and amylase decreased to 205 U/L. His anterior and posterior pituitary function showed almost no change. Urine free cortisol was 26.3 μg/day (IRMA). The ACTH, TSH, and PRL responses to provocative tests were normal, whereas the LH and FSH responses were delayed. Although the GH response to GH-releasing peptide-2 decreased, IGF-1 was 112 ng/mL. Serum Na was 138 mEq/L, and the urine osmolality was 548 mOsm/kg H2O after an overnight water deprivation test. The same degree of pituitary stalk thickening was observed on an MRI brain scan in November 2016.
Discussion
The present case is noteworthy because it elucidates certain aspects of the natural history of IgG4-related hypophysitis and raises for discussion the issue of the indications for glucocorticoid therapy.
Leporati et al. (3) suggested three criteria for the diagnosis of IgG4-related hypophysitis: 1) mononuclear cell infiltration in the biopsied pituitary gland with more than 10 IgG4-positive cells per HPF; 2) IgG4-positive lesions in other biopsied organs with typical pituitary MRI findings, such as a pituitary mass and a thickened pituitary stalk; and 3) typical pituitary MRI findings, elevated serum IgG4 level (>140 mg/dL), and rapid improvement following the administration of glucocorticoids. Our patient met the second criterion, and IgG4-related hypophysitis was diagnosed. However, because a pituitary biopsy had not been performed, we were unable to exclude other etiologies of the hypophysitis definitively.
The first-line therapy for IgG4-related hypophysitis is glucocorticoid therapy, which shrinks the swollen stalk, treats symptoms associated with mass effect, and in some cases restores the anterior pituitary function. There have been no reports of the recovery of the posterior pituitary function. Generally, the response to glucocorticoid therapy is good, but relapses sometimes occur. More than half of the published cases were treated with high-dose prednisolone (0.5-1.0 mg/kg per day), while the others were treated with a lower dose of prednisolone or a replacement dose of hydrocortisone (4). There is as yet no agreement on a standard dosage for glucocorticoids, and excessive doses are not advised for elderly patients due to the possibility of unwanted side effects (5,6).
The indications for glucocorticoid therapy for IgG4-related hypophysitis have not been discussed in the literature. According to a review of pituitary autoimmune disease by Glezer et al. (7), glucocorticoids should be administrated to patients with headache, a visual deficit, or other symptoms due to mass effect. They also mentioned hormonal replacement as the only feasible treatment option for hypopituitarism without these symptoms. Our patient showed a normal visual function and subtle hypopituitarism limited to the pituitary-gonadal axis and GH secretion. Although the peak cortisol value on a provocative test in 2016 was slightly lower than in the previous two years, the normal ACTH response, the normal urine free cortisol values, and the absence of clinical symptoms denied pituitary adrenocortical insufficiency requiring cortisol replacement. The patient did not consent to an insulin tolerance test due to his concerns about the risk of seizure and ischemic heart disease. His normal urine concentration capacity and urine output were incompatible with diabetes insipidus. Although his serum ADH level was significantly low, it could have been a false negative, considering the poor accuracy of the ADH assay in Japan. Indeed, the absence of a posterior pituitary bright spot on MRI in a patient without clinical diabetes insipidus was reported by Ngaosuwan et al (8), who speculated that the decrease in ADH secretion was not severe enough to have caused symptoms. Because our patient was elderly and suffered from Parkinson's disease as well as diabetes mellitus, which had been treated with glibenclamide for over 20 years, glucocorticoid therapy would have worsened his glycemic control, necessitated insulin injections, and exposed him to an increased risk of infectious diseases, falls, and fractures due to muscular atrophy and osteoporosis (9). For the patient, these risks were deemed to be more serious than those posed by the future loss of the pituitary function.
According to the consensus guidelines for autoimmune pancreatitis by Kamisawa et al. (10), our patient did not meet the criteria for glucocorticoid therapy, such as the presence of obstructive jaundice, abdominal pain, back pain, or sclerosing cholangitis. Furthermore, androgen replacement therapy and recombinant human GH therapy were not indicated due to symptomatic benign prostatic hyperplasia and diabetes mellitus. Therefore, we decided to follow the patient carefully.
The CT findings in 2012 show that his hypophysitis was already present at the time, indicating that his anterior and posterior pituitary continued to function for over four years without any treatment for the hypophysitis. No previous reports have described the natural course of IgG4-related hypophysitis. All previously reported cases were associated with symptoms of pituitary mass effect or severe hypopituitarism and treated by glucocorticoid and/or hormonal replacement therapy immediately after the diagnosis.
In conclusion, we presented the first known case of IgG4-related hypophysitis with subtle hypopituitarism, normal visual function, and diabetes mellitus. The patient was followed without glucocorticoid or hormonal replacement therapy for over four years, suggesting that the natural course of IgG4-related hypophysitis may be stable. Clinicians should exercise caution when considering glucocorticoid therapy, especially for elderly patients with diabetes mellitus, given the potential side effects.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Mr. James R. Valera for his editorial assistance. We would also like to thank the Tama Society of Clinical Endocrinology and Metabolism for their insight and helpful advice on the management of this patient.
References
- 1. Shimatsu A, Oki Y, Fujisawa I, et al. Pituitary and stalk lesions (infundibulo-hypophysitis) associated with immunoglobulin G4-related systemic disease: an emerging clinical entity. Endocr J 56: 1033-1041, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Shikuma J, Kan K, Ito R, et al. Critical review of IgG4-related hypophysitis. Pituitary 20: 282-291, 2017. [DOI] [PubMed] [Google Scholar]
- 3. Leporati P, Landek-Salgado MA, Lupi I, et al. IgG4-related hypophysitis: a new addition to the hypophysitis spectrum. J Clin Endocrinol Metab 96: 1971-1980, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iseda I, Hida K, Tone A, et al. Prednisolone markedly reduced serum IgG4 levels along with the improvement of pituitary mass and anterior pituitary function in a patient with IgG4-related infundibulo-hypophysitis. Endocr J 61: 195-203, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Sosa GA, Bell S, Christiansen SB, et al. Histologically confirmed isolated IgG4-related hypophysitis: two case reports in young women. Endocrinol Diabetes Metab Case Rep 2014: 140062, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harano Y, Honda K, Akiyama Y, et al. A case of IgG4-related hypophysitis presented with hypopituitarism and diabetes insipidus. Clin Med Insights Case Rep 8: 23-26, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glezer A, Bronstein MD. Pituitary autoimmune disease: nuances in clinical presentation. Endocrine 42: 74-79, 2012. [DOI] [PubMed] [Google Scholar]
- 8. Ngaosuwan K, Trongwongsa T, Shuangshoti S. Clinical course of IgG4-related hypophysitis presenting with focal seizure and relapsing lymphocytic hypophysitis. BMC Endocr Disord 15: 64-71, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McDonough AK, Curtis JR, Saag KG. The epidemiology of glucocorticoid-associated adverse events. Curr Opin Rheumatol 20: 131-137, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Kamisawa T, Okazaki K, Kawa S, et al. Japanese consensus guidelines for management of autoimmune pancreatitis. J Gastroenterol 45: 471-477, 2010. [DOI] [PubMed] [Google Scholar]