Abstract
We herein describe the first known case of pleuritis caused by Mycobacterium kyorinense without pulmonary involvement. A 48-year-old man undergoing immunosuppressant therapy presented with cough and dyspnea. An accumulation of pleural fluid was noted; however, computed tomography revealed no pulmonary lesions. Cultures of the fluid yielded non-tuberculous mycobacteria, which was identified as Mycobacterium kyorinense. The patient recovered after 6 months of therapy with clarithromycin and moxifloxacin. Clinicians should be aware that Mycobacterium kyorinense can cause pleuritis without pulmonary involvement. When mycobacterial species are isolated from the pleural fluid, precise identification and drug susceptibility testing are warranted.
Keywords: Mycobacterium kyorinense, pleuritis, clarithromycin, moxifloxacin
Introduction
Mycobacterium kyorinense is a non-pigmented, slowly growing mycobacterium that was first described in 2009 by Okazaki et al. in Japan (1). Thus far, 15 cases of M. kyorinense infection have been described in the literature (2-6). Most of the reported cases involved pulmonary infections; however, two patients with extra-pulmonary infections have been described: one had lymphadenitis and the other arthritis. Pleuritis is a rare presentation of non-tuberculous mycobacteriosis, and there have been no reports of pleuritis caused by M. kyorinense or its phylogenetically related species, M. celatum and M. branderi.
The purpose of this article is to report the first known case of pleuritis caused by M. kyorinense without pulmonary involvement and its successful treatment with clarithromycin and moxifloxacin.
Case Report
A 48-year-old man presented to our emergency department with cough and dyspnea lasting for 5 days. The patient, who had been diagnosed with follicular lymphoma (grade 1-2) 10 years earlier, had undergone chemotherapy as a treatment for lymphoma. Three years later, he achieved a complete response after undergoing unrelated allogeneic bone marrow transplantation, which was complicated by invasive Aspergillosis and disseminated Herpes zoster. The patient had received prednisolone (20 mg/day) and cyclophosphamide (20 mg/day) for 3 years as treatment for chronic graft versus host disease (GVHD) with skin lesions and chronic bilateral pleural effusion. The amount of pleural effusion remained unchanged until one month prior to his presentation.
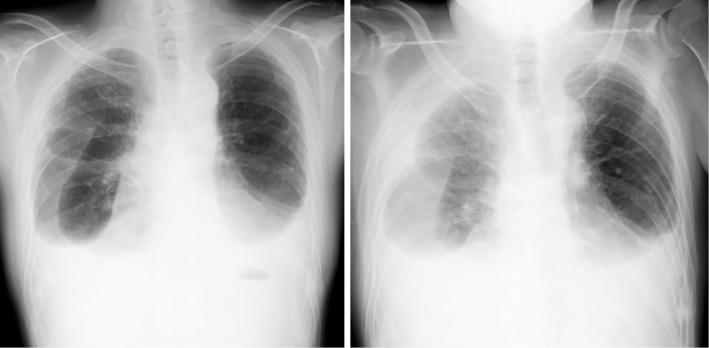
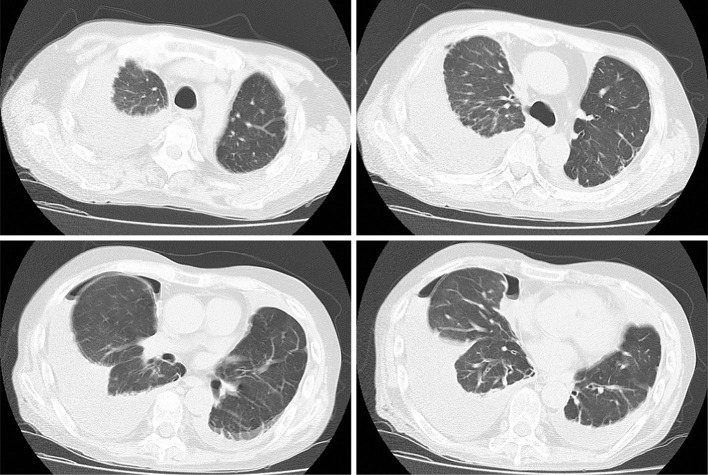
The patient weighed 57 kg and was 169 cm tall. His vital signs on admission were as follows: temperature, 37.8℃; pulse rate, 130 beats per minute; blood pressure, 185/127 mmHg; respiratory rate, 30 per minute; and oxygen saturation, 93% in room air. Auscultation revealed that the patient's breath sounds were weak. He had pitting edema on his lower limbs. Chest X-ray revealed that the accumulation of right pleural effusion had increased compared with a chest X-ray film obtained one month earlier (Fig. 1). Computed tomography of the chest demonstrated no pulmonary lesion (Fig. 2). Laboratory tests showed an increased white blood cell count (12,300/mm3), a decreased lymphocyte count (676/mm3), an elevated C-reactive protein level (10.16 mg/dL), a high creatinine concentration (1.23 mg/dL), and a high blood urea nitrogen concentration (33 mg/dL). An interferon-γ (IFN-γ) release assay (T-SPOT. TB; Oxford Immunotec, Marlborough, USA) was negative. The results of the other laboratory tests, including tests of the patient's liver enzyme, serum glucose, and electrolyte levels, were all within normal ranges.
Figure 1.
Chest X-ray films: the amount of right pleural effusion at admission (right) was increased in comparison to one month earlier (left).
Figure 2.
Computed tomography showing bilateral pleural effusion. Note that no pulmonary lesions were detected throughout the lung fields, including in the images shown here.
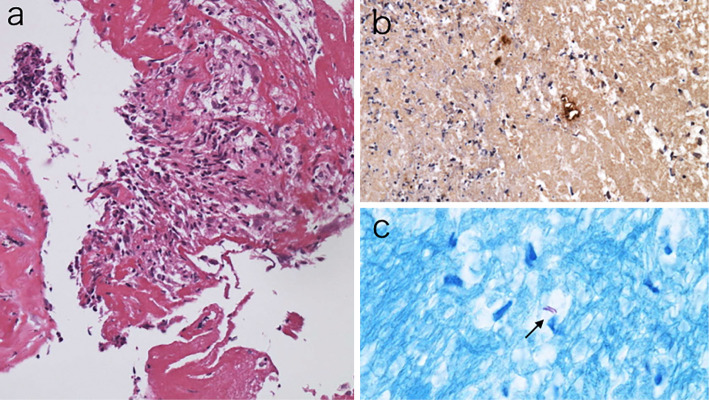
The right pleural fluid was yellowish and clear. An analysis of the fluid revealed a high white blood cell count (13,300 /μL) with a neutrophil predominance (94%), increased levels of protein (3.2 g/dL) and lactate dehydrogenase (LDH, 4,658 IU/L; serum level was 407 IU/L), a decreased level of glucose (11 mg/dL), and an increased level of adenosine deaminase (51.5 U/L). The results of Gram staining and bacterial cultures were negative. A cytological examination of the fluid detected no malignant cells. Three consecutive pleural fluid samples were positive for acid-fast staining, and the cultures of the fluid yielded mycobacteria. Thoracoscopy performed under local anesthesia revealed thickened, hyperemic, and edematous pleura with multiple areas of adhesion, fibrotic septa, and necrosis (Fig. 3). Histopathology of the biopsy specimen of the parietal pleura showed granulomas surrounded by neutrophil infiltration and fibrous changes (Fig. 4). Acid-fast staining of the biopsy specimen was positive, and the cultures of the specimen yielded mycobacteria. Polymerase chain reactions (PCRs) for M. tuberculosis, M. avium, and M. intracellulare were all negative, and no strains were identified using a commercially available DNA-DNA hybridization (DDH) method (DDH Mycobacteria ‘Kyokuto’, Kyokuto Pharmaceutical, Tokyo, Japan).
Figure 3.
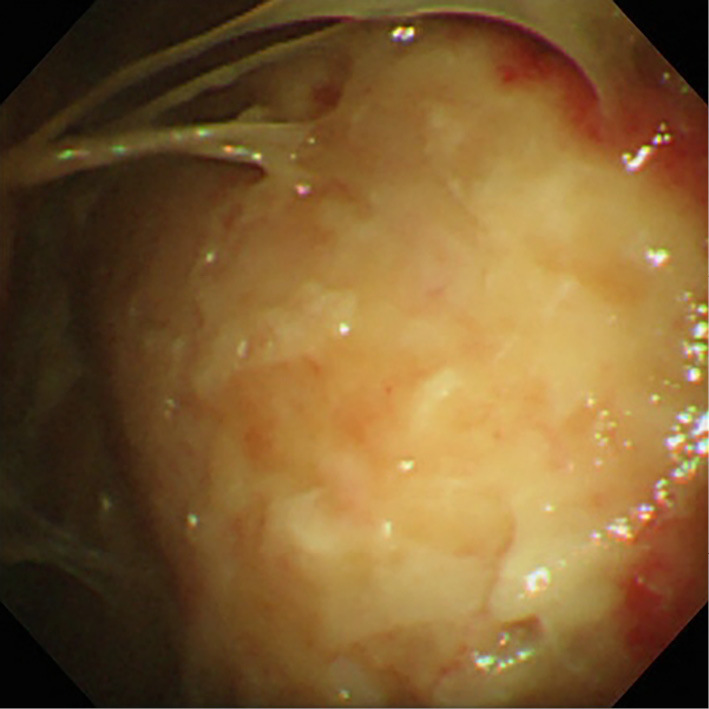
A photograph obtained during a thoracoscopic examination revealing thickened, hyperemic, and edematous pleura with multiple areas of adhesion, fibrotic septa, and necrosis.
Figure 4.
Photomicroscopies of the pleural biopsy specimen of the right parietal pleura showing granulomas surrounded by neutrophil infiltration and fibrous changes (a). Anti-Bacillus Calmette-Guerin (BCG) immunostaining (b) and Ziel-Neelsen staining (c) of the biopsy materials were positive for acid-fast bacilli.
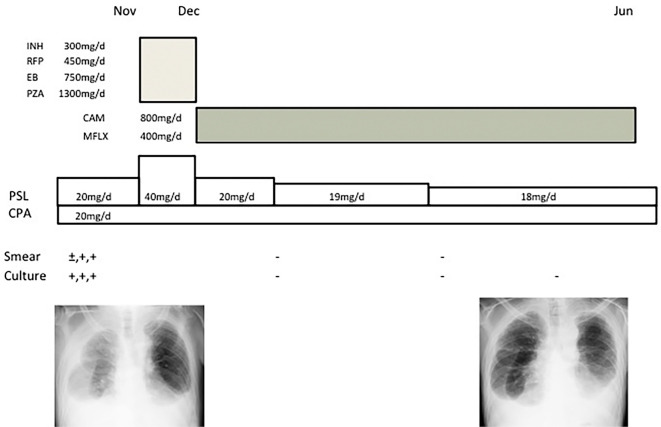
We made a tentative diagnosis of tuberculous pleuritis, and the patient received empirical therapy with isoniazid (300 mg/day), rifampin (450 mg/day), ethambutol (750 mg/day) and pyrazinamide (1,300 mg/day) for a month but showed no improvement (Fig. 5). Phylogenetic analyses of the 16S rRNA and hsp65 of the isolates from the first effusion sample were consistent with the gene sequences of the standard strain of M. kyorinense (1). Based on these results, the patient was diagnosed with pleuritis due to M. kyorinense. Drug susceptibility testing (Table) of the same isolates using the proportion test method (Vite Spectrum SR; Kyokuto Pharmaceutical, Tokyo, Japan) and the micro-dilution test for non-tuberculous mycobacteria (BrothMIC NTM; Kyokuto Pharmaceutical, Tokyo, Japan) revealed that the strain was sensitive to streptomycin, ethionamide, kanamycin, levofloxacin, clarithromycin, and amikacin and resistant to isoniazid, rifampin, and ethambutol. We therefore decided to change the therapeutic regimen to a combination of clarithromycin (800 mg/day) and moxifloxacin (400 mg/day). Moxifloxacin was selected instead of levofloxacin as a fluoroquinolone due to the patient's chronic renal failure. After six months of therapy with these drugs, the patient recovered with no adverse effects from the therapeutic agents.
Figure 5.
The clinical course. (INH: isoniazid, RFP: rifampin, EB: ethambutol, PZA: pyrazinamide, CAM: clarithromycin, MFLX: moxifloxacin, PSL: prednisolon, CPA: cyclophosphamide)
Table.
Drug Susceptibility Testings.
| Drug | Susceptibility | Drug | MIC(μg/dL) | |
|---|---|---|---|---|
| SM 10 | S | SM | 0.25 | |
| PAS 0.5 | R | EB | 2 | |
| INH 0.2 | R | KM | 0.25 | |
| INH 1.0 | R | RFP | 32 | |
| RFP 40 | R | RFB | 1 | |
| TH 20 | S | LVFX | 0.06 | |
| KM 20 | S | CAM | ≤0.03 | |
| EVM 20 | S | TH | ≤0.5 | |
| EB 2.5 | R | AMK | ≤0.5 | |
| CS 30 | R | (Micro-dilusion method) | ||
| LVFX 1.0 | S | |||
| (Proportion test method) | ||||
SM: streptomycin, PAS: para-aminosalicylic acid, INH: isoniazid, RFP: rifampin, TH: ethionamide, KM: kanamycin, EVM: enviomycin, EB: ethambutol, CS: cycloserine, LVFX: levofloxacin, CAM: clarithromycin, AMK: amikacin
Discussion
We herein describe the first case of pleuritis caused by M. kyorinense without evidence of pulmonary involvement in which antimicrobial treatment (clarithromycin and moxifloxacin) resulted in a favorable outcome.
Mycobacterial pleuritis is commonly caused by M. tuberculosis, while non-tuberculous mycobacteria (NTM) account for only 4-10% of patients with the disease (7-9). Among the causative species of NTM pleuritis, the most prevalent is Mycobacterium avium-intracellulare complex (MAC). A study from Taiwan reported that MAC accounted for 47% of NTM pleuritis cases, following rapidly growing mycobacteria (M. fortuitum, M. chelonae and M. abscessus; 29%) and M. kansasii (9%) (7). In contrast, in Japan, where rapidly growing mycobacteria are less prevalent, the causative species is almost exclusively MAC (8,9). In addition to these dominant species, however, rare NTM species, including M. xenopi, M. flavescens (7), M. scrofulaceum (10), M. lentiflavum (11), and M. ulcerans (12), have also been reported as the causative pathogens of pleural infection.
Pleural tuberculosis occurs by direct extension when a subpleural caseous focus is discharged into the pleural space, or through hematogenous seeding (13). As hematogenous seeding is confined to patients with severely impaired immunity, including HIV infection, the former mechanism is thought to be the main pathogenesis in NTM pleuritis. NTM pleuritis is uncommon, and primary NTM pleuritis without apparent pulmonary involvement is particularly rare, with only four cases reported in the literature (14-17). It has been suggested that MAC infection initially occurs in the centriacinar region in the lung and develops into air space consolidation and bronchial wall involvement with a low incidence of lymphatic abnormality (18). Consequently, subpleural caseous focus formation, or the lymphogenous spread of bacilli to the pleura may be relatively uncommon in NTM. The pathogenesis of M. kyorinense pleuritis in our patient is unknown; however, most patients with pulmonary M. kyorinense infection are immunocompetent, whereas patients with extrapulmonary disease have co-existing diseases, such as myelodysplastic syndrome and rheumatoid arthritis, which suppress the cellular immune system-although not as severely as HIV infection. We hypothesize that the impairment of cellular immunity by immunosuppressant agents was one of the contributing factors that resulted in primary pleural infection in the present case.
Our patient had neutrophil-dominant pleural effusion with an elevated LDH level and a decreased glucose level, which suggested a bacterial infection. In patients with NTM and tuberculous pleuritis, lymphocytes are usually dominant in the pleural effusion. However, Bai et al. reported that the effusion of most cases (90%) of tuberculous empyema showed neutrophilic leukocytosis with a high LDH and a low glucose concentration (19). They noted the presence of a concomitant bacterial infection in about 30% of cases, most of which had hydropneumothorax. In our case, the appearance of the pleural fluid was not pus-like, instead showing characteristics consistent with bacterial empyema, including neutrophil-dominant leukocytosis associated with elevated LDH and decreased glucose levels. A bacterial co-infection was excluded because no bacteria other than mycobacteria were obtained from repeatedly drawn fluid samples and there was no evidence of hydropneumothorax. In a case report of a patient with smear-positive M. avium pleuritis, the pleural effusion was neutrophil-dominant (96%) with an elevated LDH (5,986 IU/L) and a decreased glucose (37 mg/dL) concentration (20). We speculated that an increased mycobacterial burden in the pleural cavity induced the development of neutrophilic inflammation associated with high LDH and low glucose levels mimicking empyema. Alternatively, long-term immunosuppressing treatment, especially cyclophosphamide, may suppress lymphocyte proliferation and migration (21).
The optimal therapy for M. kyorinense infection is unclear, as the data are based on case reports. Ohnishi et al. reported that, among seven patients, treated with first-line anti-tuberculosis drugs (mainly rifampin, isoniazid, and ethambutol), no patients recuperated, whereas the infections of 5 of 6 patients who received combinations of antimicrobial drugs (including macrolides and fluoroquinolones) were subdued without recurrence (2). The MICs of rifampin (>32 μg/mL), isoniazid (0.5-32 μg/mL), and ethambutol (1-128 μg/mL) were relatively high, while the MICs of macrolides (0.03-0.125 μg/mL), aminoglycosides (0.5-1 μg/mL), and fluoroquinolones (0.06-0.25 μg/mL) were relatively low. Note that the MIC of rifampin is consistently high. For the strain infecting our patient, the MICs of rifampin (32 μg/mL) and ethambutol (2 μg/mL) were high, whereas those of clarithromycin (<0.03 μg/mL), amikacin (<0.5 μg/mL), and levofloxacin (0.06 μg/mL) were low. Furthermore, macrolides and fluoroquinolones (especially moxifloxacin) were shown to penetrate the empyemic pleural effusion well (22-24), while the penetration of rifampin was poor (25). Therefore, from a pharmacokinetic perspective, a therapeutic regimen that includes clarithromycin and moxifloxacin seems reasonable for the treatment of pleuritis caused by M. kyorinense.
Clinicians should bear in mind that rare species of NTM like M. kyorinense can cause pleuritis in the absence of any apparent pulmonary involvement. Note that M. kyorinense is consistently resistant to first-line anti-tuberculosis agents, especially rifampin. When mycobacterial species are isolated from the pleural fluid, then precise identification and drug susceptibility testing are warranted.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful to Dr. Kiyofumi Ohkusu, a professor in the Department of Microbiology, Tokyo Medical University, for performing the identification of the isolate.
References
- 1. Okazaki M, Ohkusu K, Hata H, et al. Mycobacterium kyorinense sp. nov., a novel, slow-growing species, related to Mycobacterium celatum, isolated from human clinical specimens. Int J Syst Evol Microbiol 59: 1336-1341, 2009. [DOI] [PubMed] [Google Scholar]
- 2. Ohnishi H, Yonetani S, Matsushima S, et al. Mycobacterium kyorinense infection. Emerg Infect Dis 19: 508-510, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kobashi Y, Mouri K, Obase Y, Kato S, Oka M. Pulmonary Mycobacterium kyorinense disease showed clinical improvement following combined therapy with clarithromycin and levofloxacin. Intern Med 51: 1923-1926, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Terada Y, Takeshita K, Baba R, Hiraoka R, Suzuki Y. Case report; a case of Mycobacterium kyorinense pulmonary infection. Nihon Naika Gakkai Zasshi (J Jpn Soc Intern Med) 101: 2301-2303, 2012. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 5. Sakakibara Y, Kishimoto K, Kojima K, Fujie T, Inase N. Fatal nontuberculous mycobacterial lung disease caused by Mycobacterium kyorinense: a case report with five years of follow-up. Kekkaku 89: 509-513, 2014. (in Japanese). [PubMed] [Google Scholar]
- 6. Muruganandan S, Jayaram L, Wong JS, Guy S. Pulmonary cavitary Mycobacterium kyorinense (M. kyorinense) infection in an Australian woman. IDCases 2: 37-39, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shu CC, Lee LN, Wang JT, Chien YJ, Wang JY, Yu CJ; Taiwan Anti-Mycobacteria Investigation (TAMI) Group Non-tuberculous mycobacterial pleurisy: an 8-year single-centre experience in Taiwan. Int J Tuberc Lung Dis 14: 635-641, 2010. [PubMed] [Google Scholar]
- 8. Ichiki H, Ueda S, Watanabe A, Sato C, Abe M. Nontuberculous pulmonary mycobacteriosis complicated by pleuritis. Nihon Kokyuki Gakkai Zasshi (Ann Jpn Respir Soc) 49: 885-889, 2011. (in Japanese). [PubMed] [Google Scholar]
- 9. Sado T, Nakamura Y, Kita H. Clinical analysis of nontuberculous mycobacterial infection complicated by pleurisy. Kekkaku 89: 821-824, 2014. (in Japanese). [PubMed] [Google Scholar]
- 10. Fusegawa H, Ookubo Y, Nishiumi M, Fujino T. A case of pulmonary Mycobacterium scrofulaceum infection presented as pleurisy. Kekkaku 80: 469-473, 2005. (in Japanese). [PubMed] [Google Scholar]
- 11. Lee YC, Kim SB, Gang SJ, Park SY, Kim SR. Acute necrotizing pneumonia combined with parapneumonic effusion caused by Mycobacterium lentiflavum: a case report. BMC Infect Dis 15: 354, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kibadi K. Mycobacterium ulcerans infection treated by Rifater, pyrazynamide, Myambutol, and surgery: a case report with a 6-year follow-up. Med Mal Infect 38: 156-158, 2008. (in French). [DOI] [PubMed] [Google Scholar]
- 13. Ellner JJ. Tuberculosis. In: Goldman-Cecil Medicine. 324: 25th ed. Goldman L, Schafer AI, Eds. Elsevier, Amsterdam, 2016: 2030-2039. e2. [Google Scholar]
- 14. Okada Y, Ichinose Y, Yamaguchi K, Kanazawa M, Yamasawa F, Kawashiro T. Mycobacterium avium-intracellulare pleuritis with massive pleural effusion. Eur Respir J 8: 1428-1429, 1995. [DOI] [PubMed] [Google Scholar]
- 15. Nagaia T, Akiyama M, Mita Y, Tomizawa T, Dobashi K, Mori M. Mycobacterium avium complex pleuritis accompanied by diabetes mellitus. Diabetes Res Clin Pract 48: 99-104, 2000. [DOI] [PubMed] [Google Scholar]
- 16. Kuraoka T, Sakamoto N, Arita K. Development of a case of Mycobacterium avium complex disease from right pleural effusion. Nihon Kokyuki Gakkai Zasshi (Ann Jpn Respir Soc) 38: 706-709, 2000. (in Japanese). [PubMed] [Google Scholar]
- 17. Ishikawa S, Yano S, Tokuda Y, Kobayashi K, Ikeda T, Takeyama H. A case of Mycobacterium intracellulare pleurisy without active lung lesion. Kekkaku 83: 27-31, 2008. (in Japanese). [PubMed] [Google Scholar]
- 18. Kawamoto H. A computed tomography-based study of features developmental patterns: Mycobacterium avium complex without predisposing conditions. Nihon Kokyuki Gakkai Zasshi (Ann Jpn Respir Soc) 36: 928-933, 1998. (in Japanese). [PubMed] [Google Scholar]
- 19. Bai KJ, Wu IH, Yu MC, et al. Tuberculous empyema. Respirology 3: 261-266, 1998. [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi K, Yano S, Kato K, Saito S, Tokushima T. [A case of Mycobacterium avium pulmonary disease accompanied with pleural effusion]. Kekkaku 77: 725-728, 2002. (in Japanese). [PubMed] [Google Scholar]
- 21. Balow JE, Parrillo JE, Fauci AS. Characterization of the direct effects of cyclophosphamide on cell-mediated immunological responses. Immunology 32: 899-904, 1977. [PMC free article] [PubMed] [Google Scholar]
- 22. Liapakis IE, Light RW, Pitiakoudis MS, et al. Penetration of clarithromycin in experimental pleural empyema model fluid. Respiration 72: 296-300, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Chatzika K, Manika K, Kontou P, et al. Moxifloxacin pharmacokinetics and pleural fluid penetration in patients with pleural effusion. Antimicrob Agents Chemother 58: 1315-1319, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liapakis IE, Kottakis I, Tzatzarakis MN, et al. Penetration of newer quinolones in the empyema fluid. Eur Respir J 24: 466-470, 2004. [DOI] [PubMed] [Google Scholar]
- 25. Jutte PC, Rutgers SR, Van Altena R, Uges DR, Van Horn JR. Penetration of isoniazid, rifampicin and pyrazinamide in tuberculous pleural effusion and psoas abscess. Int J Tuberc Lung Dis 8: 1368-1372, 2004. [PubMed] [Google Scholar]