Abstract
Interleukin 17 (IL-17) is increasingly recognized as a key factor that contributes to the pathogenesis of multiple sclerosis (MS) and its experimental mouse autoimmune encephalomyelitis (EAE) model. However, the roles and regulatory mechanisms of IL-17-induced pro-inflammatory cytokine production in EAE mice remain largely unclear. In this study, the expression of IL-17, hypoxia inducible factor-1α (HIF-1α), IL-1β, IL-6 and microRNA-497 (miR-497), as well as their intrinsic associations, was investigated using EAE model mice and cultured astrocytes exposed to IL-17 in vitro. We observed markedly increased production of IL-17, HIF-1α, IL-1β and IL-6 in the brain tissues of EAE mice, while the expression and secretion of HIF-1α, IL-1β and IL-6 were also significantly increased when cultured primary astrocytes from mice were stimulated with IL-17. Meanwhile, the expression of miR-497 was downregulated both in vivo and in vitro. Subsequent in vitro experiments revealed that IL-17 induced the production of IL-1β and IL-6 in astrocytes through the upregulation of HIF-1α as a transcriptional factor, indicating that IL-17-mediated downregulation of miR-497 enhanced HIF-1α expression. Furthermore, astrocyte-specific knockdown of IL-17RA and HIF-1α or astrocyte-specific overexpression of miR-497 by infection with different lentiviral vectors containing an astrocyte-specific promotor markedly decreased IL-1β and IL-6 production in brain tissues and alleviated the pathological changes and score of EAE mice. Collectively, these findings indicate that decreased miR-497 expression is responsible for IL-17-triggered high HIF-1α expression and consequent IL-1β and IL-6 production by astrocytes in EAE mice.
Keywords: astrocytes, experimental autoimmune encephalomyelitis, HIF-1α, interleukin-17, miR-497
INTRODUCTION
Multiple sclerosis (MS), an organ-specific autoimmune disease, is characterized by chronic inflammatory demyelination within the central nervous system (CNS) of humans.1, 2, 3 It is well known that autoreactive CD4+ T cells, especially Th17 cells and their secretion of interleukin-17 (IL-17), play important pathogenic roles in the inflammatory response and in demyelinating lesions within the CNS of MS patients.4, 5, 6, 7, 8, 9 In addition, interleukin-1β (IL-1β) and interleukin-6 (IL-6), two pro-inflammatory cytokines, are also related to the CNS damage that occurs in MS patients.10, 11 Reportedly, IL-17 not only recruits immune cells but also increases the production of IL-lβ and IL-6 in astrocytes, macrophages and other cells, leading to aggravation of CNS injury. Neutralizing antibody against IL-17 or IL-1 receptor antagonist (IL-1Ra) partly decreases CNS damage in MS patients.12, 13, 14, 15 Nevertheless, the regulatory role and mechanism of IL-17 in IL-1β and IL-6 production in the CNS of patients with MS are still largely unclear.
Mouse experimental autoimmune encephalomyelitis (EAE) is a widely used animal model for studying human MS.16, 17, 18 It has been revealed that, similar to human MS, Th17 cells are recruited into the CNS of EAE mice, and the levels of pro-inflammatory cytokines, such as IL-17, IL-1β and IL-6, are markedly elevated in the CNS and related to CNS damage in EAE mice.19, 20, 21, 22, 23, 24, 25 Notably, IL-17 is a trigger that can also elevate the secretion of IL-1β and IL-6 from mouse astrocytes,26, 27, 28 indicating that IL-17 is involved in the initiation and development of inflammatory injuries in EAE mice. However, the molecular mechanism by which the astrocytes of EAE mice contribute to the production of these cytokines in response to IL-17 stimulation needs to be investigated.
Increasing evidence shows that IL-17 is able to induce various biological responses in target cells through the induction of various transcriptional factors, such as Krüppel-like factor 2 (KLF2), CCAAT/enhancer-binding protein β (C/EBPβ), early growth response-1 (Egr-1) and hypoxia inducible factor-1α (HIF-1α).29, 30, 31 HIF-1α is the alpha subunit of HIF-1, which is a transcription factor that responds to the changes in available oxygen.32, 33 As a master transcriptional regulator, HIF-1α can activate the transcription of over 40 genes, including cytokines or other factors, and it is also involved in inflammation.34, 35, 36, 37 Hence, whether the level of HIF-1α can be upregulated by IL-17 stimulation and whether the transcription of IL-lβ and IL-6 genes can be directly triggered by HIF-1α to increase EAE damage remains unknown and needs to be elucidated.
MicroRNAs (miRNAs) belong to a large family of endogenous small noncoding RNAs that regulate gene expression at the post-transcriptional level. Deregulated expression of specific miRNAs is also involved in many pathological processes, such as viral infection, cancer and autoimmune diseases, including EAE.20, 38, 39, 40, 41, 42, 43 Our previous study reported that IL-17 stimulation induces upregulation or downregulation of specific miRNAs in mouse astrocytes, and among them, miR-873 can regulate the synthesis of inflammatory cytokines in mouse astrocytes by targeting A20 through NF-kB in EAE mice.44 In the present study, because miR-497 was demonstrated to be downregulated both in the CNS of EAE mice (in vivo) and in the astrocytes exposed to IL-17 (in vitro), and miR-497 was also predicted to bind to the 3′UTR of HIF-1α mRNA, we selected miR-497 from the several downregulated miRNAs to explore its regulatory function.
In the current study, the induction of IL-17, HIF-1α, IL-1β, IL-6 and miR-497 in the CNS of EAE mice, as well as the production of these parameters in cultured primary mouse astrocytes exposed to IL-17, were examined. Subsequently, the role of HIF-1α in the synthesis of IL-1β and IL-6, including its upstream regulation by miR-497, was determined in IL-17-treated astrocytes. Finally, the effects of the IL-17-miR-497-HIF-1α axis on the production of IL-1β and IL-6, as well as the pathological changes and behavior of EAE mice, were observed using astrocyte-specific lentiviral vectors to overexpress or silence the above-mentioned genes.
MATERIALS AND METHODS
Reagents
Oligodendrocyte glycoprotein (MOG) 35–55 amino-acid peptide (MOG35–55, MEVGWYRSPFSRVVHLYRNGK) and complete Freund’s adjuvant (CFA) were purchased from CL Bio-scientific Co. Ltd (Xian, China) and Sigma-Aldrich (St Louis, MO, USA), respectively. Mouse IL-17 protein was from R&D Systems (Minneapolis, MN, USA). Dulbecco’s modified Eagle’s medium (DMEM) and DMEM/F12 were obtained from HyClone (San Angelo, TX, USA). Fetal bovine serum (FBS) was from Gibco (Carlsbad, CA, USA). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). TaqMan microRNA reverse transcription kits, miR-497 TaqMan assay kits, TaqMan universal master mix II and SYBR select master mix were from Applied Biosystems (Foster City, CA, USA). A 2 × Taq Master Mix was purchased from Vazyme Biotech (Nanjing, China). PrimeScript RT reagent kits with gDNA eraser were from Takara Bio Inc. (Tokyo, Japan). RIPA lysis buffer and BCA protein assay kits were purchased from Beyotime (Nantong, China). Rabbit polyclonal antibodies against IL-17RA and HIF-1α were from Abcam (Cambridge, UK) and Bioworld Technology (St Louis, MN, USA), respectively. Mouse monoclonal antibodies against HIF-1α (H1alpha67, ChIP Grade) and β-actin were from Abcam and Boster (Wuhan, China), respectively. HRP-labeled goat anti-rabbit IgG was purchased from Cell Signaling Technology (Danvers, MA, USA). HRP-labeled goat anti-mouse IgG was obtained from Sigma-Aldrich. Enhanced chemiluminescence (ECL) western blotting substrate was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Mouse IL-17, IL-1β and IL-6 ELISA kits were from BioLegend (San Diego, CA, USA) and eBioscience (San Diego, CA, USA), respectively. A chromatin immunoprecipitation (ChIP) assay kit was obtained from Millipore (Bedford, MA, USA). The luciferase reporters pGL3-Basic, pGL3-Promoter and pRL-SV40 were purchased from Promega (Madison, WI, USA). A Neon transfection system was provided by Invitrogen. GenEscort III was from Wisegen (Nanjing, China). Polybrene was from Sigma-Aldrich.
Animals, EAE induction and score
C57BL/6 mice were purchased from Nanjing Medical University Laboratory Animal Center (Nanjing, China). All mice were maintained under specific pathogen-free conditions. All animal experiments were performed in compliance with the guidelines for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Ethics Committee of Nanjing Medical University. EAE was induced in female C57BL/6 mice at 8 weeks of age, and EAE scores were evaluated as described previously.44
Cell culture and IL-17 stimulation
Primary mouse astrocytes were obtained and cultured in DMEM/F12 with 10% FBS as described previously.44 After different treatments, the astrocytes were stimulated with IL-17 (100 ng/ml) for different time points. In addition, HEK293T cells were obtained from the American Tissue Culture Collection (ATCC, Manassas, VA, USA) and maintained in DMEM supplemented with 10% FBS (vol/vol), 100 μg/ml penicillin G and 100 μg/ml streptomycin.
Plasmid construction
The mouse HIF-1α expression plasmid pCMV/HIF-1α was a gift from Professor Fan Pan (Johns Hopkins University, USA). The mouse IL-17RA shRNA expression plasmid pSilencer2.1-U6/IL-17RA shRNA (shIL-17RA, 5′-GCACCTACGTTGTTTGCTA-3′) and scrambled control shRNA (shCTR) expression plasmid were a gift from Professor Dongqing Li (Wuhan University, China).
The luciferase reporters pGL3-Basic/IL-1β and pGL3-Basic/IL-6 were constructed by inserting the 1.169 kb mouse IL-1β promoter (−1142 to +27 nt) and the 0.736 kb mouse IL-6 promoter (−667 to +69 nt), respectively, into a pGL3-Basic vector at the kpn I restriction enzyme site. In addition, the luciferase reporter pGL3-Promoter/HIF-1α was constructed by inserting the 1.522 kb 3′UTR of mouse HIF-1α mRNA (NM_010431) into a pGL3-Promoter vector at the Xba I restriction enzyme site. The primer sequences for PCR are shown in Supplementary Table 1.
miRNA mimic and inhibitor synthesis
All of the miRNA mimics and a universal negative control miRNA mimic (CTR mimic) were purchased from GenePharma (Shanghai, China). The sequences of different miRNA mimics are shown in Supplementary Table 2.
A miRCURY LNA microRNA inhibitor, namely LNA-anti-miR-497 (4101347-000, 5′-CAAACCACAGTGTGCTGCT-3′), and a universal negative control LAN, namely LNA-anti-miR-CTR (199006-000, 5′-TAACACGTCTATACGCCCA-3′), were purchased from Exiqon (Vedbæk, Denmark).
siRNA synthesis
To silence the mouse HIF-1α gene, an siRNA against mouse HIF-1α mRNA (siHIF-1α, 5′-GAACTAACTGGACACAGTGTGTT-3′) was synthesized by GenePharm (Shanghai, China). In addition, a universal scrambled control siRNA (siCTR, 5′-UUCUCCGAACGUGUCACGUTT-3′) was also produced by GenePharm.
Cellular transfection
Primary mouse astrocytes were transfected with corresponding miRNAs, siRNAs or plasmids using a Neon transfection system.45, 46 For miRNA or siRNA transfection, 4 × 105 cells were resuspended in 100 μl of resuspension buffer that included 100 nM miRNA or siRNA and then electroporated at 1100 V (30 ms, 1 time). For plasmid transfection, 4 × 105 cells were resuspended in 100 μl of resuspension buffer that included 100 nM plasmid and then electroporated at 1350 V (20 ms, 2 times). After transfection, the astrocytes (4 × 105 cells per well) were transferred to six-well cell culture plates pre-coated with 1 mg/ml poly-L-lysine and then incubated for 48 h. The transfection efficiency was examined by observing the fluorescence of GFP (Supplementary Figure 1).
HEK293T cells were transfected with corresponding miRNAs or plasmids using GenEscort III according to the manufacturer’s instructions. Briefly, cells were seeded in 24-well cell culture plates 24 h before transfection. The medium was changed with new DMEM plus 10% FBS (500 μl per well) 30 min before transfection. Next, 1 μg of plasmid or 0.5 μg of plasmid plus 100 nmol of miRNA were mixed with 50 μl of serum-free DMEM, and then, 3 μl of GenEscort III mixed with 50 μl of serum-free DMEM was added and incubated for 10 min. Finally, 100 μl of the resultant mixture was added to each well.
Real-time PCR
Total RNA was extracted from the primary mouse astrocytes and the mouse brain tissues with TRIzol reagent. For miRNA detection, cDNA was synthesized with TaqMan microRNA reverse transcription kits. The expression level of miR-497 was assayed with miR-497 TaqMan assay kits and the TaqMan universal master mix II. The reaction program included an initial step for denaturation at 95 °C for 10 min and then 40 cycles of denaturation at 95 °C for 15 s and annealing at 60 °C for 1 min. The level of miR-497 was normalized to the level of the U6 gene. For mRNA detection, cDNA was synthesized using PrimeScript RT reagent kits with gDNA Eraser. The expression levels of mouse IL-17, IL-1β and IL-6 mRNA were quantified by real-time PCR with SYBR select master mix. Primer sequences are listed in Supplementary Table 3. The expression levels of mouse IL-17, IL-1β and IL-6 mRNA were normalized to the expression level of GAPDH mRNA. Amplification of cDNA was performed on an ABI Prism 7300 (Applied Biosystems) system with a reaction program that included an initial step at 50 °C for 2 min and 95 °C for 10 min, and then 40 cycles of denaturation at 95 °C for 15 s and annealing at 60 °C for 1 min. Each sample was assayed in triplicate. Relative gene expression levels were obtained using the formula 2−△△Ct.
Western blotting
Extracted protein (50 μg per well) was loaded into a 12% SDS polyacrylamide gel for electrophoresis and then transferred onto polyvinylidene difluoride (PVDF) membranes with a PowerPacTM Basic system (Bio-Rad, Hercules, CA, USA). The PVDF membranes were incubated in blocking buffer (5% skim milk in TBS-T buffer) at room temperature (RT) for 1 h and then incubated with antibodies to HIF-1α and β-actin at 4 °C overnight. β-actin expression in each sample was identified as the internal standard. After being washed five times with TBST-T, the PVDF membranes were further incubated with HRP-conjugated anti-rabbit IgG and HRP-conjugated anti-mouse IgG at RT for 1 h. The bands were visualized by regular X-ray film using an ECL detection system after washing the PVDF membranes five times. Finally, the density of the radiographic bands on the PVDF membranes was analyzed using Quantity One software (Bio-Rad).
ELISA
The protein levels of IL-17, IL-1β and IL-6 in the supernatant of cultured astrocytes and mouse sera and in the homogenate of mouse brain tissues (20 mg/ml in PBS) were measured with ELISAs according to the manufacturer’s instructions.
Luciferase assays
For IL-1β and IL-6 promoter reporter assays, HEK293T cells in each well of 24-well cell culture plates were transfected with a mixture of 0.5 μg pGL3 luciferase vector (pGL3-Basic/IL-1β or pGL3-Basic/IL-6), 10 ng pRL-SV40 and 0.5 μg pCMV expression plasmid (pCMV/HIF-1α or pCMV) by using GenEscort III. For the HIF-1α 3′UTR reporter assay, HEK293T cells in each well of 24-well cell culture plates were transfected with a mixture of 0.5 μg pGL3-Promoter/HIF-1α, 2.5 ng pRL-SV40 and 100 nmol miRNA mimic (miR-195, miR-292-5p, miR-322, miR-497, miR-497 mutant or miR-CTR mimic) by using GenEscortIII. Cells were lysed 48 h after transfection, and then, the luciferase activity of IL-1β and IL-6 promoters as well as HIF-1α 3′UTR reporter was measured through a dual-luciferase reporter gene assay. The ratio of firefly luciferase to Renilla luciferase was calculated for each cellular sample.
ChIP assays
ChIP assays were performed with antibody against HIF-1α as mentioned previously.44 The captured genomic DNA was further used for real-time PCR analysis with SYBR select master mix and for regular PCR analysis with 2 × Taq Master Mix according to the manufacturer’s instructions. The primers used for detection of the IL-1β promoter (−544 to −352 nt) were 5′-CTGTGTGTGCCCTGACCCA-3′ (forward) and 5′-GGATGTGCGGAACAAAGGTAG-3′ (reverse). The primers used for detection of the IL-6 promoter (−499 to −300 nt) were 5′-CAGACATACAAAAGAATCCTAGCCTC-3′ (forward) and 5′-CTTGAGCATGTCTTGATGGGAA-3′ (reverse).
Lentivirus construction and in vivo experimental design
Different lentiviral vectors encoding shIL-17RA, miR-497 mimic, miR-497 sponge, shHIF-1α and shCTR (namely, LV-shIL-17RA, LV-miR-497 mimic, LV-miR-497 sponge, LV-shHIF-1a and LV-shCTR, respectively) were purchased from GeneChem (Shanghai, China). The sequences for mouse IL-17RA and HIF-1α gene knockdown and for the miR-497 mimic were the same as those used in vitro. The sequence of the miR-497 sponge was 5′-TAGCAGCATTAGAGCAAGAAAGCATATGTAGCAGCAGACTGTGTTGCCCCAGATCTTAGCAGCACATTAGCAACTTCT-3′. For all of these lentiviral vectors, an astrocyte-specific promoter of glial fibrillary acidic protein was used to confirm astrocyte-specific knockdown or overexpression in vivo.47
To explore the roles of IL-17, miR-497 and HIF-1α in IL-1β and IL-6 production and CNS damage in EAE, mice were divided into six groups as follows: (1) LV-CTR+CFA, (2) LV-CTR+EAE, (3) LV-shIL-17RA+EAE, (4) LV-miR-497 mimic+EAE, (5) LV-miR-497 sponge+EAE, (6) LV-shHIF-1a+EAE. For lentivirus infection in vivo, C57BL/6 mice were anesthetized before microinjection. Subsequently, a microsyringe was inserted 2.0 mm lateral and 1.0 mm caudal to the bregma and 2.0 mm below the skull surface, and then, 5 μl of lentivirus (0.5 × 107 IU) with 10 μg/ml polybrene was administered to each mouse at the speed of 1 μl/min. Transfection efficiency and organ specificity were determined by observing the GFP expression in the main organs of the LV-CTR-infected mice (Supplementary Figure 2). The brain tissues of mice in different groups were obtained for detection of mRNA, miRNA and protein expression as well as for histopathology on day 20 after EAE induction.
Histopathology
To assess inflammation and demyelination, the 4-μm-thick paraffin-embedded sections of mouse brain tissues and spinal cords were stained with hematoxylin and eosin (H&E) and Luxol fast blue (LFB), and observed with light microscopy. For H&E staining, 30 randomly distributed fields (original magnification: × 200) within the brain tissues were captured from different sections of each of the mice. The inflammatory cells were counted using ImageJ software (https://imagej.nih.gov/ij). For LFB staining, 30 randomly distributed fields (original magnification: × 200) within the white matter of the spinal cords were captured from different sections of each of the mice. The area of the white matter and the area covered by the LFB stain were quantified using ImageJ software. The degree of demyelination was calculated with the following formula: (white matter area−LFB area)/white matter area × 100%. In addition, ultrathin sections of spinal cords were stained with uranyl acetate and lead citrate and examined with electron microscopy. For demyelination quantification, the G-ratio, the value of the axon diameter divided by the entire fiber diameter of the axon plus myelin sheath was measured with ImageJ software (300 axons per mouse).48
Statistics
One-way analysis of variance was used to determine significant differences among groups. Where significant differences were found, individual comparisons were made between groups using the t-statistic and adjusting the critical value according to the Bonferroni method. In addition, a Mann–Whitney test for nonparametric data (EAE score) was used. Values of P<0.05 were considered significant.
RESULTS
The production of IL-17, HIF-1α, IL-1β and IL-6 increases in the brain tissues of EAE mice
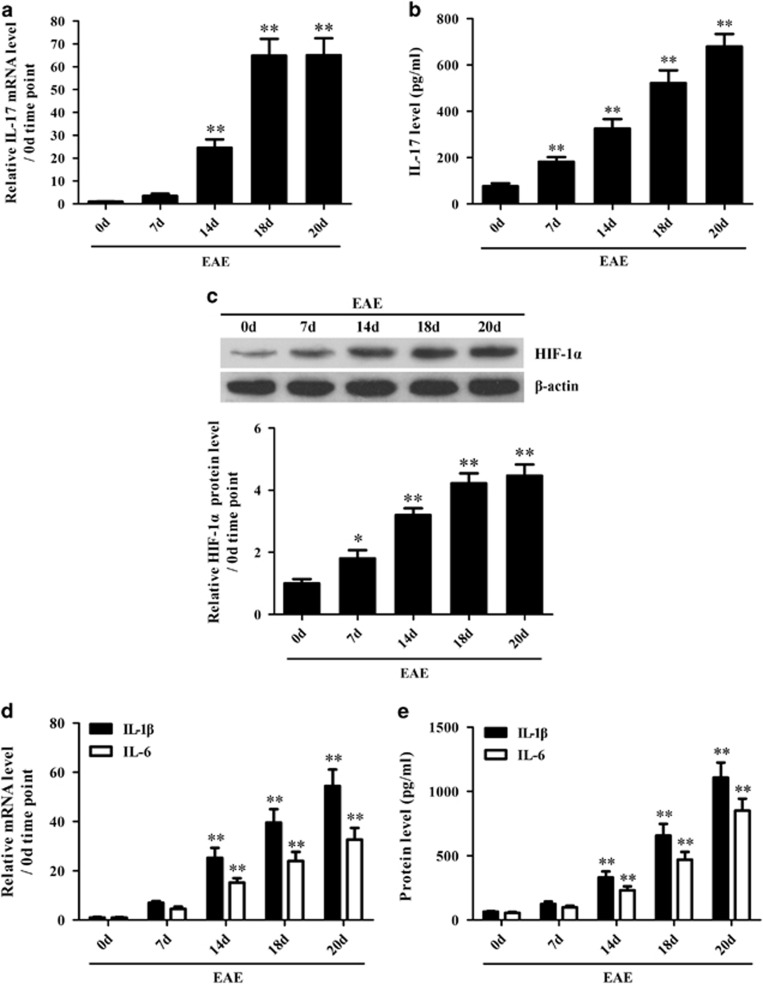
To investigate the potential roles of IL-17 in HIF-1α, IL-1β and IL-6 induction in the CNS of EAE mice, the expression of IL-17, HIF-1α, IL-1β and IL-6 at the mRNA and/or protein level was assessed in the brain tissues and/or in sera of EAE mice. Time course studies in vivo showed that IL-17 mRNA in brain tissues (Figure 1a) and IL-17 protein in the sera (Figure 1b) were noticeably elevated in mice with EAE, in which the time-dependent tendency was consistent with our previous study.44 Meanwhile, the protein level of HIF-1α was also markedly increased in the mouse brain tissues from day 7 to day 20 after EAE induction (Figure 1c). In addition, the mRNA levels of IL-1β and IL-6 in the brain tissues (Figure 1d) as well as the protein levels of IL-1β and IL-6 in the sera of EAE mice (Figure 1e) were obviously elevated in a time-dependent manner. Notably, the expression time points of IL-17, HIF-1α, IL-1β and IL-6 were consistent with each other, suggesting that IL-17 might regulate HIF-1α, IL-1β and IL-6 production in the CNS of EAE mice.
Figure 1.
The expression of interleukin (IL)-17, hypoxia inducible factor-1α (HIF-1α), IL-1β and IL-6 both in the brain tissues and the sera of experimental autoimmune encephalomyelitis (EAE) mice. (a, b) The messenger RNA (mRNA) and protein levels of IL-17 in the brain tissues (a) and in the sera (b) of EAE mice were measured with real-time PCR and ELISAs, respectively. (c) The expression of HIF-1α protein in the brain tissues of EAE mice was analyzed with a western blot. (d, e) The expression of IL-1β and IL-6 at the mRNA level in the brain tissues (d) and at the protein level in the sera (e) of EAE mice was detected using real-time PCR and ELISAs, respectively. *P<0.05, **P<0.01 versus the day 0 (0 d) time point. The results from one representative experiment out of three are shown. Data are presented as the means±s.d. (n=6 in each time point).
IL-17 stimulates astrocytes to produce HIF-1α, IL-1β and IL-6
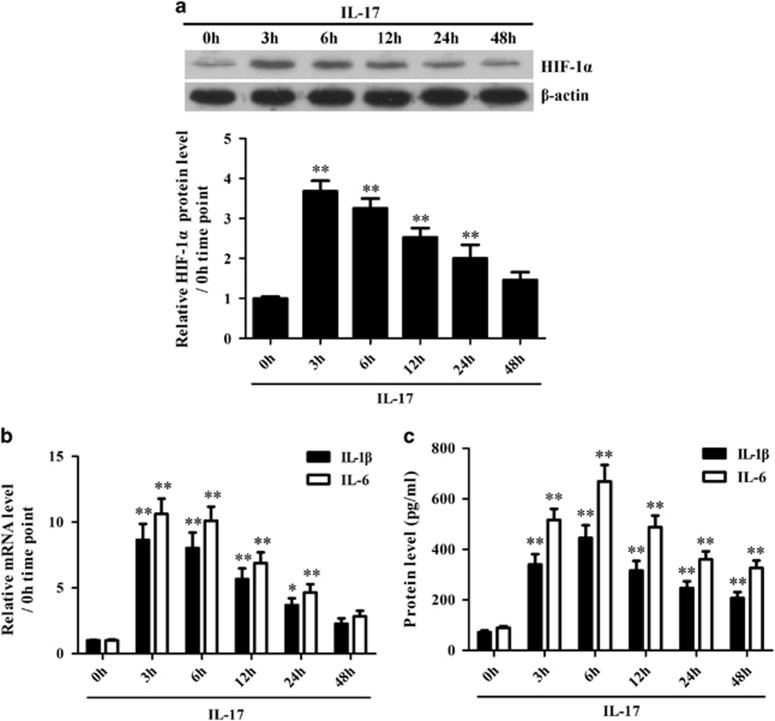
To explore the possible roles of IL-17 in HIF-1α, IL-1β and IL-6 production in astrocytes, cultured primary mouse astrocytes were stimulated with IL-17 in vitro, and then, the mRNA and/or protein levels of HIF-1α, IL-1β and IL-6 were detected in the cells or supernatant. Time course studies showed that the expression levels of HIF-1α, IL-1β and IL-6 were upregulated simultaneously in primary mouse astrocytes stimulated with IL-17 (Figure 2). Further experiments showed that IL-17RA knockdown obviously inhibited the expression of HIF-1α, IL-1β and IL-6 in primary mouse astrocytes exposed to IL-17 (Supplementary Figure 3), indicating that IL-17 induces the expression of these genes in astrocytes through its receptor IL-17RA.
Figure 2.
The expression of hypoxia inducible factor-1α (HIF-1α), interleukin (IL)-1β and IL-6 in astrocytes induced by IL-17. Primary mouse astrocytes were incubated with IL-17 for different time points. (a) The protein expression of HIF-1α in the astrocytes was detected with a western blot. (b) The mRNA levels of IL-1β and IL-6 in the astrocytes were measured with real-time PCR. (c) The protein levels of IL-1β and IL-6 in the supernatant of astrocytes were determined by ELISA. *P<0.05; **P<0.01 versus the 0 h time point. The results from one representative experiment out of three are shown. Data are expressed as the means±s.d. (n=3 in each time point).
HIF-1α mediates the production of IL-1β and IL-6 in astrocytes in response to IL-17
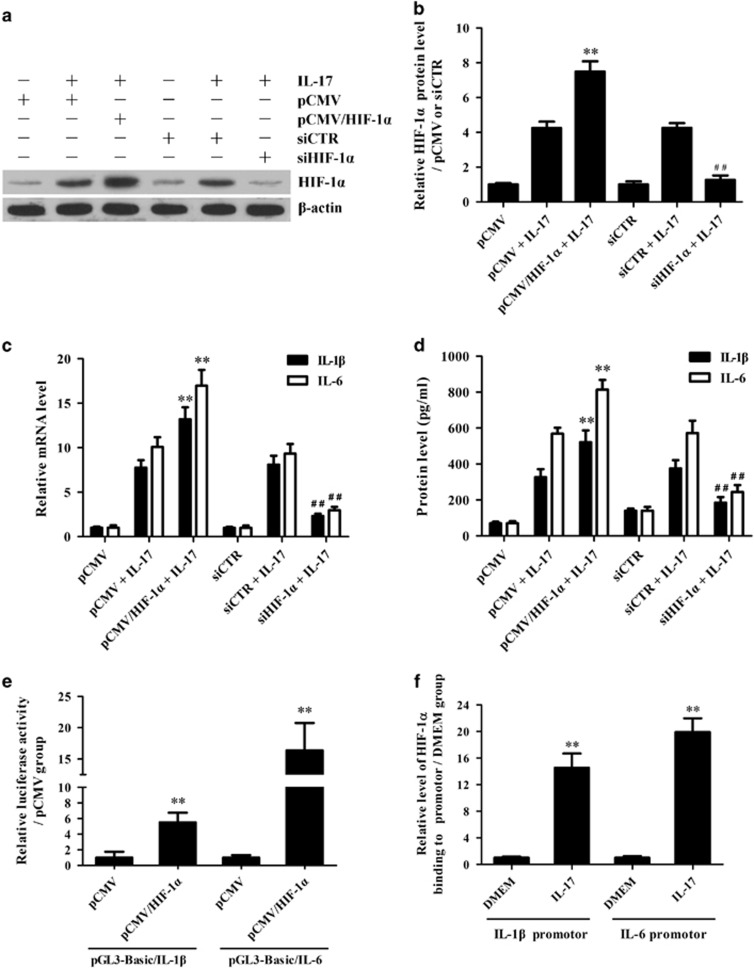
Since our present studies revealed that IL-17 stimulation in vitro could induce the expression of HIF-1α, IL-1β and IL-6 in primary mouse astrocytes, and HIF-1α was reported to mediate the production of pro-inflammatory cytokines,34, 35, 36 overexpression and knockdown experiments were further performed to explore the effects of HIF-1α on IL-1β and IL-6 expression in primary mouse astrocytes exposed to IL-17. The results showed that HIF-1α overexpression obviously enhanced IL-1β and IL-6 production in astrocytes induced by IL-17, and HIF-1α gene silencing markedly decreased IL-1β and IL-6 production in the astrocytes stimulated with IL-17 (Figures 3a–d). These data indicate that IL-17-mediated upregulation of HIF-1α contributes to the induction of IL-1β and IL-6 in astrocytes.
Figure 3.
The effects of hypoxia inducible factor-1α (HIF-1α) on interleukin (IL)-1β and IL-6 production in astrocytes exposed to IL-17. (a–d) Primary mouse astrocytes were transfected with pCMV/HIF-1α, pCMV, siHIF-1α and siCTR, separately, for 48 h and then treated with or without IL-17 for 3 and 6 h. The protein level of HIF-1α (a, b) in the astrocytes 3 h after IL-17 stimulation was measured with western blotting. In addition, the mRNA levels of IL-1β and IL-6 in the astrocytes at 3 h (c) as well as the protein levels of IL-1β and IL-6 in the supernatant of astrocytes at 6 h (d) were analyzed separately with real-time PCR and ELISAs. **P<0.01 versus the pCMV+IL-17 group; ##P<0.01 versus the siCTR+IL-17 group. (e) HEK293T cells were transfected with pGL3-Basic/IL-1β or pGL3-Basic/IL-6 accompanied by pCMV/HIF-1α, pCMV, siHIF-1α or siCTR. The luciferase activity of IL-1β and IL-6 promoters was detected 48 h after transfection. **P<0.01 versus pCMV. (f) Primary mouse astrocytes were cultured with or without IL-17 for 3 h. A ChIP assay was performed with anti-HIF-1α antibody to pull down the DNA–protein complex, and then immunoprecipitated DNA was amplified using real-time PCR with two pairs of primers for the proximal promoters of the IL-1β or IL-6 gene. **P<0.01 versus the DMEM group. The results from one representative experiment out of three are shown. Data are presented as the means±s.d. (n=3 in each group).
HIF-1α directly promotes gene transcription of IL-1β and IL-6 in astrocytes exposed to IL-17
Reportedly, HIF-1α is able to trigger gene transcription by binding to the hypoxia response element (HRE) sequence (R'CGTG) within the promoter of target genes.37, 49 Therefore, to clarify the mechanism by which HIF-1α upregulates IL-1β and IL-6 expression, luciferase experiments were performed to assess the effects of HIF-1α on IL-1β and IL-6 gene promotion in HEK293T cells. The results showed that HIF-1α over-expression markedly enhanced the transcription of IL-1β and IL-6 genes (Figure 3e). Since TFsearch predicted that two HRE elements and one HRE element were, respectively, located in the promoters of IL-1β and IL-6 genes, a ChIP assay was further designed to discover whether HIF-1α protein can bind to the promoters of these two genes. As we expected, HIF-1α protein bound to the HRE element-containing regions within IL-1β and IL-6 gene promotors (Figure 3f and Supplementary Figure 4). These results indicate that IL-17-mediated upregulation of HIF-1α could activate both IL-1β and IL-6 genes through binding to the promotors of these two genes.
Identification of downregulated miRNAs targeting HIF-1α mRNA
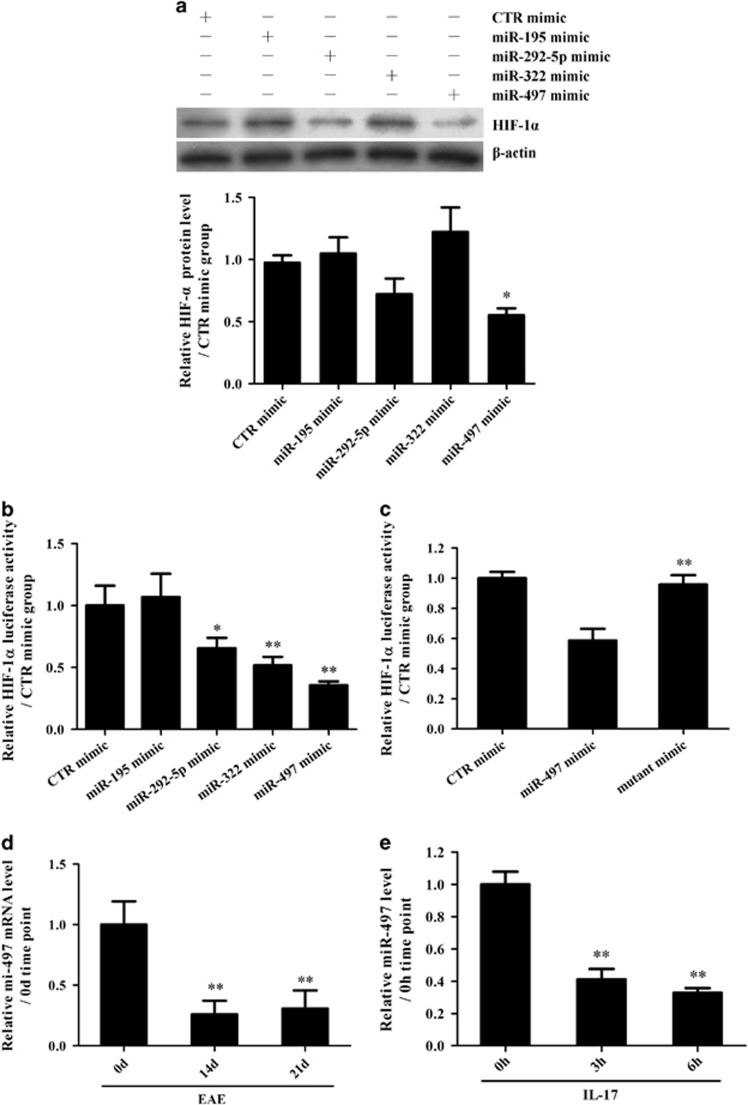
Our previous study reported changes in miRNA expression both in the brain tissues of EAE mice (in vivo) and in IL-17-treated astrocytes (in vitro).44 Since downregulation of miRNAs against HIF-1α mRNA is supposed to increase HIF-1α expression, a bioinformatics assay was used to find the potential downregulated miRNAs involved in HIF-1α upregulation in astrocytes stimulated with IL-17 in EAE mice, and, finally, four downregulated miRNAs (miR-195, miR-292-5p, miR-322 and miR-497) were predicted to bind to the 3′UTR of HIF-1α mRNA.
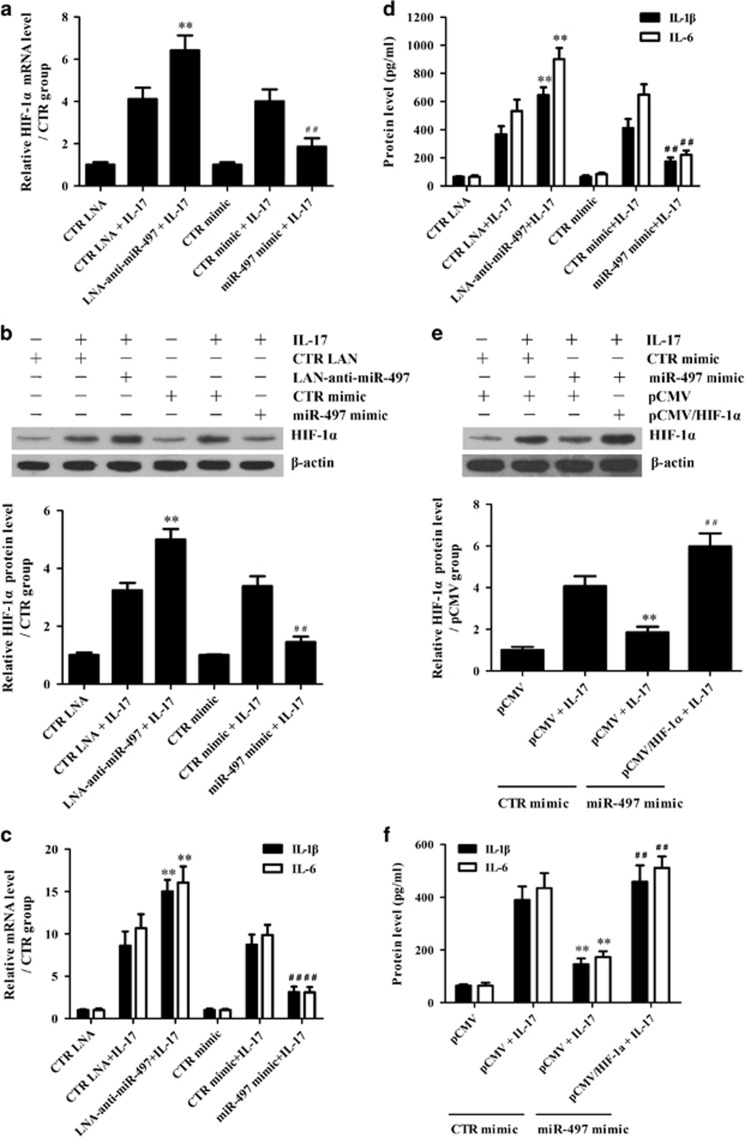
To further find which downregulated miRNA is possibly responsible for HIF-1α upregulation, overexpression experiments were performed through transfection of different miRNA mimics. We observed that although both miR-292-5p and miR-497 mimics decreased HIF-1α expression, the miR-497 mimic had a more significant inhibitory role (Figure 4a). A luciferase activity assay showed that the mimics of miR-292-5p, miR-322 and miR-497, especially miR-497, directly targeted the 3′UTR of HIF-1α mRNA (Figure 4b). Furthermore, a miR-497 mimic with a mutant seed-region did not target the 3′UTR of HIF-1α mRNA (Figure 4c), indicating that the inhibitory effect of the miR-497 mimic is sequence specific. Subsequent assays confirmed that miR-497 expression was markedly downregulated both in vivo (Figure 4d) and in vitro (Figure 4e), while IL-17RA knockdown obviously upregulated miR-497 expression in primary mouse astrocytes exposed to IL-17 (Supplementary Figure 5). Notably, Lindberg et al.50 reported that miR-497 expression was reduced in the peripheral blood lymphocytes of MS patients, indicating that miR-497 downregulation might contribute to CNS damage in MS patients. However, the role of miR-497 in the pathological CNS changes during MS, including IL-17-inducing pro-inflammatory cytokine production and its regulatory mechanism, remain largely unclear. Therefore, further functional studies on miR-497 were performed both in astrocytes exposed to IL-17 and in the EAE mouse model.
Figure 4.
Effects of potential miRNAs on hypoxia inducible factor-1α (HIF-1α) in interleukin (IL)-17-stimulated astrocytes. (a) Primary mouse astrocytes were transfected with different mimics of miR-195, miR-292-5p, miR-322, miR-497 and CTR, and the protein level of HIF-1α in the astrocytes was detected with western blotting 48 h after transfection. *P<0.05 versus the CTR mimic group. (b) HEK293T cells were transfected with a mixture of pGL3-Promoter/HIF-1α and pRL-SV40 as well as one of the miRNA mimics (miR-195, miR-292-5p, miR-322, miR-497 or CTR). Subsequently, the luciferase activity of the HIF-1α 3′UTR reporter was measured 48 h after transfection. *P<0.05; **P<0.01 versus the CTR mimic group. (c) HEK293T cells were transfected with a mixture of pGL3-Promoter/HIF-1α and pRL-SV40 as well as a miR-497 mimic or miR-497 mutant mimic. Then, the luciferase activity of the HIF-1α 3′UTR reporter was examined 48 h after transfection. **P<0.01 versus the miR-497 mimic group. (d, e) Real-time PCR was used to measure the level of miR-497 in the brain tissues of mice on 0 d, 14 d and 21 d after experimental autoimmune encephalomyelitis (EAE) induction (d, **P<0.01 versus the 0 d time point) or in the astrocytes at 0, 3 and 6 h after IL-17 stimulation (e, **P<0.01 versus the 0 h time point). The results from one representative experiment out of three are shown. Data are shown as the means±s.d. (n=3 in each group).
Downregulation of miR-497 enhances production of IL-1β and IL-6 through upregulation of HIF-1α expression in astrocytes induced by IL-17
To study whether miR-497 downregulation promotes IL-1β and IL-6 production through HIF-1α upregulation in IL-17-stimulated astrocytes, LNA-anti-miR-497 (as a miR-497-specific inhibitor) and miR-497 mimic were transfected into primary mouse astrocytes, separately, and then, HIF-1α, IL-1β and IL-6 expression was detected after IL-17 stimulation. The expression of miR-497 was clearly downregulated by LNA-anti-miR-497 and upregulated by miR-497 mimic (Supplementary Figure 6). Furthermore, LNA-anti-miR-497 enhanced IL-17-induced HIF-1α, IL-1β and IL-6 expression in the astrocytes, while the miR-497 mimic exhibited the opposite effects (Figures 5a–d). More importantly, HIF-1α overexpression in the astrocytes reversed the IL-6 and IL-1β downregulation caused by the miR-497 mimic in IL-17-stimulated astrocytes (Figures 5e and f), confirming the upstream and downstream relationship between miR-497 and HIF-1α. Taken together, our in vitro studies indicate that IL-17-mediated downregulation of miR-497 induces high HIF-1α expression and promotes IL-1β and IL-6 production via HIF-1α in astrocytes.
Figure 5.
The role of miR-497 in the expression of hypoxia inducible factor-1α (HIF-1α), interleukin (IL)-1β and IL-6 in astrocytes stimulated with IL-17. Primary mouse astrocytes were transfected with miR-497 mimic, CTR mimic, LNA-anti-miR-497 or CTR LNA for 48 h and then incubated with or without IL-17 for 3 and 6 h. (a, b) The mRNA and protein levels of HIF-1α were determined with real-time PCR (a) and western blotting (b) respectively, 3 h after IL-17 stimulation. **P<0.01 versus the CTR LNA+IL-17 group; ##P<0.01 versus the CTR mimic+IL-17 group. (c, d) The mRNA and protein levels of IL-1β and IL-6 were analyzed at 3 h in the astrocytes treated with IL-17 (c, real-time PCR) and at 6 h in the supernatant of astrocytes stimulated with IL-17 (d, ELISA). **P<0.01 versus the CTR LNA+IL-17 group; ##P<0.01 versus the CTR mimic+IL-17 group. (e, f) Primary mouse astrocytes were transfected with CTR mimic, miR-497 mimic, pCMV and pCMV/HIF-1α separately or in combination for 48 h and then cultured with or without IL-17 for 3 and 6 h. Finally, the protein levels of HIF-1α in the astrocytes (e, 3 h) as well as IL-1β and IL-6 in the supernatant of astrocytes (f, 6 h) were measured using western blotting and ELISAs, respectively. **P<0.01 versus the CTR mimic+pCMV+IL-17 group; ##P<0.01 versus the miR-497 mimic+pCMV+IL-17 group. The results from one representative experiment out of three are shown. Data are presented as the means±s.d. (n=3 in each group).
The IL-17-miR-497-HIF-1α axis contributes to the production of IL-1β and IL-6, as well as to pathological changes and behavior of EAE mice
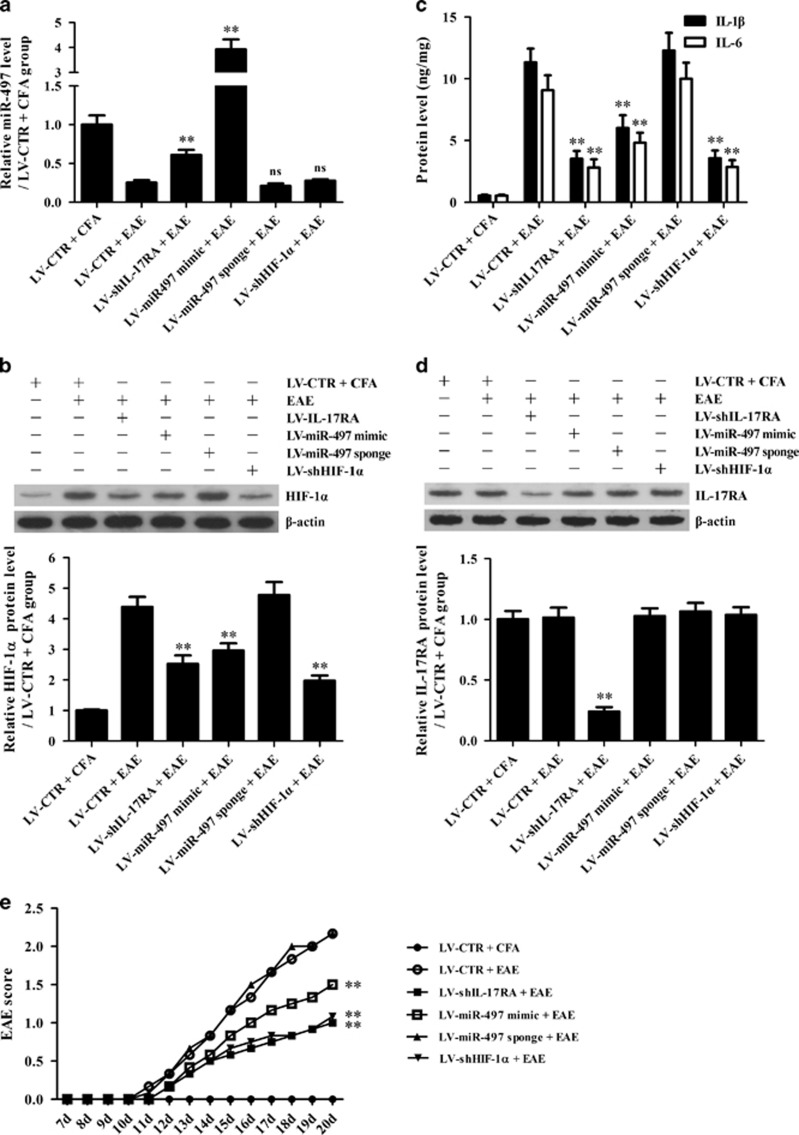
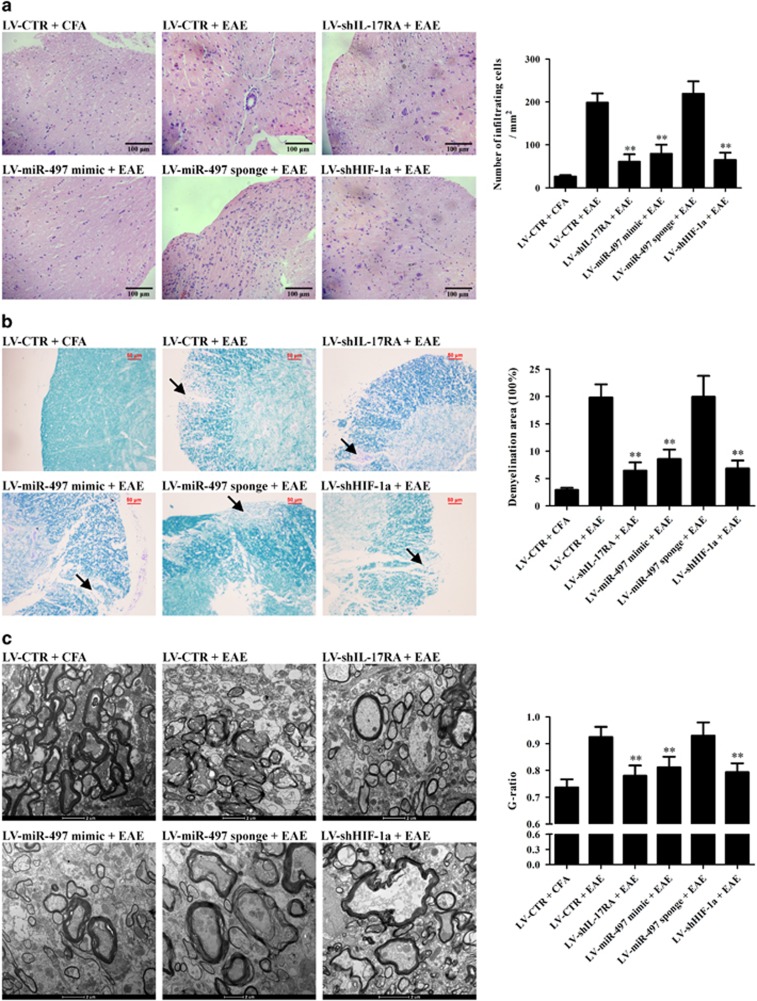
To verify the roles of the IL-17-miR-497-HIF-1α axis in the production of IL-1β and IL-6 in astrocytes, as well as the pathological changes and behavior of EAE mice, corresponding in vivo experiments were subsequently performed. Different astrocyte-specific lentiviral vectors encoding shIL-17RA, miR-497 mimic, miR-497 sponge, shHIF-1α and shCTR were used to overexpress or knockdown corresponding target genes in mouse astrocytes in vivo. EAE was induced on day 7 after lentivirus injection, and the mice were killed on day 20 after EAE induction. Mice infected with LV-shIL-17RA, LV-miR-497 mimic and LV-shHIF-1α exhibited significantly decreased HIF-1α, IL-1β and IL-6 expression (Figures 6a–c) as well as lymphocyte infiltration and demyelination in the brain tissues and spinal cords (Figures 7a–c). LV-miR-497 mimic, LV-miR-497 sponge and LV-shHIF-1α had no effect on IL-17RA expression (Figure 6d). In addition, the mice were scored according to the degree of paralysis from day 7 to day 20 after EAE induction, and the mice infected with LV-shIL-17RA, LV-miR-497 mimic and LV-shHIF-1α exhibited much lower scores than the mice infected with LV-miR-497 sponge and LV-CTR (Figure 6e). Our in vivo experiments indicated that IL-17-mediated downregulation of miR-497 increases HIF-1α expression and IL-1β and IL-6 production in astrocytes and aggravates CNS inflammation in EAE mice.
Figure 6.
The roles of the interleukin (IL)-17–miR-497–HIF-1α axis in the production of IL-1β and IL-6 as well as in the behavior of experimental autoimmune encephalomyelitis (EAE) mice. Mice were injected with different recombinant astrocyte-specific lentiviruses (LV-CTR, LV-shIL-17RA, LV-miR-497 mimic, LV-miR-497 sponge, LV-shHIF-1a) for 7 days and then immunized with MOG (35–55) for 20 days (n=6 each group). (a) The miR-497 level in the brain tissues of mice was detected with real-time PCR on day 20 after EAE induction. (b–d) The protein levels of IL-17RA, HIF-1a, IL-1β and IL-6 in the brain tissues of mice on day 20 after EAE establishment were measured using western blotting (b, HIF-1a; d, IL-17RA) and ELISAs (c, IL-1β and IL-6). (e) The EAE score was evaluated from day 7 to day 20. **P<0.01 versus the LV-CTR+EAE group; NSP>0.05 versus the LV-CTR+EAE group. The results from one representative experiment out of two are shown. Data are expressed as the means±s.d.
Figure 7.
The roles of the interleukin (IL)-17–miR-497–HIF-1α axis in the pathological changes in experimental autoimmune encephalomyelitis (EAE) mice. Mice in different groups (n=6 each group) were treated as mentioned in Figure 6. The histopathology of mouse brain tissues and spinal cords was observed using light microscopy (a, b) and electron microscopy (c) on day 20 after EAE induction. (a) H&E staining (original magnification: × 200) was performed to evaluate the inflammatory cell infiltration into brain tissues. The number of infiltrated inflammatory cells in the mice of the LV-shIL-17RA+EAE, LV-miR-497 mimic+EAE and LV-shHIF-1a+EAE groups was less than that of the LV-CTR+EAE and LV-miR-497 sponge+EAE groups. (b) LFB staining (original magnification: × 200) was used to observe pathological changes in spinal cords. The demyelination degree in the mice of the LV-shIL-17RA+EAE, LV-miR-497 mimic+EAE and LV-shHIF-1a+EAE groups was less than that in the mice of the LV-CTR+EAE and LV-miR-497 sponge+EAE groups. Arrows indicate areas of severe demyelination. (c) The spinal cords in the mice of the LV-shIL-17RA+EAE, LV-miR-497 mimic+EAE and LV-shHIF-1a+EAE groups viewed with EM exhibited reduced disruption or mild loosening of the medullary sheath and lower G-ratio compared with the mice of the LV-CTR+EAE and LV-miR-497 sponge+EAE groups. The results from one representative experiment out of two are shown. Data are expressed as the means±s.d. **P<0.01 versus the LV-CTR+EAE group.
DISCUSSION
As a critical pro-inflammatory cytokine, IL-17 is involved in various chronic autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis (RA) and MS.7, 51, 52 In EAE mice, IL-17 can aggravate the damage of the CNS by promoting inflammatory responses in multiple types of cells.53, 54 Astrocytes, which can secrete various inflammatory cytokines, have been shown to play an indispensable role in the pathogenesis of EAE.25, 55, 56 Our present studies demonstrated that the production of IL-17, HIF-1α, IL-1β and IL-6 was increased in the brain tissues of EAE mice in a time-dependent manner, with a relatively consistent time point tendency. Furthermore, IL-17 stimulation in vitro markedly upregulated IL-1β and IL-6 synthesis in primary mouse astrocytes, and IL-17RA knockdown clearly downregulated IL-1β and IL-6 production. In addition, our in vivo experiment showed that knockdown of IL-17RA in astrocytes with LV-shIL-17RA markedly decreased IL-1β and IL-6 expression and reduced lymphocyte infiltration and demyelination in the brain tissues or spinal cords of EAE mice. These data indicate that IL-17 can trigger the production of IL-1β and IL-6 in astrocytes and promote CNS damage in EAE mice, and blockade of the IL-17 signal by shIL-17RA can decrease the expression of IL-6 and IL-1β both in vitro and in vivo.
HIF-1α has been confirmed to play a regulatory role in the inflammatory response, and most investigations have focused on the involvement of HIF-1α in downstream cytokine signaling.57, 58 However, there is little research concerning the activity of HIF-1α in regulating pro-inflammatory cytokine production at the transcriptional level through binding to the core DNA sequence 5'-[A/G]CGTG-3' within the HRE of promoters.34, 35, 36 Because upregulation of HIF-1α is involved in pathological CNS changes in both MS patients and EAE model mice,59, 60, 61 studies were designed to explore the underlying molecular mechanism by which HIF-1α regulates IL-17-induced synthesis of IL-1β and IL-6 in astrocytes of EAE mice. Our present experiments revealed that the production of HIF-1α was increased in brain tissues of mice with EAE and in cultured astrocytes stimulated with IL-17. Overexpression and knockdown experiments in vitro demonstrated that IL-17-mediated upregulation of HIF-1α promoted the expression of IL-1β and IL-6 genes in the astrocytes. Meanwhile, we determined that HIF-1α could bind to the HRE element within the IL-1β and IL-6 gene promotor and then directly trigger the transcription of these two genes in astrocytes. In addition, astrocyte-specific knockdown of HIF-1α in vivo obviously inhibited the expression of IL-1β and IL-6 in the CNS of EAE mice. Collectively, our in vivo and in vitro studies indicate that IL-17-induced HIF-1α promotes the production of IL-1β and IL-6 in astrocytes of EAE mice.
Many miRNAs such as miR-23b, miR-124 and miR-326 have been demonstrated to play roles in EAE.62, 63, 64 Our previous study reported changes in miRNA expression profiles both in brain tissues of EAE mice (in vivo) and in IL-17-treated astrocytes (in vitro), and we found that upregulation of miR-873 is involved in the pathogenesis of EAE by targeting A20 ubiquitin-editing enzyme.44 However, in addition to miRNA upregulation, microRNA downregulation also plays pathogenic roles in several autoimmune diseases.65 Given that HIF-1α expression was increased both in vivo and in vitro, a subsequent bioinformatics assay was used to predict which downregulated microRNA could target HIF-1α mRNA. The results showed that four miRNAs (miR-195, miR-292-5p, miR-322 and miR-497) among the downregulated miRNAs in the expression profile were predicted to bind to the 3′UTR of HIF-1α mRNA. Further function experiments confirmed that only a miR-497 mimic could target the 3′UTR of HIF-1α mRNA and markedly suppress the expression of HIF-1α, IL-1β and IL-6 in astrocytes. In contrast, LNA-anti-miR-497 enhanced the production of HIF-1α, IL-1β and IL-6 in astrocytes induced by IL-17. Moreover, HIF-1α overexpression was able to reverse the reduction in IL-6 and IL-1β caused by the miR-497 mimic in astrocytes upon IL-17 stimulation. Furthermore, our in vivo studies also revealed that astrocyte-specific miR-497 overexpression reduced the expression of HIF-1α, IL-1β and IL-6 as well as the infiltration of lymphocytes and demyelination in the brain tissues of EAE mice. It is worth mentioning that LV-miR-497 sponge treatment did not aggravate EAE pathological changes in vivo as we expected, although LNA-anti-miR-497 did enhance IL-17-induced production of IL-1β and IL-6 in astrocytes in vitro. We think that this difference might be interpreted by considering that the downregulation of miR-497 in vivo was much more significant than that in vitro, and if miR-497 had been downregulated to a very low level in vivo, the miR-497 sponge could not reduce miR-497 expression to a lower level. Our present studies indicate that IL-17-triggered downregulation of miR-497 results in high HIF-1α expression and consequent IL-1β and IL-6 production by astrocytes in EAE mice. Notably, a recent study has revealed that miR-497 expression is decreased in the peripheral blood lymphocytes of MS patients.50 Therefore, miR-497 downregulation could contribute to CNS damage in MS patients, and thus, miR-497 might be a new therapeutic target in clinical trials.
In summary, our current studies revealed that the production of IL-17, HIF-1α, IL-1β and IL-6 was markedly increased in the brain tissues of EAE mice (in vivo), and the production of HIF-1α, IL-1β and IL-6 in cultured primary mouse astrocytes was also greatly induced by IL-17 stimulation (in vitro). Meanwhile, the expression of miR-497 was decreased both in vivo and in vitro. In addition, in vitro experiments showed that IL-17 could induce the expression of IL-1β and IL-6 genes in astrocytes through upregulation of HIF-1α as a transcriptional factor. Furthermore, IL-17 promoted HIF-1α-mediated production of IL-1β and IL-6 via miR-497 downregulation in astrocytes, indicating that miR-497 downregulation could induce high HIF-1α expression. Moreover, our experiments in vivo demonstrated that astrocyte-specific knockdown of IL-17RA or HIF-1α or astrocyte-specific overexpression of miR-497 could reduce IL-1β and IL-6 production in brain tissues and alleviate the pathological changes and score of EAE mice. These results not only confirm the above-mentioned molecular mechanism discovered in vitro but also reveal the pathogenetic role of the IL-17-miR-497-HIF-1α axis in mouse EAE and provide new insight into the pathogenesis of human MS.
Acknowledgments
We thank Professor Fan Pan (Johns Hopkins University, USA) for sending us pCMV/HIF-1α as a gift. We thank Professor Dongqing Li (Wuhan University, China) for giving us the pSilencer2.1-U6/IL-17RA shRNA as a gift. We also thank Mr Gospel Asonye, a doctoral student at Molecular Medicine/Case Western Reserve University, and Mr Jordan Mclntee, a medical clinical scientist at the Clinical Laboratory/Beloit Health System, for editing the language of the paper. The study was supported by the grants from National Natural Science Foundations of China (31470853 and 81471626) and grants from Natural Science Foundations of Jiangsu Province in China (BK20131386 and BK20151168). The study was also supported by grants from Jiangsu Province Key Lab of Neurodegeneration (No.SJ11KF07), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, Excellent Young or Middle-aged Teachers Project of Nanjing Medical University and Xuzhou Technology Bureau Foundation (KC14SH074).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Ledford H. Drug that boosts nerve signals offers hope for multiple sclerosis. Nature 2015; 520: 417. [DOI] [PubMed] [Google Scholar]
- Absinta M, Vuolo L, Rao A, Nair G, Sati P, Cortese IC et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology 2015; 85: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente D, Ortega MC, Arenzana FJ, de Castro F. FGF-2 and Anosmin-1 are selectively expressed in different types of multiple sclerosis lesions. J Neurosci 2011; 31: 14899–14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara D, D'Alessandro M, Rizzello A, De Riccardis L, Lunetti P, Del Boccio P et al. A lipidomic approach to the study of human CD4(+) T lymphocytes in multiple sclerosis. BMC Neurosci 2015; 16: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang H, Long Y, Lu Z, Hu X. Increased memory Th17 cells in patients with neuromyelitis optica and multiple sclerosis. J Neuroimmunol 2011; 234: 155–160. [DOI] [PubMed] [Google Scholar]
- Zhang X, Tao Y, Chopra M, Dujmovic-Basuroski I, Jin J, Tang Y et al. IL-11 induces Th17 cell responses in patients with early relapsing-remitting multiple sclerosis. J Immunol 2015; 194: 5139–5149. [DOI] [PubMed] [Google Scholar]
- Babaloo Z, Aliparasti MR, Babaiea F, Almasi S, Baradaran B, Farhoudi M. The role of Th17 cells in patients with relapsing-remitting multiple sclerosis: interleukin-17A and interleukin-17F serum levels. Immunol Lett 2015; 164: 76–80. [DOI] [PubMed] [Google Scholar]
- Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008; 172: 146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BV, Pan F. The role of nuclear receptors in regulation of Th17/Treg biology and its implications for diseases. Cell Mol Immunol 2015; 12: 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Studer V, Motta C, Germani G, Macchiarulo G, Buttari F et al. Cerebrospinal fluid detection of interleukin-1beta in phase of remission predicts disease progression in multiple sclerosis. J Neuroinflammation 2014; 11: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Long SA, Cerosaletti K, Ni CT, Samuels P, Kita M et al. In active relapsing-remitting multiple sclerosis, effector T cell resistance to adaptive T(regs) involves IL-6-mediated signaling. Sci Transl Med 2013; 5: 170ra115. [DOI] [PubMed] [Google Scholar]
- Elain G, Jeanneau K, Rutkowska A, Mir AK, Dev KK. The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A-induced levels of IL6 in human astrocytes. Glia 2014; 62: 725–735. [DOI] [PubMed] [Google Scholar]
- Chen J, Liao MY, Gao XL, Zhong Q, Tang TT, Yu X et al. IL-17A induces pro-inflammatory cytokines production in macrophages via MAPKinases, NF-kappaB and AP-1. Cell Physiol Biochem 2013; 32: 1265–1274. [DOI] [PubMed] [Google Scholar]
- Havrdova E, Belova A, Goloborodko A, Tisserant A, Wright A, Wallstroem E et al. Activity of secukinumab, an anti-IL-17A antibody, on brain lesions in RRMS: results from a randomized, proof-of-concept study. J Neurol 2016; 263: 1287–1295. [DOI] [PubMed] [Google Scholar]
- Burger D, Molnarfi N, Weber MS, Brandt KJ, Benkhoucha M, Gruaz L et al. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1beta in human monocytes and multiple sclerosis. Proc Natl Acad Sci USA 2009; 106: 4355–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan GK, Dev KK. Targeting S1P receptors in experimental autoimmune encephalomyelitis in mice improves early deficits in locomotor activity and increases ultrasonic vocalisations. Sci Rep 2014; 4: 5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel K, Schuhmann MK, Pankratz S, Stegner D, Herrmann AM, Braun A et al. Phospholipase D1 mediates lymphocyte adhesion and migration in experimental autoimmune encephalomyelitis. Eur J Immunol 2014; 44: 2295–2305. [DOI] [PubMed] [Google Scholar]
- Lanz TV, Becker S, Osswald M, Bittner S, Schuhmann MK, Opitz CA et al. Protein kinase Cbeta as a therapeutic target stabilizing blood-brain barrier disruption in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 2013; 110: 14735–14740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chen B, Song JH, Zhen T, Wang BY, Li X et al. Eriocalyxin B ameliorates experimental autoimmune encephalomyelitis by suppressing Th1 and Th17 cells. Proc Natl Acad Sci USA 2013; 110: 2258–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyan G, da Cunha AP, Ajay AK, Joller N, Garo LP, Kumaradevan S et al. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest 2015; 125: 1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Jin J, Chang M, Nakaya M, Hu H, Zou Q et al. TPL2 mediates autoimmune inflammation through activation of the TAK1 axis of IL-17 signaling. J Exp Med 2014; 211: 1689–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandolesi G, Musella A, Gentile A, Grasselli G, Haji N, Sepman H et al. Interleukin-1beta alters glutamate transmission at purkinje cell synapses in a mouse model of multiple sclerosis. J Neurosci 2013; 33: 12105–12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile A, Fresegna D, Federici M, Musella A, Rizzo FR, Sepman H et al. Dopaminergic dysfunction is associated with IL-1beta-dependent mood alterations in experimental autoimmune encephalomyelitis. Neurobiol Dis 2015; 74: 347–358. [DOI] [PubMed] [Google Scholar]
- Savarin C, Hinton DR, Valentin-Torres A, Chen Z, Trapp BD, Bergmann CC et al. Astrocyte response to IFN-gamma limits IL-6-mediated microglia activation and progressive autoimmune encephalomyelitis. J Neuroinflammation 2015; 12: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erta M, Giralt M, Jimenez S, Molinero A, Comes G, Hidalgo J. Astrocytic IL-6 influences the clinical symptoms of EAE in mice. Brain Sci 2016; 6: E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic V, Stosic-Grujicic S, Samardzic T, Markovic M, Miljkovic D, Ramic Z et al. Interleukin-17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J Neuroimmunol 2001; 119: 183–191. [DOI] [PubMed] [Google Scholar]
- Ma X, Reynolds SL, Baker BJ, Li X, Benveniste EN, Qin H. IL-17 enhancement of the IL-6 signaling cascade in astrocytes. J Immunol 2010; 184: 4898–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong S, Li K, Zeng G, Fang Y, Zhao J. The effects of interleukin-17 (IL-17)-related inflammatory cytokines and A20 regulatory proteins on astrocytes in spinal cord cultured in vitro. Cell Physiol Biochem 2016; 38: 1100–1110. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Gaffen SL. IL-17 inhibits adipogenesis in part via C/EBPalpha, PPARgamma and Kruppel-like factors. Cytokine 2013; 61: 898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SH, Kim HJ, Jang Y, Ryu WI, Lee H, Kim JH et al. Egr-1 is a key regulator of IL-17A-induced psoriasin upregulation in psoriasis. Exp Dermatol 2014; 23: 890–895. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang Y, Qian Y, Zhang H, Guo S, Sunagawa M et al. Interleukin-17A promotes rheumatoid arthritis synoviocytes migration and invasion under hypoxia by increasing MMP2 and MMP9 expression through NF-kappaB/HIF-1alpha pathway. Mol Immunol 2013; 53: 227–236. [DOI] [PubMed] [Google Scholar]
- Cavadas MA, Mesnieres M, Crifo B, Manresa MC, Selfridge AC, Scholz CC et al. REST mediates resolution of HIF-dependent gene expression in prolonged hypoxia. Sci Rep 2015; 5: 17851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakudo N, Morimoto N, Ogawa T, Taketani S, Kusumoto K. Hypoxia enhances proliferation of human adipose-derived stem cells via HIF-1a activation. PLoS One 2015; 10: e0139890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013; 496: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden VI, Lenio S, Kuick R, Ramakrishnan SK, Shah YM, Bachman MA. Bacterial siderophores that evade or overwhelm lipocalin 2 induce hypoxia inducible factor 1alpha and proinflammatory cytokine secretion in cultured respiratory epithelial cells. Infect Immun 2014; 82: 3826–3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh MV, Ramakrishnan SK, Thomas B, Machado-Aranda D, Bi Y, Talarico N et al. Activation of hypoxia-inducible factor-1alpha in type 2 alveolar epithelial cell is a major driver of acute inflammation following lung contusion. Crit Care Med 2014; 42: e642–e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Uttarwar L, Gao B, Charbonneau M, Shi Y, Chan JS et al. High glucose up-regulates ADAM17 through HIF-1alpha in mesangial cells. J Biol Chem 2015; 290: 21603–21614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Choi H, Kim H, Lee SK. MicroRNA miR-BART20-5p stabilizes Epstein-Barr virus latency by directly targeting BZLF1 and BRLF1. J Virol 2014; 88: 9027–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zheng Z, Wang J, Sun J, Wang P, Cheng X et al. Different miRNA expression profiles between human breast cancer tumors and serum. Front Genet 2014; 5: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Zhang T, Liu C, Badeaux MA, Liu B, Liu R et al. miRNA-128 suppresses prostate cancer by inhibiting BMI-1 to inhibit tumor-initiating cells. Cancer Res 2014; 74: 4183–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin Q, Li J, Dang J, Bian X, Shan S, Yuan J et al. miR-155 deficiency ameliorates autoimmune inflammation of systemic lupus erythematosus by targeting S1pr1 in Faslpr/lpr mice. J Immunol 2015; 194: 5437–5445. [DOI] [PubMed] [Google Scholar]
- Ichii O, Otsuka-Kanazawa S, Horino T, Kimura J, Nakamura T, Matsumoto M et al. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS One 2014; 9: e110383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi H et al. miR-141 regulates colonic leukocytic trafficking by targeting CXCL12beta during murine colitis and human Crohn's disease. Gut 2014; 63: 1247–1257. [DOI] [PubMed] [Google Scholar]
- Liu X, He F, Pang R, Zhao D, Qiu W, Shan K et al. Interleukin-17 (IL-17)-induced microRNA 873 (miR-873) contributes to the pathogenesis of experimental autoimmune encephalomyelitis by targeting A20 ubiquitin-editing enzyme. J Biol Chem 2014; 289: 28971–28986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunus MA, Chung LM, Chaudhry Y, Bailey D, Goodfellow I. Development of an optimized RNA-based murine norovirus reverse genetics system. J Virol Methods 2010; 169: 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JC, Atze K, Yeung PL, Toro-Ramos AJ, Camarillo C, Thompson K et al. Efficient, high-throughput transfection of human embryonic stem cells. Stem Cell Res Ther 2010; 1: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M, Kisseberth WC, Su Y, Besnard F, Messing A. GFAP promoter directs astrocyte-specific expression in transgenic mice. J Neurosci 1994; 14: 1030–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SM, Khalaj AJ, Kumar S, Winchester Z, Yoon J, Yoo T et al. Multiple functional therapeutic effects of the estrogen receptor beta agonist indazole-Cl in a mouse model of multiple sclerosis. Proc Natl Acad Sci USA 2014; 111: 18061–18066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Kanemoto S, Cui X, Kaneko M, Asada R, Matsuhisa K et al. OASIS modulates hypoxia pathway activity to regulate bone angiogenesis. Sci Rep 2015; 5: 16455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg RL, Hoffmann F, Mehling M, Kuhle J, Kappos L. Altered expression of miR-17-5p in CD4+ lymphocytes of relapsing-remitting multiple sclerosis patients. Eur J Immunol 2010; 40: 888–898. [DOI] [PubMed] [Google Scholar]
- Vincent FB, Northcott M, Hoi A, Mackay F, Morand EF. Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis Res Ther 2013; 15: R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellner H. Targeting interleukin-17 in patients with active rheumatoid arthritis: rationale and clinical potential. Ther Adv Musculoskelet Dis 2013; 5: 141–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sarma J, Ciric B, Marek R, Sadhukhan S, Caruso ML, Shafagh J et al. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J Neuroinflammation 2009; 6: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol 2006; 177: 566–573. [DOI] [PubMed] [Google Scholar]
- Pagenstecher A, Lassmann S, Carson MJ, Kincaid CL, Stalder AK, Campbell IL. Astrocyte-targeted expression of IL-12 induces active cellular immune responses in the central nervous system and modulates experimental allergic encephalomyelitis. J Immunol 2000; 164: 4481–4492. [DOI] [PubMed] [Google Scholar]
- Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H et al. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 2010; 32: 414–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Chen Z, Zhang L, Tian D, Wang D, Fan D et al. Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology 2015; 149: 1053–1067. [DOI] [PubMed] [Google Scholar]
- Remels AH, Gosker HR, Verhees KJ, Langen RC, Schols AM. TNF-alpha-induced NF-kappaB activation stimulates skeletal muscle glycolytic metabolism through activation of HIF-1alpha. Endocrinology 2015; 156: 1770–1781. [DOI] [PubMed] [Google Scholar]
- Davies AL, Desai RA, Bloomfield PS, McIntosh PR, Chapple KJ, Linington C et al. Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann Neurol 2013; 74: 815–825. [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Ludwin S, Tabira T, Guseo A, Lucchinetti CF, Leel-Ossy L et al. Tissue preconditioning may explain concentric lesions in Balo's type of multiple sclerosis. Brain 2005; 128: 979–987. [DOI] [PubMed] [Google Scholar]
- Zeis T, Graumann U, Reynolds R, Schaeren-Wiemers N. Normal-appearing white matter in multiple sclerosis is in a subtle balance between inflammation and neuroprotection. Brain 2008; 131: 288–303. [DOI] [PubMed] [Google Scholar]
- Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S et al. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 2009; 10: 1252–1259. [DOI] [PubMed] [Google Scholar]
- Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-alpha-PU.1 pathway. Nat Med 2011; 17: 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y et al. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med 2012; 18: 1077–1086. [DOI] [PubMed] [Google Scholar]
- Pan W, Zhu S, Dai D, Liu Z, Li D, Li B et al. MiR-125a targets effector programs to stabilize Treg-mediated immune homeostasis. Nat Commun 2015; 6: 7096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.