Do environmental cues, and commensal bacteria in particular, have an impact on autoimmunity, and can we modulate the production of gut microbial metabolites to re-equilibrate immune responses as well as restrain autoimmune diseases? Mariño et al.1 elegantly addressed these issues in a very recent Nature Immunology paper. They report that diets designed to release large amounts of the microbial metabolites acetate and butyrate finely tune autoreactive immune responses, which lends weight to the fascinating hypothesis that medicinal foods and metabolites might contribute to treating autoimmune diseases.
With an incidence of 3–5% in the worldwide population and a prevalence of ~1 in 300 in more industrialized countries among individuals who are at least 18 years of age, type 1 diabetes (which is also known as insulin-dependent diabetes mellitus, or IDDM) is a social and economic hurdle that requires awareness from clinicians and researchers alike.
IDDM is a metabolic disease resulting from irreversible autoimmune destruction of insulin-producing pancreatic β-cells. Thus, insulin is no longer available, and patients experience high blood sugar levels accompanied by acute (for example, ketoacidosis, coma and eventually death) or chronic complications that lead to end-organ damage (for example, heart, brain, kidney and eye).
While genetic variants at loci controlling immune responses correlate with the pathogenesis of IDDM, genetic alterations alone cannot explain the increased rate of the disease in the last few decades.2 Thus, the research focus has been pointed at environmental factors, such as hygiene, antibiotic use and diet, which are all factors that influence the composition of the intestinal microbiota, that is, the trillions of microorganisms that make their home in our gut.3 This issue was originally investigated in non-obese diabetic (NOD) mice that spontaneously develop IDDM within 15 weeks of age. Wen et al.4 showed that NOD mice lacking Myd88 (NOD.Myd88−/−), which is an adaptor for multiple innate immune receptors recognizing microbial stimuli, were protected from diabetes when housed in a specific pathogen-free (SPF) animal facility, but not if they were housed in germ-free conditions or after antibiotic treatment, which confirms the protective role of commensal bacteria in IDDM. More recently, studies have focused on the composition of the gut microbiota and how its ability to produce metabolites influences the immune response. Microbiota comparisons between children with at least two IDDM-associated autoantibodies and autoantibody-negative children matched for human leucocyte antigen risk genotype, sex, age and early feeding history showed a low abundance of butyrate-producing and lactate-producing species, and there is an increased abundance of Bacteroides in the children with β-cell autoimmunity.5
Butyrate, acetate and propionate are short-chain fatty acids (SCFAs) produced within the colon through bacterial fermentation of dietary fibers. SCFAs are energy sources for epithelial cells in the colon and contribute to the maintenance of colonic epithelium integrity.6 SCFAs can also expand and increase the function of regulatory T cells (Tregs).7 Finally, SCFAs can influence gene transcription through inhibition of histone deacetylase activity.8
On the basis of this evidence, Mariño and colleagues aimed to investigate whether diets yielding large amounts of acetate and/or butyrate have an impact on diabetes in NOD mice. They started by confirming that SPF NOD Myd88−/− female mice fed a conventional, non-purified diet developed diabetes less frequently than SPF NOD mice, and protection from diabetes was associated with an increased concentration of butyrate and acetate in the peripheral blood and portal vein blood in the mice. Additionally, NOD females fed acetate from 5 weeks of age showed significant protection from diabetes, which confirmed that SCFAs impact diabetes occurrence. Because adult NOD females had a significantly lower concentration of both butyrate and acetate than age-matched NOD males, these findings explain, at least in part, the higher propensity of NOD females to develop diabetes compared to NOD males. It will be interesting to investigate whether the same trend occurs in human females with at least two IDDM-associated autoantibodies compared to males with similar autoantibody positivity.
To more precisely investigate the role of butyrate and acetate in IDDM and more closely mimic bacterial fermentation in the colon, NOD mice were fed either high-amylose maize starch (HAMS) that has been acetylated (HAMSA) or butyrylated (HAMSB), which is a powerful strategy for assessing the effects of specific SCFAs (that is, acetate versus butyrate) on intestinal biology. Five weeks of a HAMSA- or HAMSB-supplemented diet in NOD females between 5 and 15 weeks of age was enough to prevent pancreatic islet infiltration and to significantly delay diabetes onset. Interestingly, this difference was maintained for up to 30 weeks, and both HAMSA and HAMSB diets were effective against diabetes regardless of when or how long they were given.
Mechanistically, a HAMSA diet and, to a lesser extent, a HAMSB diet suppressed proliferation of autoreactive CD8+ and CD4+ T cells, whose frequency and number decreased in both the spleen and pancreatic lymph nodes of treated mice relative to mice fed the control diet. Additionally, the number, differentiation, gene transcription and surface phenotype of B cells were substantially affected by SCFAs and acetate in particular, which suggests that B cells are relevant antigen-presenting cells in diabetes, and SCFAs negatively impact the antigen-presenting capacity of these cells. Finally, the HAMSB diet increased the number, gene expression and function of Treg cells, which restrained diabetes onset in T-cell transfer experiments. Because IDDM onset can occur in the first years of life, it would be interesting to know if such SCFA-mediated mechanisms also influenced immune tolerance within the thymus.
An SCFA-rich diet also impacted gut permeability. Specifically, the HAMSB diet augmented the expression of tight junctions in the colon of NOD females, and both the HAMSA and HAMSB diets decreased the concentration of lipopolysaccharide in the peripheral blood of NOD mice. In parallel, the two diets lowered the concentration of the pro-diabetogenic cytokine interleukin (IL)-21 and enhanced the serum levels of IL-22, which is known as a homeostatic cytokine for the intestinal epithelium.
As expected, the HAMSA diet shaped the microbiota composition in NOD mice and specifically enriched the microbial flora in Bacteroides. Of relevance, the microbiota shaped by the HAMSA diet was responsible per se for the protection from diabetes onset when transplanted in germ-free NOD mice and correlated with very high concentrations of acetate in hepatic portal blood and cecal contents.
On the basis of the different and yet complementary pathways induced by acetate and butyrate to restrain pancreatic islet infiltration, the authors exposed NOD mice to a combination of HAMSA and HAMSB diets, which showed that treated mice were completely protected from diabetes. Interestingly, half of the dose of the combined diet was ineffective in preventing diabetes onset. These findings agree with the observation that the ‘western diet’, which is characterized by an average uptake of half of the recommended dose of fiber,9 is associated with asthma, allergy and autoimmune diseases.
All together, these findings1 show that fiber supplementation impacts the composition of the gut microbiota, which expands SCFA-producing bacteria. On the one hand, SCFAs, especially acetate and butyrate, act on the colonic epithelium, which induced production of IL-22 and increased the expression of occludin to eventually preserve epithelial gut integrity. On the other hand, SCFAs modulate the immune response to protect the host from diabetes and likely other autoimmune diseases as well (Figure 1). Fiber supplementation has already been tested in children with IDDM.10 Mariño et al.1 extended this concept by suggesting the use of HAMSA and HAMSB supplements, which have also been tested in the clinic for gastrointestinal tract disorders.11
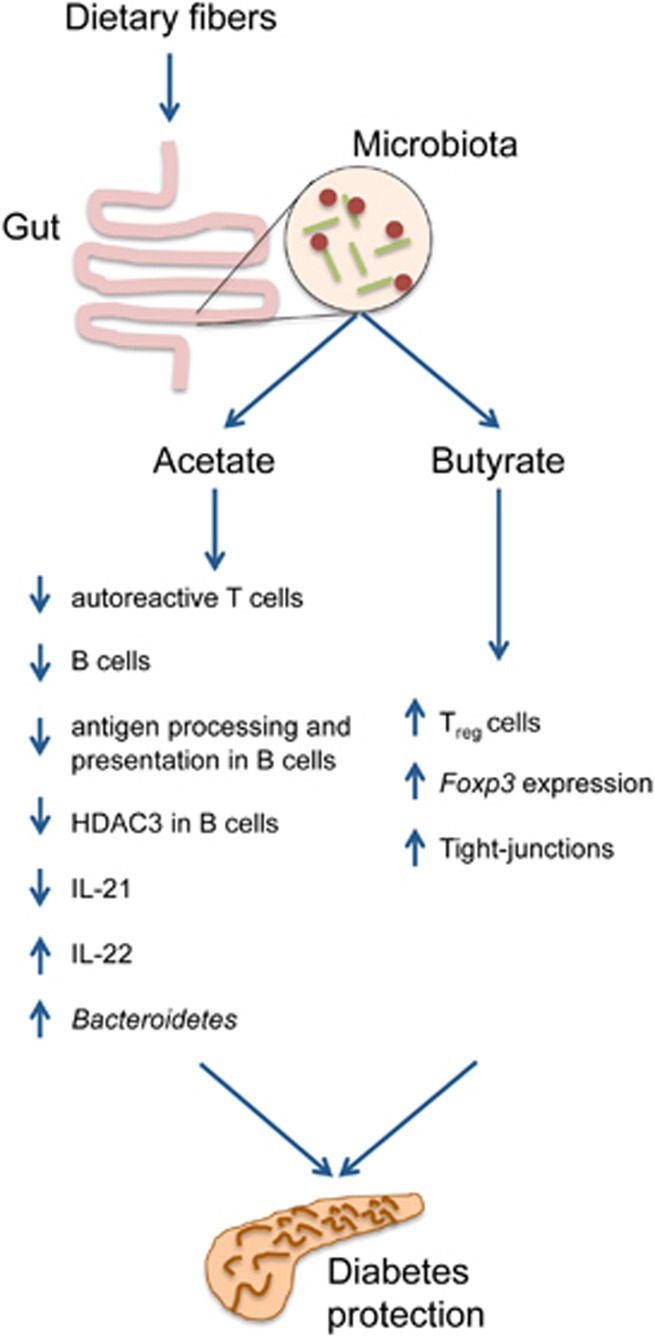
Figure 1.
Effects of acetate and butyrate on the intestinal epithelium and the immune system. The figure depicts the most relevant effects exerted by acetate and butyrate on the gut epithelium and the immune system, which impact diabetes, as identified by Marino et al.1 The blue arrows to the left of each target indicate whether acetate and butyrate cause an increase or decrease in the target.
The mechanistic findings of this study go well beyond diabetes because dietary metabolites have already been shown to contrast food allergies, colitis and colon cancer. Additionally, the modulating effects of the microbiota on the immune system are both local and systemic because modifications to the microbiota composition contribute to autoimmune demyelination, arthritis, graft versus host disease and responses to chemotherapy and immunotherapy.12, 13 What is particularly fascinating is the relationship between commensal bacteria and cancer. Despite early evidence that antimicrobial therapy prevented solid tumor development in mice,14 the mechanisms through which commensal bacteria impact extra-mucosal cancer have yet to be clarified.15 For most of the extra-mucosal tumors, we aim to expand tumor-specific CD4+ and CD8+ T cells while restraining Treg cells, and high acetate and butyrate might even be detrimental. In the context of developing precision medicine, we predict the next decade will lead to several studies directed at identifying diets and supplements tailored to individual patients.
Acknowledgments
The work was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC; grant no. IG16807 to M Bellone). Arianna Brevi conducted this study in partial fulfillment of her PhD requirements at San Raffaele University.
Footnotes
The authors declare no conflict of interest.
References
- Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 2017; 18: 552–562. [DOI] [PubMed] [Google Scholar]
- Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet 2014; 383: 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knip M, Siljander H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat Rev Endocrinol 2016; 12: 154–167. [DOI] [PubMed] [Google Scholar]
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008; 455: 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goffau MC, Luopajarvi K, Knip M, Ilonen J, Ruohtula T, Harkonen T et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes 2013; 62: 1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol 2011; 12: 5–9. [DOI] [PubMed] [Google Scholar]
- Tanoue T, Atarashi K, Honda K. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 2016; 16: 295–309. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013; 504: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DE, Mainous AG 3rd, Lambourne CA. Trends in dietary fiber intake in the United States, 1999-2008. J Acad Nutr Diet 2012; 112: 642–648. [DOI] [PubMed] [Google Scholar]
- Nader N, Weaver A, Eckert S, Lteif A. Effects of fiber supplementation on glycemic excursions and incidence of hypoglycemia in children with type 1 diabetes. Int J Pediatr Endocrinol 2014; 2014: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Leu RK, Winter JM, Christophersen CT, Young GP, Humphreys KJ, Hu Y et al. Butyrylated starch intake can prevent red meat-induced O6-methyl-2-deoxyguanosine adducts in human rectal tissue: a randomised clinical trial. Br J Nutr 2015; 114: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg JB, Sonnenberg GF. Host-microbiota interactions shape local and systemic inflammatory diseases. J Immunol 2017; 198: 564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Daillere R, Roberti MP, Routy B, Kroemer G. Anticancer effects of the microbiome and its products. Nat Rev Microbiol 2017; e-pub ahead of print 22 May 2017; doi:10.1038/nrmicro.2017.44. [DOI] [PubMed]
- Enzler T, Gillessen S, Manis JP, Ferguson D, Fleming J, Alt FW et al. Deficiencies of GM-CSF and interferon gamma link inflammation and cancer. J Exp Med 2003; 197: 1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowski MR, Conejo-Garcia JR. Size does not matter: commensal microorganisms forge tumor-promoting inflammation and anti-tumor immunity. Oncoscience 2015; 2: 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]