Abstract
Osthole, an active component of Chinese herbal medicines, reportedly possesses various pharmacological properties and has potential therapeutic applications. This study explored the anti-allergic effects of osthole in asthmatic mice and investigated the immunomodulatory actions of osthole on dendritic cells (DCs) and T cells. Herein, we show that oral administration of osthole to BALB/c mice after ovalbumin (OVA) sensitization ameliorated all of the cardinal features of T helper 2 (Th2)-mediated allergic asthma; namely, the production of OVA-specific immunoglobulin E, airway hyperresponsiveness, airway inflammation and the production of Th2-type cytokines including interleukin (IL)-4, IL-5 and IL-13. Surprisingly, IL-10 production was not inhibited and was even enhanced by osthole treatment. We observed a significant increase in the percentages of IL-10-producing DCs and forkhead box P3-positive regulatory T (Treg) cells in osthole-treated asthmatic mice. Additionally, in vitro analyses revealed that osthole-treated bone-marrow-derived DCs had a partial maturation phenotype, secreting large amounts of IL-10 and low levels of proinflammatory cytokines, such as IL-12, IL-6 and tumor necrosis factor-α, and displaying reduced levels of MHC class II surface molecules. These DCs displayed immunosuppressive capacity by directly inhibiting effector T-cell responses or inducing Treg cells. In addition, osthole directly inhibited the activated CD4+ T-cell proliferation and Th1/Th2-type cytokine production in this system. Collectively, these results suggest that DCs and T cells are potential target cells responsible for the action of osthole against allergic asthma.
Keywords: asthma, dendritic cell, osthole, T cell
Introduction
Asthma is a complex, heterogeneous chronic inflammatory disease with multiple phenotypes. Allergic asthma, a type of T helper 2 (Th2)-associated asthma, is increasingly prevalent in developed countries, and responses to allergic asthma are characterized by the involvement of allergen-specific immunoglobulin E (IgE) and Th2 cells.1, 2 Inhaled antigens are taken up by dendritic cells (DCs) in the airway lumen, after which activated DCs migrate to regional thoracic lymph nodes (TLNs), where antigen presentation to naive CD4+ T cells occurs. These antigen-activated DCs express costimulatory molecules and cytokines that provide key signals for the differentiation of naive CD4+ T cells into Th1, Th2, Th17 or regulatory T (Treg) cells.3, 4 Previous studies have demonstrated that the development of Th2 cells and the cytokines they produce, such as interleukin (IL)-4, IL-5 and IL-13, strongly contribute to eosinophilic inflammation, airway mucus production, airway hyperresponsiveness (AHR) and airway remodeling concomitant with high circulating IgE levels in patients with allergic asthma.5, 6 By contrast, Treg cells, which suppress the activation and proliferation of effector T cells, help limit ongoing lung inflammation.7, 8, 9 Thus, DCs play crucial roles in promoting allergic airway inflammation and protecting the lungs from overt damage, and represent novel targets for directed therapy for asthma.
Treg cell subsets are generally categorized into two groups: thymus-derived Treg (tTreg) cells and peripherally derived Treg (pTreg) cells.10 tTreg cells are characterized by constitutive expression of forkhead box P3 (Foxp3) and CD25. However, depending on whether Treg cells stably express Foxp3, pTreg cells may further divide into Foxp3+ Treg cells and Foxp3− type 1 regulatory T (Tr1) cells.11 The mechanisms of suppressive functions for Treg cells have been identified; they include the secretion of inhibitory cytokines (IL-10 and TGF-β), downmodulation of antigen-presenting cells (APCs) by cell-cell contact (cytotoxic T-lymphocyte antigen 4, CTLA-4), release of cytolytic molecules (granzymes) and IL-2 deprivation through CD25.12 In studies of allergen immunotherapy, the appearance of Treg cells expressing Foxp3, IL-10 or both molecules was found to be associated with reduced allergic airway inflammation in humans.13 However, in patients with asthma, there is a breakdown in Treg-regulatory mechanisms that results in the development of airway inflammation.14, 15
Osthole, 7-methoxy-8-(3-methyl-2-butenyl) coumarin, is a pure compound that is isolated from seeds of Cnidium monnieri (L.) Cusson and is widely used in traditional Chinese medicine. Osthole has received considerable attention because it has a variety of biological and pharmacological properties, including anti-cancer, anti-inflammatory, immunomodulatory, anti-hepatitis, neuroprotective, osteogenic and anti-allergic effects.16 Our previous study showed that osthole exerted an antitumor effect in a P-388 D1 tumor-bearing mouse model.17 Other animal studies have also demonstrated that osthole attenuates immune inflammatory diseases such as autoimmune encephalomyelitis, IgA nephropathy and contact dermatitis.18, 19, 20 Experimental evidence revealed that osthole exhibited immunomodulatory and anti-inflammatory activity by decreasing NF-κB activation, inhibiting the phosphorylation of p38 mitogen-activated protein kinase and c-Jun N-terminal kinase 1/2 (JNK1/2), and reducing tumor necrosis factor (TNF)-α, nitric oxide (NO) and cyclooxygenase expression.21 Additionally, osthole prevented anti-Fas antibody-induced hepatitis in mice.22 Another attractive finding was its suppression of eotaxin, an IL-4-induced eosinophil-specific C-C chemokine, in bronchial epithelial BEAS-2B cells.23 Thus, we propose that the bioactivities of osthole might influence immune responses and provide a new alternative for relieving the symptoms of allergic asthma. However, to date, the anti-allergic effects of osthole against allergic asthma and its modulatory effects on DCs and T cells remain unknown.
In the present study, we examined whether osthole treatment can suppress allergic Th2 responses in an ovalbumin (OVA)-induced asthma model and achieve anti-allergic activities against the development of airway syndromes. Furthermore, the immunoregulatory effects of osthole on DCs and T cells were explored. Herein, we provide new evidence for an anti-inflammatory role of osthole, expanding the potential use of osthole as an immunomodulatory adjuvant to treat Th2-mediated allergic inflammation.
Materials and methods
Preparation of osthole
Osthole (purity ⩾99.5%, as determined through high-performance liquid chromatography) was isolated from the fruit of C. monnieri using previously described purification methods.17 A stock solution was prepared by dissolving osthole in dimethyl sulfoxide (DMSO), and it was stored at 4 °C until use.
Animals
Female BALB/c mice and DO11.10 mice expressing a transgenic T-cell receptor specific to amino acids 323–339 of OVA were purchased from the National Laboratory Animal Center and Laboratory Animal Center of National Taiwan University (Taipei, Taiwan) and maintained at the Animal Center of Taipei Medical University. Animals were used at 5–8 weeks of age and were randomly housed in individually ventilated cages, which were maintained in a temperature- and humidity-controlled room on a 12-h light-dark cycle. Laboratory pellet chow and water were freely available. The animal care and handling protocols were approved by the Animal Research Ethics Board of the College of Medicine, Taipei Medical University.
Administration of osthole to allergen-sensitized mice
Female BALB/c mice (n=6 per group) at 6 weeks of age were sensitized with an intraperitoneal (i.p.) injection of 50 μg of OVA (Sigma-Aldrich, St Louis, MO, USA) plus 4 mg of alum (Thermo Scientific, Rockford, IL, USA) on day 1. On days 14 and 28, mice were boosted with OVA (30 μg) with the same dosage of alum. On days 34 and 35, they were intranasally (i.n.) challenged with 100 μg OVA. Then, they were challenged with 5% OVA in 0.9% NaCl by inhalation administration for 3 consecutive days (days 36–38) for 30 min daily. Finally, on day 39, the AHR of the mice was measured and they were killed. To examine the effects of various osthole dosages (5, 25 and 50 mg/kg of body weight), three groups of mice were orally fed osthole dissolved in a 0.5% sodium carboxymethyl cellulose (CMC) solution (Sigma-Aldrich) once per day on days 15–38. OVA-sensitized and -challenged mice were fed a volume of 0.5% CMC, equivalent to that fed to the asthmatic positive control (PC). Naive mice that were not sensitized or challenged with OVA served as the negative control (NC).
Measurement of serum OVA-specific IgE
Serum samples were collected from mice on day 39. OVA-specific IgE serum antibody titers were measured using an ELISA kit (Becton Dickinson Biosciences, San Jose, CA, USA). All samples were compared to OVA-specific IgE standard serum. The concentration of standard serum was arbitrarily assigned as 1 ELISA unit (EU). The results are expressed as EU=(Absorbancesample−Absorbanceblank)/(Absorbancestandard−Absorbanceblank).
Measurement of AHR
AHR was measured at 24 h after the last OVA instillation. As previously described,24 responsiveness to aerosolized methacholine (MCh, Sigma-Aldrich) and the resulting AHR were measured by lung resistance (RL) in tracheostomized and anesthetized mice. Mice were placed in a ventilator (model 683; Harvard Apparatus, South Natick, MA, USA) with breathing controlled at 150 breaths/min, a tidal volume of 0.3 ml and a positive end-expiratory pressure of 2–4 cmH2O. Increasing doses (1–32 mg/ml) of MCh aerosol were administered, and the RL was continuously computed (Labview, National Instruments, Dallas, TX, USA) by fitting the flow, volume and pressure to an equation of motion.
Analysis of bronchoalveolar lavage fluid and lung histology
Mice were killed after the AHR was measured, and the lungs were immediately lavaged via a tracheal cannula with 3 × 1 ml of Hank’s balanced salt solution (HBSS) without ionized calcium and magnesium. The lavage fluid was centrifuged at 400g for 10 min at 4 °C. Supernatants were collected for the chemokine and cytokine assays. Cells were resuspended in 1 ml of RPMI-1640 medium and combined with 2% fetal bovine serum (FBS) after washing. Total cell counts were determined by counting at least 200 cells of the cytocentrifuged preparations in a hemocytometer with Liu’s stain (Chi I Pao, Taipei, Taiwan). Cells were classified as macrophages, eosinophils, neutrophils and lymphocytes based on standard morphological criteria. The lungs were immediately removed and fixed in 10% buffered formalin after lavage, routinely processed and embedded in paraffin. Five-micrometer sections were prepared and stained with hematoxylin and eosin (H&E). Additionally, periodic acid-Schiff (PAS) staining was performed to identify mucus production by epithelial cells. To quantify the degree of histological inflammation and mucus production, stained slices were scanned with a digital camera and analyzed using ImageJ software. Inflammatory changes and mucus production, respectively, are presented as the percentage of the inflamed area and PAS-positive area. Analyses of BALF cells and lung histology were performed in a blinded manner.
Determination of cytokine and chemokine levels
Levels of IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, eotaxin, TNF-α and interferon (IFN)-γ were analyzed using ELISA kits; namely, eotaxin, IL-5, IL-10 and IFN-γ kits (R&D Systems, Minneapolis, MN, USA) and IL-1β, IL-4, IL-6, IL-12, IL-13, and TNF-α kits (eBioscience, San Diego, CA, USA).
Analysis of CD4+ T-cell responses in the spleen
The spleens from each group of mice were collected and ground into single-cell suspensions. Red blood cells were removed using an ACK lysis buffer and washed three times with HBSS. For the cytokine assay, 107 splenocytes were stimulated with OVA (50 μg/ml) in a 24-well plate. Supernatants were collected after 72 h of culturing, and cytokine levels were detected with an ELISA kit. In the proliferation assay, 3 × 105 splenocytes were seeded into each well of a 96-well plate and cultured with OVA (50 μg/ml). After 5 days of culturing, the cells were pulsed with [3H]-thymidine for another 18 h. The cells were collected, and the counts per minute (cpm) were measured with a β-counter.
Detection of IL-10-producing cells ex vivo
Flow cytometric analysis was used to identify IL-10-producing populations in the lungs and TLNs ex vivo. For flow cytometry, lung lobes from different experimental groups were collected and enzymatically digested with 4 mg/ml collagenase A (Sigma-Aldrich) for 90 min at 37 °C. Single-cell suspensions were generated by resuspending digested tissue through a 0.07-mm nylon cell strainer. After centrifugation, the erythrocytes were removed using an ACK lysis buffer. TLNs were also collected and mechanically minced. The cell suspension from the lungs or TLNs was washed, and cell counts were determined. To identify T populations, single-cell suspensions were stimulated with 20 ng/ml phorbol 13-myristate 12-acetate (PMA; Sigma-Aldrich) plus 1 μg/ml ionomycin (Sigma-Aldrich) for 6 h. The cells were then stained for surface and intracellular markers. The following molecules were used: anti-CD11c-FITC, anti-CD4-PE-Cy5, anti-CD11b-PE-Cy5, anti-IL-10-PE, anti-Foxp3-FITC and anti-CD25-PE (sources are from eBioscience); anti-IL-4-PE, anti-c-kit (CD117)-PE-Cy5, anti-CCR3 (CD193)-PE-Cy5.5, anti-F4/80-PE-Cy5, anti-CD68-PE and anti-IL-10-FITC (sources are from Biolegend, San Diego, CA, USA); Siglec-F-PE and anti-FcεRI-PE (sources are from Thermo Fisher, Rockford, IL, USA). Flow cytometry was performed using a FACSCalibur or a FACSCanto II (Becton Dickinson, Mountain View, CA, USA), and the data were analyzed using Cellquest pro software or FACSDiva software (Becton Dickinson).
Culture and characterization of bone marrow-derived DCs
Bone marrow cells were collected from 6–8-week-old BALB/c mice and cultured in RPMI-1640 combined with 5% FBS complete medium with granulocyte-macrophage colony-stimulating factor (GM-CSF) (200 U/ml) on day 0. On day 2, fresh complete medium containing GM-CSF was added to replace the old medium. On day 5, nonadherent cells were transferred to a new plate with GM-CSF (100 U/ml) and IL-4 (200 U/ml) to decrease contamination by macrophages. To perform the MTT assay, day 8 culture bone marrow-derived DCs (105 cells/well) were treated with various concentrations of osthole for 48 h. Untreated DCs were cultured as the control. Cells were pulsed with 10 μl of 5 mg/ml MTT and incubated for another 4 h. Then, cells were solubilized in DMSO, and the value was determined using a microplate reader at 570 nm. After determining the optimal concentration by the MTT assay, 106 DCs were treated with or without osthole (1, 5 and 10 μg/ml) and then activated with 100 ng/ml lipopolysaccharide (LPS; Sigma-Aldrich). DCs and supernatants were collected after 24 h of incubation. Cytokine levels in the supernatants were analyzed using ELISA kits. Cells were stained with antibodies and analyzed by flow cytometry. DCs were gated according to the standard forward- and side-scatter profiles of CD11c+ large cells. Anti-CD11c-FITC, anti-CD40-FITC, anti-CD86-FITC, anti-CD11c-PE, anti-I-A/I-E (MHC class II)-PE and anti-CD80-PE monoclonal antibodies (mAbs) were purchased from eBioscience.
DC:T-cell coculture in vitro
For the mixed lymphocyte reaction (MLR), 106 day 8 bone marrow-derived DCs from BALB/c mice were treated with LPS (100 ng/ml), osthole (10 μg/ml), LPS plus osthole or medium only. After 24 h of culturing, DCs were irradiated with 3000 rad (from a 137Cs source). Then, allogeneic CD4+ T cells (2 × 105 cells/well) from C57BL/6 mice were cultured with different proportions of irradiated DCs (104, 2 × 104 and 4 × 104 cells/well). T cells were cultured for 72 h and then pulsed with [3H]-thymidine for another 18 h. The cells were collected, and cpm values were measured with a β-counter. For the cytokine IFN-γ assay, culture supernatants were collected at 72 h. In another experiment, to assess the effects of osthole-treated DCs on syngeneic T cells, 106 cells per well of purified naive DO11.10 CD4+ T cells were cocultured with osthole (10 μg/ml)-treated DCs, OVA (100 μg/ml)-activated DCs or OVA plus osthole-treated DCs, at a ratio of 1:2.5 (DC:T cells) in the presence of IL-2 (2 ng/ml). After 7 days of culturing, the T cells were restimulated with 4 × 105 irradiated OVA (100 μg/ml)-pulsed DCs. Cytokine levels in the culture supernatants from these T cells were assayed after 48 h using ELISA kits. To assay T-cell proliferative activity, 2 × 105 cells/well of DO11.10 CD4+ T cells were generated following two rounds of stimulation by differently treated DCs and then stimulated with 4 × 105 irradiated splenocytes and an OVA323-339 peptide (4 μg/ml) in 96-well plates. After 56 h of culturing, T-cell proliferation was measured by [3H]-thymidine incorporation and is expressed as c.p.m.
Measurement of CD4+ T-cell responses in vitro
To assess the direct effect of osthole on T cells, 2 × 105 CD4+ T cells from BALB/c mice were cultured in 96-well plates and treated with different doses of osthole (0.5, 1 and 5 μg/ml) in the presence of anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) antibody stimulation. After 56 h of culturing, cell proliferation was measured based on the [3H]-thymidine incorporation. For the cytokine assays, 5 × 105 CD4+ T cells were cultured in 48-well plates and stimulated with anti-CD3/anti-CD28 antibodies in the presence of osthole. The culture supernatants were collected at 72 h, and cytokine production was determined using ELISA kits.
Data analysis
Results are expressed as the mean±standard error of the mean (s.e.m.). Statistical analyses were performed using one-way analysis of variance followed by Dunnett’s post hoc test. The results with P<0.05 were considered statistically significant.
Results
Osthole reduced anti-OVA IgE production in serum
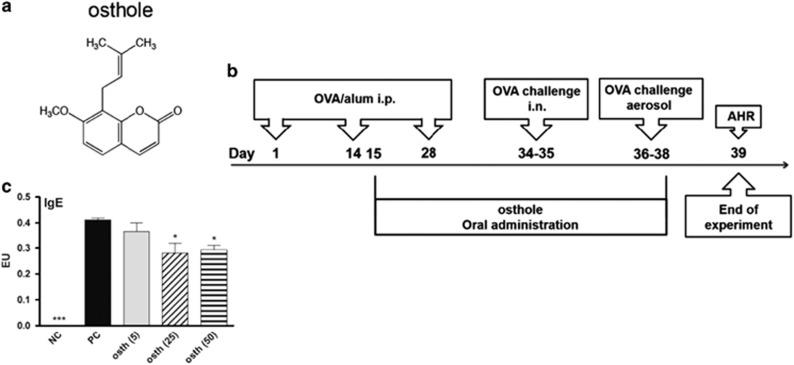
We evaluated the therapeutic effects of osthole in an OVA-induced asthmatic animal model. The structure of osthole and the treatment protocol are shown in Figures 1a and b. OVA-sensitized mice were orally fed with different doses of osthole at 5, 25 and 50 mg/kg and abbreviated as osth (5), (25) and (50), respectively. The PC group was fed a 0.5% CMC solution instead of osthole. NC mice were neither sensitized with OVA nor fed osthole. Serum samples were collected on day 39, and levels of an OVA-specific IgE antibody in the serum were assessed. Animals sensitized and challenged with OVA (PC group) had markedly elevated OVA-specific IgE production, which was significantly inhibited by administration of osthole at 25 and 50 mg/kg (Figure 1c).
Figure 1.
Osthole reduced OVA-specific IgE antibody production in sera. (a) The structure of osthole, which has the chemical formula C15H16O3 and a molar mass of 244.29 g/mol. (b) Curative protocol. Mice were randomized into five groups. On days 1, 14 and 28, all groups were sensitized with an i.p. injection of OVA allergen. Three groups of mice (osth (5), osth (25) and osth (50)) were orally fed 5, 25 and 50 mg/kg, respectively, of osthole on days 15–38. PC mice were administered a CMC solution instead of osthole. Mice were then i.n. challenged with OVA on days 34 and 35. Finally, they were exposed to OVA aerosols for 3 days, and the AHR was measured 1 day after the last challenge. BALF was collected after the AHR was measured. NC mice were neither sensitized with OVA nor administered osthole treatment. (c) OVA-specific IgE levels in sera. The results are expressed as the mean±s.e.m. of six mice per group. *P<0.05, **P<0.01, ***P<0.001 vs the PC group.
Osthole attenuated allergen-induced AHR and airway inflammation
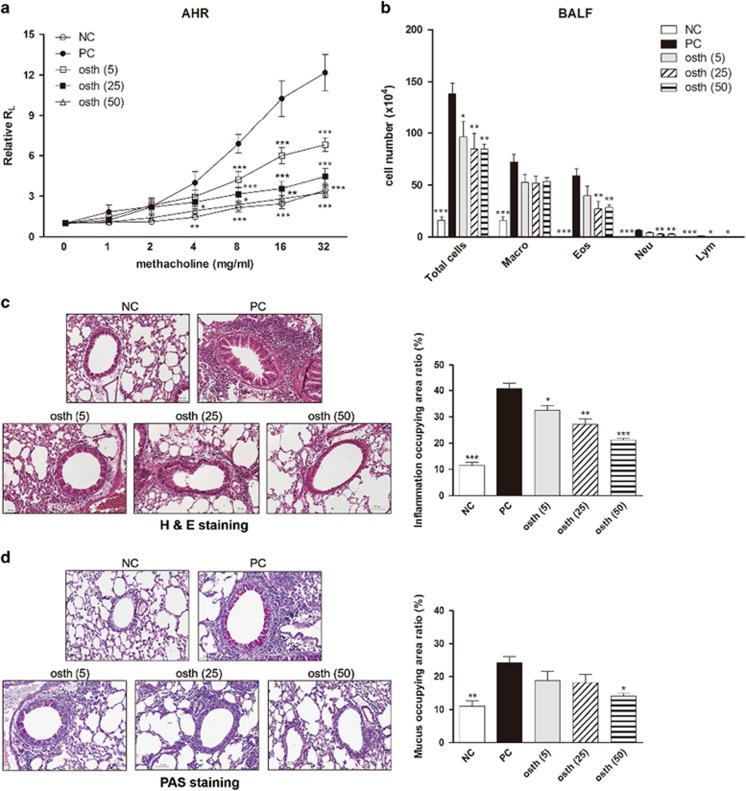
To evaluate the anti-allergic effects of osthole on asthma, the AHR and infiltration of inflammatory cells in the lungs were further investigated. As illustrated in Figure 2a, after exposure to increasing concentrations of methacholine (1–32 mg/ml), the degree of AHR dramatically increased in the PC group, whereas this upregulation was effectively reduced in a dose-dependent manner in osthole-treated mice. In addition, PC mice exhibited an elevated number of inflammatory cells (that is, total cells, macrophages, eosinophils and neutrophils) in BALF (Figure 2b). By contrast, oral treatment of mice with medium (osth (25)) and high doses (osth (50)) of osthole markedly reduced the infiltration of these inflammatory cells, particularly eosinophils. H&E-stained lung sections from the PC group showed an accumulation of inflammatory cells around the bronchioles (Figure 2c), whereas the extent of inflammatory cell infiltration was markedly diminished in the osthole-treated groups. Moreover, mucus overproduction from goblet cells was clearly indicated in violet in the bronchial airways of the PC group (Figure 2d), unlike in the osthole-treated groups. Osthole administration suppressed goblet cell hyperplasia in the lungs. Overall, our data show that osthole attenuated allergen-induced airway inflammation in mice with OVA-induced asthma.
Figure 2.
Osthole reduced airway hyperresponsiveness and airway inflammation. (a) Oral administration of osthole suppressed the development of AHR in mice with OVA-induced asthma. After OVA challenge, the airway resistance of treated mice was measured in response to increasing concentrations of methacholine (1–32 mg/ml) through invasive body plethysmography. The results are expressed as the pulmonary resistance in the ratio of lung resistance (RL) after PBS nebulization. (b) Numbers of pulmonary eosinophils and neutrophils were reduced in osthole-treated mice. After the pulmonary function parameters were measured, BALF was collected and stained with Liu’s stain. Cells from BALF were counted and classified as macrophages (Macro), eosinophils (Eos), neutrophils (Neu) and lymphocytes (Lym). (c) Lung sections were stained with H&E to measure infiltrated inflammatory cells. (d) Lung sections were stained with PAS to measure mucus hypersecretion. Inflammatory changes and mucus production are, respectively, presented as percentages of inflamed areas and PAS-positive areas in different experimental groups. The results are expressed as the mean±s.e.m. of six mice per group. *P<0.05, **P<0.01, ***P<0.001 vs the PC group.
Osthole suppressed the production of OVA-induced chemokine and cytokine secretion
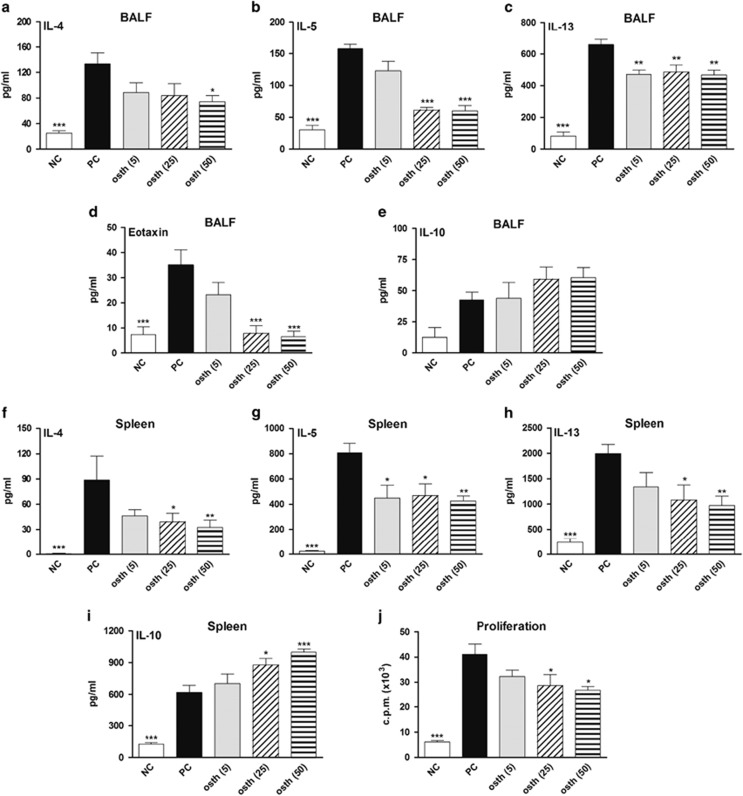
The high expression level of IgE in these mice reflects the elicited Th2 response, in which IL-4 and IL-13 act on B cells by driving immunoglobulin class switching toward the production of IgE.25 To further confirm whether osthole treatment inhibits the OVA-induced Th2-predominant immune reaction in the lungs, levels of Th2-type cytokines and eotaxin in BALF were analyzed. The BALF from PC mice was found to have notably increased Th2 cytokine (IL-4, IL-5 and IL-13) and eotaxin secretion, whereas those of mice fed the medium (osth (25)) and high doses (osth (50)) of osthole exhibited significantly decreased production of these mediators (Figures 3a–d). However, IL-10 levels in BALF did not statistically differ among these groups (Figure 3e). Similar Th2-type cytokine patterns were shown in the culture supernatants of OVA-restimulated splenocytes in these mice (Figures 3f–h). Notably, osthole treatments at 25 and 50 mg/kg markedly increased IL-10 production in the culture supernatants of OVA-stimulated splenocytes (Figure 3i). In the effector T-cell proliferation assay, osthole-treated mice showed a significantly lower proliferative capacity for OVA-specific effector T cells than did PC mice (Figure 3j). In addition, we investigated the preventive effects of osthole in mice with OVA-induced asthma. The protocol for osthole treatment is shown in Supplementary Figure S1a. We found that administration of osthole at 50 mg/kg attenuated the inflammatory response in mice, including decreased severity of AHR, a smaller extent of airway inflammation, and lower Th2 cytokine production in comparison to the PC group (Supplementary Figures S1b–g). Collectively, these findings indicate that osthole was able to modulate the magnitude of Th2-mediated responses during the development of OVA-induced allergic asthma.
Figure 3.
Osthole reduced Th2 immune responses in mice with OVA-induced asthma. (a–e) BALF from each group of mice was collected and analyzed for IL-4, IL-5, IL-13, eotaxin and IL-10 contents using ELISA kits. (f–i) Splenocytes (107 cells/well) were stimulated with OVA for 3 days. Levels of IL-4, IL-5, IL-13 and IL-10 in supernatants were determined using ELISA kits. (j) OVA-specific T-cell proliferation was reduced in osthole-treated mice. Splenocytes (3 × 105 cells/well) were stimulated with OVA for 5 days. The cells were then pulsed with [3H]-thymidine for 16–18 h. The c.p.m. values were measured with a β-counter. The results are expressed as the mean±s.e.m. of six mice per group. *P<0.05, **P<0.01, ***P<0.001 vs the PC group.
Osthole increased the presence of IL-10-expressing DCs and Treg cells in mice with OVA-induced asthma
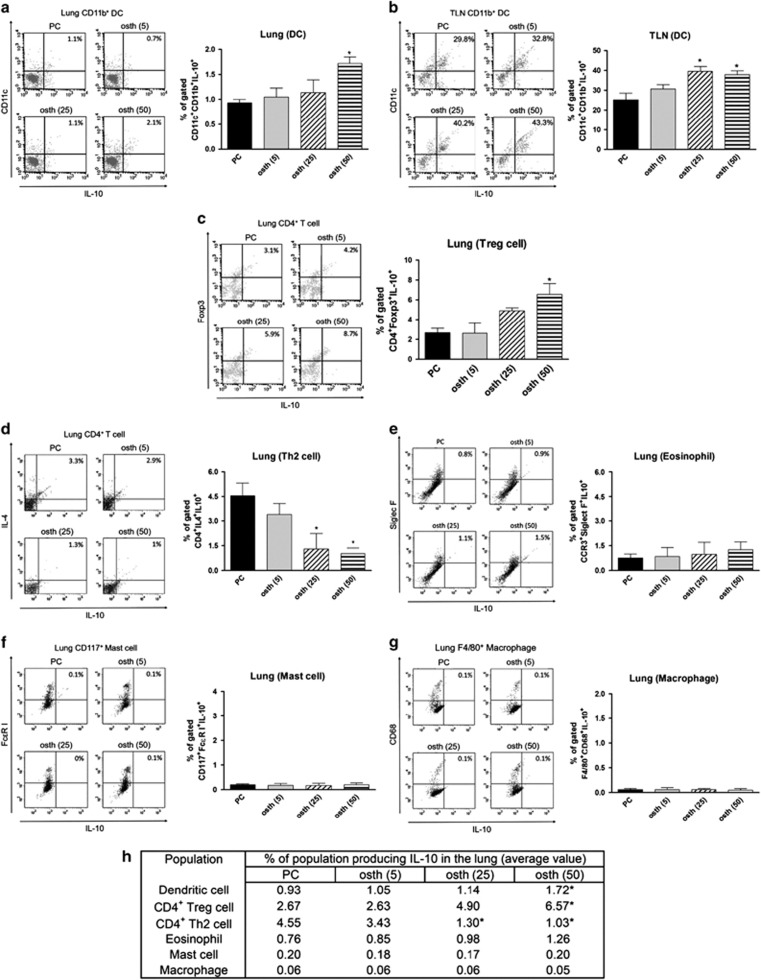
IL-10 is an inducible anti-inflammatory cytokine and can be produced by several cell types, including Th2, Treg, mast cells, eosinophils, DCs and macrophages.26, 27, 28 Flow cytometry was performed to clarify the source of this cytokine in the lungs and TLNs of osthole-treated mice. As shown in Figures 4a and b, the osthole-treated groups presented significantly increased percentages of IL-10-producing CD11c+CD11b+ DCs in both the lungs and TLNs compared with the PC group. Moreover, the frequency of IL-10-producing CD4+Foxp3+ Treg cells was increased in the lungs following treatment with a high dose of osthole (Figure 4c). In addition, PC mice expressed an increased percentage of CD4+IL4+ Th2 cells producing IL-10 compared with the osthole-treated mice (Figure 4d). We could not detect appreciable IL-10 production from other cells in the lung, such as eosinophils, mast cells or macrophages (Figures 4e–g). Collectively, these results show that osthole treatment promoted an increased frequency of IL-10-producing DCs and enhanced Foxp3+ Treg differentiation in vivo (Figure 4h).
Figure 4.
Osthole treatment enhanced the generation of IL-10-producing DCs and Treg cells. (a–g) Frequencies of IL-10-producing cells in the lung or TLNs. Lungs were digested with collagenase A, and single-cell suspensions from lungs or TLNs were acquired as described in ‘Materials and Methods’ section. Cell phenotypes determined by staining as follows: CD11b+CD11c+ (DCs), CD4+Foxp3+ (Treg cells), CD4+IL-4+ (Th2 cells), CCR3+Siglec F+ (eosinophils), CD117+FcεRI+ (mast cells) and F4/80+CD68+ (macrophages). Data are representative dot plots of digested cells with IL-10. Percentages in the top right quadrants refer to percentages of cells expressing IL-10. Values are expressed as the mean±s.e.m. of four mice per group. (h) Data are expressed as cell types producing IL-10 in the median range. *P<0.05 vs the PC group.
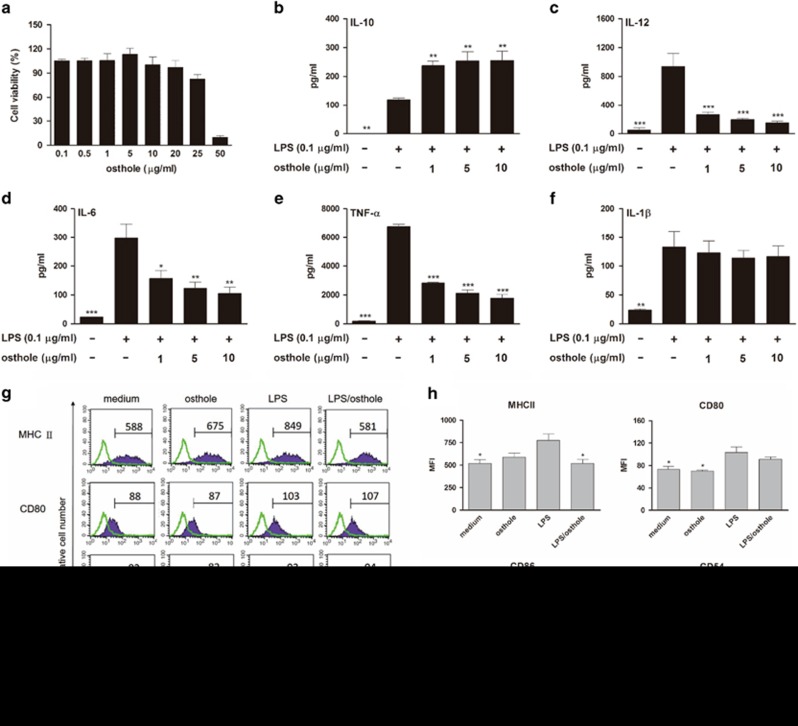
Osthole inhibited LPS-induced maturation in bone marrow-derived DCs
IL-10-producing tolerogenic DCs are semimature DCs with reduced levels of surface molecules (MHC II, CD80, CD86, CD54 and CD40) and stimulatory cytokines (IL-6 and IL-12).13 Since tolerogenic DCs have the capacity to produce IL-10, which may function to promote IL-10-producing Treg cells from naive T cells, we hypothesized that osthole might regulate the differentiation route of T cells by affecting the mode of DC maturation and function. Because the effects of osthole on DCs had not yet been evaluated, the cytotoxic effect of osthole on bone marrow-derived DCs from BALB/c mice was initially examined. After 48 h of DC treatment with different doses of osthole (0.1–50 μg/ml), cell viability was detected using an MTT assay (Figure 5a). The results revealed that osthole had no cytotoxicity in DCs at concentrations up to 20 μg/ml; thus, concentrations of up to 10 μg/ml osthole were used for subsequent experiments. Next, we investigated the effect of osthole on cytokine production by DCs. DCs were treated with different concentrations of osthole (1, 5 and 10 μg/ml) following LPS (100 ng/ml) stimulation. As shown in Figure 5b, IL-10 production by LPS-stimulated DCs was significantly elevated in the presence of osthole treatment. Conversely, IL-12 production was clearly decreased in osthole-treated DCs (Figure 5c). Moreover, our study showed that osthole treatment significantly suppressed IL-6 and TNF-α production in these activated DCs, although no such change in the IL-1β level was observed (Figures 5d–f). We also analyzed the phenotype profiles of DCs. Compared with LPS-activated DCs, osthole (10 μg/ml) plus LPS-treated DCs showed no differences in the expression of the costimulatory molecules, CD54, CD80 or CD86, but a marked reduction in the level of MHC II was observed (Figures 5g and h). Collectively, these findings indicate that osthole treatment suppressed the maturation of DCs.
Figure 5.
Cytokine pattern and phenotype of osthole-treated bone marrow-derived DCs. (a) Cytotoxic effect of osthole on DCs, which were treated with different doses of osthole for 48 h. Cell viability was detected by the MTT assay. (b–f) Levels of IL-10, IL-12 and proinflammatory cytokines (IL-6, TNF-α and IL-1β) from osthole-treated DCs with LPS stimulation. DCs were treated with various concentrations of osthole or medium alone and then activated with LPS. Supernatants were collected and analyzed using ELISA kits. (g) Expression levels of MHC class II, CD54, CD80 and CD86 on DCs. DCs were treated with medium, osthole (10 μg/ml), LPS (100 ng/ml) or osthole plus LPS. After incubation, the cells were analyzed by flow cytometry. Values shown in the flow cytometric profiles are the mean fluorescence intensity (MFI). DCs were gated on CD11c, and the incidence of CD11c+ cells expressing the surface marker is indicated within each histogram. (h) The MFI was calculated. The results are expressed as the mean±s.e.m. from three independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs LPS-treated DCs (LPS group).
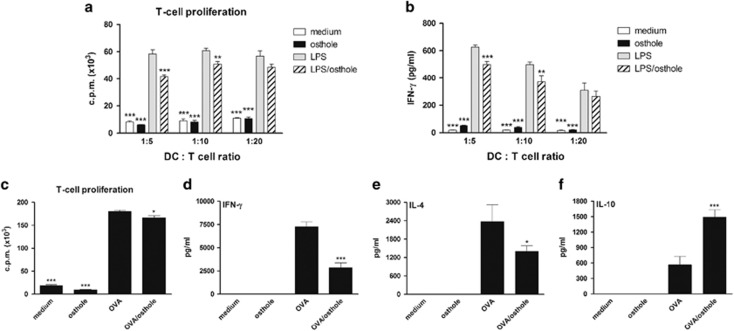
Osthole treatment of DCs reduced T-cell proliferation and induced IL-10-producing Treg cells
Because osthole treatment of DCs induced high levels of IL-10 production and a low proinflammatory cytokine pattern, we speculated that osthole-treated DCs would possess immunosuppressive ability, inhibiting effector T-cell responses. To examine the suppressive capacity of osthole-treated DCs on T-cell responses, differently treated DCs were cocultured with allogeneic CD4+ T cells at various ratios in an MLR. Upon stimulation of LPS-activated DCs, the T cells showed a strong proliferative capacity and further differentiated into Th1 cells that secreted high levels of IFN-γ (Figures 6a and b). In comparison to DCs stimulated with LPS alone, LPS plus osthole-treated DCs exhibited a significantly weakened ability to stimulate T-cell proliferation and IFN-γ production. Furthermore, we assessed whether osthole-treated DCs might act as tolerogenic DCs to promote the differentiation of Treg cells in vitro. OVA-specific, naive DO11.10 CD4+ T cells were repeatedly cocultured with OVA-, osthole- or OVA plus osthole-treated DCs, and the T-cell proliferative response was measured after two rounds of stimulation. As shown in Figure 6c, DO11.10 CD4+ T-cell populations driven by OVA plus osthole-treated DCs showed a slightly lower proliferative response than those driven by OVA-pulsed DCs. In addition, after naive DO11.10 CD4+ T cells were cultured with different treatment-conditioned DCs for 7 days, the T cells were restimulated with irradiated OVA-pulsed DCs and cytokine profiles were detected. In comparison to OVA-pulsed DCs, OVA plus osthole-treated DCs exhibited significant suppression of IFN-γ and IL-4 production but markedly promoted IL-10 production in T cells (Figures 6d–f). These results suggest that osthole tends to induce a proportion of antigen-specific IL-10-producing Treg cells but not Th1 (IFN-γhigh) or Th2 (IL-4high) cells through osthole-modulated DCs.
Figure 6.
Immunomodulatory effects of osthole-treated DCs on T-cell proliferation and cytokine production in vitro. (a and b) In an allogeneic mixed lymphocyte reaction, DCs from BALB/c mice were treated with medium alone, osthole (10 μg/ml), LPS (100 ng/ml) or LPS plus osthole for 24 h. Naive CD4+ T cells from C57BL/6 mice were then cocultured with γ-irradiated DCs at DC:T-cell ratios of 1:5, 1:10 and 1:20 for 72 h. T-cell proliferation was determined by [3H]-thymidine uptake, and the results are expressed as the c.p.m. (c–f) For the T-cell proliferative assay, naive DO11.10 CD4+ T cells were cultured with different treatment-conditioned DCs from BALB/c mice for 7 days as one culture cycle. After the second cycle, T cells were stimulated with irradiated splenocytes and OVA323-339 peptide. [3H]-Thymidine uptake was measured after 3 days of culturing. For the cytokine assays, T cells were culture with differently treated DCs for 7 days and further restimulated with γ-irradiated OVA-pulsed DCs. Supernatants were collected, and cytokine levels were analyzed using ELISA kits. The results are expressed as the mean±s.e.m. of three independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs LPS-treated DCs (LPS group).
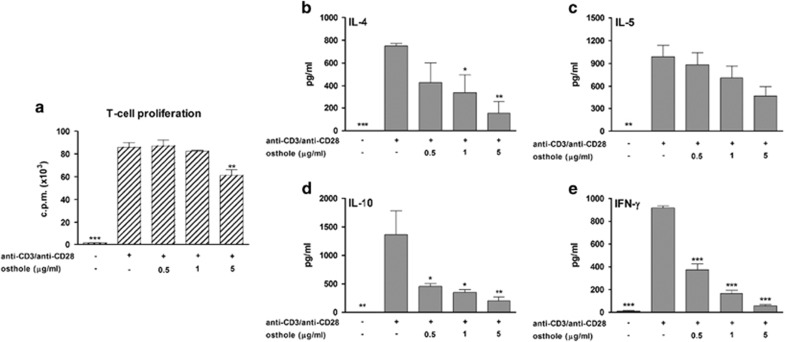
Osthole directly suppressed T-cell activation
We also investigated whether osthole could directly affect T-cell proliferation and differentiation. CD4+ T cells were treated with osthole (0.5, 1 and 5 μg/ml) and activated by plate-bound anti-CD3/anti-CD28 antibodies. As shown in Figure 7a, compared with T cells stimulated by anti-CD3/anti-CD28 antibodies alone, osthole at 5 μg/ml significantly reduced T-cell proliferation. In addition, cytokines (IL-4, IL-5, IL-10 and IFN-γ) produced by T cells were significantly inhibited by osthole treatment (Figures 7b–e). These results indicated that osthole directly reduced the effector T-cell response; however, it did not cause differentiation of T cells into IL-10-producing Treg cells.
Figure 7.
Direct effect of osthole on CD4+ T cells. (a) The direct effect of osthole on T-cell proliferation. T cells (2 × 105 cells/well) were treated with different doses (0.5, 1 and 5 μg/ml) of osthole and stimulated with anti-CD3 (1 μg/ml)/anti-CD28 (1 μg/ml) antibodies for 56 h. T cells were stimulated with anti-CD3/anti-CD28 alone as the control. Proliferative ability was determined using a [3H]-thymidine incorporation assay. (b–e) The direct effect of osthole on T-cell cytokine production. T cells (5 × 105 cells/well) were cultured in 48-well plates and stimulated with anti-CD3/anti-CD28 antibodies in the presence of osthole for another 72 h. Culture supernatants were collected and analyzed by ELISA. The results from three independent experiments are shown and expressed as the mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001 vs the control (anti-CD3/anti-CD28 stimulation) group.
Discussion
Allergic asthma is a common and chronic respiratory disorder, and conventional drugs, such as corticosteroids, are used as the first line of treatment. Corticosteroids are the most effective class of drugs for preventing asthma attacks. However, prolonged use of corticosteroids can cause serious side effects in children. In recent years, the pathology of allergic asthma has become clearer, leading to the exploration of new therapeutic reagents for this disorder. Natural products used in traditional Chinese medicine are applicable for the development of effective and safe alternative therapies for asthma.
Osthole, a natural coumarin derivative isolated from several medicinal plants and herbs such as C. monnieri and Peucedanum ostruthium, has a long history of clinical use for the treatment of eczema and cutaneous pruritus. Although osthole possesses diverse biological activities, data are scarce concerning its effect on allergic asthma. We investigated the immunomodulatory effects of osthole in an asthmatic murine model for the first time. Osthole at 5-50 mg/kg of body weight did not show adverse side effects on the outward appearance or organ weights of treated mice (Supplementary Table S1). Our results clearly show that treatment of allergic mice with osthole attenuated the production of OVA-specific IgE in the serum, airway inflammation in the lungs and Th2-type cytokine responses in the BALF and spleen. Overall, these data suggest that osthole can attenuate OVA-induced Th2 responses, thereby limiting the immunopathology in allergic mice.
In allergic asthma, Th2-type cytokines (IL-4, IL-5 and IL-13) are mainly involved in the development of an inflammatory phenotype, and poor clinical control is associated with alveolar Th2 responses.29 IL-4 and IL-13 are switching factors for the production of IgE by B lymphocytes.30, 31 IL-4 plays the most important role in inducing a Th2 cytokine response, but IL-13 has a stronger effect on the induction of pathological symptoms such as AHR, eosinophilic infiltration and airway mucus secretion compared with IL-4.32 In addition, IL-5 is generally recognized as critical for stimulating eosinophil differentiation, maturation, activation and recruitment.33 Based on the preceding information, the therapeutic strategy of inhibiting Th2 immunity may lead to improvements in the control of allergic asthma. Osthole ameliorated the severity of allergic asthma in treated mice by inhibiting the Th2 response. Additionally, osthole could directly reduce the proliferation and cytokine secretion of the activated T-cells, as confirmed in vitro.
We further clarified whether the suppressive effect of osthole on Th2 responses was partially due to the induction of Treg cells in osthole-treated mice. Our results revealed that IL-10 production was upregulated in the spleen but not in the lungs. IL-10 is produced by various cell types. Thus, the levels of IL-10 in the BALF showed no differences among these groups, likely because the combination of all the cells in the lungs for the IL-10 analysis diluted the Treg cell effect. Therefore, flow cytometry analysis as applied to further assess the ability of osthole treatment to enhance CD4+IL-10+Foxp3+ Treg cell and reduce CD4+IL-10+IL-4+ Th2 cell populations in the lungs. Previous studies have noted that IL-10-producing Treg cells play a key role in immune tolerance, preventing uncontrolled inflammation and immunopathology.34, 35, 36, 37 These Treg cells can inhibit effector T-cell proliferation and cytokine production, reduce IgE production, suppress airway eosinophilia and downregulate the release of proinflammatory cytokines from mast cells by mechanisms involving IL-10 in allergic diseases.38, 39, 40, 41 Collectively, our findings suggest that osthole treatment dampened effector Th2 cell responses to limit allergen-induced immunopathology, at least in part by augmenting the numbers of IL-10+Foxp3+ Treg cells.
DCs are key APCs that determine whether the response to an inhaled antigen will entail allergic inflammation or tolerance. We found that osthole treatment induced the development of DCs that produced IL-10 in asthmatic mice. Furthermore, osthole plus LPS-treated bone marrow-derived DCs exerted reduced levels of proinflammatory cytokines such as IL-6, TNF-α and IL-12 in vitro. This anti-inflammatory action of osthole was observed in LPS-stimulated J774A.1 macrophages, in which osthole suppressed TNF-α expression by inhibiting activation of the NF-κB, p38 and JNK1/2 pathways.21 In addition, osthole was reported as a 5-lipoxygenase (5-LO) inhibitor.42 5-LO is a rate-limiting enzyme in the synthesis of leukotrienes (LTs). As LTs are proinflammatory lipid mediators involved in many inflammatory diseases, the ability of osthole to inhibit 5-LO is beneficial for reducing inflammatory responses. Moreover, the results from a carrageenan-induced model suggested that osthole-mediated suppression of inflammatory factors was associated with cyclic adenosine monophosphate and cyclic guanosine monophosphate elevation.43 Importantly, this is the first study to provide evidence that osthole-treated, LPS-stimulated DCs exhibit comparatively higher amounts of IL-10 production and lower levels of MHC class II expression. IL-10 is a major immunosuppressive cytokine that can downregulate MHC class II molecules of DCs, inhibit DC secretion of IL-12 and counteract the development and function of Th1 cells.44, 45 Although it is interesting to discuss the intracellular signaling pathway involved in modulating IL-10 expression in DCs in response to osthole, additional evidence is not available. Previous reports have indicated that both the NF-κB and MAPK pathways, including p38, ERK, JNK, are essential for LPS-induced IL-10 expression in mouse macrophages.45, 46 They predicted that these pathways were responsible for the increased levels of CCAAT/enhancer-binding protein δ (C/EBPδ), which is required for gene activation of IL-10. Thus, it is possible that osthole promotes IL-10 expression by regulating the NF-κB and MAPK signaling pathways, and further experiments are needed to understand the intracellular mechanisms of osthole on the induction of IL-10 synthesis in DCs. Alternatively, the secretion of functionally active IL-12 by DCs could be the crucial differentiation factor for Th1 cells.47 In mice with experimentally induced autoimmune encephalomyelitis, one study demonstrated that the increased secretion of IFN-γ from Th1 cells was inhibited by osthole treatment.18 Therefore, based on these results, we propose that osthole may inhibit effector T-cell responses by suppressing DC activation and maturation. However, previous studies have demonstrated that DCs expressing the polarizing cytokine IL-10 are particularly important for inducing Treg cells.48, 49 In contrast to fully mature DCs that express high levels of MHC class II, CD80 and CD86 and large amounts of proinflammatory cytokines to drive Th1 cell activation, osthole-treated DCs more closely resemble partially mature DCs that display poor T-cell stimulatory activity.3 In fact, antigen presentation by partially mature IL-10-producing DCs has been previously shown to induce the formation of IL-10-producing Foxp3+ Treg cells, which protect the host against allergy.48 The present in vitro experimental data imply that osthole-treated DCs play a role as IL-10-secreting tolerogenic DCs that induce the development of Treg cells to further suppress effector T-cell responses. Thus, an osthole-treated DC transfer experiment will verify whether osthole-modified DCs are truly able to suppress allergic asthma.
In the MLR, responding cells are allogeneic CD4+ T cells that further differentiate into Th1 cells in the presence of DC stimulation. Notably, the data show that osthole-treated DCs have the immunoregulatory ability to directly prohibit T-cell proliferation in vitro. Furthermore, we attempted to coculture osthole-treated DCs and OVA-specific CD4+ T cells from TCR-transgenic DO11.10 mice to induce Treg cells in vitro. However, these osthole-treated DC-priming heterogeneous T-cell populations, which contained IL-10-secreting Treg cells and effector T cells, could only exert a slight suppressive capacity on effector T-cell proliferation. Thus, although a small number of Treg cells can be induced by osthole-treated DCs, it seems that these Treg cells are insufficient to dampen effector T-cell responses. Due to their low proliferative capacity, these Treg cells are difficult to isolate under standard culture conditions, and their functions are not easily tested using a suppressive assay. In addition, the phenotype and suppressive mechanisms of these Treg cells must be further investigated.
In summary, our results demonstrate that treatment with osthole significantly attenuates the severe symptoms of Th2-mediated asthma in mice. Our data show that osthole treatment generates semimature DCs upon antigen stimulation, limiting the magnitude of the immune response by inhibiting T-cell proliferation and directing Treg differentiation. Additionally, osthole directly inhibits T-cell responses. These results provide a scientific foundation for applying osthole to treat Th2-mediated allergic diseases.
Acknowledgments
This study was supported by research grants from the Ministry of Science and Technology, Taiwan (MOST 103-2320-B-038-032-MY3) and Wan Fang Hospital, Taipei, Taiwan (104TMU-WFH-14).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 2012; 18: 716–725. [DOI] [PubMed] [Google Scholar]
- Gallelli L, Busceti MT, Vatrella A, Maselli R, Pelaia G. Update on anticytokine treatment for asthma. Biomed Res Int 2013; 2013: 104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB. Induction of CD4+ regulatory and polarized effector/helper T cells by dendritic cells. Immune Netw 2016; 16: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vroman H, van den Blink B, Kool M. Mode of dendritic cell activation: the decisive hand in Th2/Th17 cell differentiation. Implications in asthma severity? Immunobiology 2015; 220: 254–261. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 2008; 454: 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd CM, Saglani S. T cells in asthma: influences of genetics, environment, and T-cell plasticity. J Allergy Clin Immunol 2013; 131: 1267–1274. [DOI] [PubMed] [Google Scholar]
- Akdis CA. Therapies for allergic inflammation: refining strategies to induce tolerance. Nat Med 2012; 18: 736–749. [DOI] [PubMed] [Google Scholar]
- Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol 2012; 30: 243–270. [DOI] [PubMed] [Google Scholar]
- Ma C, Ma Z, Liao XL, Liu J, Fu Q, Ma S. Immunoregulatory effects of glycyrrhizic acid exerts anti-asthmatic effects via modulation of Th1/Th2 cytokines and enhancement of CD4+CD25+Foxp3+ regulatory T cells in ovalbumin-sensitized mice. J Ethnopharmacol 2013; 148: 755–762. [DOI] [PubMed] [Google Scholar]
- Gregori S, Passerini L, Roncarolo MG. Clinical outlook for type-1 and FOXP3+ T regulatory cell-based therapy. Front Immunol 2015; 6: 593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Zhang R, Jin B, Chen L. Type 1 regulatory T cells: a new mechanism of peripheral immune tolerance. Cell Mol Immunol 2015; 12: 566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noval Rivas M, Chatila TA. Regulatory T cells in allergic diseases. J Allergy Clin Immunol 2016; 138: 639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavkaytar O, Akdis CA, Akdis M. Modulation of immune responses by immunotherapy in allergic diseases. Curr Opin Pharmacol 2014; 17: 30–37. [DOI] [PubMed] [Google Scholar]
- Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. J Allergy Clin Immunol 2007; 119: 1258–1266. [DOI] [PubMed] [Google Scholar]
- Provoost S, Maes T, van Durme YM, Gevaert P, Bachert C, Schmidt-Weber CB et al. Decreased FOXP3 protein expression in patients with asthma. Allergy 2009; 64: 1539–1546. [DOI] [PubMed] [Google Scholar]
- Zhang ZR, Leung WN, Cheung HY, Chan CW. Osthole: a review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid Based Complement Alternat Med 2015; 2015: 919616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SY, Hsu CS, Wang KT, Wang MC, Wang CC. Antitumor effects of osthol from Cnidium monnieri: an in vitro and in vivo study. Phytother Res 2007; 21: 226–230. [DOI] [PubMed] [Google Scholar]
- Chen X, Pi R, Zou Y, Liu M, Ma X, Jiang Y et al. Attenuation of experimental autoimmune encephalomyelitis in C57 BL/6 mice by osthole, a natural coumarin. Eur J Pharmacol 2010; 629: 40–46. [DOI] [PubMed] [Google Scholar]
- Hua KF, Yang SM, Kao TY, Chang JM, Chen HL, Tsai YJ et al. Osthole mitigates progressive IgA nephropathy by inhibiting reactive oxygen species generation and NF-κB/NLRP3 pathway. PLoS One 2013; 8: e77794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H, Tomohiro N, Ido Y, Kubo M. Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri and its major component, osthol. Biol Pharm Bull 2002; 25: 809–812. [DOI] [PubMed] [Google Scholar]
- Liao PC, Chien SC, Ho CL, Wang EI, Lee SC, Kuo YH et al. Osthole regulates inflammatory mediator expression through modulating NF-κB, mitogen-activated protein kinases, protein kinase C, and reactive oxygen species. J Agric Food Chem 2010; 58: 10445–10451. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Kawasaki T, Hino O. Osthole prevents anti-Fas antibody-induced hepatitis in mice by affecting the caspase-3-mediated apoptotic pathway. Biochem Pharmacol 2003; 65: 677–681. [DOI] [PubMed] [Google Scholar]
- Chiu PR, Lee WT, Chu YT, Lee MS, Jong YJ, Hung CH. Effect of the Chinese herb extract osthol on IL-4-induced eotaxin expression in BEAS-2B cells. Pediatr Neonatol 2008; 49: 135–140. [DOI] [PubMed] [Google Scholar]
- Huang HM, Hsiao G, Fan CK, Lin CL, Leu SJ, Chiang BL et al. Notch ligand delta-like 4-pretreated dendritic cells alleviate allergic airway responses by enhancing IL-10 production. PLoS One 2013; 8: e63613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaia G, Vatrella A, Gallelli L, Renda T, Cazzola M, Maselli R et al. Respiratory infections and asthma. Respir Med 2006; 100: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell mediated immune regulation. Immunol Rev 2008; 226: 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabat R, Grütz G, Warszawska K, Kirsch S, Witte E, Wolk K et al. Biology of interleukin-10. Cytokine Growth Factor Rev 2010; 21: 331–344. [DOI] [PubMed] [Google Scholar]
- Duan W, Croft M. Control of regulatory T cells and airway tolerance by lung macrophages and dendritic cells. Ann Am Thorac Soc 2014; 11 (Suppl 5): S306–S313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergqvist A, Andersson CK, Mori M, Walls AF, Bjermer L, Erjefalt JS. Alveolar T-helper type-2 immunity in atopic asthma is associated with poor clinical control. Clin Sci 2015; 128: 47–56. [DOI] [PubMed] [Google Scholar]
- Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol 2003; 3: 721–732. [DOI] [PubMed] [Google Scholar]
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol 2008; 8: 205–217. [DOI] [PubMed] [Google Scholar]
- Bagnasco D, Ferrando M, Varricchi G, Passalacqua G, Canonica GW. A critical evaluation of anti-IL-13 and anti-IL-4 strategies in severe asthma. Int Arch Allergy Immunol 2016; 170: 122–131. [DOI] [PubMed] [Google Scholar]
- Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol 2006; 24: 147–174. [DOI] [PubMed] [Google Scholar]
- Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol 2005; 5: 271–283. [DOI] [PubMed] [Google Scholar]
- Jin JO, Han X, Yu Q. Interleukin-6 induces the generation of IL-10-producing Tr1 cells and suppresses autoimmune tissue inflammation. J Autoimmun 2013; 40: 28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JY, Im KI, Lee ES, Kim N, Nam YS, Jeon YW et al. Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis. Sci Rep 2016; 6: 26851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Hsiao G, Wang CC, Lee YL. Imperatorin exerts antiallergic effects in Th2-mediated allergic asthma via induction of IL-10-producing regulatory T cells by modulating the function of dendritic cells. Pharmacol Res 2016; 110: 111–121. [DOI] [PubMed] [Google Scholar]
- Koulis A, Robinson DS. The anti-inflammatory effects of interleukin-10 in allergic disease. Clin Exp Allergy 2000; 30: 747–750. [DOI] [PubMed] [Google Scholar]
- Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin-10 dependent. J Exp Med 2005; 202: 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akdis CA, Akdis M. Mechanisms and treatment of allergic disease in the big picture of regulatory T cells. J Allergy Clin Immunol 2009; 123: 735–746. [DOI] [PubMed] [Google Scholar]
- Böhm L, Maxeiner J, Meyer-Martin H, Reuter S, Finotto S, Klein M et al. IL-10 and regulatory T cells cooperate in allergen-specific immunotherapy to ameliorate allergic asthma. J Immunol 2015; 194: 887–897. [DOI] [PubMed] [Google Scholar]
- Resch M, Steigel A, Chen ZL, Bauer R. 5-Lipoxygenase and cyclooxygenase-1 inhibitory active compounds from Atractylodes lancea. J Nat Prod 1998; 61: 347–350. [DOI] [PubMed] [Google Scholar]
- Li Z, Ji H, Song X, Han N, Chen N. Osthole attenuates the development of carrageenan-induced lung inflammation in rats. Int Immunopharmacol 2014; 20: 33–36. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Gregori S, Bacchetta R, Battaglia M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr Top Microbiol Immunol 2014; 380: 39–68. [DOI] [PubMed] [Google Scholar]
- Ma X, Yan W, Zheng H, Du Q, Zhang L, Ban Y et al. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Res 2015; 4: F1000 Faculty Rev-1465. [Google Scholar]
- Liu YW, Chen CC, Tseng HP, Chang WC. Lipopolysaccharide-induced transcriptional activation of interleukin-10 is mediated by MAPK- and NF-κB-induced CCAAT/enhancer-binding protein δ in mouse macrophages. Cell Signal 2006; 18: 1492–1500. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmarkers. Nat Immunol 2012; 13: 722–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yang A, Huang H, Zhang X, Town J, Davis B et al. Induction of type 2T helper cell allergen tolerance by IL-10-differentiated regulatory dendritic cells. Am J Respir Cell Mol Biol 2010; 42: 190–199. [DOI] [PubMed] [Google Scholar]
- Volz T, Skabytska Y, Guenova E, Chen KM, Frick JS, Kirschning CJ et al. Nonpathogenic bacteria alleviating atopic dermatitis inflammation induce IL-10-producing dendritic cells and regulatory Tr1 cells. J Invest Dermatol 2014; 134: 96–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.