Abstract
Immunoproteasome activation in immune cells is involved in the modulation of immune responses. Increasing evidence indicates that proteasome inhibitors show beneficial effects in treating autoimmune diseases, but it remains unclear whether proteasome inhibition is an effective approach for suppressing autoimmune development in Sjögren’s syndrome (SS). Our previous work has demonstrated a critical role for Th17 cells in the development of experimental SS (ESS) in mice. In this study, we detected high levels of low-molecular-weight protein 7 (LMP7), a key subunit of the immunoproteasome, in Th17 cells from ESS mice. Moreover, treatment with bortezomib (BTZ), a proteasome inhibitor, markedly suppressed Th17 differentiation in both murine and human naive T cells in culture. Furthermore, ESS mice treated with BTZ displayed significantly higher saliva flow rates and a reduction in tissue destruction in the salivary glands compared with vehicle-treated ESS mice. Notably, BTZ-treated ESS mice showed markedly decreased Th17 cells, germinal center B cells and plasma cells in the peripheral lymphoid organs. In addition, adoptively transferred wild type naive CD4+ T cells rapidly differentiated into Th17 cells and induced salivary dysfunction in IL-17-deficient mice immunized for ESS induction. However, BTZ treatment profoundly suppressed the donor T-cell-derived Th17 response and ameliorated the reduction in salivary secretion in IL-17-deficient recipient mice. Taken together, our findings demonstrate that proteasome inhibition can effectively ameliorate ESS by suppressing the Th17 response, which may contribute to the development of a novel therapeutic strategy for the treatment of SS.
Keywords: proteasome inhibition, Sjögren’s syndrome, Th17 cells
Introduction
Sjögren’s syndrome (SS) is an autoimmune disease characterized by xerostomia and keratoconjunctivitis sicca associated with lymphocytic infiltration and tissue destruction in the salivary glands (SGs) and lacrimal glands.1 Histological examination of labial minor salivary gland biopsies of patients with SS revealed accumulated focal lymphocytic aggregates, while CD4+ T cells and B cells were identified as major populations in the lymphocytic focus.2, 3, 4, 5 Recent studies have revealed the involvement of various cell populations and cytokines during SS development, which indicates complex mechanisms for the pathogenesis of SS.6, 7 Recently, Th17 cells have been implicated in the pathogenesis of autoimmune diseases,8 and therapies targeting Th17 cells have exhibited beneficial effects on disease progression.9, 10, 11 Increasing evidence also indicates that Th17 cells are important players in the autoimmune inflammation of SS. Using a murine model, we previously identified a critical role for Th17 cells in the development of experimental SS (ESS).12 Moreover, B cells have been increasingly recognized as the major effector cell population in the disease pathology of SS. Recently, biological therapies targeting B cells, including rituximab, epratuzumab and belimumab, have shown promising efficacy for the treatment of SS.13, 14, 15 Although extensive studies have been performed, there is still a lack of effective therapies for patients with SS compared with other autoimmune diseases.
In addition to their success in cancer therapies, proteasome inhibitors have been used as experimental therapeutics for treating autoimmune diseases.16 The proteasome is a non-lysosomal protein complex for degrading the proteins involved in cell cycle control, differentiation and metabolism of all eukaryotic cells. During inflammation, the constitutive subunits of the proteasome are replaced by inducible subunits including low-molecular-weight protein 7 (LMP7, also called β5i) in cytokine-stimulated immune cells, which leads to the formation of the immunoproteasome. Several studies have suggested that proteasome inhibitors elicit suppressive effects on immune cells, including B cells and T cells.16 Protein-secreting immune cells, especially plasma cells, are highly sensitive to proteasome inhibitors due to the activation of the terminal unfolded protein response.17 Bortezomib (BTZ) is the first approved proteasome inhibitor for treating patients with multiple myeloma by targeting plasma cells. Emerging evidence indicates that BTZ and other proteasome inhibitors are effective for the treatment of various autoimmune diseases in animal models including collagen-induced arthritis18, 19 and murine lupus.20, 21 Recently, BTZ administration significantly ameliorated refractory systemic lupus erythematosus (SLE) in patients in a phase II clinical study.22 It has been reported that specific inhibition of LMP7, a subunit of the immunoproteasome, can suppress Th17 and Th1 cell responses in mice with experimental autoimmune encephalomyelitis (EAE).23 Interestingly, dysregulated expression levels of catalytic proteasome subunits have been detected in peripheral blood mononuclear cells (PBMCs) from patients with primary SS (pSS).24 Moreover, the expression of LMP7 was significantly increased in the minor salivary glands of patients with SS.25 Although previous studies have indicated the involvement of proteasome activation in SS development, it remains largely unknown whether inhibition of the proteasome possesses any therapeutic benefits for the treatment of SS.
In this study, we detected high levels of LMP7 expression in Th17 cells from ESS mice, whereas proteasome inhibition by BTZ potently suppressed Th17 cell differentiation in culture. Furthermore, we found that BTZ treatment markedly inhibited Th17 cell response and ameliorated SG pathology in ESS mice, which suggests that proteasome inhibition may represent a promising therapeutic strategy for the treatment of SS.
Materials and methods
Mice
Female C57BL/6 (CD45.2) and B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) mice between 6 and 8 weeks of age were purchased from Jackson Laboratory (Bar Harbor, ME, USA). IL-17 knockout (KO) mice on a C57BL/6 background were kindly provided by Dr Yoichiro Iwakura at The University of Tokyo, Japan. All mice were maintained in a specific pathogen-free animal facility at The University of Hong Kong. All animal experiments were approved by the Committee on the Use of Live Animals in Teaching and Research at The University of Hong Kong.
Human CD4+ T-cell isolation
PBMCs from healthy blood donors were obtained from the Hong Kong Red Cross Blood Transfusion Service with institutional approval. Obtained whole-blood samples were diluted in phosphate-buffered saline (PBS) with 2% human AB serum. The diluted samples were carefully layered on prepared Lymphoprep medium, followed by centrifugation at 800g for 20 min at room temperature with the brake off. The buffy coat was then collected for further purification. Naive CD4+ T cells were purified from PBMCs using a Naive CD4+ T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) following the manufacturer’s instructions. Briefly, the PBMCs were incubated with the Naive CD4+ T Cell Biotin-Antibody Cocktail II for 5 min, followed by incubation with Naive CD4+ T Cell MicroBead Cocktail II for 10 min. The cells were passed through a magnetic column, and the flow-through containing naive CD4+ T cells was collected.
ESS induction
ESS induction was performed as previously described.26 Briefly, SGs from naive C57BL/6 mice were isolated and homogenized in PBS. The supernatant was collected after centrifugation, and the protein concentration was determined using a bicinchoninic acid assay (Sigma-Aldrich, St Louis, MO, USA). Wild type (WT) C57BL/6 or IL-17 KO mice were immunized with 400 μg SG protein emulsified in Freund’s complete adjuvant (Difco, Sparks, MD, USA) on day 0 and boosted with 400 μg SG protein emulsified in Freund’s incomplete adjuvant (Difco, Sparks, MD, USA) on day 14 via subcutaneous injection for ESS induction. WT mice immunized with adjuvant alone served as naive controls.
Bortezomib treatment
BTZ (Selleck Chemicals LLC, Houston, TX, USA) was dissolved in DMSO at 5 mg/ml and further diluted in PBS for administration. Mice received BTZ treatment via intravenous injection twice a week for 7 weeks at a dosage of 0.75 mg/kg body weight, starting on day 3 after the first immunization. The control group was administered an equal volume of vehicle (DMSO diluted in PBS).
Saliva flow rate measurement
Saliva flow rates were measured as previously described.26 Briefly, mice were anesthetized and intraperitoneally injected with pilocarpine (Sigma-Aldrich) at a dose of 5 mg/kg body weight. Saliva was collected immediately after pilocarpine injection for 15 min using a 20-μl pipet tip from the oral cavity.
Histological analysis
SGs were collected and frozen sections were prepared, followed by hematoxylin and eosin (H&E) staining. A widely accepted scoring system27 based on the density and number of infiltrated lymphocytic foci was used to evaluate the severity of the tissue damage according to the results of the H&E staining. A lymphocytic focus was defined as a group of >50 lymphocytes. The focus score (FS) was classified as: FS=0: no lymphocytic infiltration; FS=1: <1 lymphocytic focus per 4 mm2 (0<FS<1); FS=2: <2 lymphocytic foci per 4 mm2; FS=3: two or more lymphocytic foci per 4 mm2.
Immunofluorescence microscopy
Frozen sections of SGs were fixed with 4% PFA for 15 min and blocked with 1% BSA for 30 min at room temperature. The sections were then stained with FITC-conjugated anti-CD4 (clone GK1.5, BioLegend, San Diego, CA, USA, 5 μg/ml) and Alexa Fluor 647-conjugated anti-CD19 (clone 6D5, BioLegend, 5 μg/ml) at 4 °C overnight. Nuclei were counterstained with Hoechst 33258 (CalBioChem, San Diego, CA, USA, 1 μg/ml) for 5 min at room temperature. The sections were observed under a confocal microscope (Zeiss LSM 700, Carl Zeiss, Oberkochen, Germany).
Flow cytometric analysis
Intracardial perfusion was performed on the mice before tissue collection. Spleens and cervical lymph nodes were isolated from mice and ground in culture medium (RPMI 1640 medium with 10% FCS). The suspensions were filtered through 70-μm cell strainers. SGs were isolated and digested in collagenase IV (Sigma-Aldrich, 0.3 mg/ml) at 37 °C for 15 min. After digestion, the cells were filtered through 70-μm cell strainers, while red blood cells were lysed by ACK (ammonium–chloride–potassium) buffer. The following antibodies were used to identify surface markers and intracellular molecules: anti-CD45 (clone 30-F11), anti-CD4 (clone GK1.5), anti-CD19 (clone 6D5), anti-GL-7 (clone GL7), anti-Fas (clone 15A7), anti-CD138 (clone 281-2), anti-CD45.1 (clone A20), anti-interferon-γ (anti-IFN-γ, clone XMG1.2), anti-IL-17 (clone TC11-18H10.1) and the isotype-matched control antibodies from BioLegend. Anti-Foxp3 (clone FHK-16s) was purchased from eBioscience (San Diego, CA, USA). Rabbit anti-proteasome 20S LMP7 (clone EPR14482(B)) was purchased from Abcam (Cambridge, UK). All of the above-mentioned antibodies are monoclonal according to the manufacturer’s description.
Intracellular and intranuclear staining was performed as previously described.28 Briefly, cells were stimulated with phorbol myristate acetate (50 ng/ml; Sigma-Aldrich), ionomycin (500 ng/ml; Sigma-Aldrich) and monensin (2 μM; BioLegend) for 5 h. Intracellular cytokines and 20S proteasome LMP7 were stained in cells fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences, San Diego, CA, USA). For the staining of LMP7, a secondary antibody, DyLight donkey anti-rabbit IgG (BioLegend), was used. Intranuclear staining of Foxp3 was performed using Foxp3/Transcription Factor Staining Buffer Set (eBioscience). FMO groups were defined when performing flow cytometric analysis. Cells were also stained with isotype antibodies as a control.
Enzyme-linked immunosorbent assay
Serum levels of IgG against Sjögren’s syndrome-related antigen A (SSA) and M3 muscarinic receptor (M3R) were examined by a standard sandwich enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well MaxiSorp plates were coated with antigen peptides (5 μg/ml) at 4 °C overnight. Plates were washed and incubated with blocking buffer (0.5% gelatin, 0.5% bovine serum albumin and 0.05% Tween 20 in PBS) at room temperature for 1 h. Serum samples were diluted (1:100) and incubated in plates for 2 h at room temperature, followed by incubation of biotin-conjugated anti-mouse IgG (BioLegend, 0.5 μg/ml) for 1 h. After washing, HRP Streptavidin (BioLegend, 1:1000) was added, and the plates were incubated for 30 min. Then, plates were washed and freshly prepared TMB substrate (BioLegend, 50 μl) was added. After 10 min, 20 μl stop solution (3 M H2SO4) was added and absorbance at 450 nm was measured using a Sunrise microplate reader (Tecan, Männedorf, Switzerland). Antigenic peptides of SSA (AVALREYRKKMDIPA) and M3R (VLVNTFCDSCIPKTYWNLGY) were synthesized chemically by a solid-phase approach and purified by high-performance liquid chromatography (SBS Genetec Co., Ltd., Beijing, China).
Cell culture and carboxyfluorescein diacetate succinimidyl ester labeling
Murine Th17 cell differentiation was performed as previously described.29 Purified naive CD4+ T cells from human PBMCs were cultured in anti-CD3 and anti-CD28 pre-coated culture plates and stimulated with recombinant human IL-6 (R&D System, Minneapolis, MN, USA, 20 ng/ml), TGF-β (R&D System, 3 ng/ml) and IL-23 (R&D System, 20 ng/ml). The cells were treated with vehicle or 10 nM BTZ. After 72 h of culture, cells were collected for further analysis. For the proliferation assay, murine CD4+ T cells were labeled with 2 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma-Aldrich) in a 37 °C water bath for 10 min before cell culture. Cell divisions were determined by measuring the CFSE fluorescence intensity by flow cytometry.
Adoptive transfer of CD4+ T cells
Naive CD4+ T cells from the spleen and cervical lymph nodes (CLN) of naive BoyJ mice, a C57BL/6 strain with an alternative CD45 alloantigen (CD45.1), were purified by a Naive CD4+ T Cell Isolation Kit (Miltenyi Biotec). Purified CD44loCD62LhiCD4+ T cells (1 × 107 cells) were intravenously transferred into recipient IL-17 KO mice 10 days after the first immunization with SG protein. Recipient mice received vehicle or BTZ treatment three times every 3 days after the transfer. On day 14 after the first immunization, mice received a boost immunization with SG protein emulsified in IFA. Saliva flow rate measurement and flow cytometric analysis were performed at 20 days after the first immunization.
Data analysis and statistics
Data are presented as the means±s.d. Statistical significance was determined by Student’s t-test and two-way ANOVA. P-value <0.05 was considered statistically significant.
Results
BTZ treatment suppresses Th17 generation in vitro
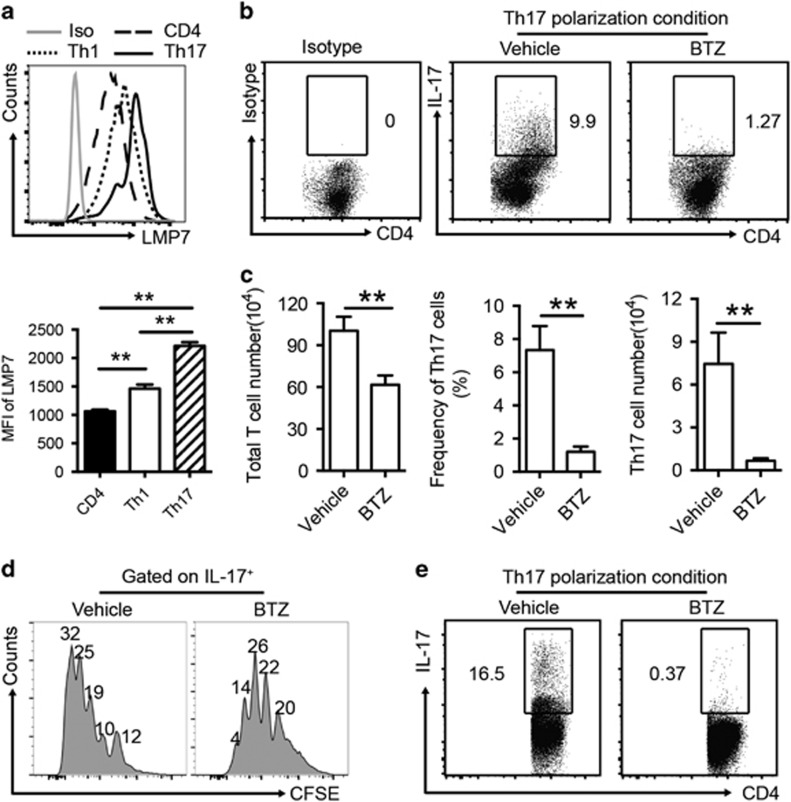
To characterize the proteasome activation in CD4+ T cells, we examined the expression of LMP7, a subunit of the immunoproteasome, in Th1 and Th17 cells from the draining CLN of ESS mice by flow cytometry. As shown in Figure 1a, CD4+ T helper cells expressed LMP7. Notably, Th17 cells expressed significantly higher levels of LMP7 than Th1 cells (Figure 1a).
Figure 1.
BTZ inhibits Th17 generation and proliferation in vitro. (a) Single-cell suspensions from CLN of ESS mice were prepared for the measurement of expression levels of LMP7 in CD4+ T cells, Th1 cells and Th17 cells by flow cytometry. Median fluorescence intensity values of LMP7 in T-cell populations were measured (n=3 per group, mean±s.d., **P<0.01). (b) Purified naive murine CD4+ T cells were cultured under Th17 polarization conditions in the presence or absence of BTZ (10 nM) for 3 days. Representative flow cytometric profiles showing the frequencies of Th17 cells are presented. (c) After 3 days in culture, the total numbers of CD4+ T cells were quantified. Both the frequency and number of Th17 cells were analyzed (n=3 per group, mean±s.d., **P<0.01). (d) CFSE-labeled CD4+ T cells were cultured in Th17 polarization conditions for 3 days and treated with or without BTZ (10 nM). Flow cytometric analysis was performed on gated IL-17+ Th17 cells. The indicated numbers are the percentages of Th17 cells in each division (n=3 per group, mean±s.d., *P<0.05). (e) Purified human naive CD4+ T cells were cultured under Th17 polarization conditions in the presence or absence of BTZ (10 nM) for 3 days. All results are representative of three independent experiments. BTZ, bortezomib; CLN, cervical lymph nodes; ESS, experimental Sjögren’s syndrome.
To further determine whether immunoproteasome inhibition affects Th17 differentiation in vitro, sorting-purified naive CD4+ T cells were cultured under Th17 polarization conditions with BTZ or vehicle for 72 h. Compared with vehicle-treated controls, the total yield of CD4+ T cells after culture showed a moderate 1.5-fold decrease in the presence of BTZ. However, BTZ treatment resulted in a marked fivefold reduction in the frequencies of IL-17+ Th17 cells (Figures 1b and c). Consistently, BTZ treatment markedly suppressed RORγt expression in cultured T cells (data not shown). To examine whether BTZ inhibits Th17 cell expansion by blocking their proliferation, we cultured CFSE-labeled CD4+ T cells in the presence or absence of BTZ under polarization conditions for Th17 induction. Flow cytometric analysis revealed that BTZ treatment significantly inhibited Th17 cell proliferation (Figure 1d). Consistently, the suppressive function of BTZ on Th17 differentiation was also observed in the culture of human naive CD4+ T cells (Figure 1e). Thus, these results demonstrated that BTZ treatment can suppress Th17 cell differentiation and proliferation in vitro, which provided a rationale for BTZ-mediated targeting of Th17 cells in the development of ESS.
BTZ treatment significantly ameliorates ESS development in mice
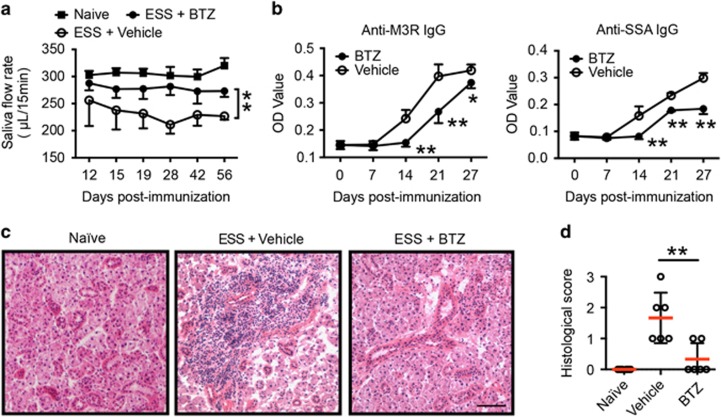
To examine the potential therapeutic effects of BTZ in ESS development, SG protein-immunized mice were treated with either BTZ or vehicle twice a week for 7 weeks, starting at day 3 after the first immunization. To assess ESS development, saliva flow rates were measured to evaluate glandular function in immunized mice. Compared with vehicle-treated controls, BTZ-treated mice displayed significantly higher saliva flow rates as the disease progressed (Figure 2a). Moreover, BTZ-treated ESS mice exhibited significantly lower levels of serum autoantibodies, including anti-SSA and anti-M3R IgG 2 weeks after the first immunization compared with their vehicle-treated counterparts (Figure 2b). Histological examination revealed extensive tissue destruction with severe lymphocytic infiltration in the SGs of vehicle-treated mice at 15 weeks post immunization, but only mild tissue inflammation with little lymphocyte infiltration was observed in the SGs of BTZ-treated mice (Figure 2c). Consistently, BTZ-treated mice showed significantly decreased histological scores of SG destruction compared with vehicle-treated controls (Figure 2d). Together, these results indicated that BTZ treatment effectively suppressed disease development in ESS mice.
Figure 2.
BTZ treatment ameliorates ESS development. ESS mice were induced with immunization of SG proteins and injected with either vehicle or BTZ intravenously twice a week for 7 weeks, starting at 3 days after the first immunization. (a) The saliva flow rates were monitored in naive or SG immunized mice treated with vehicle or BTZ. Mice immunized with adjuvant alone served as naive controls. Values between the vehicle- and BTZ-treated groups were compared, with an asterisk (*) indicating the differences (n=5–11 per group, mean±s.d., *P<0.05, **P<0.01). (b) Levels of IgG against M3R (left) and SSA (right) in the serum of vehicle- or BTZ-treated mice were measured by an ELISA assay (n=5~8 per group, mean±s.d., *P<0.05, **P<0.01). (c, d) Immunized mice were treated with vehicle or BTZ for 7 weeks. The mice were killed for histological analysis at 15 weeks after the first immunization. (c) Representative images of H&E staining of SG tissue sections from naive, vehicle- or BTZ-treated mice showing glandular infiltration (scale bar=20 μm). (d) Histological scores were assessed based on lymphocytic infiltration in the SG (**P<0.01). BTZ, bortezomib; ESS, experimental Sjögren’s syndrome; SG, salivary gland; SSA, Sjögren’s syndrome-related antigen A.
BTZ treatment inhibits lymphocytic infiltration in the SG of ESS mice
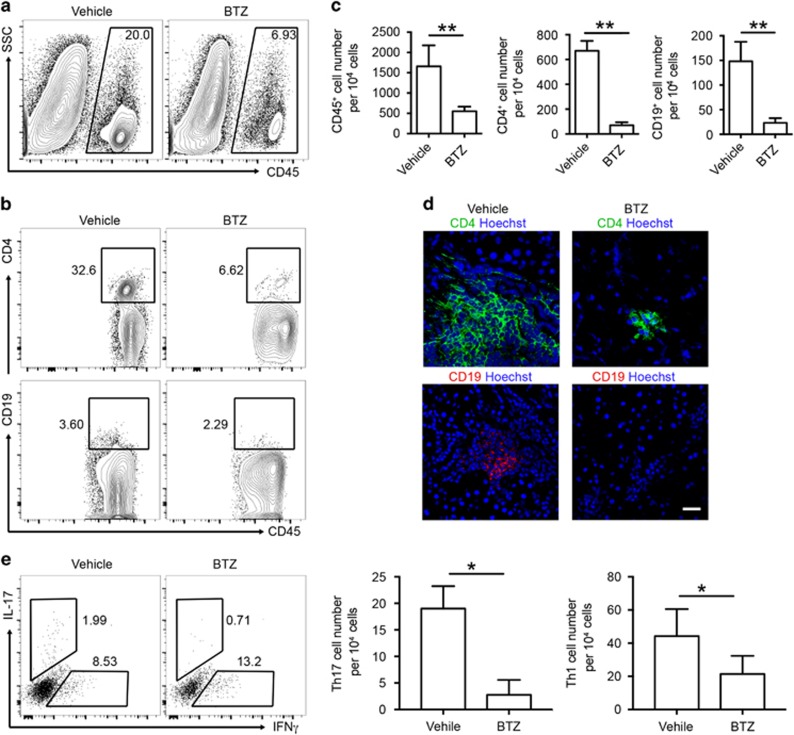
In both human and murine SS, CD4+ T cells are the major population identified in the infiltrates of SG, while B-cell compartments are associated with disease severity.30 To evaluate how T- and B-cell responses were affected by BTZ treatment, we examined the lymphocytic infiltrates within the SG by flow cytometric analysis and immunofluorescence microscopy. Consistent with the histological observations (Figures 2c and d), we detected a significantly lower number of CD45+ leukocytes in the SG of BTZ-treated ESS mice (Figures 3a and c). A marked reduction in both the frequencies and numbers of CD4+ T cells and B cells was observed in the SG of BTZ-treated ESS mice (Figures 3b and c). Further examination by immunofluorescence microscopy revealed predominant accumulation of CD4+ T cells in the SG infiltrates in vehicle-treated controls, which was profoundly diminished in the SG of BTZ-treated ESS mice (Figure 3d). Flow cytometric analysis of glandular infiltrating CD4+ T cells revealed significantly elevated Th1 and Th17 cells in the SG of ESS mice, while the cell numbers of both subsets were substantially decreased in BTZ-treated mice (Figure 3e). In addition, glandular infiltrating B cells mainly exhibited the CD23+ follicular-like phenotype, whereas B cells were hardly detected in the SG of ESS mice with BTZ treatment (Figure 3d and Supplementary Figure 1). Although CD11c+ dendritic cells were also detected in the infiltrates, there was no obvious difference in their frequencies between BTZ- and vehicle-treated mice (data not shown). Thus, BTZ treatment inhibited lymphocytic infiltration and attenuated the disease pathology in the SG of ESS mice.
Figure 3.
BTZ treatment reduces the infiltration of CD4+ T cells and B cells in the SG. ESS mice treated with vehicle or BTZ were killed for examination at 15 weeks after the first immunization. (a) Representative flow cytometric profiles of infiltrating CD45+ leukocytes in the SG. (b) Infiltrating CD4 T cells and B cells in the SG were analyzed by flow cytometry (gated on CD45+). (c) Quantification of infiltrating leukocytes, CD4+ T cells and B cells in the SG of ESS mice (n=5 per group, mean±s.d., **P<0.01). (d) Representative images from immunofluorescence microscopy showing infiltrating CD4 T cells and B cells in the SG (scale bar=20 μm). (e) Infiltrating Th1 cells and Th17 cells in the SG were analyzed by flow cytometry, and the cell numbers were quantified (n=3 per group, mean±s.d., *P<0.05). BTZ, bortezomib; ESS, experimental Sjögren’s syndrome; SG, salivary gland.
BTZ treatment markedly suppresses Th17 response in ESS mice
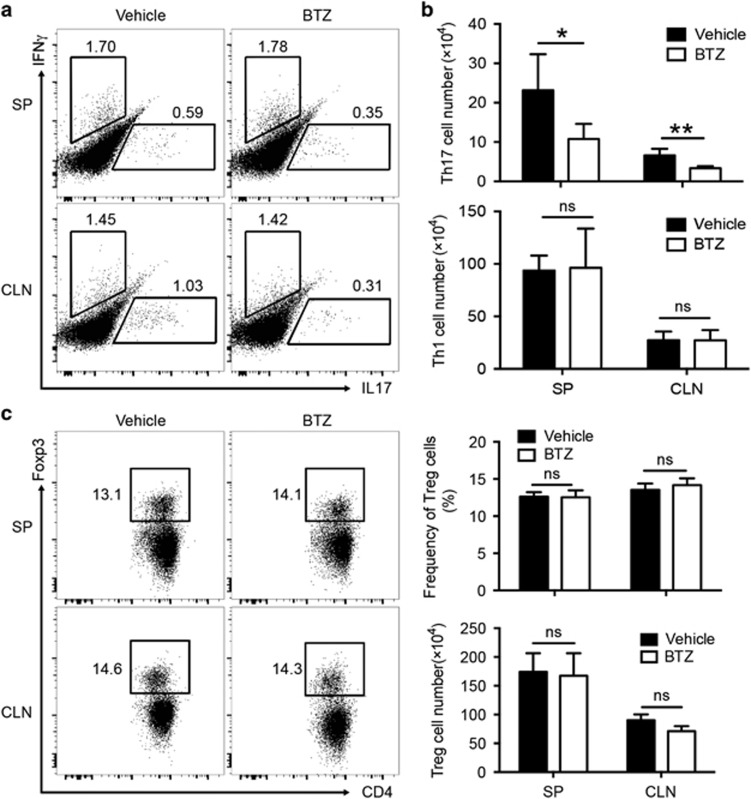
To further characterize the effector T- and B-cell subsets that respond to BTZ treatment during ESS development, we performed phenotypic analysis in the secondary lymphoid organs, including the draining CLN and spleen (SP). Compared with vehicle-treated controls, BTZ treatment resulted in significantly lower frequencies of Th17 cells in both the CLN and SP in ESS mice, and the differences were even more pronounced when cell numbers were enumerated, showing a threefold reduction in the Th17 population (Figures 4a and b). In contrast, both the frequencies and cell numbers of Th1 cells remained unchanged upon BTZ treatment (Figures 4a and b), which indicated that BTZ preferentially suppressed Th17 cells.
Figure 4.
BTZ treatment impairs Th17 responses in ESS mice. ESS mice treated with either vehicle or BTZ were killed for examination at 3 weeks after the first immunization. (a) Representative flow cytometric profiles showing the frequencies of Th1 cells and Th17 cells (gated on the CD4+ population) in the SP and CLNs. (b) The total cell numbers of Th1 cells and Th17 cells were quantified (n=6 per group, mean±s.d., *P<0.05, **P<0.01). (c) Representative flow cytometric profiles showing Foxp3+ Treg cells and quantifications of the frequencies and numbers of Treg cells in both the SP and CLN (n=5 per group, mean±s.d.). BTZ, bortezomib; CLN, cervical lymph node; ESS, experimental Sjögren’s syndrome; SG, salivary gland; SP, spleen; SSA, Sjögren’s syndrome-related antigen A.
The balance between Th17 and regulatory T (Treg) cells has been shown to modulate the progression of autoimmune diseases.31 However, the suppressive functions of Treg cells in the peripheral lymphoid organ from ESS mice (3 weeks after the first immunization) were equivalent to those of naive mice (data not shown). To further investigate whether the diminished number of Th17 cells in BTZ-treated mice was due to enhanced Treg cell responses, we examined the expression of the transcriptional factor forkhead box P3 (Foxp3) in CD4+ T cells by flow cytometry. However, there were no significant differences in the frequencies or numbers of Foxp3+ Treg cells in the peripheral lymphoid organs between BTZ- and vehicle-treated groups (Figure 4c).
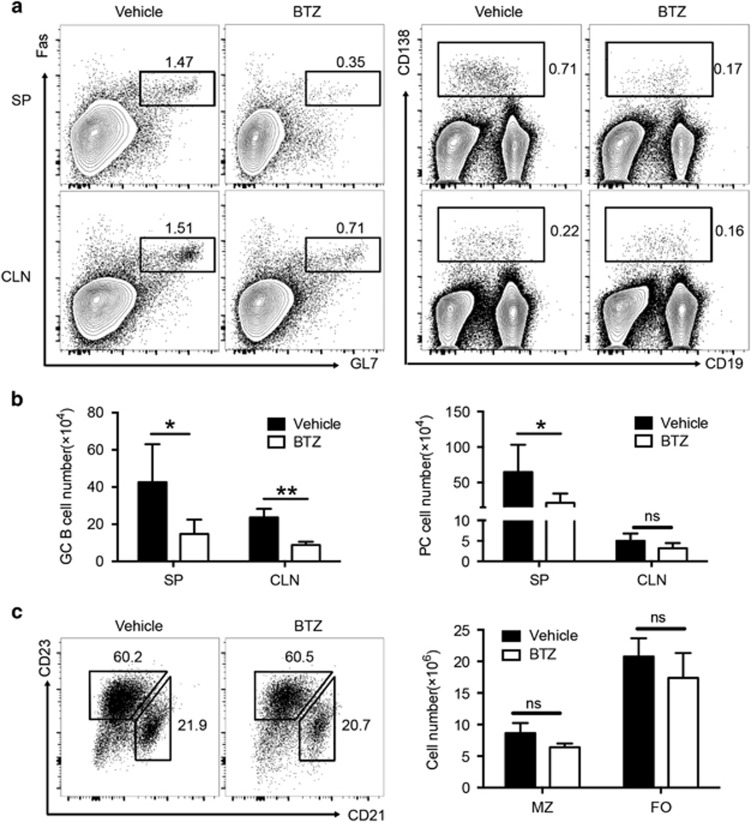
Consistent with the results of the reduced serum autoantibodies (Figure 2), ESS mice exhibited a markedly decreased number of plasma cells in the SP after BTZ treatment (Figures 5a and b). Moreover, a significant reduction of germinal center (GC) B cells was also observed in both the SP and CLN of BTZ-treated ESS mice (Figures 5a and b). In contrast, both the frequencies and numbers of marginal zone (MZ) B cells (CD19+CD21+CD23lo) and follicular (FO) B cells (CD19+CD21loCD23+) were unaltered in BTZ-treated ESS mice (Figure 5c). Together, the data demonstrated that BTZ treatment resulted in attenuation of the humoral immune response in ESS mice.
Figure 5.
BTZ treatment inhibits B-cell responses in ESS mice. ESS mice treated with either vehicle or BTZ were killed for examination at 3 weeks after the first immunization. (a) Representative flow cytometric profiles showing Fas+GL-7+ GC B cells (left panel, gated on the CD19+ population) and CD138+ PCs (right panel) in the SP and CLNs. (b) The total numbers of GC B cells and plasma cells were analyzed (n=6 per group, mean±s.d., *P<0.05, **P<0.01). (c) Representative flow cytometric profiles showing CD21+CD23low MZ B cells and CD21lowCD23+ FO B cells (gated on the CD19+ population) in the spleen of ESS mice. Total numbers of MZ B cells and FO B cells were calculated (n=5 per group, mean±s.d.). BTZ, bortezomib; CLN, cervical lymph node; ESS, experimental Sjögren’s syndrome; FO, follicular; GC, germinal center; MZ, marginal zone; PC, plasma cells; SP, spleen.
BTZ suppresses Th17-mediated ESS development in IL-17 KO mice
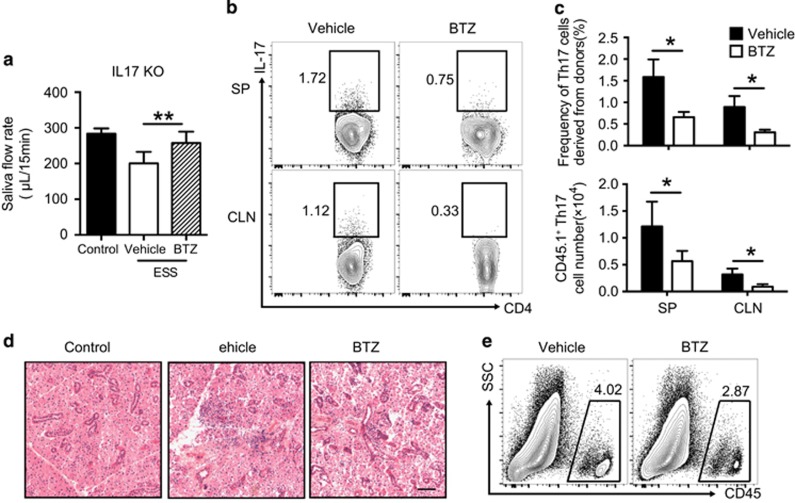
To verify the therapeutic effects of BTZ by targeting Th17 cells in ESS, CD4+ T cells from congenic CD45.1 BoyJ mice were used to examine Th17 generation and function during disease progression in CD45.2 IL-17 KO mice. Sorting-purified CD4+ T cells from naive BoyJ mice were transferred into immunized IL-17 KO mice, followed by vehicle or BTZ treatment. Transfer of WT CD4+ T cells induced saliva secretion dysfunction in vehicle-treated control mice, whereas BTZ treatment significantly attenuated the reduction of saliva flow rates (Figure 6a). Moreover, BTZ-treated recipient mice showed ameliorated tissue damage and reduced lymphocytic infiltration (Figures 6d and e).
Figure 6.
BTZ attenuates the Th17 response and ESS development in IL-17 KO mice. Naive CD4+ T cells from naive BoyJ mice were transferred into immunized IL-17 KO mice. Recipient mice received vehicle or BTZ treatment three times every 3 days. (a) Saliva flow rates of vehicle- or BTZ-treated mice were measured (n=6 per group, mean±s.d., *P<0.01). (b) Representative flow cytometric profiles showing Th17 cells derived from donor cells (gated on CD45.1+CD4+). (c) Statistical analysis of both the frequencies (upper panel) and total cell numbers (lower panel) of Th17 cells derived from donor cells in IL-17 KO mice (n=6 per group, mean±s.d., *P<0.05). (d, e) The vehicle or BTZ-treated recipient mice were killed at 15 weeks after the first immunization for analysis. (d) Representative images of H&E staining of the SG tissue sections showing glandular infiltration (scale bar=20 μm). (e) Representative flow cytometric analysis of infiltrating immune cells in the SG. BTZ, bortezomib; ESS, experimental Sjögren’s syndrome; KO, knockout.
Notably, donor CD4+ T cells rapidly differentiated into Th17 cells in both the CLN and SP in control mice but were significantly decreased in both frequency and number in the lymphoid organs after BTZ treatment (Figures 6b and c). However, the frequencies and cell numbers of Th1 cells derived from donor naive T cells in the two groups showed no significant differences (data not shown). Together, the data showed that BTZ suppressed the Th17 response and ESS development in IL-17 KO mice with transferred WT CD4+ T cells.
Discussion
In this study, we first detected high levels of LMP7 expression in Th17 cells from ESS mice. Moreover, BTZ treatment markedly inhibited Th17 differentiation from both murine and human naive T cells in culture. Importantly, BTZ treatment effectively suppressed the Th17 response and attenuated ESS development in mice. Thus, these results suggest that proteasome inhibition may represent a promising strategy for treating patients with SS.
Extensive studies have identified the proteasome as an effective target for the treatment of multiple myeloma and mantle B-cell lymphoma.32 There is compelling evidence that the immunoproteasome plays a critical role in the differentiation and survival of T-helper cells.33 Recent studies have shown that specific inhibition of LMP7 can suppress Th17 and Th1 cell responses in mice with EAE.23 LMP7 inhibition suppressed the phosphorylation of STAT3 and RORγt in Th17 polarization conditions and thus greatly affected the signaling pathway involved in Th17 differentiation.34 In addition, LMP7 KO mice displayed impaired Th17 cell responses in a colitis model.35 Moreover, the administration of BTZ resulted in significantly decreased IL-17 concentrations but only a mild change in IFN-γ concentrations in serum.35 In this study, Th17 cells derived from ESS mice were shown to express significantly higher levels of LMP7 than Th1 cells, which suggests a higher sensitivity of Th17 cells in response to proteasome inhibition. Although BTZ suppressed the differentiation of both Th1 and Th17 cells in vitro (Figure 1 and Supplementary Figure 2), BTZ-treated ESS mice did not show any significant reduction in the total numbers of Th1 and Treg cell subsets but exhibited an approximate threefold decrease in both the frequency and absolute number of Th17 cells in lymphoid organs, which indicated that BTZ treatment preferentially suppresses the Th17 response in vivo. Although multiple cell populations and complex cytokine networks are involved in the pathogenesis of SS,6 recent studies have indicated a key role for IL-17-producing T cells in the pathogenesis of SS in both humans and mice. In patients with SS, both systemic and local IL-17 levels are closely related with the disease activity of SS.36 Moreover, patients with SS at the early stage also display an increased frequency of Th17 cells in peripheral blood.37 Several studies indicated that IL-17 may be involved in both salivary gland and lacrimal gland dysfunction.38, 39, 40 In addition, humoral responses are also critical for the disease pathology of SS. Autoantibodies, including IgGs against M3R and SSA, are highly prevalent in patients with pSS and play a pathophysiological role in the hypofunction of salivary gland epithelial cells.41, 42, 43 In the ESS model, mice exhibited highly activated Th17 responses and humoral responses at 3 weeks after the first immunization, which was associated with saliva secretion impairment. Similar to patients, the immunized mice at this stage are defined as early onset in accordance with the newly published criteria for SS.44 Further, the histopathological changes of SG can be clearly observed in ESS mice at 15 weeks after the first immunization, while the tissue damage was mild in BTZ-treated mice. In this study, BTZ-treated mice displayed higher saliva flow rates and attenuated tissue destruction with less lymphocytic infiltration in the SG compared with vehicle-treated ESS controls. It is evident that BTZ treatment also suppressed the humoral response and autoantibody production in ESS mice, which may indicate a direct effect of BTZ on the inhibition of the B-cell response. Interestingly, both MZ and FO B-cell subsets were unaffected by BTZ treatment, while GC B cells and plasma cells were significantly decreased in BTZ-treated mice, which may suggest that the attenuated GC response and autoantibody production are mainly due to the suppression of the Th17 cell response by BTZ treatment, a notion supported by previous studies that indicated a key role for Th17 cells in driving the GC reaction and humoral autoimmunity.45, 46 Previous studies also indicated that Th17 cells may promote autoreactive B-cell responses in the ESS model,12 and IL-17 signaling can enhance plasmatic differentiation.47 Apart from the well-recognized effect of BTZ in the targeting of B cells, our current findings from the experiments with the transfer of WT CD4+ T cells into immunized IL-17 KO mice have provided strong evidence that BTZ can potently suppress the Th17 response in vivo. Together, these results suggest that either IL-17 or Th17 may represent a promising target for treating patients with SS. Nevertheless, further studies are warranted to elucidate the molecular mechanisms by which BTZ affects Th17 differentiation and function.
Recent studies have shown that proteasome inhibitors antagonize immune responses under autoimmune conditions.16 BTZ and other proteasome inhibitors can affect the functions of macrophages, T cells, B cells and dendritic cells in the inflammatory response.16 Selective inhibition of LMP7 leads to impaired differentiation of Th1 and Th17 cells both in vivo and in vitro.34 Notably, LMP7 KO mice display reduced numbers of Th1 and Th17 cells in mice with DSS-induced colitis.35 Recent evidence indicates that LMP7 inhibition suppressed Th1 and Th17 cells in EAE mice,23 which indicated a strong functional implication for the proteasome in the regulation of T-helper cell differentiation and function. Although it has been reported that LMP7 inhibition promotes Treg generation in culture,34 we did not observe any obvious changes in both the frequency and number of Treg cells in the lymphoid organs of BTZ-treated mice.
Several studies have shown that LMP7 inhibition significantly ameliorates clinical symptoms in mice with experimentally induced arthritis,18 systemic lupus erythematosus,21 experimental autoimmune encephalomyelitis23 and inflammatory bowel disease.48 Consistent with these published studies, we did not observe any obvious side effects from BTZ treatment in ESS mice, which may suggest that BTZ treatment preferentially targets the immunoproteasome in activated immune cells at the site of inflammation. Although there are gaps in research between animal models and human diseases, animal models that recapitulate the key symptoms of human diseases may serve as useful tools for pre-clinical studies. The ESS mice exhibit similar clinical manifestations compared to patients with pSS, including salivary hypofunction and autoantibody production. Previous studies demonstrated a critical role for Th17 cells in ESS development. In this study, we targeted Th17 cells through proteasome inhibition. Explorations of new therapies, including optimization of BTZ dosages and combination with other medications including glucocorticoids, may provide novel insights for disease management. Recently, improvements in patients with SLE after BTZ treatment have been reported in a clinical trial.22 In addition, a case report showed the beneficial effects of BTZ administration on general symptoms in a patient with pSS refractory to rituximab therapy.49 Although both the risks and side effects of BTZ in clinical treatments have been extensively studied,50 further delineation of the human proteasome structure in high resolution will facilitate the development of next-generation proteasome inhibitors with higher efficiency and fewer side effects.51 Thus, more clinical studies are needed to validate the immunoproteasome as a new target for treating autoimmune diseases.
In summary, our findings have demonstrated that BTZ treatment can effectively attenuate the development of ESS, which suggests that immunoproteasome inhibition may represent a novel strategy for targeting SS.
Acknowledgments
We thank the technical support and service of the Medical Faculty Core Facility and Laboratory Animal Unit in The University of Hong Kong. This study was supported by grants from the National Natural Science Foundation of China (81373195, 91442116, 81601424 and 31300739), the National Basic Research Program (No. 2014CB541904), the Natural Science Foundation of Jiangsu (Grant No. BK20150533), the General Research Fund, Hong Kong Research Grants Council (No. 17114515; 17149716); and the Hong Kong Croucher Foundation (260960116).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Fox RI. Sjögren’s syndrome. Lancet 2005; 366: 321–331. [DOI] [PubMed] [Google Scholar]
- Adamson TC, Fox R, Frisman D, Howell F. Immunohistologic analysis of lymphoid infiltrates in primary Sjogren’s syndrome using monoclonal antibodies. J Immunol 1983; 130: 203–208. [PubMed] [Google Scholar]
- Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM. Characteristics of the minor salivary gland infiltrates in Sjogren’s syndrome. J Autoimmun 2010; 34: 400–407. [DOI] [PubMed] [Google Scholar]
- Fox RI, Carstens SA, Fong S, Robinson CA, Howell F, Vaughan JH. Use of monoclonal antibodies to analyze peripheral blood and salivary gland lymphocyte subsets in Sjögren’s syndrome. Arthritis Rheum 1982; 25: 419–426. [DOI] [PubMed] [Google Scholar]
- Talal N, Sylvester RA, Daniels TE, Greenspan JS, Williams RC Jr. T and B lymphocytes in peripheral blood and tissue lesions in Sjögren’s syndrome. J Clin Invest 1974; 53: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youinou P, Pers J-O. Disturbance of cytokine networks in Sjögren’s syndrome. Arthritis Res Ther 2011; 13: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youinou P, Devauchelle-Pensec V, Pers JO. Significance of B cells and B cell clonality in Sjögren’s syndrome. Arthritis Rheum 2010; 62: 2605–2610. [DOI] [PubMed] [Google Scholar]
- Dong C. Targeting Th17 cells in immune diseases. Cell Res 2014; 24: 901–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR et al. Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORgammat activity. Nature 2011; 472: 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Huang S, Yuan X, Liang J, Xu R, Yao G et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol 2015. e-pub ahead of print 5 October 2015 doi:10.1038/cmi.2015.89. [DOI] [PMC free article] [PubMed]
- Rui K, Zhang Z, Tian J, Lin X, Wang X, Ma J et al. Olfactory ecto-mesenchymal stem cells possess immunoregulatory function and suppress autoimmune arthritis. Cell Mol Immunol 2016; 13: 401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Rui K, Deng J, Tian J, Wang X, Wang S et al. Th17 cells play a critical role in the development of experimental Sjogren’s syndrome. Ann Rheum Dis 2015; 74: 1302–1310. [DOI] [PubMed] [Google Scholar]
- Mariette X, Seror R, Quartuccio L, Baron G, Salvin S, Fabris M et al. Efficacy and safety of belimumab in primary Sjögren’s syndrome: results of the BELISS open-label phase II study. Ann Rheum Dis 2013; 74: 526–531. [DOI] [PubMed] [Google Scholar]
- Meijer JM, Meiners PM, Vissink A, Spijkervet FK, Abdulahad W, Kamminga N et al. Effectiveness of rituximab treatment in primary Sjogren’s syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2010; 62: 960–968. [DOI] [PubMed] [Google Scholar]
- Steinfeld SD, Tant L, Burmester GR, Teoh NK, Wegener WA, Goldenberg DM et al. Epratuzumab (humanised anti-CD22 antibody) in primary Sjögren’s syndrome: an open-label phase I/II study. Arthritis Res Ther 2006; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge SE, Scheper RJ, Lems WF, de Gruijl TD, Jansen G. Proteasome inhibitors as experimental therapeutics of autoimmune diseases. Arthritis Res Ther 2015; 17: 1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006; 107: 4907–4916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchamuel T, Basler M, Aujay MA, Suzuki E, Kalim KW, Lauer C et al. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med 2009; 15: 781–787. [DOI] [PubMed] [Google Scholar]
- Lee S-W, Kim J-H, Park Y-B, Lee S-K. Bortezomib attenuates murine collagen-induced arthritis. Ann Rheum Dis 2009; 68: 1761–1767. [DOI] [PubMed] [Google Scholar]
- Neubert K, Meister S, Moser K, Weisel F, Maseda D, Amann K et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med 2008; 14: 748–755. [DOI] [PubMed] [Google Scholar]
- Ichikawa HT, Conley T, Muchamuel T, Jiang J, Lee S, Owen T et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum 2012; 64: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander T, Sarfert R, Klotsche J, Kühl AA, Rubbert-Roth A, Lorenz H-M et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis 2015; 74: 1474–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M, Mundt S, Muchamuel T, Moll C, Jiang J, Groettrup M et al. Inhibition of the immunoproteasome ameliorates experimental autoimmune encephalomyelitis. EMBO Mol Med 2014; 6: 226–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Gamboa L, Lesemann K, Kuckelkorn U, Scheffler S, Ghannam K, Hahne M et al. Gene expression of catalytic proteasome subunits and resistance toward proteasome inhibition of B lymphocytes from patients with primary Sjögren syndrome. J Rheumatol 2013; 40: 663–673. [DOI] [PubMed] [Google Scholar]
- Egerer T, Martinez-Gamboa L, Dankof A, Stuhlmüller B, Dörner T, Krenn V et al. Tissue-specific up-regulation of the proteasome subunit β5i (LMP7) in Sjögren’s syndrome. Arthritis Rheum 2006; 54: 1501–1508. [DOI] [PubMed] [Google Scholar]
- Lin X, Rui K, Deng J, Tian J, Wang X, Wang S et al. Th17 cells play a critical role in the development of experimental Sjogren’s syndrome. Ann Rheum Dis 2014; 74: 1302–1310. [DOI] [PubMed] [Google Scholar]
- Scardina GA, Spanò G, Carini F, Spicola M, Valenza V, Messina P et al. Diagnostic evaluation of serial sections of labial salivary gland biopsies in Sjögren s syndrome. Med Oral Patol Oral Cir Bucal 2007; 12: 565–568. [PubMed] [Google Scholar]
- Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ et al. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol 2010; 184: 3321–3325. [DOI] [PubMed] [Google Scholar]
- Yang M, Deng J, Liu Y, Ko KH, Wang X, Jiao Z et al. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am J Pathol 2012; 180: 2375–2385. [DOI] [PubMed] [Google Scholar]
- Costa S, Schutz S, Cornec D, Uguen A, Quintin-Roue I, Lesourd A et al. B-cell and T-cell quantification in minor salivary glands in primary Sjogren’s syndrome: development and validation of a pixel-based digital procedure. Arthritis Res Ther 2016; 18: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 2014; 13: 668–677. [DOI] [PubMed] [Google Scholar]
- Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012; 120: 947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groettrup M, Kirk CJ, Basler M. Proteasomes in immune cells: more than peptide producers? Nat Rev Immunol 2010; 10: 73–78. [DOI] [PubMed] [Google Scholar]
- Kalim KW, Basler M, Kirk CJ, Groettrup M. Immunoproteasome subunit LMP7 deficiency and inhibition suppresses Th1 and Th17 but enhances regulatory T cell differentiation. J Immunol 2012; 189: 4182–4193. [DOI] [PubMed] [Google Scholar]
- Schmidt N, Gonzalez E, Visekruna A, Kühl AA, Loddenkemper C, Mollenkopf H et al. Targeting the proteasome: partial inhibition of the proteasome by bortezomib or deletion of the immunosubunit LMP7 attenuates experimental colitis. Gut 2010; 59: 896–906. [DOI] [PubMed] [Google Scholar]
- Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjögren’s syndrome immunopathogenesis. Am J Pathol 2009; 175: 1167–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alunno A, Carubbi F, Bistoni O, Caterbi S, Bartoloni E, Bigerna B et al. CD4(−)CD8(−) T-cells in primary Sjogren’s syndrome: association with the extent of glandular involvement. J Autoimmun 2014; 51: 38–43. [DOI] [PubMed] [Google Scholar]
- De Paiva C, Chotikavanich S, Pangelinan S, Pitcher J, Fang B, Zheng X et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol 2009; 2: 243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Yin H, Lee BH, Carcamo W, Chiorini J, Peck A. Pathogenic effect of interleukin-17A in induction of Sjögren’s syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Res Ther 2010; 12: R220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjögren’s syndrome-like ocular surface disease in thrombospondin-1 deficient'mice. Am J Pathol 2009; 175: 1136–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Matsumoto I, Wakamatsu E, Goto D, Sugiyama T, Matsumura R et al. Muscarinic acetylcholine receptor autoantibodies in patients with Sjögren’s syndrome. Ann Rheum Dis 2005; 64: 510–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagliusi P, D’Amore M, D’Amore S, Scagliusi A. Sjogren’s syndrome: apoptosis by anti-SSA and anti-SSB antibodies. Reumatismo 2006; 58: 165–166. [DOI] [PubMed] [Google Scholar]
- Sumida T, Tsuboi H, Iizuka M, Asashima H, Matsumoto I. Anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjögren’s syndrome. Mod Rheumatol 2013; 23: 841–845. [DOI] [PubMed] [Google Scholar]
- Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2016; 76: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman-Brecks CL, Racz JL, Meyer DM, LaBranche TP, Allen PM. Th17 cells can provide B cell help in autoantibody induced arthritis. J Autoimmun 2011; 36: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci USA 2008; 105: 14993–14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ma K, Chen M, Ko KH, Zheng BJ, Lu L. IL-17A promotes pulmonary B-1a cell differentiation via induction of Blimp-1 expression during influenza virus infection. PLoS Pathog 2016; 12: e1005367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M, Dajee M, Moll C, Groettrup M, Kirk CJ. Prevention of experimental colitis by a selective inhibitor of the immunoproteasome. J Immunol 2010; 185: 634–641. [DOI] [PubMed] [Google Scholar]
- Jakez-Ocampo J, Atisha-Fregoso Y, Llorente L. Refractory primary sjögren syndrome successfully treated with bortezomib. J Clin Rheumatol 2015; 21: 31–32. [DOI] [PubMed] [Google Scholar]
- Chen D, Frezza M, Schmitt S, Kanwar J, Dou Q. Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives. Curr Cancer Drug Targets 2011; 11: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J, Henneberg F, Mata RA, Tittmann K, Schneider TR, Stark H et al. The inhibition mechanism of human 20S proteasomes enables next-generation inhibitor design. Science 2016; 353: 594–598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.