Abstract
Background:
Contemporary neurobiology, periodontal medicine, and immunology are now focusing on the relationship between chronic periodontitis and systemic diseases, which also include Alzheimer’s disease (AD). However a causative relationship between dementia and periodontitis has yet to be confirmed.
Objective:
The aim of the study was to determine whether periodontal health status and cognitive abilities are correlated with the relative changes in systemic measures of pro- and anti-inflammatory cytokines as a reflection of systemic inflammation. We hypothesized that poor periodontal health status may be associated with cognitive impairment and dementia via the exacerbation of systemic inflammation.
Methods:
Based on the periodontal and psychiatric examinations and the cytokine levels produced by unstimulated and LPS-stimulated PBL isolated from 128 participants, we have examined if the coexisting of these two clinically described conditions may have influence on the systemic inflammation. Mini-Mental State Examination (MMSE) and Bleeding on Probing (BoP) test results were combined into the one mathematical function U, which determines the severity of specific condition, called Cognitive and periodontal impairment state. Similarly, the levels of cytokines were combined into the one mathematical function V, whose value determines the level of Inflammatory state. The correlation between U and V was determined.
Results:
These results confirm that the presence of cognitive decline and the additional source of pro-inflammatory mediators, like periodontal health problems, aggravate the systemic inflammation.
Conclusion:
It is most likely that the comorbidity of these two disorders may deepen the cognitive impairment, and neurodegenerative lesions and advance to dementia and AD.
Keywords: Cognitive impairment, periodontal disease, dementia, systemic inflammation, cytokines, Alzheimer’s disease
1. INTRODUCTION
A possible link between cognitive impairment and dementia, and systemic inflammatory diseases like periodontitis are postulated and investigated from over decade. Dementias are a set of multi-etiologic brain disorders characterized by the acquired behavioral and cognitive deficits. It primarily affects the elderly, but unfortunately neurodegenerative changes can commence at any age. Dementia has many origins, among which Alzheimer’s disease (AD) is the major cause. AD is multifactorial disease, concerned as systemic diseases, because of affect both central nervous system (CNS) and systemic processes [1]. AD is characterized by early neuronal loss and associated with multiple risk factors. It is the most common type of dementia (50 to 75% of cases) in elderly above 60 years old and it represents one of the main socio-health problems in almost every country in the world [2-4]. Among the most important AD risk factors, age, sex, and genetic abnormalities are non-modifiable. There is no effective approaches that can slow, stop or reverse the AD process, therefore the identification of altered risk factors is important to prevent AD. The increasing studies suggest that gingivitis and periodontitis may be the modifiable risk factors of AD [5]. In elderly, periodontitis is common because of reduced ability to take care of oral hygiene, which can stimulate recurrent chronic oral infection [6, 7]. The correlation between periodontitis and the prevalence of cardiovascular diseases and diabetes has been shown. [8, 9], but the relationship between periodontitis and dementia is still needed to clarify.
One of the most important features of AD neuropathology is neuroinflammation, an inflammatory process pending in the brain, which can be enhanced by systemic inflammation. β-amyloid (Aβ) plagues and tau neurofibrillary tangles are associated with initiation of the immune response in the brain tissue [10, 11]. The key mediators of activation and maintaining the inflammatory processes, are cytokines produced by glia cells and leukocytes infiltrating the brain from periphery. Inflammation and pro-inflammatory mediators, blood vessel damage, and oxidative stress have been found to be capable of inducing neurodegeneration, resulting in neuronal loss and brain injury [10, 12-14]. A number of reports indicating elevated levels of pro-inflammatory cytokines, like TNF-α, IL-1α/β, IL-6 or IL-8 in the brains, blood and cerebrospinal fluid (CSF) of AD patients [15-17]. In the brain, inflammation may be caused by a local central nervous system insult and/or by peripheral infections. Currently, it is postulated that infections elsewhere in the body might aggravate inflammatory processes in the brain. Among the microorganisms suspected in AD, human herpesvirus type 1 (HHV-1), but also Candida species and several non-oral and oral bacteria are intensively investigated [18-20]. Periodontitis is a peripheral chronic infection caused by certain oral pathogens, that elicits a significant systemic inflammatory response [21]. The bacterial antigens from the biofilm that cover the surface of the oral mucosa affect the processes responsible for the local tolerance and immune and systemic response. Thus, periodontal disease may be an important source of systemic inflammatory molecules [22, 23]. TNF-α, IL-1β, IL-6, IL-8 and IL-10 are associated with the inflammatory process induced by LPS of periodontal microorganisms, present in the oral cavity or infiltrating into the blood [24, 25]. Moreover, as it is suggested, LPS can access the AD brain during life and elevated antibodies of periodontal pathogens have been found in AD patients as well as in chronic periodontitis patients [19, 24, 26]. It is postulated that the pro-inflammatory mediators have an effect on local inflammatory processes, but also support systemic inflammation in distant organs. Moreover, it is known that systemic inflammation and chronic exposure to pro-inflammatory mediators may exacerbate the neurodegenerative environment by stimulation of the production of amyloid-β (Aβ) and tau protein [17, 27, 28]. These findings became the basis of the hypothesis that clinical periodontitis is associated with incidence and progression of AD [27].
Recently it was reported that there is an association between oral health status and cognitive decline in AD [29], and there are suggestions that chronic oral infection promotes inflammation, which can add to the inflammatory pool by contributing several pro-inflammatory mediators, and lead to confusion and dementia [19, 30]. However there is still not enough convincing data about the relationship between periodontal and cognitive impairment and systemic inflammation. The study objective is to determine whether periodontal health status and cognitive abilities are correlated with the relative changes in systemic measures of pro- and anti-inflammatory cytokines as a reflection of systemic inflammation. We hypothesized that poor periodontal health status may be associated with cognitive impairment and dementia via the exacerbation of systemic inflammation.
2. MATERIALS AND METHODS
2.1. Participants
The study comprised of 128 participants (83 females, 45 males) aged 55-90 years. Participants include: patients under the care of Department of Periodontology of the Wroclaw Medical University and Department of Psychiatry of the Wroclaw Medical University in Wroclaw, Poland and Alzheimer’s Disease Center in Ścinawa near Wroclaw and volunteers recruited from Medical University hospital crew, IIET PAS and Medical University employee and from their families.
2.2. Periodontal Examination
The periodontal examination of all the subjects was manually performed with a mirror and University of North Carolina (UNC)-15 periodontal probe (Hu Friedy, Chicago, IL, USA) by a single researcher (A.S-J). Number of teeth and measures of probing depth in mm (PD), clinical attachment level in mm (CAL), and gingival inflammation measured as bleeding on probing (yes/no) (BoP) and oral hygiene status by approximal plaque index (API) were recorded at 6 sites (at the disto-, mid-, and mesio-buccal as well as the lingual positions) on all teeth except the third molars. The clinical diagnosis of moderate and severe periodontitis was based on Page & Eke classification [31]. All the participants did not receive treatment for periodontal disease in the past 6 months.
2.3. Psychiatric Examination
The baseline examination included psychiatric and neurological examinations, as well as laboratory tests, electroencephalographic examinations (EEG) and computer tomography (CT) or MRI structural studies. Mini-mental State Examination (MMSE) was used for the screening of dementia. All patients enrolled in the study met DSM-V and NINCDA-ADRDA criteria for probable AD dementia. The exclusion criteria for all evaluated subjects were severe head injury, headache, street drug or oral steroid use, current alcohol abuse or dependence, loss of 25% of the weight in the past year. Subjects with cerebral vascular damage or vascular risk factors were also excluded from the study. The additional exclusion criterion was any history of major psychiatric or central nervous system illness. Patients were excluded if they did not agree to respond to the test questions and/or if they had life-threatening diseases other than AD.
2.4. Standard Protocol Approval
These studies have been approved by the Bioethics Committee of the Wroclaw Medical University (approval number 350/2013) with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
2.5. Isolation of Peripheral Blood Leukocytes (PBL)
PBL from participants included into the study were isolated as previously described [32].
2.6. PBL Culture and Stimulation
Freshly isolated PBL (ex vivo) were divided into two parts. One part of the PBL (2x106 cells mL-1) was suspended in PRMI medium with 2% of fetal bovine serum (FBS) and incubated 24 h at 37 ºC/ 5% CO2 (unstimulated leukocytes). The second part of the PBL (2x106 cells mL-1) was suspended in PRMI medium with 2% FBS and stimulated with 1 µg mL-1 of LPS (Sigma, Poland). Next cells were incubated 24 h at 37 ºC/ 5% CO2. Samples of medium above the PBL were collected after 24h of incubation and stored at -80 ºC for cytokines determination.
2.7. Cytokine Assay
Cytokine levels were determined in the supernatants using ELISA kits (Biomedical Laboratories, USA; BD Biosciences, USA) for human IL-1β, IL-6, IL-10, TNF-α according to the producers' instructions. The optical density was measured at 450 nm using a Multiskan RC spectrophotometric reader (Thermo Labsystems, USA). Cytokine concentrations were expressed in pg/ml.
2.8. Statistical Methods
Let Mi be a score of the MMSE test for i-th person and let Pi be a result of the periodontal BoP test for the same person. Similarly, let Cij be a concentration of the j-th cytokine measured in i-th person, j = 1, 2, 3, 4 and i = 1, 2, ... , 128. Three-element random vector Z(1)i = (Mi, Pi, Mi, × Pi)' codes a cognitive and periodontal status of the i-th person, where variable Mi×Pi reflects interaction between cognitive and periodontal results. Similarly, vector Zi(2)=(C i, ?i)' codes concentrations of four investigated cytokines, where ? i is a vector of six interactions between all pairs of four cytokines in the vector C i.
Let’s introduce a linear function and let’s call it Cognitive and periodontal impairment state. Similarly, let’s introduce a linear function and let’s call it Inflammatory state. The main task is to summarize the association between Cognitive and periodontal impairment state and Inflammatory state. To do this we find coefficient vectors and such that Pearson’s linear correlation between U and V is as large as possible. Mentioned associations are described by coefficients and as well as by Pearson’s linear correlations,, between U and every variable in Z(1) (i.e. MMSE score and BoP score) and between V and every variable in Z(2). Additionally, percent of observed variability among U, which is explained by variability among V, is computed, and vice-versa. Strength of relation between Cognitive and periodontal impairment state and Inflammatory state is measured with Pearson’s correlation coefficient, . Chi-square test is used to test hypothesis opposite to with. All variables (MMSE, BoP, cytokine levels) are standardized to mean = 0 ,variance = 1, and additionally are adjusted to age and sex of investigated persons, to remove eventual influence of these concomitant variables. Standardization of the investigated variables automatically implicates standardized scales of the Cognitive and periodontal impairment state and Inflammatory state.
Data presented in Table 1 were summarized with positional statistics: first (Q1), second (median) and third quartile (Q3) as well as with variability coefficient . Relationship between cytokine levels, Cj, and score results of both MMSE and BoP test was summarized, at first, by conditional averages given the test’s result, i.e. conditional average level of j-th cytokine is respectively, where,,, are limits of intervals in Tables 2 and 3. To show these relationships more understandable (Fig. 1) respective curves were fitted to the obtained points.
Table 1.
Sample size parameters.
| Variable | Q1 | Median | Q3 | W [%] |
|---|---|---|---|---|
| Age | 61 | 70 | 77 | 11.6 |
| MMSE | 18 | 22 | 28 | 21.7 |
| BoP | 34.8 | 51.2 | 67.6 | 32.0 |
| TNF | 22 | 62 | 175 | 77.6 |
| LPS-induced TNF | 572 | 962 | 1618 | 47.7 |
| IL-6 | 2074 | 4693 | 10618 | 67.3 |
| LPS-induced IL-6 | 22227 | 31077 | 43452 | 32.3 |
| IL-10 | 28 | 67 | 158 | 69.7 |
| LPS-induced IL-10 | 894 | 1314 | 1932 | 36.7 |
| IL-1 | 90 | 182 | 369 | 60.7 |
| LPS-induced IL-1 | 1280 | 2008 | 3149 | 42.2 |
Q1, Q3 - 1st and 3rd quartile (25%, 75%), respectively;
W - variability coefficient; lower W → lower variability.
Table 2.
The conditional averages of cytokines levels, given that the BoP test results take a value from particular interval. Original concentrations are presented in pg/ml, as well as relative concentrations, with minimal concentrations as a baselines (minimal concentrations taken as 1).
| Cytokine | BoP Score Interval [%] | |||||
|---|---|---|---|---|---|---|
| 0-20 | 20-40 | 40-60 | 60-80 | 80-100 | ||
| TNF | pg/ml | 29* | 55 | 67 | 69 | 69 |
| Relative** | 1* | 1.9 | 2.31 | 2.38 | 2.38 | |
| IL-6 | pg/ml | 3162 | 4686 | 5085 | 5432 | 5516 |
| Relative | 1 | 1.48 | 1.61 | 1.72 | 1.74 | |
| IL-10 | pg/ml | 50 | 53 | 63 | 77 | 92 |
| Relative | 1 | 1.06 | 1.26 | 1.54 | 1.84 | |
| IL-1 | pg/ml | 101 | 161 | 222 | 251 | 251 |
| Relative | 1 | 1.59 | 2.2 | 2.49 | 2.49 | |
* Minimal concentration taken as baseline.
** Relative = concentration/minimal concentration.
Table 3.
The conditional averages of cytokines levels given that the MMSE test results take a value from particular interval. Original concentrations are presented in pg/ml, as well as relative concentrations, with minimal concentrations as a baselines (minimal concentrations taken as 1).
| Cytokine | MMSE Score Interval | ||||
|---|---|---|---|---|---|
| 10-14 | 15-19 | 20-24 | 25-30 | ||
| TNF | pg/ml | 95 | 64 | 47 | 43* |
| Relative** | 2.21 | 1.49 | 1.09 | 1* | |
| IL-6 | pg/ml | 5319 | 4793 | 4424 | 3522 |
| Relative | 1.51 | 1.36 | 1.26 | 1 | |
| IL-10 | pg/ml | 90 | 69 | 60 | 49 |
| Relative | 1.84 | 1.41 | 1.22 | 1 | |
| IL-1 | pg/ml | 71 | 65 | 64 | 53 |
| Relative | 1.34 | 1.23 | 1.21 | 1 | |
* Minimal concentration taken as baseline.
** Relative = concentration/minimal concentration.
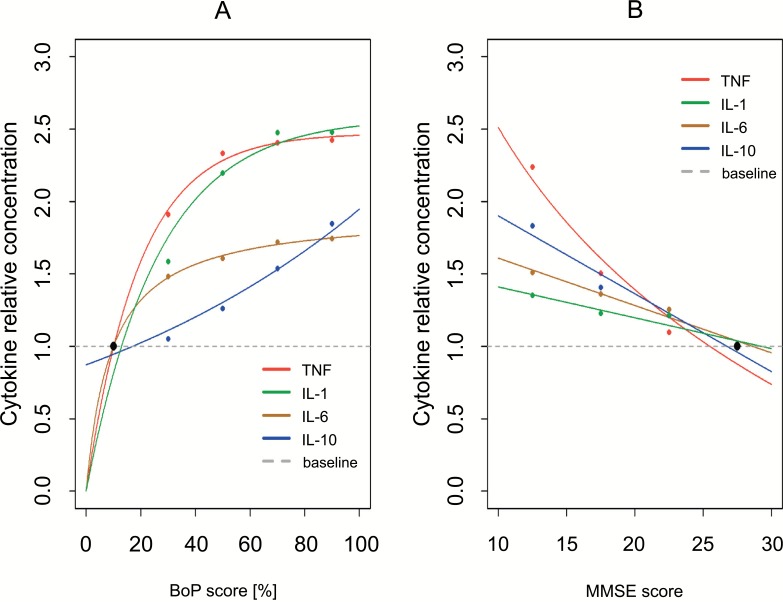
Fig. (1).
Association between the averages of cytokines levels and clinical parameters BoP score (A) and MMSE score (B).
Average concentrations are presented in relative scales, with minimal concentrations as a baselines (minimal concentrations taken as 1). Data presented in Table 1 and Table 2. The conditional averages of cytokines levels (points). Fitted curves were included (lines).
3. RESULTS
3.1. Summary Characteristics of the Parameters Analyzed in the Study
Table 1 contains characteristics of the parameters investigated in this study. It was shown that the median of age is Med = 70 years, 75% of cases were older then Q1= 61 years, 25% of cases were older than Q3 = 77 years. Similarly, the average level of TNF produced by unstimulated PBL was Med = 62 pg/ml, in 75% of cases TNF level was higher than Q1 = 22 pg/ml, and 25% of cases were higher than Q3 = 175 pg/ml. The average level of TNF produced by LPS-induced PBL was Med = 962 pg/ml, in 75% of cases TNF level was higher than Q1 = 572 pg/ml, and 25% of cases were higher than Q3 = 1618 pg/ml. Characteristics of the remaining parameters similarly as above. All participants were brought under thorough periodontal and psychiatric examination, and for our studies BoP and MMSE results were chosen. BoP is an indicator of gingival inflammation and is effective in monitoring of periodontal disease (33). It does not allow for diversity between periodontitis and gingivitis, but in our study participants with diagnosed periodontitis (according to CAL and PD, see Materials and methods) and not only gingivitis were included. MMSE were used as a standard test for screening of cognitive impairment and dementia.
3.2. Cytokine Profile and Clinical Parameters
Firstly, we have investigated if the level of the cytokines IL-1β, IL-6, IL-10 and TNF-α, as a marker of systemic inflammation, is associated with periodontal health status and cognitive impairment. For this purpose PBL from blood samples from 128 participants were isolated. After 24-hour incubation, supernatants above PBL for cytokine determination were collected.
In Table 2 we showed the averages of cytokine levels among participants divided into five groups, 0-20; 20-40; 40-60; 60-80 and 80-100, based on the result of the BoP test. The average level of TNF-α produced by PBL from participants with no more than 20% of BoP score, [0-20], was 29 pg/ml. Similarly, the average level of TNF-α in the group [80-100] was 69 pg/ml. The concentrations of the investigated cytokines were produced by the PBL ex vivo in different orders of magnitude, so to better understand their dynamic and to show the kinetics of all cytokines on the one graph, relative concentrations were calculated. As baseline, minimal concentrations of every cytokine were taken. For example, relative concentration of TNF in the BoP score group [20-40] was (concentration 29 pg/ml was taken as the baseline in this group). The analysis of the results presented in (Table 2) shows, that there is a positive relation between BoP score and average level of the investigated cytokines IL-1β, Il-6, IL-10 and TNF-α. The average concentration of TNF-α in the group [80-100] is 2.38 times higher than the average concentration in the baseline group [0-20]. Characteristics of the relationship of remaining cyto-kines and BoP test show similar results as above. In Table 3 we showed the averages of cytokines levels among participants divided into four groups, 0-14; 15-19; 20-24, and 25-30, based on the result of the MMSE test. Characteristics of the relationship of investigated cytokines levels and MMSE test show results same as above.
Summarizing, the average level of every investigated cytokine, produced by unstimulated PBL ex vivo, raised with the increase in BoP test, what is connected with worse periodontal health status, and with fall in MMSE test, what is correlated with cognitive decline and dementia. Fig. (1) presents the relative concentrations of investigated cytokines, presented in the Table 2 and Table 3, points with fitted curves (lines). Obtained results were good starting points for in-depth analysis of this research problem, when we take into consideration all cytokines together, with an interaction between them, and examine the relationship between inflammatory state and cognitive and periodontal impairment.
3.3. Relationship between Cognitive and Periodontal Impairment and Systemic Inflammation
Next, we have attempted to explain if periodontal and cognitive impairment contribute to the increase in systemic inflammation, and, if the presence of these two disorders is connected with higher inflammatory state compared to the presence of only one of these conditions. For this purpose MMSE and BoP test results were treated as one specific condition, that we called Cognitive and periodontal impairment state. Both scores were included into the single function, to better describe the clinical state of every investigated participant. This state was next compared to the Inflammatory state, measured by the levels of cytokines IL-1β, IL-6, IL-10 and TNF-α, produced by unstimulated and LPS-stimulated PBL.
Table 4 presents all the results including cytokine levels from unstimulated PBL. It is shown that. Scores of BoP are standardized, so it means, that if BoP score increases by one standard deviation from the mean, the Cognitive and periodontal impairment state, measured as function U, increases by 0.57 units. Similarly, coefficient for MMSE score . It means, that when MMSE score decreases by one standard deviation, Cognitive and periodontal impairment state increases by 0.51 units. This is comprehensible, because less MMSE points mark on the cognitive decline. Moreover, it is investigated, that there is influential interaction between this two measurements. Coefficient for interaction between MMSE and BoP scores is . It simplifies, that the Cognitive and periodontal state cannot be correlated with Inflammatory state as a simple sum of the MMSE and BoP results. Beta coefficients describe relative effect of every of two variable, i.e. taking into consideration the second measurement. To better understand the relation between MMSE score, BoP score and Cognitive and periodontal impairment state (see Statistical methods) correlation coefficients between MMSE and U as well as between BoP and U are presented in Table 4. We can see, that correlation between BoP score and Cognitive and periodontal impairment state is . It means, that the higher the BoP score the higher Cognitive and periodontal impairment state. Similarly, the lower the MMSE score the higher the Cognitive and periodontal impairment state. In Table 4 it is presented that about 10% (9.7% exactly) of all variability observed in Cognitive and periodontal impairment state can be explained by the Inflammatory state, i.e. by the levels of investigated cytokines. The effect is not strong, but taking into consideration the nature of the investigated problem, this effect is substantial. Based on 95% confidence interval, CI95% (5.4, 17.6), we conclude, that the possibility cannot be excluded, that in general population almost 17% of the observed variability of the Cognitive and periodontal impairment state can be explained by Inflammatory state.
Table 4.
Correlation between Cognitive and periodontal impairment state and Inflammation state. Correlations between Cognitive and periodontal impairment state and it’s measures: MMSE, BoP. Correlations between Inflammatory state and it’s measures: cytokines levels. Coefficients β of the linear functions U and V defining Cognitive and periodontal impairment state (U) and Inflammatory state (V) in PBL isolated from all participants.
| Cognitive and Periodontal Impairment State Measures |
Coefficients |
Correlation |
|---|---|---|
| BoP.mod2 | 0.57 | 0.61 |
| MMSE | -0.51 | -0.65 |
| BoP x MMSE | 0.51 | 0.62 |
| Variability explained by inflammation state [%] |
9.7 CI95% =(5.4, 17.6) |
|
| Inflammatory state measures |
coefficients | correlation |
| TNF | 0.33 | 0.56 |
| IL-6 | -0.17 | 0.44 |
| IL-10 | 0.3 | 0.57 |
| IL-1 | 0.2 | 0.59 |
| TNF x IL-6 | -0.45 | -0.18 |
| TNF x IL-10 | -0.17 | -0.22 |
| TNF x IL-1 | -0.47 | -0.21 |
| IL- 6 x IL-10 | -0.39 | 0.05 |
| IL- 6 x IL-1 | 0.5 | 0.26 |
| IL 10 x IL-1 | 0.8 | 0.34 |
| Variability explained by cognitive and periodontal state [%] |
3.2 CI95% (1, 7.1) |
|
|
correlation between U and V |
0.51 CI95% (0.39; 0.63) |
|
| Test for | ||
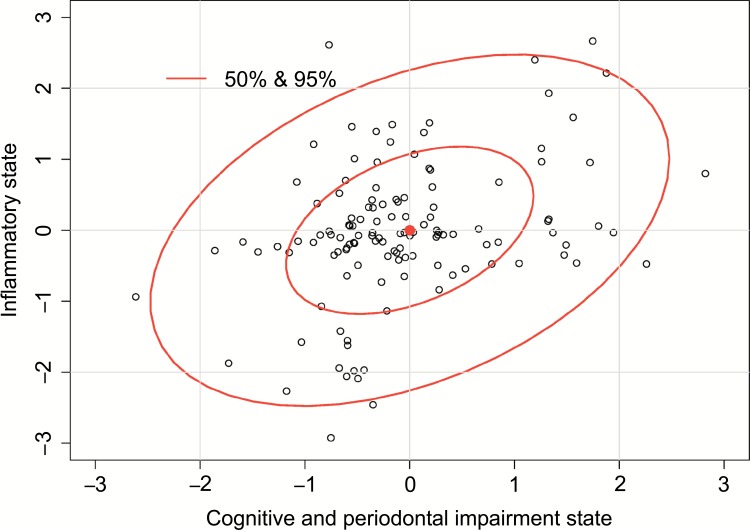
The relative effects of the cytokines levels, described by the coefficients β(2) , on the Inflammatory state have been shown. Coefficient for TNF-α is βTNF, which means that when TNF-α increases by one standard deviation from the mean level, the Inflammatory state increases by 0.33 units. But the coefficients for interaction effects between TNF-α and the three other interleukins, IL-1β, IL-6 and IL-10, are negative. It has been shown, that TNF-α effect on the Inflammatory state is modified by other investigated cytokines (and vice versa). Similarly, the higher level of IL-10 and IL-1β means the higher Inflammatory state. Both are positive as well as coefficient for the interaction between these cytokines . This relatively high interaction effect simplifies that Inflammatory state cannot be described as a sum of IL-10 and IL-1β single effects. Their common effect, in relation to Inflammatory state, is substantial, and stronger than a sum of their single effects. Similarly, the effect of interaction is observed for IL-1 β and IL-6. Negative β(2) for some interactions show, that the relations between cytokines levels and Inflammatory state are not additive, i.e., relation of one cytokine with Inflammatory state depends on the level of the other cytokines. Generally, to better understand discussed relations the simple Pearson’s correlation coefficients between the levels of every cytokine and the level of the Inflammatory state was investigated. For example, correlation between TNF-α level and Inflammatory state is (Table 4). It means that participants with higher level of TNFα have higher level of Inflammatory state. But the main question is: Do people with higher level of Inflammatory state have higher level of Cognitive and periodontal impairment state? In Table 4 we showed the correlation coefficient between these two states, measured as function (Cognitive and periodontal state) and (Inflammatory state). This correlation is and it is quite substantial. Based on the confidence interval at 95% level we conclude that we can be almost sure, that in general population this correlation is at least , and it is not impossible that it is even as high as .. This is not surprising that only about 3% of observed variability of cytokines levels can be explained by Cognitive and periodontal impairment state. Fig. (2) presents correlation between Cognitive and periodontal impairment state (U) as a function of MMSE and BoP (see Statistical methods) and Inflammatory state (V), as a function of cytokine levels produced by cultured and unstimulated PBL.
Fig. (2).
Correlation between Cognitive and periodontal impairment state (U), measured as the function of MMSE and BoP (see Statistical methods), and Inflammatory state (V) as a function of cytokines levels produced by unstimulated PBL ex vivo.
Therefore, higher level of BoP score, which correlates with periodontal disease, is connected with higher Cognitive and periodontal impairment state, and thus with higher level of the Inflammatory state, as a reflection of systemic inflammation measured by the levels of cytokines produced by unstimulated PBL. Similarly, lower MMSE result, which correlates closely with the presence of cognitive impairment and dementia, is connected with higher Cognitive and periodontal impairment state. What’s more, the presence of high BoP (periodontitis) scores and low MMSE (cognitive impairment and dementia) scores together mean higher Cognitive and periodontal impairment state and thus higher Inflammatory state, compared to the prevalence of only one of these conditions.
Table 5 presents the results of cytokines levels produced by LPS-stimulated PBL. Based on the β(1) coefficients it is presented, that the highest impact on the correlation between Cognitive and periodontal impairment state and Inflamma-tory state has the MMSE test results. Coefficient is more than two-fold higher than coefficient for BoP test results, where , and is negative, because less points in MMSE test means the worse cognitive functions and dementia. It has been shown that about 9% of the observed variability among Cognitive and periodontal impairment state can be explained by the Inflammatory state, i.e. by the levels of cytokines IL-1β, IL-6, IL-10 and TNF-α. From Table 5 we get coefficients which describe the impact of the particular cytokines levels on the correlation between Cognitive and periodontal impairment state and Inflammatory state. It is presented that the highest impact have TNF-α, and IL-1β . Based on the coefficients β(2) for interaction components it is observed that the effect of the TNF-α level cannot be described without consideration of the other cytokines. The effect of an interaction between TNF-α and other cytokines in creating the Inflammatory state has a reflection in the value of the correlation coefficient between TNF-α level and the value of the Inflammatory state, . This value, practically zero, shows, that there is no simple correlation between TNF-α and Inflammatory state if the levels of other cytokines are ignored. The highest impact on the correlation between Cognitive and periodontal impairment state and Inflammatory state has IL-1β. The coefficient for this cytokine is and its influence is in positive interaction with the level of the IL-10. The coefficient for this interaction component is and it means that if we consider the correlation between Inflammatory state and Cognitive and periodontal impairment state, the higher level of IL-1β and IL-10 correlates with higher level of Inflammatory state, but their common effects are more than additive. Based on the simple correlation coefficients it is investigated, that all the cytokines are positively correlated with Inflammatory state, even if the presence and levels of the other cytokines and interactions between them are ignored. For example, correlation between IL-1β level and Inflammatory state is , it simplifies that if the level of the rest of cytokines is ignored, the higher level of the IL-1β always will be connected with higher level of the Inflammatory state, etc. In the Table 5 we find the correlation between Cognitive and periodontal impairment state (variable U, see Statistical methods) and Inflammatory state (variable V). Confidence interval for this correlation coefficient is CI95% (0.38, 0.63) and it shows that in general population this correlation is at least about and not higher than . This observed correlation is significant at . The correlation between Cognitive and periodontal impairment state (U), measured as a function of MMSE and BoP (see Statistical methods) and Inflammatory state (V), as a function of the levels of cytokines produced by LPS-stimulated PBL it is presented on Fig. (3).
Table 5.
Correlation between Cognitive and periodontal impairment state and Inflammation state. Correlations between Cognitive and periodontal impairment state and it’s measures: MMSE, BoP. Correlations between Inflammatory state and it’s measures: cytokines levels. Coefficients β of the linear functions U and V defining Cognitive and periodontal impairment state (U) and Inflammatory state (V) in LPS-induced PBL isolated from all participants.
| Cognitivel and Periodontal Impairment State Measures | Coefficients | Correlation |
|---|---|---|
| BoP.mod2 | 0.36 | 0.42 |
| MMSE | -0.81 | -0.89 |
| BoP x MMSE | 0.28 | 0.44 |
| Variability explained by inflammation state [%] |
9.2 CI95% =(4.9, 16.4) |
|
| Inflammatory state measures |
coefficients | correlation |
| TNF | -0.54 | -0.03 |
| IL-6 | 0.05 | 0.51 |
| IL-10 | 0.12 | 0.36 |
| IL-1 | 0.86 | 0.67 |
| TNF x IL-6 | 0.47 | -0.35 |
| TNF x IL-10 | -0.44 | -0.09 |
| TNF x IL-1 | -0.44 | -0.5 |
| IL- 6 x IL-10 | 0.36 | 0.16 |
| IL- 6 x IL-1 | -0.35 | -0.38 |
| IL 10 x IL-1 | 0.43 | 0.12 |
| Variability explained by cognitive and periodontal state [%] | 2.8 CI95%(0.9, 6.5) |
|
| correlation between U and V | 0.5 CI95%(0.38; 0.63) |
|
| Test for | ||
x - interaction between two measures;
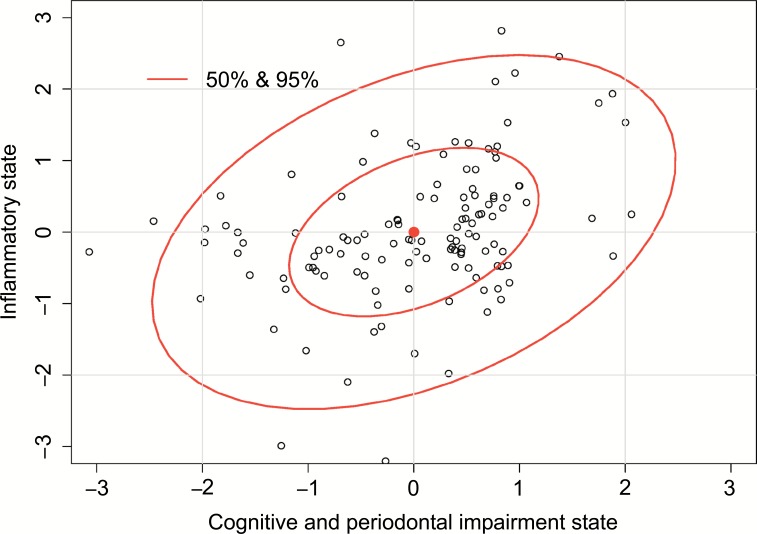
Fig. (3).
Correlation between Cognitive and periodontal impairment state (U), measured as the function of MMSE and BoP (see Statistical methods), and Inflammatory state (V) as a function of cytokines levels produced by LPS-stimulated PBL ex vivo.
Therefore, worse periodontal health status (higher BoP score) as well as cognitive decline (lower result in MMSE test) (via negative value of the coefficient) are connected with the higher level of the Cognitive and periodontal impairment state (via positive ) and higher Inflammatory state, as a reflection of systemic inflammation measured by the levels of cytokines produced by LPS-stimulated PBL. According to the obtained results we have seen that unstimulated as well as LPS-stimulated PBL ex vivo respond similarly by producing the elevated levels of systemic inflammatory markers IL-1β, IL-6, IL-10 and TNF-α. Moreover, all the cytokines are positively correlated with the inflammatory state. Thus, it confirms that poor periodontal health status and cognitive decline aggravate the inflammatory response.
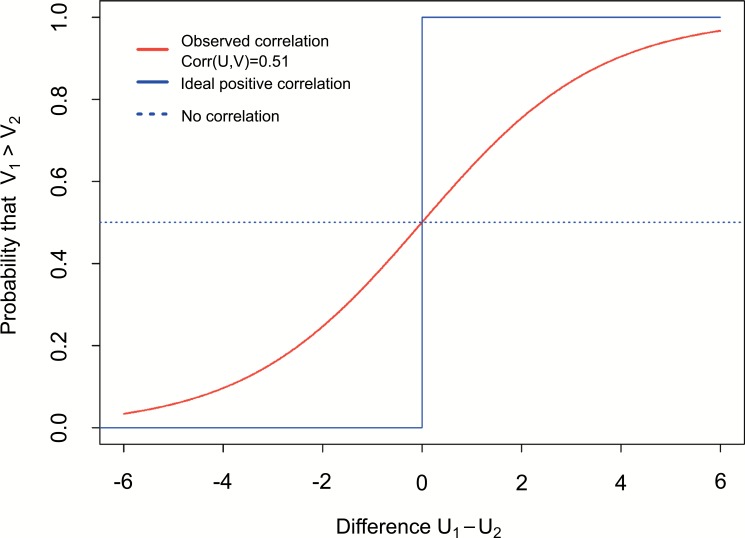
Based on the results presented in Tables 4 and 5 as well as on the (Figs. 2 and 3) the relationship between Cognitive and periodontal impairment state (U) and Inflammatory state (V) is showed. The interesting question is: What is the probability, that first person has higher Inflammation state than the second one, if the first one has higher Cognitive and periodontal impairment state? According to the notation introduced in Statistical methods we ask about probability . Figure 4 presents functional relation between π and difference . Firstly, if there is no correlation between U and V, expected curve takes the same probability π = 0.5 for every difference (dot blue line on Fig. 4). Secondly, if the correlation between U and V is ideal positive then the probability will take π = 0 for every for every (solid blue line on Fig. 4). This observed correlation (PBL) is (Table 4). This correlation is reflected by the red curve on Fig. (4). Based on the results presented on the (Fig. 4) we can conclude, that if person number one have lower Cognitive and periodontal impairment state than the person number two (negative difference ), there is low probability, that person number one have higher Inflammatory state than person number two. Otherwise, if person number one has higher Cognitive and periodontal impairment state comparing to person number two (positive difference ), then it is more probable, that person number one also has higher Inflammatory state.
Fig. (4).
Association between Cognitive and periodontal impairment state (U), and Inflammatory state (V) as the probability, that first person have higher Inflammatory state than the second one, if the first person has higher or lower Cognitive and periodontal impairment state than the second one. This probability is showed as a function of difference in Cognitive and periodontal impairment states between this two persons.
DISCUSSION
Still increasing number of studies suggest the relationship between periodontitis and cognitive decline and AD [3, 27, 29, 30, 34] but the link is elusive and the exact mechanism is still unclear. Periodontitis can lead to the AD progression, and two probable mechanisms are proposed: periodontitis preceding systemic inflammation/infection and bacterial and viral influence [35]. The chronic inflammatory response that occurs in AD is a complex process that involves innate and adaptive cells and their produced inflammatory mediators (TNFα, IL-1, IL-6, IL-7, IL-10, IL-15 and IL-18) [36]. Cytokines IL-1, IL-6, IL-10 and TNF-α are involved in the pathogenesis of periodontitis and are a good biomarkers of nonsurgical treatment of chronic periodontitis [37-39].
While the influence of inflammatory processes in the pathogenesis of periodontitis and AD is reported, this study addressed whether there is a relationship between pro- and anti-inflammatory cytokine profile, that plays an important role in the immune response in periodontal disease and AD, and the results of the BoP test and MMSE test, as a reflection of periodontal health status and cognitive abilities. It has been shown that the level of pro-inflammatory cytokines IL-1, IL-6 and TNF-α but also anti-inflammatory IL-10, produced by PBL ex vivo, increases with worsened periodontal status (increase of BoP score) and cognitive decline (fall of MMSE score). These results confirmed early observations that the systemic inflammatory markers are elevated in diagnosed cognitive impairment and AD patients with periodontitis. For example, it has been shown that in AD patients with periodontitis the antibodies of common periodontal bacteria and plasma levels of TNF-α are increased [40]. Three folds higher level of TNF-α in serum of AD patients with chronic periodontitis was also presented [41]. Ide et al. investigated an association between periodontitis and cognitive decline in AD by the measurement of serum level of pro- and anti-inflammatory markers. They show a relative increase in the levels of CRP and TNF-α and decrease in IL-10 over a six month follow up period in AD participants with periodontitis, but the results are not informative because of the small numbers of participants and because of the large data scatter. Thus, the future investigations that could confirm these results are needed.
It was presented earlier that PBMCs from patients with chronic periodontitis have suppressed anti-inflammatory cytokine production (IL-10) [42] and that the IL-10 production was restored after blocking the pro-inflammatory cytokine release by peripheral blood mononuclear cells (PBMC) from chronic periodontitis patients in response to P. gingivalis LPS. We showed an increase in IL-10 production by PBL, therefore it seems that the cytokine profile, as well as the inflammatory state associated with the presence of both periodontitis and cognitive impairment, change the leukocyte response, which replay with the increased production of both pro and anti-inflammatory cytokines. Moreover IL-10 is known as a down-regulator of IL-1 production, so higher level of pro-inflammatory cytokine (IL-1β, IL-6 and TNF-α) may lead to an increase in IL-10 release. The elevated levels of both pro- and anti-inflammatory cytokines were also observed in patients with periodontitis and other systemic diseases, like rheumatoid arthritis or inflammatory bowel disease [43, 44].
To test the hypothesis that the coexisting of poor periodontal status and cognitive impairment may exacerbate the systemic inflammation, we have measured the level of pro- (IL-1β, IL-6 and TNF-α) and anti-inflammatory IL-10 systemic markers produced by unstimulated and LPS-stimulated PBL. All participants were brought under thorough periodontal and psychiatric examination, but for our analysis BoP and MMSE tests results were chosen. The reason was that the BoP is effective in the diagnosis and monitoring of active periodontal diseases but also is a reliable indicator of gingival inflammation [33]. However, measurement of the PoB does not allow for diversity between periodontitis and gingivitis, but we confirm that in our study, participants diagnosed with periodontitis and not only gingivitis were included. MMSE is a standard test used for the evaluation of cognitive decline (screening of cognitive impairment and dementia). There are some limitations to the current research that may affect the results. One could not expect that there will be a simple correlation between the level of particular cytokines and the number of points in the MMSE test. Inflammation is a complex process and there is no simple relationship between inflammation and the level of individual cytokine level and the relationship between cytokine level and BoP or MMSE results. Therefore, in this study we have used such-and-such methodology, where the MMSE and BoP test results are combined into the one value (U) with a particular function, whose the result determines the severity of specific condition, that we collectively called Cognitive and periodontal impairment state. Similarly, the levels of cytokines were combined into the one function (V) whose value (the result of adding cytokine concentrations and multiplied by their coefficients βΜ) determines the level of Inflammatory state, as a reflection of systemic inflammation.
It was suggested previously that the higher prevalence of both conditions (periodontitis and dementia) in elderly could imply that their association might be coincidental [34]. We have shown the evidence that the higher BoP result (worsened bleeding), which means periodontal health problems, the higher U-function value (Cognitive and periodontal impairment state), because βP for the BoP is positive and significantly higher than zero. Likewise, we presented that the less points in MMSE test, which indicates on the cognitive impairment and dementia, means, again, the higher U-function value because of the negative βΜ for the MMSE. It means that the cognitive impairment and dementia (fall in MMSE test) and periodontal disease (high BoP), when coexist together, lead to the higher systemic inflammation compared to the prevalence of only one of these conditions. Thus, we confirmed early proposed mechanism, where inflammatory mediators of periodontitis may contribute to, exacerbate, and share risk factors with dementia [45]. These observations are significant to better understand the mechanisms involved in the pathogenesis of neuroinflammatory diseases, like AD. The increase of systemic inflammation leads to the rise of pro-inflammatory cytokines that may enter into the brain through the more permeable blood brain barrier (BBB) and stimulate the glial cells to synthesize additional pro-inflammatory cytokines and evoke neuroinflammation. Periodontitis is a significant source of systemic inflammatory molecules, that causes or promotes other chronic systemic inflammatory diseases, and an association between periodontal disease and brain Aβ load in humans was shown [23, 46].
Therefore it is reasonable to consider periodontal health problems as a modifiable risk factor of cognitive impairment and dementia, and early treatment of periodontitis may limit severity and progression of cognitive lesions. Future research using more accurate, especially psychiatric, examinations, and designation of other systemic inflammatory markers are most welcome and warranted to support this investigation.
CONCLUSION
The inflammatory processes change along with the disease process and the impact of numerous biomarkers, like cytokines still produced by immune cells, may be different at different points in the disease. The prevalence of the various neuropsychiatric symptoms and neurodegenerative changes alter over the course of the disease and it is very likely that the presence of additional source of pro-inflammatory mediators, like periodontal disease, might not initiate AD but exacerbate the systemic inflammation and thus deepen the neurodegenerative lesions.
Ethics Approval and Consent to Participate
We had the approval of the Bioethics Committee of the Wroclaw Medical University (approval number 350/2013).
Consent for Publication
Not applicable.
ACKNOWLEDGEMENTS
Project supported by Wroclaw Centre of Biotechnology, programme The Leading National Research Centre (KNOW) for years 2014-2018. Special thanks for Bogna Jatczak, Ph.D., Agnieszka Wiśniewska, MSc, and Iwona Siemieniec from Laboratory of Virology IIET PAS for technical support.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Morris J.K., Honea R.A., Vidoni E.D., Swerdlow R.H., Burns J.M. Is Alzheimer’s disease a systemic disease? Biochim. Biophys. Acta. 2014;1842(9):1340–1349. doi: 10.1016/j.bbadis.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dá Mesquita S., Ferreira A.C., Sousa J.C., Correia-Neves M., Sousa N., Marques F. Insights on the pathophysiology of Alzheimer’s disease: The crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci. Biobehav. Rev. 2016;68:547–562. doi: 10.1016/j.neubiorev.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Gil-Montoya J.A., Sanchez-Lara I., Carnero-Pardo C., Fornieles F., Montes J., Vilchez R., et al. Is periodontitis a risk factor for cognitive impairment and dementia? A case-control study. J. Periodontol. 2015;86(2):244–253. doi: 10.1902/jop.2014.140340. [DOI] [PubMed] [Google Scholar]
- 4.Leszek J., Sochocka M. Services and developments around the world: Poland. In: De Waal H., et al., editors. Designing and Delivering Dementia Services. W: John Wiley & Sons, Ltd; 2013. pp. 257–260. [Google Scholar]
- 5.Stewart R., Weyant R.J., Garcia M.E., Harris T., Launer L.J., Satterfield S., et al. Adverse oral health and cognitive decline: the health, aging and body composition study. J. Am. Geriatr. Soc. 2013;61(2):177–184. doi: 10.1111/jgs.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatipoglu M.G., Kabay S.C., Güven G. The clinical evaluation of the oral status in Alzheimer-type dementia patients. Gerodontology. 2011;28(4):302–306. doi: 10.1111/j.1741-2358.2010.00401.x. [DOI] [PubMed] [Google Scholar]
- 7.Syrjälä A-M., Ylöstalo P., Ruoppi P., Komulainen K., Hartikainen S., Sulkava R., et al. Dementia and oral health among subjects aged 75 years or older. Gerodontology. 2012;29(1):36–42. doi: 10.1111/j.1741-2358.2010.00396.x. [DOI] [PubMed] [Google Scholar]
- 8.AlJehani Y.A. Risk factors of periodontal disease: review of the literature. Int. J. Dent. 2014;2014:182513. doi: 10.1155/2014/182513. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Links between atherosclerotic and periodontal disease. Exp. Mol. Pathol. 2016;100(1):220–235. doi: 10.1016/j.yexmp.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Heneka M.T., Carson M.J., Khoury J.E., Landreth G.E., Brosseron F., Feinstein D.L., et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heneka M.T., Golenbock D.T., Latz E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015;16(3):229–236. doi: 10.1038/ni.3102. [DOI] [PubMed] [Google Scholar]
- 12.Hong H., Kim B.S., Im H-I. Pathophysiological role of neuroinflammation in neurodegenerative diseases and psychiatric disorders. Int. Neurourol. J. 2016;20(Suppl. 1):S2–S7. doi: 10.5213/inj.1632604.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leszek J., Barreto G.E., Gąsiorowski K., Koutsouraki E., Ávila-Rodrigues M., Aliev G. Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: role of brain innate immune system. CNS Neurol. Disord. Drug Targets. 2016;15(3):329–336. doi: 10.2174/1871527315666160202125914. [DOI] [PubMed] [Google Scholar]
- 14.Sochocka M., Koutsouraki E.S., Gasiorowski K., Leszek J. Vascular oxidative stress and mitochondrial failure in the pathobiology of Alzheimer’s disease: a new approach to therapy. CNS Neurol. Disord. Drug Targets. 2013;12(6):870–881. doi: 10.2174/18715273113129990072. [DOI] [PubMed] [Google Scholar]
- 15.Rubio-Perez J.M., Morillas-Ruiz J.M. A review: inflammatory process in Alzheimer’s disease, role of cytokines. Sci. World J. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith J.A., Das A., Ray S.K., Banik N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012;87(1):10–20. doi: 10.1016/j.brainresbull.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyss-Coray T., Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012;2(1):a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miklossy J., McGeer P.L. Common mechanisms involved in Alzheimer’s disease and type 2 diabetes: a key role of chronic bacterial infection and inflammation. Aging. 2016;8(4):575–588. doi: 10.18632/aging.100921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olsen I., Singhrao S.K. Can oral infection be a risk factor for Alzheimer’s disease? J. Oral Microbiol. 2015;7:29143. doi: 10.3402/jom.v7.29143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singhrao S.K., Harding A., Poole S., Kesavalu L., Crean S. Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s disease. Mediators Inflamm. 2015;2015:137357. doi: 10.1155/2015/137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015;15(1):30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polepalle T., Moogala S., Boggarapu S., Pesala D.S., Palagi F.B. Acute phase proteins and their role in periodontitis: a review. J. Clin. Diagn. Res. 2015;9(11):ZE01–ZE05. doi: 10.7860/JCDR/2015/15692.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z., Nakanishi H. Connection between periodontitis and Alzheimer’s disease: possible roles of microglia and leptomeningeal cells. J. Pharmacol. Sci. 2014;126(1):8–13. doi: 10.1254/jphs.14r11cp. [DOI] [PubMed] [Google Scholar]
- 24.Carvalho-Filho P.C., Gomes-Filho I.S., Meyer R., Olczak T., Xavier M.T., Trindade S.C. Role of Porphyromonas gingivalis HmuY in Immunopathogenesis of Chronic Periodontitis. Mediators Inflamm. 2016;2016:7465852. doi: 10.1155/2016/7465852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trindade S.C., Olczak T., Gomes-Filho I.S., Moura-Costa L.F., Cerqueira E.M., Galdino-Neto M., et al. Induction of interleukin (IL)-1β, IL-10, IL-8 and immunoglobulin G by Porphyromonas gingivalis HmuY in humans. J. Periodontal Res. 2012;47(1):27–32. doi: 10.1111/j.1600-0765.2011.01401.x. [DOI] [PubMed] [Google Scholar]
- 26.Poole S., Singhrao S.K., Kesavalu L., Curtis M.A., Crean S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers Dis JAD. 2013;36(4):665–677. doi: 10.3233/JAD-121918. [DOI] [PubMed] [Google Scholar]
- 27.Kamer A.R., Craig R.G., Dasanayake A.P., Brys M., Glodzik-Sobanska L., de Leon M.J. Inflammation and Alzheimer’s disease: possible role of periodontal diseases. Alzheimers Dement J Alzheimers Assoc. 2008;4(4):242–250. doi: 10.1016/j.jalz.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Lim S.L., Rodriguez-Ortiz C.J., Kitazawa M. Infection, systemic inflammation, and Alzheimer’s disease. Microbes Infect. 2015;17(8):549–556. doi: 10.1016/j.micinf.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Ide M., Harris M., Stevens A., Sussams R., Hopkins V., Culliford D., et al. Periodontitis and cognitive decline in Alzheimer’s disease. PLoS One. 2016;11(3):e0151081. doi: 10.1371/journal.pone.0151081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbayya K., Puthanakar N.Y., Naduwinmani S., Chidambar Y.S. Association between periodontitis and Alzheimer’s disease. N. Am. J. Med. Sci. 2015;7(6):241–246. doi: 10.4103/1947-2714.159325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Page R.C., Eke P.I. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007;78(7) Suppl.:1387–1399. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 32.Sochocka M., Zaczyńska E., Taboł A., Czarny A., Leszek J., Sobczyński M. The influence of donepezil and EGb 761 on the innate immunity of human leukocytes: effect on the NF-κB system. Int. Immunopharmacol. 2010;10(12):1505–1513. doi: 10.1016/j.intimp.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Highfield J. Diagnosis and classification of periodontal disease. Aust. Dent. J. 2009;54(Suppl. 1):S11–S26. doi: 10.1111/j.1834-7819.2009.01140.x. [DOI] [PubMed] [Google Scholar]
- 34.Gaur S., Agnihotri R. Alzheimer’s disease and chronic periodontitis: is there an association? Geriatr. Gerontol. Int. 2015;15(4):391–404. doi: 10.1111/ggi.12425. [DOI] [PubMed] [Google Scholar]
- 35.Gurav A.N. Alzheimer’s disease and periodontitis--an elusive link. Rev. Assoc. Med. Bras. 2014;60(2):173–180. doi: 10.1590/1806-9282.60.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Hall J.R., Wiechmann A.R., Johnson L.A., Edwards M., Barber R.C., Winter A.S., et al. Biomarkers of vascular risk, systemic inflammation, and microvascular pathology and neuropsychiatric symptoms in Alzheimer’s disease. J Alzheimers Dis JAD. 2013;35(2):363–371. doi: 10.3233/JAD-122359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reis C.D., Costa A.V., Guimarães J.T., Tuna D., Braga A.C., Pacheco J.J., et al. Clinical improvement following therapy for periodontitis: Association with a decrease in IL-1 and IL-6. Exp. Ther. Med. 2014;8(1):323–327. doi: 10.3892/etm.2014.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taba M, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. 2005. [DOI] [PMC free article] [PubMed]
- 39.Yucel-Lindberg T., Båge T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013;15:e7. doi: 10.1017/erm.2013.8. [DOI] [PubMed] [Google Scholar]
- 40.Kamer A.R., Craig R.G., Pirraglia E., Dasanayake A.P., Norman R.G., Boylan R.J., et al. TNF-alpha and antibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J. Neuroimmunol. 2009;216(1-2):92–97. doi: 10.1016/j.jneuroim.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farhad S.Z., Amini S., Khalilian A., Barekatain M., Mafi M., Barekatain M., et al. The effect of chronic periodontitis on serum levels of tumor necrosis factor-alpha in Alzheimer disease. Dent. Res. J. 2014;11(5):549–552. [PMC free article] [PubMed] [Google Scholar]
- 42.Berker E., Kantarci A., Hasturk H., Van Dyke T.E. Blocking proinflammatory cytokine release modulates peripheral blood mononuclear cell response to Porphyromonas gingivalis. J. Periodontol. 2013;84(9):1337–1345. doi: 10.1902/jop.2012.120422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Javed F., Ahmed H.B., Mikami T., Almas K., Romanos G.E., Al-Hezaimi K. Cytokine profile in the gingival crevicular fluid of rheumatoid arthritis patients with chronic periodontitis. J. Investig. Clin. Dent. 2014;5(1):1–8. doi: 10.1111/jicd.12066. [DOI] [PubMed] [Google Scholar]
- 44.Menegat J.S., Lira-Junior R., Siqueira M.A., Brito F., Carvalho A.T., Fischer R.G., et al. Cytokine expression in gingival and intestinal tissues of patients with periodontitis and inflammatory bowel disease: An exploratory study. Arch. Oral Biol. 2016;66:141–146. doi: 10.1016/j.archoralbio.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 45.Rai B., Kaur J., Anand S.C. Possible relationship between periodontitis and dementia in a North Indian old age population: a pilot study. Gerodontology. 2012;29(2):e200–e205. doi: 10.1111/j.1741-2358.2010.00441.x. [DOI] [PubMed] [Google Scholar]
- 46.Kamer A.R., Pirraglia E., Tsui W., Rusinek H., Vallabhajosula S., Mosconi L., et al. Periodontal disease associates with higher brain amyloid load in normal elderly. Neurobiol. Aging. 2015;36(2):627–633. doi: 10.1016/j.neurobiolaging.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]