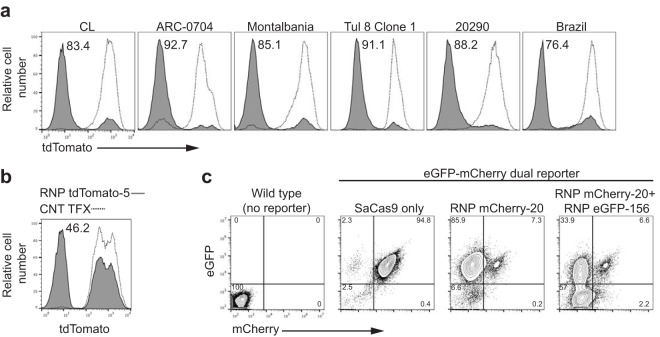
FIG 3 .
SaCas9/sgRNA-mediated gene knockout in multiple strains and life cycle stages of T. cruzi. (a) Epimastigotes of different T. cruzi strains, each expressing tdTomato, were electroporated with RNP complexes containing SaCas9 and sgRNA tdTomato-5, targeting tdTomato (gray filled). Negative controls were electroporated with water (dashed lines). Loss of tdTomato fluorescence was determined 7 days posttransfection by flow cytometry. (b) tdTomato-expressing trypomastigotes from T. cruzi strain CL were electroporated in the presence of either water (dashed line) or SaCas9/sgRNA tdTomato-5, targeting tdTomato (gray filled), and then incubated in flasks with a nearly confluent monolayer of noninfected Vero cells. Twenty-four hours after electroporation, the flasks containing Vero cells and trypomastigotes were washed to eliminate the parasites that had not adhered or invaded the host cells. Seven days posttransfection, trypomastigotes were harvested and loss of tdTomato fluorescence was determined by flow cytometry. (c) T. cruzi epimastigotes expressing mCherry and eGFP were electroporated with SaCas9 protein alone or with RNP complexes targeting only mCherry (>90% efficiency) or both mCherry and eGFP (~90% mCherry knockout and 55% double knockout). All experiments were replicated 2 times with similar results.