Abstract
Surgeons have cited difficulties in identifying the parathyroid glands (PG) during thyroidectomy. To overcome the limitation of naked eye, many studies on near-infrared fluorescence imaging of PGs have been introduced and suggested that fluorescence imaging is useful for both localizing PGs and evaluating their function. This imaging technique has been reported in two ways: (I) imaging using a fluorescent material called indocyanine green (ICG); and (II) autofluorescence using intrinsic fluorophores. These innovative and novel techniques are expected to have a significant impact on performing thyroid or parathyroid surgery. In this article, current papers that describe ICG fluorescence and autofluorescence imaging of PG during thyroid and parathyroid surgery are reviewed.
Keywords: Parathyroid gland (PG), autofluorescence, near-infrared
Introduction
Thyroidectomy is one of the most frequently performed endocrine operations. As the prognosis of patients with well-differentiated thyroid cancer is excellent, preventing or minimizing post-thyroidectomy complications such as recurrent laryngeal nerve (RLN) injury or parathyroid gland (PG) hypofunction remains challenges for surgeons (1,2). PG is the critical structure to be preserved very carefully to reduce the postoperative complications in every thyroid surgery. This needs careful dissection as well as early exact identification. Though some techniques were introduced, they were not often widely accepted. So far, visual inspection and palpation, which can be gained through experience, have been the major tools of surgeons. Recently, biomedical engineering techniques have been studied to overcome the limitations of the subjective identification and reduce the incidence of postoperative hypocalcemia.
Basically, the first step to preserve PG and prevent hypofunction of this organ is proper identification. However, PGs are very small and embedded within paratracheal fat tissues, and have a color similar to surrounding tissues. Therefore, it may not be easy even for experienced surgeons to find out all PGs with the naked eye during surgery (3-5). Thus, various methods have been studied to localize PGs during surgery. Ultrasonography and Sestamibi scan have been used for hyperparathyroidism, with sensitivities of 69–75% and 49–70%, respectively (6). However, these imaging techniques are not useful for identifying a normal PG. Intraoperative frozen section examination is a definitive method to confirm the parathyroid tissue histologically, but this method can cause vascular damage by removing a part of PG and is associated with additional cost and waiting time (7). In 1971, methylene blue was introduced for the identification of diseased and normal PGs (8). However, the surgical outcome did not show improved results compared to visual identification by the operators. Furthermore, the technique can induce side effects from the dye or injection procedure, such as neurotoxicity (9,10). Though aminolevulinic acid, a photosensitizer that accumulates in the PG, was used in 2006, the results were not constant (50% failure rate) and it needed isolation of the patient from light for 48 hours (11). Radio-guided techniques using 99 m technetium-methoxyisobutylisonitrile have been described to identify PG. This method was not useful and was associated with severe radiation exposure (12). Recently, Hyun et al. (13) reported that certain synthetic fluorophores are useful for identifying PGs in animal models. However, these exogenous agents require intravenous injection and the feasibility of using these fluorophores in human thyroid surgery has not yet been verified. Despite the descriptions of these various techniques for identifying PGs, most of these methods are not currently useful and are not actually applied to clinical practice.
Recently, some innovative techniques with the use of near-infrared (NIR) fluorescence, which may overcome the current limitations in the parathyroid localization, were reported. In this article, we review the novel emerging technologies with ICG fluorescence imaging and autofluorescence imaging of PG with NIR.
Indocyanine green (ICG) fluorescence imaging of PG
ICG in medical diagnostics
NIR fluorescence has been used in intraoperative angiography, extrahepatic cholangiography, coronary artery bypass graft, intestinal reanastomosis, lymph node mapping (14). NIR wavelengths are attractive in biomedicine because of the high penetration depth, low scattering and absorption in the tissue. The clinical applications of NIR fluorescence have mainly been performed by exogenous contrast agents, mostly by ICG (15). Recently, application of the ICG has expanded to the imaging of parathyroid localization (2,14). Identification of PGs by fluorescence is a newly introduced field combining medicine and optics.
ICG was approved by the FDA in 1959 and has been used as a useful contrast agent. ICG is a water soluble anionic amphiphilic tricarbocyanine dye. When injected, it binds tightly to lipoproteins and is trapped in the vascular space, which allows it to act a real-time intravascular contrast agent. ICG is taken up by hepatocytes and excreted into the bile (15-18). Incidence of serious adverse events with ICG injection in a large series was reported at a rate of 0.05%. As ICG has well known safety profiles, it has become increasingly popular as an exogenous contrast dye (19) (Figure 1).
Figure 1.
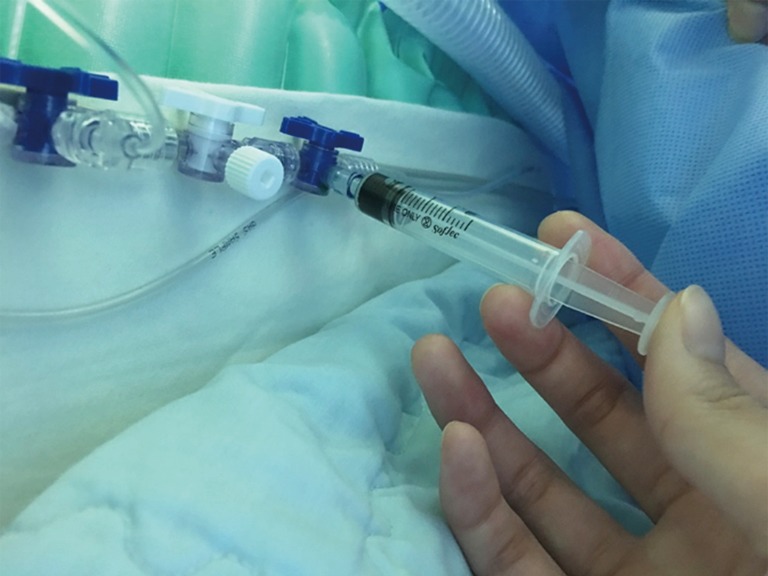
Preparation of indocyanine green. ICG was mixed with 10 mL of sterile water, and 3–5 mL was administrated intravenously. The injection can be repeated until a maximum dose of 5 mg/kg/day is reached. This figure was kindly provided by courtesy of Professor Yoon Woo Koh, Department of Otolaryngology-Head and Neck Surgery of Yonsei University, Korea. ICG, indocyanine green.
ICG fluorescence imaging of normal PGs
Distinguishing PGs from surrounding tissues, such as thyroid gland, fat, and connective tissues has been a challenge for surgeons. For less experienced surgeons, it is always tough to identify and preserve the gland. Even for highly-experienced surgeons, inadvertent parathyroid excision has been reported to be up to 15% of thyroidectomy cases. Moreover, the incidence of temporary hypoparathyroidism is up to 46% and that of permanent hypoparathyroidism is up to 6.6% after total thyroidectomy (3,20).
In 2015, Suh et al. (2) reported the first pre-clinical use of ICG and NIR fluorescent imaging for visualization of PGs in dogs. They described the response of fluorescence intensity according to ICG dose and showed that the method could determine the optimal concentration of ICG to visualize PGs in dogs. They proved that ICG NIR fluorescent imaging could visualize PGs and concluded that intraoperative fluorescence imaging can be used to facilitate parathyroid preservation during thyroidectomy.
Angiography with the fluorescent dye ICG has been performed in patients undergoing thyroid surgery to visualize the vascularity of the identified PGs (21,22). NIR imaging was conducted after administration of 5–8.75 mg of ICG using laparoscopic PINPOINT® system (Novadaq Technologies, Inc.). The results showed that most of the visually identified PGs had ICG uptake. Patients who had at least one PG with good perfusion maintained sufficient function after thyroid surgery. Predicting function of PGs after surgery was easier with ICG uptake than with visual evaluation. ICG angiography allows direct assessment of the PG feeding vessels that are at risk of damage during surgery. It can help make decisions if the PG needs autotransplantation.
Subsequently, Yu et al. (23) presented the integration of Firefly® technology (Intuitive Surgical, Inc.), namely intraoperative NIR imaging of ICG, into the da Vinci Si robot system to help identify and preserve PGs safely. Lang et al. (24) showed that ICG fluorescence angiography is a useful adjunct to determine the residual parathyroid function after total parathyroidectomy and to predict postoperative hypoparathyroidism risk. The SPY® Fluorescent Imaging System (Novadaq Technologies, Inc.) was used for NIR imaging. They demonstrated the relationship between the greatest and overall average fluorescence intensity of ICG in the remaining PGs and the risk of postoperative hypocalcemia after total thyroidectomy.
Although the results of ICG fluorescence imaging of normal PGs were promising, limitations were found. As PGs lie in close relation to thyroid glands, their discrimination was not clear because ICG was also absorbed by the thyroid gland. In addition, increasing ICG uptake in well vascularized non-parathyroid tissue, such as thyroid, thymus, and lymph nodes, can mislead in the interpretation of the vascularity of PG (Figure 2).
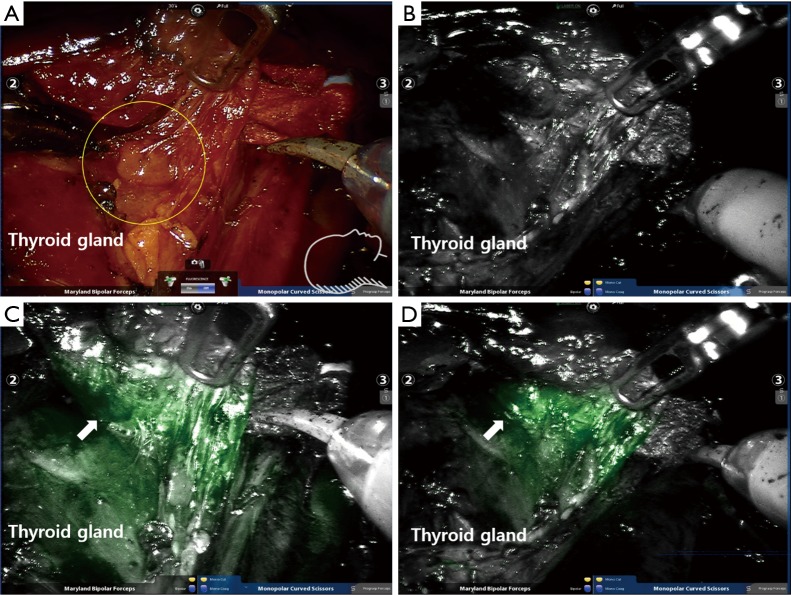
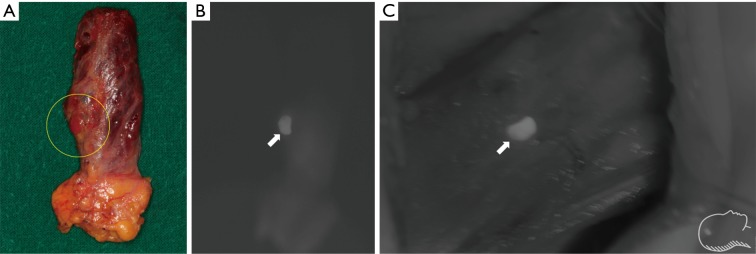
Figure 2.
Intraoperative NIR ICG images showing identification of normal parathyroid gland during retroauricular robotic thyroidectomy (DaVinci Si system, near infrared camera). (A) A right inferior parathyroid gland observed by naked eye under white light imaging (circle); (B) ahead of administration of ICG. The parathyroid gland was not clearly distinguishable with NIR mode imaging; (C) post-administration of ICG. The fluorescence was observed from the both parathyroid gland (arrow) and thyroid gland; (D) the intensity of the parathyroid gland (arrow) appeared to be higher. Distinction between the parathyroid gland and thyroid became clear as the parathyroid gland was separated from the thyroid gland. These figures were kindly provided by courtesy of Professor Yoon Woo Koh, Department of Otolaryngology-Head and Neck Surgery of Yonsei University, Korea. NIR, near-infrared; ICG, indocyanine green.
ICG fluorescence of parathyroid adenoma and hyperplasia
The use of ICG for intraoperative PG localization during reoperative parathyroid surgery in humans was reported in 2015 (25,26). In these studies, the ICG dose was 5 mg per injection with a total dose up to 10 mg per patient, fluorescence was first observed 2 min after ICG administration and was reported to last up to 20 min. ICG uptake was found in 93% of the PGs recognized by the naked eye. ICG NIR imaging guided surgery was relatively useful in those PGs separated from the thyroid gland and in reoperative surgery after thyroidectomy. ICG NIR imaging may be an assessment tool for residual PGs perfusion.
Other group reported the results on the usage of ICG in subtotal parathyroidectomy (27). The aim of the study was to evaluate the vascularity of the parathyroid remnant by intraoperative ICG angiography and to determine whether it could predict postoperative remnant function. In this study, all four glands were exposed, followed by ICG angiography, and the gland to be preserved was selected according to the degree of perfusion. As a result, all 13 patients were able to maintain normal postoperative parathyroid hormone levels, which suggest that there is a good correlation between PG perfusion and postoperative function. This technique could be applied to predict postoperative function by intraoperative evaluation of PG vascularization (Figure 3).
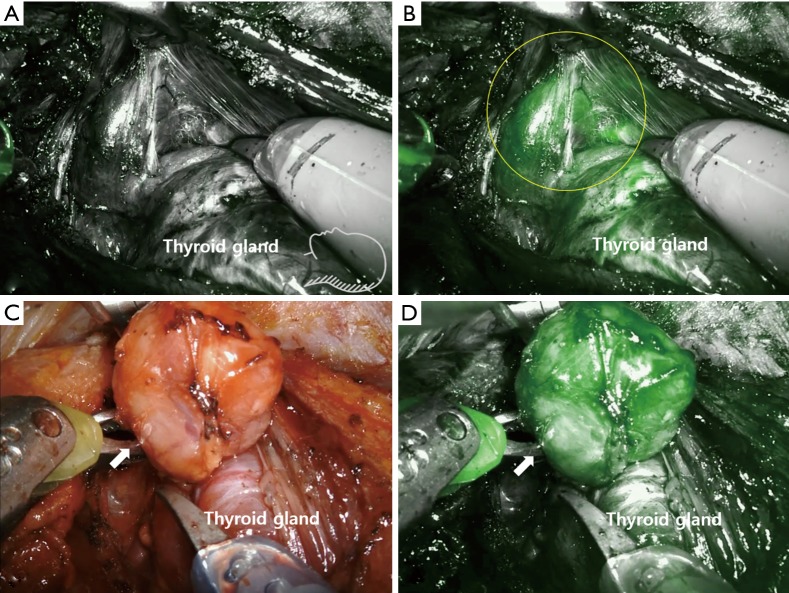
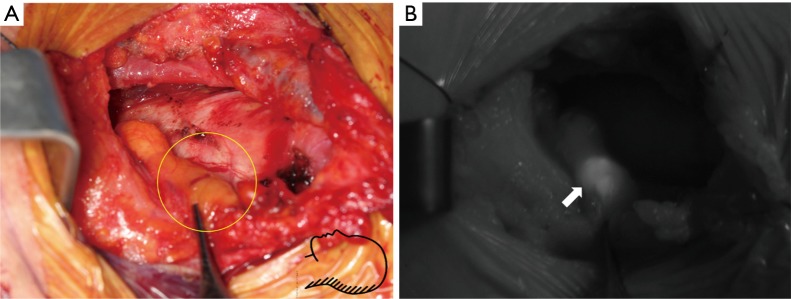
Figure 3.
NIR ICG imaging of the parathyroid adenoma during retroauricular robotic thyroidectomy (DaVinci Si system, near infrared camera). (A) Photo taken with near infrared mode light before administrating ICG; (B) the image after ICG injection. Both parathyroid (circle) and thyroid gland were stained. The parathyroid gland (arrow) was dissected with naked eye (C) and localized with ICG fluorescence imaging (D). These figures were kindly provided by courtesy of Professor Yoon Woo Koh, Department of Otolaryngology-Head and Neck Surgery of Yonsei University, Korea. NIR, near-infrared; ICG, indocyanine green.
Autofluorescence imaging of PG
Autofluorescence is the natural emission of light by intrinsic fluorophores. The term is used to distinguish the light originating from artificially added fluorescent markers (exogenous contrast dye) such as ICG. The application of this phenomenon to visualize PGs using NIR wavelength during thyroid or parathyroid surgery is the latest technique in the field. Generally, autofluorescence property occurs in the visible wavelength spectrum. However, in 2011, Paras et al. (28) suggested that PG has a fluorescent property in the NIR region and emits NIR light at a peak wavelength of 820 nm when it is illuminated with light at 785 nm. They demonstrated that the PG had the strongest autofluorescence intensity at 820 nm followed by low intensity of the thyroid gland. There was no substantial autofluorescence in the surrounding fat, muscle, and trachea. The results suggested that PGs can be distinguished from the surrounding tissues using this property (7,28).
The mechanism of autofluorescence is still unclear. However, the hypothesis is that autofluorescence is derived from calcium-sensing receptor protein which is present at highest concentration in the chief cells of PG, at lower concentration in the thyroid, and absent in other tissues of the neck (29). There is no noticeable difference in the intensity of autofluorescence between healthy and diseased PG except for secondary hyperparathyroidism which has decreased intensity due to down regulation of calcium sensing receptor (30).
Near-infrared autofluorescence imaging for the detection of PG
The imaging of PGs autofluorescence in human thyroidectomy patients was reported for the first time by McWade et al. (31) in 2014. The modified NIR fluorescence imaging system (Karl Storz, PDD camera) was used. The NIR image was taken by attaching a long-pass 808 nm filter in front of the camera, which blocked excitation light from the 785 nm diode laser while allowing passage of the emitted NIR light. Intraoperative imaging of PG was performed on six patients (three patients diagnosed with primary hyperparathyroidism, two with nontoxic nodular goiters, and one with papillary thyroid cancer), and the mean time for imaging capture was 6 minutes. The autofluorescence imaging was successfully detected in 100% of the PGs regardless of disease state. The overlaid image of parathyroid fluorescence on a white light image of actual surgical area clearly distinguished the PG from the surrounding tissues. Fluorescence intensity emitted from the PGs was 2.4 to 8.5 times higher than the surrounding tissues, such as the fat, muscle, thyroid, nerve, and trachea. This suggested that the intraoperative NIR autofluorescence imaging technique has the potential of imaging-guided thyroid or parathyroid surgery for identification of PGs in real time.
The same group also demonstrated the possibility of NIR fluorescence spectroscopy system for the intraoperative autofluorescence based optical guidance for PG detection. NIR fluorescence spectroscopy system was used to collect NIR autofluorescence spectra of 264 PGs from 137 patients. While the PG NIR autofluorescence intensity was higher than the surrounding tissue in 97% of PGs examined, the thyroid gland showed weak fluorescence. There was no fluorescence in the muscle, fat, lymph nodes, thymus, and trachea. NIR fluorescence spectroscopy showed the high accuracy (94–100%) for various disease states. The technique was capable of reliable real-time PGs detection during surgery regardless of clinical and demographic characteristics of the patient. However, it should be taken account that decreased autofluorescence in secondary hyperparathyroidism due to down regulation of calcium sensing receptor (30).
As described, based on the NIR induced PGs autofluorescence, the first real-time intraoperative autofluorescence imaging of PG from human thyroidectomy patients without using any contrast dye was reported (31). Though it was an excellent study, it was hard to know the exact location of PGs on the actual surgical field with NIR imaging only because there was no information about surrounding tissues on the image. Only PGs was visualized without showing the surrounding tissue, which means that this imaging technique has limitations in anatomical guidance in thyroidectomy. To improve this shortcoming, we developed a new imaging system with 780 nm NIR light to excite the PG and infrared light to visualize the surrounding tissues simultaneously.
In 2016, we published the result of a prospective study on a total of 16 PGs from eight patients who underwent surgery for thyroid cancer (32). In this study, our new technique was introduced for the immediate visualization of both PGs and whole surrounding tissues in a single image at the experimental level. Collimated LED (Thorlabs, Newton, NJ, USA) with an excitation filter appropriate for PG autofluorescence, an illuminator (INFRALUX-300, Daekyoung, Korea) for reflection of the entire surgical area, and a digital single-lens reflex camera (Canon, EOS REBEL T3, Japan) with camera lens (Canon EF 50 mm f/1.8 II, Japan) including emission filter were used to obtain image (Figure 4). Using our technique, all 16 PGs were detected (positive predictive value =100%). In addition, whole surgical field could be visualized together with PGs simultaneously. Moreover, the PGs that were exposed or even covered by connective tissues or blood vessels could be detected due to intense emission. Though an infrared camera is necessary to detect NIR wavelength light, there is no cost disadvantage in this tool since a commercially available digital camera was used instead (Figures 5-7).
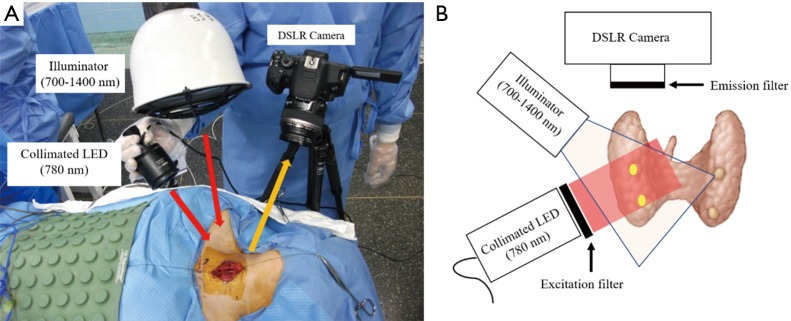
Figure 4.
Images showing how to take a picture. (A) A photo of equipment setting in the operating room; (B) schematic diagram for detecting autofluorescence of parathyroid tissue with simultaneous illumination of the background tissues.
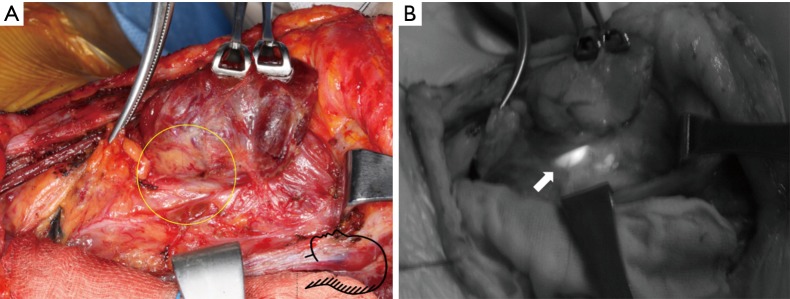
Figure 5.
Images for detecting left inferior parathyroidectomy during conventional open thyroidectomy. (A) White light image after thyroid lobe lateralization. Inferior parathyroid gland is expected to be in the circle; (B) autofluorescence with NIR illuminator image. Autofluorescence emission (arrow) coincided with inferior parathyroid gland. Whole surgical field could be visualized by NIR illumination. NIR, near-infrared;
Figure 6.
Images for detecting left inferior parathyroidectomy during conventional open thyroidectomy. (A) White light image of thyroidectomy and central neck dissection specimen. Detection of a removed parathyroid gland from the thyroidectomy specimen (circle); (B) the parathyroid gland showed autofluorescence (arrow) even when it was removed and devascularized; (C) preparation for autotransplantation of parathyroid gland (arrow) to right sternocleidomastoid muscle.
Figure 7.
Images showing unusual location of parathyroid tissue. (A) A suspicious intrathymic parathyroid gland (circle); (B) localization of intrathymic parathyroid gland by NIR autofluorescence imaging (arrow).
While the new innovative studies were introduced, several groups reported on the intraoperative PG autofluorescence imaging using commercially available medical devices. In 2016, Falco et al. (33) reported intraoperative PG autofluorescence imaging methods using Fluobeam® (Fluoptics, Grenoble, France) in 28 patients with various thyroid and parathyroid disease. They showed the equipment can be used to identify parathyroid tissues. In the same year, De Leeuw et al. (34) reported autofluorescence imaging results of Fluobeam® under the supervision a pathologist for ex vivo specimens and a surgeon in vivo during surgery. The resected specimens were evaluated by NIR imaging to determine if they included any parathyroid tissue. The positive imaging tissues were sampled and confirmed by histology. Sixteen out of 17 PGs from 28 patients were detected by NIR imaging with a sensitivity of 94.1% and a specificity of 80% in ex vivo analysis. The normalized fluorescence intensity of the PGs was on average 2.91 times greater than thyroid fluorescence, which was a similar result to those of previous reports. Moreover, autofluorescence remains stable even after one hour of resection or after formalin fixation of the tissue, which indicates that PG autofluorescence is not affected by blood flow or formalin fixation. In this study, intraoperative NIR imaging was also performed on 35 patients and a total of 81 PGs were identified by the naked eye with high confidence. The normalized fluorescence intensity of the PGs was an average of 2.93 times greater than that of thyroid fluorescence with a sensitivity of 98.8%. Although the Fluobeam® was not specifically developed for PG imaging, their coverage could be extended to thyroid and parathyroid surgery.
Ladurner et al. (35) described a study using a commercially available NIR/ICG endoscopic system (KARL STORZ, Tuttlingen, Germany) modified by interposing an additional long-pass filter. This system used a xenon light source. It can switch between white light and NIR light and emits very little green and red light to visualize the surrounding tissues in the NIR mode. Of the 42 PGs in total, 34 showed autofluorescence in the exposed state, while the remaining eight cases were unable to be visualized because these glands were embedded in adipose tissue.
As Kim et al. (32), Falco et al. (33), De Leeuw et al. (34), and Ladurner et al. (35) reported the visualization of both PG and surrounding tissues with experimental or commercialized NIR instruments at the almost same period in 2016, the authors may have considered that their reports were for the first time. Although the biological background for intrinsic fluorophores responsible for autofluorescence has not yet been proven, clinical application could be extended to more various clinical fields. Turning off the operation room light to get NIR autofluorescence images is one of the limitations. In addition, as NIR penetrates just several millimeters in depth, it is difficult to observe autofluorescence from deep seated PGs. Evaluation of perfusion of PG with autofluorescence is not well studied. We expect technical advances can overcome the limitations in the near future.
Conclusions
NIR fluorescence imaging technique with or without exogenous dye during thyroid and parathyroid surgery is a newly introduced area and will lead surgeons to predict location of PGs during surgery in real time. In particular, autofluorescence imaging with totally safe NIR light is a non-invasive, safe, and newly emerging technique. We believe this technology can help the surgeons characterize the proper dissection plane to minimize parathyroid damage.
Acknowledgements
Funding: This study was supported by grants from Marine Biotechnology Program [20150220] funded by Ministry of Oceans and Fisheries, Korea.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Hundahl SA, Fleming ID, Fremgen AM, et al. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the US, 1985-1995. Cancer 1998;83:2638-48. [DOI] [PubMed] [Google Scholar]
- 2.Suh YJ, Choi JY, Chai YJ, et al. Indocyanine green as a near-infrared fluorescent agent for identifying parathyroid glands during thyroid surgery in dogs. Surg Endosc 2015;29:2811-7. 10.1007/s00464-014-3971-2 [DOI] [PubMed] [Google Scholar]
- 3.Lee NJ, Blakey JD, Bhuta S, et al. Unintentional parathyroidectomy during thyroidectomy. Laryngoscope 1999;109:1238-40. 10.1097/00005537-199908000-00010 [DOI] [PubMed] [Google Scholar]
- 4.Lin DT, Patel SG, Shaha AR, et al. Incidence of inadvertent parathyroid removal during thyroidectomy. Laryngoscope 2002;112:608-11. 10.1097/00005537-200204000-00003 [DOI] [PubMed] [Google Scholar]
- 5.Sasson AR, Pingpank JF, Jr, Wetherington RW, et al. Incidental parathyroidectomy during thyroid surgery does not cause transient symptomatic hypocalcemia. Arch Otolaryngol Head Neck Surg 2001;127:304-8. 10.1001/archotol.127.3.304 [DOI] [PubMed] [Google Scholar]
- 6.Siperstein A, Berber E, Barbosa GF, et al. Predicting the success of limited exploration for primary hyperparathyroidism using ultrasound, sestamibi, and intraoperative parathyroid hormone: analysis of 1158 cases. Ann Surg 2008;248:420-8. [DOI] [PubMed] [Google Scholar]
- 7.McWade MA, Paras C, White LM, et al. A novel optical approach to intraoperative detection of parathyroid glands. Surgery 2013;154:1371-7. 10.1016/j.surg.2013.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley NE. Methylene blue for rapid identification of the parathyroids. Br Med J 1971;3:680-1. 10.1136/bmj.3.5776.680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel HP, Chadwick DR, Harrison BJ, et al. Systematic review of intravenous methylene blue in parathyroid surgery. Br J Surg 2012;99:1345-51. 10.1002/bjs.8814 [DOI] [PubMed] [Google Scholar]
- 10.Tummers QR, Schepers A, Hamming JF, et al. Intraoperative guidance in parathyroid surgery using near-infrared fluorescence imaging and low-dose Methylene Blue. Surgery 2015;158:1323-30. 10.1016/j.surg.2015.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prosst RL, Gahlen J, Schnuelle P, et al. Fluorescence-guided minimally invasive parathyroidectomy: a novel surgical therapy for secondary hyperparathyroidism. Am J Kidney Dis 2006;48:327-31. 10.1053/j.ajkd.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 12.Grubbs EG, Mittendorf EA, Perrier ND, et al. Gamma probe identification of normal parathyroid glands during central neck surgery can facilitate parathyroid preservation. Am J Surg 2008;196:931-5; discussion 935-6. 10.1016/j.amjsurg.2008.07.026 [DOI] [PubMed] [Google Scholar]
- 13.Hyun H, Park MH, Owens EA, et al. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat Med 2015;21:192-7. 10.1038/nm.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marshall MV, Rasmussen JC, Tan IC, et al. Near-infrared fluorescence imaging in humans with indocyanine green: a review and update. Open Surg Oncol J 2010;2:12-5. 10.2174/1876504101002020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol 2003;7:626-34. 10.1016/j.cbpa.2003.08.007 [DOI] [PubMed] [Google Scholar]
- 16.Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox I, Wood E. Indocyanine green: physical and physiologic properties. Proceedings of the staff meetings. Mayo Clinic, 1960. [PubMed] [Google Scholar]
- 18.Miwa M. The principle of ICG fluorescence method. Open Surg Oncol J 2010;2:26-8. 10.2174/1876504101002020026 [DOI] [Google Scholar]
- 19.Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due to indocyanine green. Ophthalmology 1994;101:529-33. 10.1016/S0161-6420(94)31303-0 [DOI] [PubMed] [Google Scholar]
- 20.Ahn D, Sohn JH, Kim JH, et al. Inadvertent parathyroidectomy during thyroid surgery for papillary thyroid carcinoma and postoperative hypocalcemia. J Korean Thyroid Assoc 2012;5:65-72. 10.11106/jkta.2012.5.1.65 [DOI] [Google Scholar]
- 21.Vidal Fortuny J, Belfontali V, Sadowski S, et al. Parathyroid gland angiography with indocyanine green fluorescence to predict parathyroid function after thyroid surgery. Br J Surg 2016;103:537-43. 10.1002/bjs.10101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaidi N, Bucak E, Yazici P, et al. The feasibility of indocyanine green fluorescence imaging for identifying and assessing the perfusion of parathyroid glands during total thyroidectomy. J Surg Oncol 2016;113:775-8. 10.1002/jso.24237 [DOI] [PubMed] [Google Scholar]
- 23.Yu HW, Chung JW, Yi JW, et al. Intraoperative localization of the parathyroid glands with indocyanine green and Firefly (R) technology during BABA robotic thyroidectomy. Surg Endosc 2017;31:3020-7. 10.1007/s00464-016-5330-y [DOI] [PubMed] [Google Scholar]
- 24.Lang BH, Wong CK, Hung HT, et al. Indocyanine green fluorescence angiography for quantitative evaluation of in situ parathyroid gland perfusion and function after total thyroidectomy. Surgery 2017;161:87-95. 10.1016/j.surg.2016.03.037 [DOI] [PubMed] [Google Scholar]
- 25.Sound S, Okoh A, Yigitbas H, et al. Utility of indocyanine green fluorescence imaging for intraoperative localization in reoperative parathyroid surgery. Surg Innov 2015. [Epub ahead of print]. [DOI] [PubMed]
- 26.Zaidi N, Bucak E, Okoh A, et al. The utility of indocyanine green near infrared fluorescent imaging in the identification of parathyroid glands during surgery for primary hyperparathyroidism. J Surg Oncol 2016;113:771-4. 10.1002/jso.24240 [DOI] [PubMed] [Google Scholar]
- 27.Vidal Fortuny J, Sadowski SM, Belfontali V, et al. Indocyanine Green Angiography in Subtotal Parathyroidectomy: Technique for the Function of the Parathyroid Remnant. J Am Coll Surg 2016;223:e43-e49. 10.1016/j.jamcollsurg.2016.08.540 [DOI] [PubMed] [Google Scholar]
- 28.Paras C, Keller M, White L, et al. Near-infrared autofluorescence for the detection of parathyroid glands. J Biomed Opt 2011;16:067012. 10.1117/1.3583571 [DOI] [PubMed] [Google Scholar]
- 29.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev 2001;81:239-97. [DOI] [PubMed] [Google Scholar]
- 30.McWade MA, Sanders ME, Broome JT, et al. Establishing the clinical utility of autofluorescence spectroscopy for parathyroid detection. Surgery 2016;159:193-202. 10.1016/j.surg.2015.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McWade MA, Paras C, White LM, et al. Label-free intraoperative parathyroid localization with near-infrared autofluorescence imaging. J Clin Endocrinol Metab 2014;99:4574-80. 10.1210/jc.2014-2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim SW, Song SH, Lee HS, et al. Intraoperative Real-Time Localization of Normal Parathyroid Glands With Autofluorescence Imaging. J Clin Endocrinol Metab 2016;101:4646-52. 10.1210/jc.2016-2558 [DOI] [PubMed] [Google Scholar]
- 33.Falco J, Dip F, Quadri P, et al. Cutting Edge in Thyroid Surgery: Autofluorescence of Parathyroid Glands. J Am Coll Surg 2016;223:374-80. 10.1016/j.jamcollsurg.2016.04.049 [DOI] [PubMed] [Google Scholar]
- 34.De Leeuw F, Breuskin I, Abbaci M, et al. Intraoperative Near-infrared Imaging for Parathyroid Gland Identification by Auto-fluorescence: A Feasibility Study. World J Surg 2016;40:2131-8. 10.1007/s00268-016-3571-5 [DOI] [PubMed] [Google Scholar]
- 35.Ladurner R, Sommerey S, Al Arabi N, et al. Intraoperative near-infrared autofluorescence imaging of parathyroid glands. Surg Endosc 2017;31:3140-5. 10.1007/s00464-016-5338-3 [DOI] [PubMed] [Google Scholar]