Key Clinical Message
Diagnosis of hemophagocytic syndrome remains a challenge in particular during pregnancy. Concomitant presence of clinical and biological signs, for example, fever, pancytopenia, hyperferritinemia, and hypertriglyceridemia, should alert clinicians to suspect HPS and proceed to prompt treatments.
Keywords: Case report, diagnosis, hemophagocytic syndrome, oocyte donation, pregnancy
Introduction
Macrophage activating syndrome is also called hemophagocytic syndrome (HPS). It may be primary as in familial hemophagocytic lymphohistiocytosis (HLH), Chediak–Higashi, Griscelli, and Purtilo syndromes, or secondary as in case of malignancies hemato‐oncology, oncologic diseases, infectious diseases, or autoimmune disease. HPS is rare but underdiagnosed and can be life‐threatening if undiagnosed. Its incidence is estimated between 0.8% and 4% cases per year including pediatric and adult HPS 1.
Hemophagocytic syndrome is known since the 1950s, and further described by Risdall et al. 2. The pathophysiology consists of activation of T lymphocytes and natural killer cells (HLH), either secondary to an opportunistic infection, or primary due to a deficiency of immunomodulatory mechanisms.
This HLH immune activation leads to a high production of pro‐inflammatory cytokines. These cytokines activate the monocyte–macrophage system and enhance the HLH in a positive feedback 3, 4, 5, 6, 7.
These macrophages are responsible for hemophagocytosis expressed clinically by various symptoms such as fever, lymphadenopathy, hepatosplenomegaly. Clinical presentation of these signs strongly suggests HPS.
Hemophagocytic syndrome occurrence in pregnancy is rare and there are only a few reported cases in the literature 5, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19. Here, we describe a case of HPS during the third trimester (30 weeks of gestational age (GA)) of pregnancy: its diagnosis, treatment, and fetal and maternal outcomes. This case is further compared with the literature to set forth a proposal for advancement of best clinical practices in HPS during pregnancy.
Methods
We present here the case of a primigravida 44‐year‐old woman who presented at 30 weeks GA + 4 days to the emergency room for fever of 39.4°C associated with a cough since 15 days. This patient had a history of primary infertility salpingectomy for hydrosalpinx. Raynaud syndrome with positive antinuclear antibodies (e.g., antiribonucleoproteins) and moderate peripheral thrombocytopenia have been diagnosed since 2 years. Antiphospholipid antibodies were negative. The pregnancy was achieved by in vitro fertilization with oocyte donation. Despite abnormal (i.e., dark circles on the legs and arms) skin pigmentation early in pregnancy, the diagnosis of lupus (i.e., before pregnancy onset, she has been followed up for thrombocytopenia and suspicion of autoimmune disease) or Sharp syndrome (mixed connective tissue disease) could not be confirmed. Nevertheless, given the suspicion of autoimmune disease, aspirin 75 mg/day was started.
At the emergency room, the patient presented with fever of 39.4°C, blood pressure at 99/62 mmHg, heart rate at 121/min, and oxygen saturation at 98% on room air. There was no history of infection or recent travel. The chest radiography showed some pulmonary infiltrates. Other clinical examinations were normal except for the presence of submandibular adenopathy. Blood biology workup showed moderate pancytopenia and inflammatory syndrome (Table 1). The fetal heart rate recording showed tachycardia (i.e., due to the high fever, 170 beats per min). As a result, the patient was hospitalized in gynecology–obstetrics unit (Fig. 1). Intravenous antibiotic (amoxicillin 1 g tid) was started and the baseline laboratory workups (urinary and blood bacteriological analyses) were negative.
Table 1.
Laboratory trends from baseline to Day 9
| D0 | D2 | D3 | D4 | D9 | |
|---|---|---|---|---|---|
| Temperature (°C) | 39.4 | 38.2 | – | 36.1 | 37 |
| Hemoglobin (g/dL) | 8.4 | 8.6 | 9.3 | 7.9 | 9 |
| Platelets (Giga/L) | 130 | 107 | 103 | 85 | 74 |
| Leukocytes (Giga/L) | 3.4 | 2 | 2.1 | – | 3.1 |
| Lymphocytes (Giga/L) | – | 0.37 | 0.58 | – | 0.73 |
| Polynuclear neutrophils (Giga/L) | – | 1.42 | 1.3 | 0.6 | 2.11 |
| C‐reactive Protein (mg/L) | 64 | 90.6 | 121 | 160 | 47 |
| ALAT (UI/L) | – | – | – | 51 | – |
| ASAT (UI/L) | – | – | – | 106 | – |
| Haptoglobin (g/L) | – | – | – | <0.1 | – |
| LDH (UI/L) | – | – | – | 1520 | – |
| Fibrinogen (g/L) | 4.45 | 4.45 | 4.89 | 4.89 | – |
| Cephalin clotting time | – | – | – | 1.34 | – |
| Kaolin clotting time | – | – | – | 1.16 | – |
| Triglyceridemia (mg/dL) | – | – | – | 2.85 | – |
| Ferritinemia (μg/L) | – | – | – | 1373 | 498 |
| Potassium (mmol/L) | – | – | – | 3.4 | – |
| Proteinuria (g/L) | – | – | – | 0.45 | – |
Figure 1.
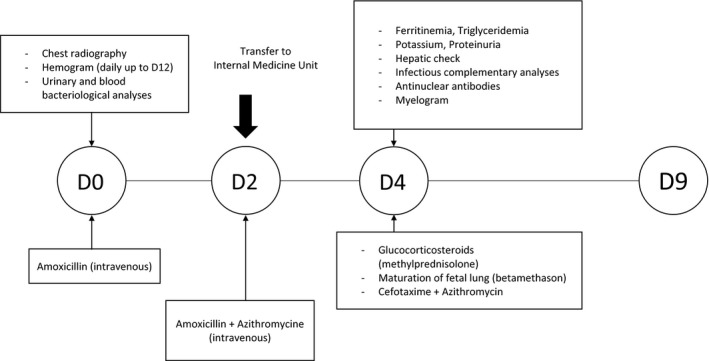
The investigative approaches and initial treatments.
Within a few hours of antimicrobial therapy, there was an improvement of pulmonary symptoms, yet a deterioration of pancytopenia.
Upon patient's arrival to the internal medicine unit, a bi‐antimicrobial therapy with azithromycin and amoxicillin was started. After 48 h of treatment, new biological deteriorations were observed (Table 1): moderate hepatic cytolysis, cholestasis, hemolysis, low potassium, hypertriglyceridemia, hyperferritinemia, inflammatory syndrome, elevated proteinuria (with normal blood pressure), and deterioration of pancytopenia.
Additional laboratory workup in search for antinuclear antibodies showed very slight amount (one positive antinuclear antibodies reading out of 320). All infectious explorations were negative (blood culture, cytobacteriological examination of urine, parvovirus B 19 serology, and PCR, searching for pneumococcus, legionella, mycoplasma pneumoniae, and chlamydiae, EBV, HCV, TPHA, and VDRL, and HIV serology, and tuberculosis). The patient had immunity against CMV, rubella, and toxoplasmosis.
Deterioration of liver function warranted an abdominal ultrasound which showed isolated and moderate hepatomegaly.
Hemophagocytic syndrome was suspected given the clinicobiological characteristics associating fever, hepatomegaly, pancytopenia, hyperferritinemia, and hypertriglyceridemia. This diagnosis was promptly confirmed by myelogram (Fig. 2).
Figure 2.
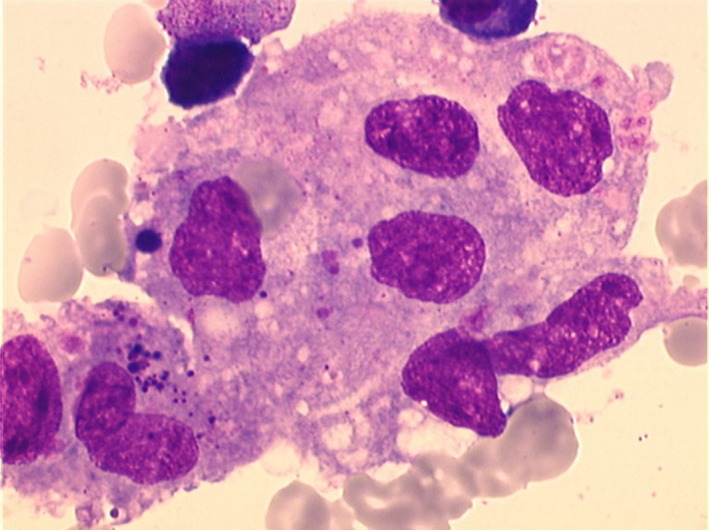
Attached macrophages forming a giant cell with multiple nuclei. Phagocytosis of red blood cells and platelets.
The myelogram did not show abnormal cells such as Sternberg, or osteoblasts, or osteoclasts. Given the HPS confirmation, parenteral glucocorticosteroids (GC) were started (methylprednisolone at 1 mg/kg). Maturation of fetal lungs was achieved. At the same time, antibiotic spectrum was again enlarged and amoxicillin was replaced with cefotaxime. The clinical and biological evolution became promptly satisfactory. Thromboprophylaxis was started.
In obstetric terms, fetal ultrasound monitoring showed intrauterine growth restriction below the 3rd percentile (i.e., fetal weight: 1548 g at 32 GA + 3 days), with normal fetal and maternal vascular ultrasound. At 38 GA + 4 days, because of the low weight for intrauterine growth restriction (i.e. 2258 g ± 15%), gestational diabetes, and detected oligohydramnios, it was decided to induce labor. The patient gave birth at 38 GA + 6 days, to a girl weighing 2380 g (1st percentile) and in good health.
During the postpartum, oral GC prednisolone was continued for 4 weeks at the dosage of 60 mg per day. A decrease in dosage was scheduled at 3 months postpartum. No HPS relapse occurred after discontinuation of GC and no new autoimmune disease symptoms were found during follow‐up. The newborn's 8‐month clinical examination showed a normal growth without any sign of neurological damage.
Results
Tables 2 and 3 display the review of HPS peri‐pregnancy data from the literature and our work.
Table 2.
Displays the results of the comparison between our case and the related literature (first part)
| Authors/[biblio] | Gestational age (weeks) | Maternal age (years) | Known risk factors | Prepartum complications | Clinical signs | Laboratory work up | HPS etiology | Study year |
|---|---|---|---|---|---|---|---|---|
| Gill et al. 11 | 18 | 30 | No | Non | Fever, hepatomegaly | Pancytopenia, cytolysis | Unclear | 1994 |
| Mihara et al. 10 | 16 | 32 | No | Non | Fever | Pancytopenia, hyperferritinemia, markedly elevated LDH | EBV | 1999 |
| Nakabayashi et al. 13 | 21 | ND | No | Preeclampsia, DIVC, IUGR | Fever | Thrombopenia, leukopenia, cytolysis | Unclear | 1999 |
| Chmait et al. 9 | 29 | 24 | No | DIVC | Adenopathy, fever | Pancytopenia, cytolysis | EBV (postmortem diagnosis) | 2000 |
| Yamagushi et al. 17 | 2nd trimester | ND | No | No | Fever, skin lesions | Pancytopenia, hypertriglycemia, hyperferritinemia, cytolysis | HSV | 2005 |
| Pérard et al. 12 | 22 | 28 | Lupus | Preeclampsia | Fever | Pancytopenia, hypertriglycemia, hyperferritinemia | Lupus | 2007 |
| Hahaoka et al. 27 | 23 | 33 | No | Lymphoma diagnosed | Fever, hepatosplenomegaly | Pancytopenia | B‐cell Lymphoma | 2007 |
| Teng et al. 8 | 23 | 28 | No | Transfusion for anemia compensation and dyspnea improvement | Fever, hepatosplenomegaly | Anemia, thrombopenia, hypertriglycemia | Autoimmune hemolytic anemia | 2009 |
| Shukla et al. 28 | 23 | 10 | No | No | Fever, hepatosplenomegaly | Pancytopenia, hypertriglycemia, hyperferritinemia | Unclear | 2011 |
| Arewa et al. 16 | 21 | 31 | No | No | Fever, jaundice, abdominal pain | Pancytopenia | HIV | 2011 |
| Hannebicque Montaigne et al. 5 | 29 | 21 | Mixed connectivitis (lupus, cryoglobulinemia, Gougerot–Sjogren) | ICU transfer at 22 GA due to vascular failure, bilateral PE, at 25 GA | Fever | Pancytopenia, hyperferritinemia, hypertriglycemia | Lupus | 2012 |
| Dunn et al. 14 | 19 | 41 | Still disease | No | Fever, skin lesions | Cytolysis, anemia, leukopenia, hypertriglycemia, hyperferritinemia | Still Disease | 2012 |
| Mayama et al. 19 | 21 | 28 | No | No | Fever | Pancytopenia hyperferritinemia | Parvovirus B 19 | 2014 |
| Tumian et al. 15 | 38 | 35 | No | No | Jaundice | Anemia, thrombopenia, hypertriglycemia, cytolysis | CMV (postmortem diagnosis) | 2015 |
| Samra et al. 18 | 16 | 36 | No | No | Fever, hepatosplenomegaly | Pancytopenia, hyperferritinemia | Unclear | 2015 |
| Current | 30 | 44 | Raynaud syndrome | Autoimmune | Fever, hepatomegaly | Pancytopenia, hyperferritinemia et hypertriglycemia, cytolysis | History of autoimmune disease | 2015 |
ND, Not documented; IUGR, intrauterine growth retardation; DIVC, disseminated intravascular coagulation; PE, pulmonary embolism; GA, gestational age; CMV, cytomegalovirus; HSV, herpes simplex virus; HIV, human immunodeficiency virus; ICU, intensive care unit; EBV, Epstein–Barr virus.
Table 3.
Displays the results of the comparison between our case and the related literature (second part)
| Authors/[biblio] | Prepartum treatments | Mortality risk factors | C‐section yes/no | Neonatal gestational age (weeks) | Neonatal outcome | Maternal outcome | Study year |
|---|---|---|---|---|---|---|---|
| Gill et al. 11 | Ig IV | Anemia + thrombopenia | No | Full‐term | Alive | Alive | 1994 |
| Mihara et al. 10 | Glucocorticoides, Ig IV, aciclovir, gabexate | DIVC, age >30 | No | 35 | Alive | Alive | 1999 |
| Nakabayashi et al. 13 | IgIV | Preeclampsia, DIVC | Yes | 29 | Alive (respiratory distress) | Alive | 1999 |
| Chmait et al. 9 | Ig IV, Aciclovir | DIVC | Yes | 30 | Alive |
Dead multi‐organ failure |
2000 |
| Yamagushi et al. 17 | Glucocorticoides, cyclosporine, aciclovir | Hyperferritinemia | Yes (breech presentation) | 37 | Alive | Alive | 2005 |
| Pérard et al. 12 | Glucocorticoides, IgIV | Anemia + thrombopenia + hyperferritinemia | No | 30 | Alive | Alive (postpartum cerebral hemorrhage) | 2007 |
| Hahaoka et al. 27 | Chemotherapy R‐CHOP, Cell transplantation | Age >30, anemia + thrombopenia | Yes | 28 (fetal distress) | Alive | Alive | 2007 |
| Teng et al. 8 | Glucocorticoides (treatment failure, improvement after birth) | Anemia + thrombopenia | Yes | 29 | Dead (respiratory distress) | Alive | 2009 |
| Shukla et al. 28 | Glucocorticoides, abortion | Anemia + thrombopenia, hyperferritinemia | No | 10 | Spontaneous miscarriage | Alive | 2011 |
| Arewa et al. 16 | Antimalaria, HAART | Age >30, anemia + thrombopenia | No | Full‐term | Alive | Alive | 2011 |
| Hannebicque Montaigne et al. 5 | Ig IV, glucocorticoides | Anemia + thrombopenia + hyperferritinemia | No | 38 | Alive (neuro postnatal follow‐up, MRI visible cerebral anoxia (asphyxial stigmata) | Alive | 2012 |
| Dunn et al. 14 | Glucocorticoides | Age >30 | Yes (IUGR + twin pregnancy) | 30 | Alive | Alive | 2012 |
| Mayama et al. 19 | Glucocorticoides | Hyperferritinemia | No | 38 | Alive | Alive | 2014 |
| Tumian et al. 15 |
Postpartum onset: glucocorticoides IgIV, cyclosporine |
Age >30, DIVC, retard diagnostic | Yes (fetal distress) | 38 | Alive | Dead multi‐organ failure | 2015 |
| Samra et al. 18 | Glucocorticoides | Age >30, hyperferritinemia | No | Full‐term | Alive | Alive | 2015 |
| Current |
Antibiotherapies glucocorticoides |
Age >30, hyperferritinemia | No | 38 | Alive | Alive | 2015 |
IUGR, intrauterine growth retardation; DIVC, disseminated intravascular coagulation; Ig IV, immunoglobulin intravenous; HAART, highly active antiretroviral therapy.
HPS diagnosis: clinical and biological symptoms
Hemophagocytic syndrome diagnosis was carried out taking into account several specific clinical signs, such as fever >38.5°C, splenomegaly, hepatomegaly, lymphadenopathy, pulmonary infiltrates, erythema, purpura, and neurological evidence. Biological abnormalities were pancytopenia, cholestasis and cytolysis, hyperferritinemia, hypertriglyceridemia, hypofibrinogenemia, and increased LDH 4, 7, 20, 21. According to the literature, fever is the most prevalent clinical sign conveying patient to seek medical help, that is, the fever is often associated with pancytopenia and cytolysis 10 (Tables 2 and 3).
HPS diagnostic tools
Gold standard diagnostic tool is myelogram which allows identification of hemophagocytosis. Our patient's myelogram showed rich, infiltrated, and benign histiocyte–macrophages. In the absence of histological evidence, a repeated myelogram should be performed 22.
Of note, hemophagocytosis is common in cases of polytransfusion or hematologic diseases and is not regarded as a pathognomonic criterion of HPS 23.
HPS etiology
Etiological evaluation led to ruling out anyneoplasia 6, 7 (solid malignant tumors, hematologic malignancy). Upon patient questioning, no deterioration of general state was reported. Clinical examination did not reveal any mass or poly lymphadenopathy syndrome. In addition, the myelogram and blood workup did not reveal neoplastic malignant cells. Given the patient's medical history, HPS secondary to autoimmune disease seemed highly likely after excluding any infectious disease. Immune deficiency‐related HPS is very common 4, 6, 15, 16, 17, 18, 19 (45%). The most prevalent pathogens responsible for immune deficiency‐related HPS were reported to be herpesviridae, in particular, CMV, EBV, and HSV. Other less prevalent pathogens were mycobacteria and parasites. Advanced stages of HPS are reported to be secondary to HIV infection 16, 24. In immune deficiency‐related HPS, there is a challenge distinguishing the symptoms induced by pathogens from those secondary to immunosuppression. Indeed, most cases of secondary HPS have been reported in chronic immunosuppression, that is, patients with renal failure, HIV, hematologic or autoimmune disease. Immune deficiency‐related HPS was ruled out given the negative infectious workup.
HPS treatments
According to the literature, autoimmune related HPS can be treated by the following therapies: GC, intravenous immunoglobulins, methotrexate, and biotherapies 11, 25, 26. The use of GC in our patient allowed reduction in disease progression and complications. Disease progression and its related complications were reduced after GC therapy.
HPS prognosis
Hemophagocytic syndrome prognosis was positive with satisfactory outcomes for the mother and the fetus. In case of autoimmune disease, the literature has reported only a few cases of positive pregnancy outcomes 10, 13, 16. Fatal pregnancy outcomes have been reported across several reports 8, 9, 15.
Discussion
HPS diagnosis
Hemophagocytic syndrome diagnosis is challenging given its rare but serious characteristics often requiring intensive care. The median age for HPS occurrence is 48 yo [35–62 yo] 20 with male predominance 7, 20. There have only been a few reported cases of HPS during pregnancy with sometimes fatal fetal and maternal outcomes 5, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19. According to the literature, the mean age for HPS onset during pregnancy is 31 yo and diagnosis is carried out at the second trimester (around 22 GA) 8, 12, 13, 16, 17, 19, 27, 28.
HPS etiology
According to the literature, autoimmune disease‐related HPS prevalence is around 7.2% 6, 12. Systemic diseases such as lupus (a prevalence of 2.4%, Wong et al. 29) or Still's disease are the most prevalent cases of secondary HPS 6, 7, 29, 30. In prepartum, our patient presented with moderate peripheral autoimmune thrombocytopenia accompanied by antiribonucleoprotein antibodies and Raynaud's syndrome. In the absence of other events, no specific treatment was started. Nevertheless, the association of the above symptoms with the hormonal changes induced by pregnancy raised the question of vascular‐placental risk. Thus, acetyl salicylate DL‐lysine was prescribed during the entire pregnancy.
Fardet et al. 31 developed a diagnostic score for the adult HPS. It consists of several items: autoimmune disease, maximum temperature, hepatomegaly, splenomegaly, levels of hemoglobin, platelets and leukocytes levels, hyperferritinemia, hypertriglyceridemia, levels of fibrinogen and transaminase, hemophagocytosis found on the bone marrow. This score is a diagnostic aid for gynecologist–obstetrician facing an uncommon but serious pathology often underdiagnosed. It is accessible on http://saintantoine.aphp.fr/score/. According to this score, there was a 96.7% probability that our patient had HPS.
HPS and pregnancy‐induced risk factors
Pregnancy is a time when the immune system is strongly stimulated and the placenta plays the role of immunological barrier between the fetus and the mother 28. However, this mechanism fails in pregnancy pathologies such as preeclampsia. Given the variable immunological disturbances during pregnancy, it becomes a favorable context to trigger HPS in the presence of additional risk factors such as systemic disease or infection 20. To date, there is no literature on oocyte donation and recipient mother's immune conflict. Nevertheless, we did set forth such likely correlation, that is, the recipient's immunological reaction seemed to be triggered against the presence of unknown genetic matter. Several studies have highlighted an increased rate of pregnancy‐induced hypertension and preeclampsia in patients who underwent in vitro fertilization by oocyte donation versus oocytes of the recipient (OR = 3.3; 95% CI [1.2–8.9]) 32, 33, 34. We believe that this higher pregnancy‐induced hypertension and preeclampsia can be explained by the recipient's immunological reaction triggered against the presence of unknown genetic matter, that is, allogeneic graft. Triggered immunological mechanism of the mother impairs placental implantation and increases maternal systemic resistance leading to preeclampsia and further complications of autoimmune reaction 35. During pregnancy, in the presence of numerous biological signs similar to those of HPS, clinicians should first preclude a differential diagnosis of preeclampsia. Preeclampsia was excluded in our patient case.
HPS prognosis
Mortality rate linked to the primary or secondary HPS is very high. In HPS, prognosis is poor in 49% of cases and patients with HIV or malignant hemopathy are at higher risk of mortality 6, 7. The literature has put forward the mean premature GA of 30 weeks 7, 11, requiring most likely a C‐section for fetal and maternal salvage in cases of preeclampsia or cerebral hemorrhage 9, 12, 13, 14, 15, 17. Kaito et al. identified the following risk factors of mortality: maternal age >30 years, intravascular disseminated coagulation, anemia associated with thrombocytopenia, cholestasis, elevated ferritin, and β2 microglobulinemia 36.
HPS treatment
To date, there is no consensus on the best management of either primary or secondary HPS. The overall aim of treatment is to resolve all hydroelectric disorders, transfuse in case of cytopenia, and manage organ failures 37. In addition, it is necessary to treat the cause of HPS: antimicrobial treatment, chemotherapy, or immunomodulators. GC have played a major role in treatment of HPS between 1994 and 2004 and this regardless of the underlying etiology 18.
Even though immunoglobulins are being regarded as the first‐line treatments for HPS, GC have been used as the first‐line treatments in most reported cases 8, 11, 16, 19. Immunoglobulins and cyclosporine have been mostly used for HPS treatment in GC‐resistant cases 18.
Conclusion
Hemophagocytic syndrome is not well known during pregnancy, yet can be fatal. Mother's and fetus's prognoses are poor and require vital emergency care. HPS diagnosis is a challenge due to variable clinical presentation and nonspecificity of the clinical and biological findings. Mortality, prognosis, and disease progression may be influenced by delay in diagnosis, treatment onset, and HPS etiology. This case and its comparison to the literature showed the absence of consensual diagnosis and management of HPS. Making the right diagnosis in a timely manner during pregnancy seems to be the most significant barrier to treatment and would offer the best prognosis for the patient. Multidisciplinary team work is mandatory to reach prompt diagnosis for such uncommon yet fatal disorder during pregnancy. Clinicians should be alerted when there is an association of clinical and biological signs such as fever, pancytopenia, hyperferritinemia, and hypertriglycemia to suspect HPS and proceed with prompt treatments. To reach consensus on diagnostic criteria for HPS, diagnostic scoring tools, for example, Fardet et al. 31 scoring, as well as novel therapies such as immune modulators combined with biotherapies should be taken into account in further observational studies.
Authorship
AR, PM, and ZA: contributed to the study design and methodology. AR, PM, ZA, AD, and ELM: contributed to the data interpretation and wrote the manuscript. AR, ELM, AD, CT, and SR: provided patient care and follow‐up, collected patient data, laboratory workup, and interpreted the data. AR and PM: performed the review of literature.
Conflict of Interest
None declared.
Clinical Case Reports 2017; 5(11): 1756–1764
References
- 1. Créput, C. , Galicier L., Oksenhendler E., and Azoulay E.. 2005. Syndrome d'activation lymphohistiocytaire : revue de la littérature, implications en réanimation. Réanimation 14:604–613. [Google Scholar]
- 2. Risdall, R. J. , McKenna R. W., Nesbit M. E., Krivit W., Balfour H. H., Simmons R. L., et al. 1979. Virus‐associated hemophagocytic syndrome: a benign histiocytic proliferation distinct from malignant histiocytosis. Cancer 44:993–1002. [DOI] [PubMed] [Google Scholar]
- 3. Emmenegger, U. , Schaer D. J., Larroche C., and Neftel K. A.. 2005. Haemophagocytic syndromes in adults: current concepts and challenges ahead. Swiss Med. Wkly 135:299–314. [DOI] [PubMed] [Google Scholar]
- 4. Gonzalez, F. V. 2009. Syndrome d'activation macrophagique d'origine infectieuse : étiologies et prise en charge. Réanimation 18:284–290. [Google Scholar]
- 5. Hannebicque‐Montaigne, K. , Le Roc'h A., Launay D., Coulon C., Deruelle P., and Langlois S.. 2012. Haemophagocytic syndrome in pregnancy: a case report. Ann. Fr. Anesthèsie Rèanimation 31:239–242. [DOI] [PubMed] [Google Scholar]
- 6. Karras, A. , and Hermine O.. 2002. Hemophagocytic syndrome. Rev. Médecine Interne. Fondée Par. Société Natl Francaise Médecine Interne 23:768–778. [DOI] [PubMed] [Google Scholar]
- 7. Ramos‐Casals, M. , Brito‐Zerón P., López‐Guillermo A., Khamashta M. A., and Bosch X.. 2014. Adult haemophagocytic syndrome. Lancet 383:1503–1516. [DOI] [PubMed] [Google Scholar]
- 8. Teng, C.‐L. , Hwang G.‐Y., Lee B.‐J., Wang R.‐C., and Chou M.‐M.. 2009. Pregnancy‐induced hemophagocytic lymphohistiocytosis combined with autoimmune hemolytic anemia. J. Chin. Med. Assoc. JCMA 72:156–159. [DOI] [PubMed] [Google Scholar]
- 9. Chmait, R. H. , Meimin D. L., Koo C. H., and Huffaker J.. 2000. Hemophagocytic syndrome in pregnancy. Obstet. Gynecol. 95(6 Pt 2):1022–1024. [DOI] [PubMed] [Google Scholar]
- 10. Mihara, H. , Kato Y., Tokura Y., Hattori Y., Sato A., Kobayashi H., et al. 1999. Epstein‐Barr virus‐associated hemophagocytic syndrome during mid‐term pregnancy successfully treated with combined methylprednisolone and intravenous immunoglobulin. Rinshō Ketsueki Jpn J. Clin. Hematol. 40:1258–1264. [PubMed] [Google Scholar]
- 11. Gill, D. S. , Spencer A., and Cobcroft R. G.. 1994. High‐dose gamma‐globulin therapy in the reactive haemophagocytic syndrome. Br. J. Haematol. 88:204–206. [DOI] [PubMed] [Google Scholar]
- 12. Pérard, L. , Costedoat‐Chalumeau N., Limal N., Hot A., Cohen J., Vauthier‐Brouzes D., et al. 2007. Hemophagocytic syndrome in a pregnant patient with systemic lupus erythematosus, complicated with preeclampsia and cerebral hemorrhage. Ann. Hematol. 86:541–544. [DOI] [PubMed] [Google Scholar]
- 13. Nakabayashi, M. , Adachi T., Izuchi S., and Sugisaki A.. 1999. Association of hypercytokinemia in the development of severe preeclampsia in a case of hemophagocytic syndrome. Semin. Thromb. Hemost. 25:467–471. [DOI] [PubMed] [Google Scholar]
- 14. Dunn, T. , Cho M., Medeiros B., Logan A., Ungewickell A., and Liedtke M.. 2012. Hemophagocytic lymphohistiocytosis in pregnancy: a case report and review of treatment options. Hematology 17:325–328. [DOI] [PubMed] [Google Scholar]
- 15. Tumian, N. R. , and Wong C. L.. 2015. Pregnancy‐related hemophagocytic lymphohistiocytosis associated with cytomegalovirus infection: A diagnostic and therapeutic challenge. Taiwan J. Obstet. Gynecol. 54:432–437. [DOI] [PubMed] [Google Scholar]
- 16. Arewa, O. P. , and Ajadi A. A.. 2011. Human immunodeficiency virus associated with haemophagocytic syndrome in pregnancy: a case report. West Afr. J. Med. 30:66–68. [DOI] [PubMed] [Google Scholar]
- 17. Yamaguchi, K. , Yamamoto A., Hisano M., Natori M., and Murashima A.. 2005. Herpes simplex virus 2‐associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet. Gynecol. 105(5 Pt 2):1241–1244. [DOI] [PubMed] [Google Scholar]
- 18. Samra, B. , Yasmin M., Arnaout S., and Azzi J.. 2015. Idiopathic hemophagocytic lymphohistiocytosis during pregnancy treated with steroids. Hematol. Rep. 7:6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mayama, M. , Yoshihara M., Kokabu T., and Oguchi H.. 2014. Hemophagocytic lymphohistiocytosis associated with a parvovirus B19 infection during pregnancy. Obstet. Gynecol. 124(2 Pt 2 Suppl 1):438–441. [DOI] [PubMed] [Google Scholar]
- 20. Rivière, S. , Galicier L., Coppo P., Marzac C., Aumont C., Lambotte O., et al. 2014. Reactive hemophagocytic syndrome in adults: a retrospective analysis of 162 patients. Am. J. Med. 127:1118–1125. [DOI] [PubMed] [Google Scholar]
- 21. Li, J. , Wang Q., Zheng W., Ma J., Zhang W., Wang W., et al. 2014. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore) 93:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henter, J. I. , Aricò M., Elinder G., Imashuku S., and Janka G.. 1998. Familial hemophagocytic lymphohistiocytosis. Primary hemophagocytic lymphohistiocytosis. Hematol. Oncol. Clin. North Am. 12:417–433. [DOI] [PubMed] [Google Scholar]
- 23. Goel, S. , Polski J. M., and Imran H.. 2012. Sensitivity and specificity of bone marrow hemophagocytosis in hemophagocytic lymphohistiocytosis. Ann. Clin. Lab. Sci. 42:21–25. [PubMed] [Google Scholar]
- 24. Fardet, L. , Lambotte O., Meynard J.‐L., Kamouh W., Galicier L., Marzac C., et al. 2010. Reactive haemophagocytic syndrome in 58 HIV‐1‐infected patients: clinical features, underlying diseases and prognosis. AIDS Lond. Engl. 24:1299–1306. [DOI] [PubMed] [Google Scholar]
- 25. Larroche, C. 2012. Hemophagocytic lymphohistiocytosis in adults: diagnosis and treatment. Joint Bone Spine 79:356–361. [DOI] [PubMed] [Google Scholar]
- 26. Jordan, M. B. , Allen C. E., Weitzman S., Filipovich A. H., and McClain K. L.. 2011. How I treat hemophagocytic lymphohistiocytosis. Blood 118:4041–4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanaoka, M. , Tsukimori K., Hojo S., Abe Y., Mutou T., Muta K., et al. 2007. B‐cell lymphoma during pregnancy associated with hemophagocytic syndrome and placental involvement. Clin. Lymphoma Myeloma. 7:486–490. [DOI] [PubMed] [Google Scholar]
- 28. Shukla, A. , Kaur A., and Hira H. S.. 2013. Pregnancy Induced Haemophagocytic Syndrome. J. Obstet. Gynaecol. India 63:203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wong, K. F. , Hui P. K., Chan J. K., Chan Y. W., and Ha S. Y.. 1991. The acute lupus hemophagocytic syndrome. Ann. Intern. Med. 114:387–390. [DOI] [PubMed] [Google Scholar]
- 30. Dhote, R. , Simon J., Papo T., Detournay B., Sailler L., Andre M.‐H., et al. 2003. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty‐six cases and literature review. Arthritis Rheum. 49:633–639. [DOI] [PubMed] [Google Scholar]
- 31. Fardet, L. , Galicier L., Lambotte O., Marzac C., Aumont C., Chahwan D., et al. 2014. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis. Rheumatol. 66:2613–2620. [DOI] [PubMed] [Google Scholar]
- 32. Le Ray, C. , Scherier S., Anselem O., Marszalek A., Tsatsaris V., Cabrol D., et al. 2012. Association between oocyte donation and maternal and perinatal outcomes in women aged 43 years or older. Hum. Reprod. 27:896–901. [DOI] [PubMed] [Google Scholar]
- 33. Serhal, P. F. , and Craft I. L.. 1989. Oocyte donation in 61 patients. Lancet 1:1185–1187. [DOI] [PubMed] [Google Scholar]
- 34. Sekhon, L. H. , Gerber R. S., Rebarber A., Saltzman D. H., Klauser C. K., Gupta S., et al. 2014. Effect of oocyte donation on pregnancy outcomes in in vitro fertilization twin gestations. Fertil. Steril. 101:1326–1330. [DOI] [PubMed] [Google Scholar]
- 35. van der Hoorn, M. L. P. , Lashley E. E. L. O., Bianchi D. W., Claas F. H. J., Schonkeren C. M. C., and Scherjon S. A.. 2010. Clinical and immunologic aspects of egg donation pregnancies: a systematic review. Hum. Reprod. Update 16:704–712. [DOI] [PubMed] [Google Scholar]
- 36. Kaito, K. , Kobayashi M., Katayama T., Otsubo H., Ogasawara Y., Sekita T., et al. 1997. Prognostic factors of hemophagocytic syndrome in adults: analysis of 34 cases. Eur. J. Haematol. 59:247–253. [DOI] [PubMed] [Google Scholar]
- 37. Buyse, S. , Teixeira L., Galicier L., Mariotte E., Lemiale V., Seguin A., et al. 2010. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 36:1695–1702. [DOI] [PubMed] [Google Scholar]


