Abstract
Background
This phase 1, dose-finding study determined the safety, maximum tolerated dose (MTD)/recommended phase 2 dose (RP2D), antitumor activity, and molecular correlates of IPI-926, a Hedgehog pathway (HhP) inhibitor, combined with cetuximab in patients with relapsed/metastatic squamous cell carcinoma of the head and neck.
Patients and Methods
Cetuximab was given with a 400 mg/m2 loading dose followed by 250 mg/m2 weekly. IPI-926 was given daily starting two weeks after cetuximab initiation. A “3+3” study design was used. Prior therapy with cetuximab was allowed. Tumor biopsies occurred prior to cetuximab initiation, prior to IPI-926 initiation, and after treatment with both drugs.
Results
Nine patients were enrolled. The RP2D was 160 mg, the same as the single-agent IPI-926 MTD. Among 9 treated, 8 evaluable patients, the best responses were 1 partial response (12.5%), 4 stable disease (50%), and 3 disease progressions (37.5%). The median progression free survival was 77 days (95% confidence interval 39–156). Decreases in tumor size were seen in both cetuximab-naïve patients (one HPV-positive, one HPV-negative). The most frequent treatment-emergent adverse events were fatigue, muscle cramps, and rash. No DLTs were observed. Tumor shrinkage and progression free survival were associated with intra-tumoral ErbB and HhP gene expression down-regulation during therapy, supporting the preclinical hypothesis.
Conclusion
Treatment with IPI-926 and cetuximab yielded expected toxicities with signs of antitumor activity. Serial tumor biopsies were feasible and revealed proof-of-concept biomarkers.
Keywords: Hedgehog signaling pathway, phase 1, cetuximab, combination therapy, head and neck squamous cell carcinoma
Introduction
Cetuximab is an anti-epidermal growth factor receptor (EGFR/ErbB) antibody whose efficacy in treating relapsed/metastatic head and neck squamous cell carcinomas (R/M HNSCC) is limited by inherent or acquired resistance [1]. Epithelial-to-mesenchymal transition (EMT) has been hypothesized as a possible cause for drug resistance and worse prognosis in HNSCC [2–4]. The Hedgehog signaling pathway (HhP) has been implicated in EMT [5]. In the HhP the sonic hedgehog (SHH) ligand activates a signaling cascade that leads to glioma-associated oncogene family zinc finger 1 (GLI1) expression, which in turn modulates numerous cancer target genes [5, 6]. Expression of HhP and GLI1 is associated with poor response to radiation in vivo and worse prognosis in HNSCC patients treated with curative intent radiation therapy [7, 8]. Preclinical data suggest that the hedgehog and EGFR pathways interact. EGFR and HhP signaling converge and/or synergize upstream of GLI1 through the MEK/ERK signaling pathway in cancer cells and during keratinocyte oncogenic transformation [9, 10]. In patient–derived tumor xenografts (PDX) inhibition of the HhP with the novel HhP inhibitor IPI-926 (Infinity Pharmaceuticals, Boston, MA) caused tumors to have a more epithelial, EGFR-dependent phenotype [11]. When HhP inhibition was combined with cetuximab, tumors were eliminated in two cases and re-growth was significantly delayed in the other two cases [11]. Expression of EMT genes TWIST and ZEB2 was increased in sensitive xenografts, suggesting a possible resistant mesenchymal population [11]. Therefore, combined inhibition of EGFR with cetuximab and the HhP pathway with IPI-926 was a rational approach in patients with R/M HNSCC.
In the first-in-human, phase 1, single-agent study of IPI-926, the recommended phase 2 dose (RP2D) was 160 mg daily [12]. The most common adverse events (AEs) were fatigue, nausea, muscle spasms, liver function abnormalities, and alopecia [12]. Given the preclinical rationale for combining HhP and EGRF inhibition, we conducted an open-label, phase 1 study combining IPI-926 and cetuximab to determine the maximal tolerated dose (MTD)/RP2D, toxicity profile, antitumor activity, and molecular correlates in patients with R/M HNSCC (NCT01255800).
Patients and Methods
Patients
Inclusion criteria included patients with: histologically/cytologically confirmed R/M HNSCC; tumors amenable to biopsy; willingness to undergo three sequential tumor biopsies; measurable disease per RECIST 1.1; age ≥18 years, life expectancy > 12 weeks; adequate hepatic, hematologic, and renal function; Eastern Cooperative Oncology Group performance status (ECOG PS) of ≤2; ability to swallow whole pills; previous treatment completed >4 weeks prior, and use of effective contraception. Prior treatment with cetuximab was allowed. Exclusion criteria included: presence of any medical/social factors affecting patient safety; pregnancy or breastfeeding; known human immunodeficiency virus; known or suspected clinically active brain metastases; venous thromboembolic disease that was symptomatic or diagnosed within the previous month; baseline QTcF >450 ms (men) or >470 (women); concurrent use of strong inducers or inhibitors of CYP3A4, PgP inhibitors, or medications that prolong the QTcF interval; and/or history of hypersensitivity reactions to cetuximab. The institutional review board granted approval and written informed consent was mandatory.
Design
This was an open-label, dose escalation study of orally administered daily IPI-926 in combination with cetuximab given in 28-day cycles. On C1D0 patients underwent a tumor biopsy and aspiration. Cetuximab was administered at 400 mg/m2 IV on C1D1 and then 250 mg/m2 IV weekly thereafter. Cetuximab was administered first to allow patients to receive an FDA-approved therapy earlier in their treatment course. Patients underwent a tumor biopsy on C1D14. IPI-926 was administered by mouth starting on C1D15 and continued once daily by mouth thereafter. Patients underwent a third biopsy on C2D14–21. Patients who developed a cetuximab-rash were treated per local standard of care
IPI-926 Dose Escalation
IPI-926 was administered at 130 or 160 mg daily to cohorts of 3 or more patients each using a standard “3+3” design. The 130 mg starting dose was chosen as representing the first dose level down from the established single-agent MTD of 160 mg in order to maximize safety. Each cohort initially enrolled up to 3 patients. Patients were considered evaluable for efficacy if they received at least four weeks of therapy unless the reason for not doing so was a dose limiting toxicity (DLT) or other IPI-926-related toxicity. Non-evaluable patients were replaced. If none of the first 3 evaluable patients experienced a DLT, then the dose of IPI-926 was escalated; if no more than 1 DLT was observed in the first 3 evaluable patients, the cohort was expanded to 6 patients. A dose was considered not tolerated if the observed rate of DLT in at least 6 patients was 33%. Patients were evaluated for efficacy by imaging using RECIST 1.1 every 8 weeks by imaging. Patients with stable disease or better received repeat cycles of treatment until progressive disease, unacceptable toxicity, or withdrawal of consent.
Safety Monitoring
Safety assessments included: vital signs, laboratory assessments, and physical exams. Adverse events were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.02. DLT included ≥grade 3 non-hematologic events considered possibly, probably, or definitely related to the combination study drug treatment, excluding untreated nausea, vomiting, or diarrhea.
Biopsy protocol and tissue analysis
Two tumor tissue core biopsies were collected using standard practices by interventional radiology and samples were transferred directly to 10% formalin for processing. One fine needle aspirate (FNA) was deposited directly into RLT lysis buffer for RNA isolation (Qiagen) using the manufacturers protocol. RNA sequencing was performed on fresh or flash frozen FNA material. Tissue samples were cut and stained with hematoxylin-eosin (H/E) and by immunohistochemistry (IHC) that has been previously described [11]. HPV status was determined by in situ hybridization.
Statistics
Sample size was determined empirically, based upon a 3+3 escalation design. Descriptive statistics were used for analyses of safety and tumor response. The bioinformatics strategy for RNAseq Analysis was previously reported [13].
Results
Demographics and Baseline Characteristics
Patient demographics and baseline characteristics are described in Table 1. Nine patients were enrolled and eight received therapy with both drugs (N=3 [130 mg], N=6 [160 mg]). The median age was 57 years and most patients were heavily pretreated. Most patients (77.8%) had received a prior EGFR-targeted therapy. A small majority of patients were HPV-positive (55.6%) and both local-regional and distant relapses were represented.
Table 1.
Patient and disease baseline characteristics.
| Demographic or Patient Characteristic |
IPI-926 Dose Cohort | ||
|---|---|---|---|
|
| |||
| 130 mg (N= 3) | 160 mg (N=6) | Total (N=9) | |
|
| |||
| Age, median (y) | 53 | 60 | 57 |
|
| |||
| Male | 2 | 5 | 7 |
|
| |||
| Female | 1 | 1 | 2 |
|
| |||
| White or Caucasian | 3 | 5 | 8 |
|
| |||
| Middle Eastern | 0 | 1 | 1 |
|
| |||
| ECOG PS | |||
| 0 | 0 | 1 | 1 |
| 1 | 3 | 5 | 8 |
|
| |||
| Smoker ≥10 pack-years | 2 | 1 | 3 |
|
| |||
| Location of primary | |||
|
| |||
| Tonsil | 1 | 3 | 4 |
|
| |||
| Oral Tongue | 1 | 1 | 2 |
|
| |||
| Base of Tongue | 1 | 1 | 2 |
|
| |||
| Unknown primary | 0 | 1 | 1 |
|
| |||
| HPV status | |||
|
| |||
| Positive | 2 | 3 | 5 |
|
| |||
| Negative | 1 | 2 | 3 |
|
| |||
| Unknown | 0 | 1 | 1 |
|
| |||
| No. of prior therapies for relapsed/metastatic cancer | 1.6 (range: 1–3) | 2.5 (range: 1–3) | 2.2 (range: 1–3) |
|
| |||
| Prior cetuximab | 2 | 5 | 7 |
|
| |||
| Site(s) of measurable disease | |||
|
| |||
| Local-regional only | 2 | 2 | 4 |
|
| |||
| Distant only | 1 | 2 | 3 |
|
| |||
| Local-regional and Distant | 0 | 2 | 2 |
Dose Escalation and Safety
IPI-926 dosing started at 130 mg and was escalated to 160 mg (single-agent MTD). No DLTs were seen in either of the 2 dose-escalation cohorts. Patients receiving at least one dose of either drug were evaluated for safety (N=9). The most frequent all-grade treatment emergent AEs attributed to IPI-926 were nausea (33%), muscle cramps (22.2%), and fatigue (22.2%) (Table 2). The most common all-grade treatment emergent AEs attributed to cetuximab were mucocutaneous (Table 2). One patient in the 160 mg cohort had a grade 3 infusion reaction to the loading dose of cetuximab and was replaced. Four patients experienced a total of four serious adverse events (SAEs); all were deemed due to concurrent illness or disease under study. One patient died while on study from a stroke related to underlying disease. There were no dose reductions in cetuximab due to AEs. Three patients in the IPI-926 160 mg cohort reduced their dose to 130 mg for fatigue, though in each case the fatigue was deemed related to disease under study and only possibly related to IPI-926. Hematologic toxicity was not observed. Study enrollment stopped when IPI-926 production ceased after a negative pancreatic cancer study.
Table 2.
Treatment-related adverse events by dose level and symptom.
| IPI-926 dose level | 130 mg | 160 mg | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Evaluable patients | 3 | 6 | 9 | ||||||
| Patients with a DLT | 0 | 0 | 0 | ||||||
| Grade | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Attributed to IPI-926 | |||||||||
| Nausea | 2 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 |
| Muscle Cramps | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 |
| Fatigue | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| Alopecia | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Dysguesia | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Elevated AST | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Attributed to Cetuximab | |||||||||
| Acneiform rash | 2 | 0 | 0 | 2 | 0 | 0 | 4 | 0 | 0 |
| Dry skin/desquamation | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| Infusion reaction | 0 | 0 | 0 | 0 | 0 | 1* | 0 | 0 | 1 |
| Diarrhea | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Mucositis | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Hypomagnesemia | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
The patient with a grade 3 infusion reaction to cetuximab was removed from study following his loading dose of cetuximab and never received IPI-926.
Efficacy
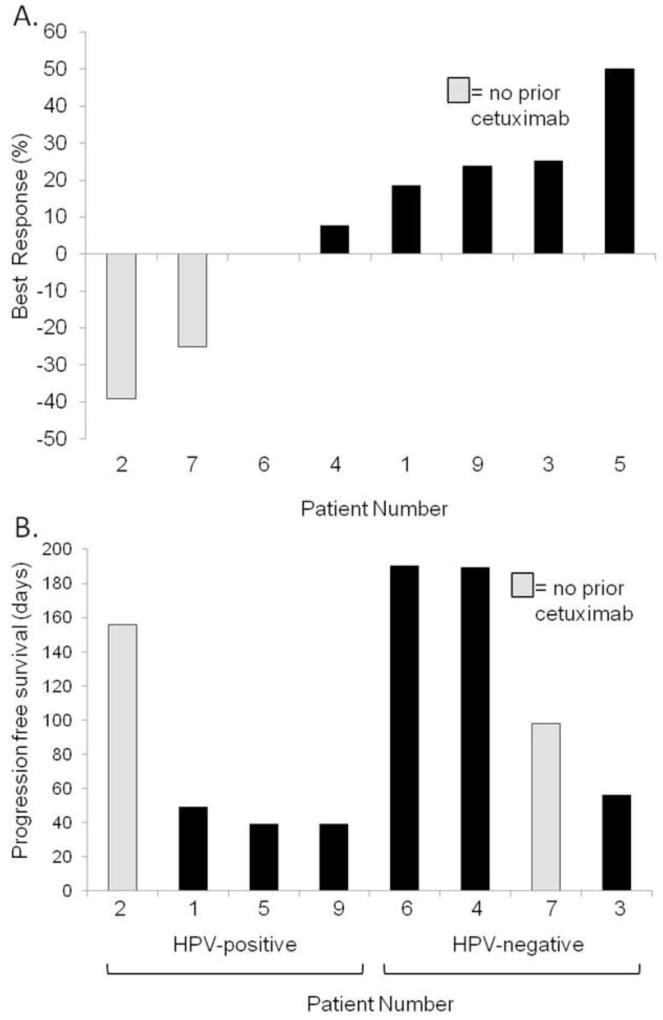
Of the 9 patients treated, 8 were evaluated following 2 or more cycles for response per RECIST 1.1 (one was not eligible for evaluation due to a grade 3 cetuximab reaction and was replaced). The best responses in evaluable patients were 1 partial response (PR) (12.5%), 4 stable diseases (SD) (50%), and 3 progressive diseases (PD) (37.5%) (Figure 1A). The two cetuximab-naïve patients had the most significant responses. Median PFS for all patients was 77 days (95% confidence interval [CI] 39–189). The median PFS for HPV-positive patients was 44 days (95% CI 39–156) versus 144 days for HPV-negative cancers (95% CI 56–190, p=0.09 by log rank test). Overall, 2 (22.2%) patients discontinued therapy due to an AE (cetuximab infusion reaction and stroke) and 7 (77.8%) patients stopped due to disease progression.
Figure 1.
Clinical responses to therapy. A) Best responses for patients treated with IPI-926 and cetuximab. B) Progression free survival by HPV status and prior cetuximab exposure. Median PFS for HPV-positive is 44 days (95% CI 39–156) versus 144 days for HPV-negative cancers (95% CI 56–190, p=0.09).
Biopsies and Molecular Correlates
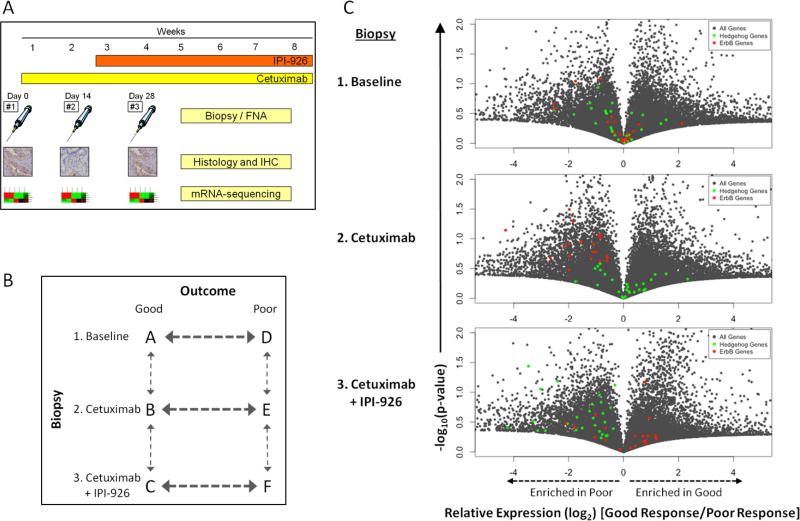
All 9 patients underwent a tumor biopsy at cycle 1 day 0 (C1D0). All eight patients remaining on study at C1D14 received study biopsies. Six patients received a biopsy at C2D14–21 (Figure 2A); two patients had discontinued therapy at that point for progression. There were no complications of sequential biopsies.
Figure 2.
(A) Clinical trial biopsy collection protocol. (B) mRNA-sequencing comparisons for collected biopsies. Arrows indicate direction of comparison. (C) Volcano plots depicting global gene expression (grey) as well as expression of selected ErbB genes (red dots) that are heavily influenced by EGFR and Hedgehog pathway genes (green dots) and. Poor outcomes (left of 0) are enriched for ErbB and Hedgehog genes (more dots) compared to good outcomes after cetuximab and cetuximab plus IPI-926, respectively.
RNAseq-based transcriptome analysis was performed on each biopsy and comparisons were made based on patient outcomes, where “good” outcomes included tumor regression and/or PFS of >150 days (patients 2, 4, 6, 7); the remaining patients were “poor” outcomes. Bivariate comparisons were completed as diagramed in Figure 2B. However, comparisons between good and poor outcomes were determined to be more robust due to larger sample sizes (Bold Arrows).
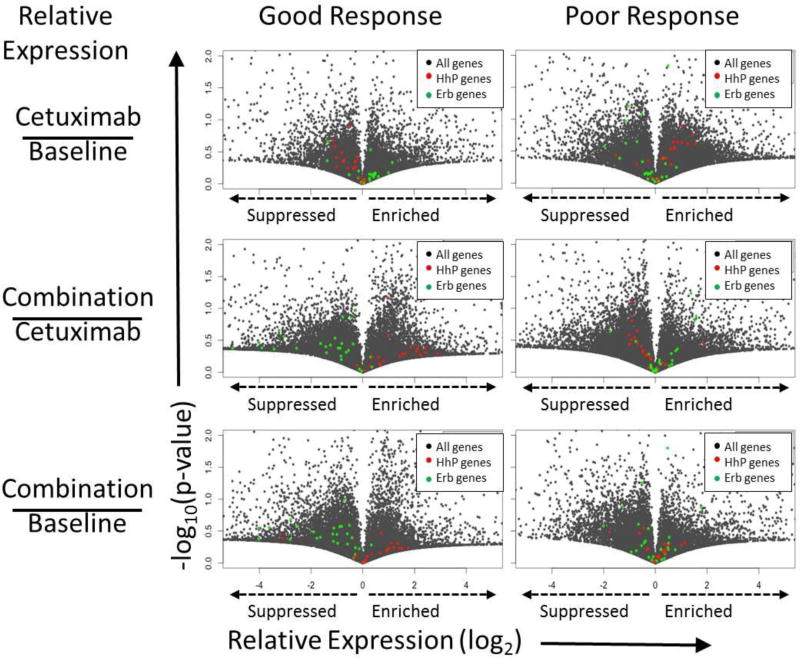
To investigate how EGFR and HhP inhibition modulate ErbB and HhP genes, volcano plots of global gene expression were generated highlighting genes in both pathways. We first compared (Figure 2B, horizontal comparison) the relative expression of genes (good/poor outcomes) at each of the three biopsies (Figure 2C). After cetuximab treatment the expression of ErbB genes (red) was suppressed in patients that had a good outcomes, shifting the population dramatically. A similar suppression and shift of HhP genes (green) in good outcomes is seen upon the addition of IPI-926 and together this supports that both cetuximab and IPI-926 successfully suppressed their targeted pathways (Figure 2C). Using the same method we next compared the effects of treatment on good and poor outcomes independently (Figure 2B, vertical comparisons). As expected, cetuximab treatment suppressed ErbB genes in good responders but had no effect on patients with a poor outcome (Figure 3). Similarly, addition of IPI-926 suppressed HhP genes when compared to baseline or cetuximab biopsies in the same patients. Importantly, HhP inhibition led to increased expression of ErbB genes. We have previously reported similar findings in HNSCC cell lines and PDX, concluding that HhP signaling is a negative regulator of EGFR signaling whose inhibition sensitizes cells to EGFR inhibition [11].
Figure 3.
Volcano plots depicting global gene expression (grey) as well as expression of selected ErbB genes (red dots) that are heavily influenced by EGFR and Hedgehog pathway genes (green dots). Good (left) and poor (right) outcomes are separated. Relative expression is depicted for the comparisons on the left. In good outcomes, ErbB genes (red) and HhP genes (green) are suppressed (shifted right to left) following cetuximab and IPI-926 treatment, respectively.
Discussion
This study demonstrates that IPI-926, an oral, small molecule inhibitor of the Hedgehog signaling pathway, can be safety combined with standard weekly cetuximab at the single-agent IPI-926 MTD of 160 mg daily. Tumor biopsies during therapy were feasible and demonstrated that transcriptome analysis of target pathways correlated with clinical outcome.
No new safety signals were observed in this trial. The most common AEs of all grades included fatigue, nausea, and muscle cramps associated IPI-926 and electrolyte and mucocutaneous reactions associated with cetuximab [12, 14]. As there were only 2 dose cohorts in this small phase 1 trial, it was difficult to assess whether IPI-926-related AEs were dose dependent in this trial, although previous experiences indicate IPI-926-related toxicities are dose-dependent [12]. The addition of IPI-926 to cetuximab did not reduce cetuximab dose intensity, as no dose reductions in cetuximab were required. The declared RP2D of 160 mg IPI-926 plus cetuximab is supported by the lack of DLTs, though the sample size limits authoritative conclusions. Moreover, when IPI-926 was combined with gemcitabine, a more toxic agent than cetuximab, in another study the RP2D was also 160 mg daily (NCT01130142). Though our study did not perform pharmacokinetic assessments, there appears to be no PK interaction with other multi-agent anti-cancer therapies [15].
Though definitive conclusions about efficacy are limited by sample size in this pilot trial, there were signs of clinical activity with this drug combination. The partial response rate of 12% is consistent with the expected response rate in cetuximab-naïve patients with R/M HNSCC, though most patients in this study were previously treated with cetuximab [14]. Both cetuximab-naïve patients but no cetuximab-pretreated patients experienced tumor regression. While tumor shrinkage may have reflected responses to cetuximab independent of IPI-926, it is also consistent with our preclinical investigations and the gene expression profiles in this study demonstrating that HhP inhibition drives tumors to a more epithelial, EGFR-sensitive phenotype [11]. However, the lack of responses in cetuximab pre-treated patients suggests that HhP inhibition may not potent enough to convert EGFR-resistant tumors back into an EGFR-sensitive state.. One could theorize that, based upon pre-clinical data, HhP inhibitors should be given prior to cetuximab in future in order to increase EGFR-dependence and cetuximab activity. Unfortunately, these data were not established when the clinical protocol was written. There was a suggestion of increased PFS in HPV-negative tumors (143 days) compared to HPV-positive (44 days), though this did not reach statistical significance (p=0.09). While HPV-positive HNSCC patients have better overall prognosis in the R/M setting, growing data suggest that EGFR inhibition may be more effective in HPV-negative cases[16]. HhP inhibition may have enhanced this effect in the HPV-negative tumors in this study. Interestingly, in preclinical studies the combination was more effective in HPV-negative tumors, and HPV-positive tumors had higher GLI1 expression when treated with combined HhP and EGFR inhibition [11].
Serial tumor analysis was feasible and yielded molecular correlations of clinical response, and supported preclinical observations. In patients with a good outcome, tumor mRNA levels of ErbB/MAPK and HhP pathways were suppressed by cetuximab and IPI-926, respectively; this pattern was not observed in poor outcomes. These findings are consistent with in vitro and PDX data showing that ErbB and HhP suppression are required for antitumor activity with the combination of cetuximab and IPI-926 [11]. This study also demonstrated that HhP inhibition increases ErbB signaling. In a future study, it would be intriguing to assess whether giving an HhP inhibitor prior to cetuximab increases responses by making tumors more EGFR dependent. Given the small sample size, it is possible that ErbB and HhP changes over time may have reflected the natural biology of the tumors rather than treatment effect. Similarly, the categorization of tumors into “good” or “bad” based upon both tumor shrinkage and PFS may have confounded the results. The necessity of effective ErbB pathway and HhP blockade for benefit, however, remains similar to preclinical models and supports these as treatment effects in patients. Unfortunately, the best predictive biomarker of benefit for HhP inhibition across all tumor is a mutation in the HhP (i.e. PTCH) and this in only seen commonly in basal cell carcinoma [17]. Overall, these data suggest that a) effective suppression of target pathways in patients was associated with improved tumor response, and b) the preclinical models used to design this trial predicted patient responses accurately.
This study has several limitations. The clearest limitation is that it is a nine patient pilot study with no expansion cohort. Though the small sample size resulted from cessation of IPI-926 production, it makes it difficult to draw definitive conclusions about toxicity and efficacy. Similarly, it diminishes the robustness of the molecular correlations, though p values for several comparisons between good and poor responders were well below 0.001. Another limitation was the inclusion of both cetuximab pre-treated and naïve patients, as this heterogeneity may have introduced a variable that makes efficacy more difficult to assess. Lastly, cetuximab may work via adaptive immunity and modulation of the tumor microenviroment [18, 19]. The roles of the immune system and tumor microenvironment were not clearly assessed in this trial.
In conclusion, this phase 1 combination study of IPI-926 and cetuximab established the RP2D as 160 mg of IPI-926 when given with cetuximab. The toxicity profile appears to be tolerable and there were encouraging signs of anti-cancer activity. Serial tumor biopsies were feasible and revealed that clinical responses may correlate with molecular down regulation of the EGFR and Hedgehog pathways. These findings are consistent with preclinical observations and support further exploration of this therapeutic combination. Dual blockade of EGFR and HhP would be particularly intriguing to assess in a study of anti-EGFR treatment naïve, HPV negative patients.
Highlights.
-
-
The hedgehog and EGFR signaling pathways interact in head and neck squamous cell carcinomas (HNSCC).
-
-
We performed a pilot study to assess the safety and efficacy of IPI-926, an oral hedgehog inhibitor, plus cetuximab in patients with relapsed/metastatic HNSCC
-
-
The combination of IPI-926 and cetuximab was safe.
-
-
Serial tumor biopsies were safe and suggested that efficacy was associated with hedgehog and EGFR pathway inhibition.
Acknowledgments
Support:
This work was supported by R21DE019712 (A.J.), the Daniel and Janet Mordecai Foundation (A.J.), the Charles C. Gates Center for Regenerative Medicine and Stem Cell Biology, and the University of Colorado Cancer Center Support Grant (P30CA046934).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest Statement:
A. Jimeno has received laboratory research support from Infinity. No other authors report conflicts of interest.
ClinicalTrials.gov Identifier:
Prior presentations:
Presented in part at the 2014 ASCO/ASTRO Head and Neck Cancer Symposium, Scottsdale AZ.
References
- 1.Burtness B, Bauman JE, Galloway T. Novel targets in HPV-negative head and neck cancer: overcoming resistance to EGFR inhibition. Lancet Oncol. 2013;14:e302–309. doi: 10.1016/S1470-2045(13)70085-8. [DOI] [PubMed] [Google Scholar]
- 2.Prince MEP, Ailles LE. Cancer Stem Cells in Head and Neck Squamous Cell Cancer. Journal of Clinical Oncology. 2008;26:2871–2875. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- 3.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pectasides E, Rampias T, Sasaki C, et al. Markers of epithelial to mesenchymal transition in association with survival in head and neck squamous cell carcinoma (HNSCC) PLoS One. 2014;9:e94273. doi: 10.1371/journal.pone.0094273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung CH, Dignam JJ, Hammond ME, et al. Glioma-associated oncogene family zinc finger 1 expression and metastasis in patients with head and neck squamous cell carcinoma treated with radiation therapy (RTOG 9003) J Clin Oncol. 2011;29:1326–1334. doi: 10.1200/JCO.2010.32.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gan GN, Eagles J, Keysar SB, et al. Hedgehog signaling drives radioresistance and stroma-driven tumor repopulation in head and neck squamous cancers. Cancer Res. 2014;74:7024–7036. doi: 10.1158/0008-5472.CAN-14-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isohata N, Aoyagi K, Mabuchi T, et al. Hedgehog and epithelial-mesenchymal transition signaling in normal and malignant epithelial cells of the esophagus. Int J Cancer. 2009;125:1212–1221. doi: 10.1002/ijc.24400. [DOI] [PubMed] [Google Scholar]
- 10.Schnidar H, Eberl M, Klingler S, et al. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keysar SB, Le PN, Anderson RT, et al. Hedgehog signaling alters reliance on EGF receptor signaling and mediates anti-EGFR therapeutic resistance in head and neck cancer. Cancer Res. 2013;73:3381–3392. doi: 10.1158/0008-5472.CAN-12-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jimeno A, Weiss GJ, Miller WH, Jr, et al. Phase I study of the Hedgehog pathway inhibitor IPI-926 in adult patients with solid tumors. Clin Cancer Res. 2013;19:2766–2774. doi: 10.1158/1078-0432.CCR-12-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keysar SB, Astling DP, Anderson RT, et al. A patient tumor transplant model of squamous cell cancer identifies PI3K inhibitors as candidate therapeutics in defined molecular bins. Mol Oncol. 2013;7:776–790. doi: 10.1016/j.molonc.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–2177. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 15.Ko AH, LoConte N, Tempero MA, et al. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas. 2015 doi: 10.1097/MPA.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vermorken JB, Stohlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 2013;14:697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 17.Amaria RN, Bowles DW, Lewis KD, Jimeno A. Vismodegib in basal cell carcinoma. Drugs Today (Barc) 2012;48:459–467. doi: 10.1358/dot.2012.48.7.1808490. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Zhang X, Mortenson ED, et al. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol Ther. 2013;21:91–100. doi: 10.1038/mt.2012.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srivastava RM, Lee SC, Andrade Filho PA, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19:1858–1872. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]