Abstract
Background:
Combating biofilm-dependent oral infections involves the use of synthetic antibiotics, which are often associated with bacterial resistance and adverse effects. As a result, herbs such as cranberry have emerged as an alternative treatment. The aim of this study was to evaluate, through an integrative literature review, the effectiveness of cranberry extract on cultures and biofilms of periodontopathogenic bacteria.
Materials and Methods:
In vitro and in vivo studies evaluating the action of cranberry extract on the growth, coaggregation and formation of periodontopathogenic bacteria and periodontal biofilm were identified. Searches were carried out in the “Cochrane Library,” “MEDLINE,” “Web of Science,” “Scopus,” “LILACS,” “Scielo,” and “Google Scholar” databases, using the terms: “vaccinium macrocarpon;” “cranberries;” “cranberry;” “biofilms;” “periodontitis;” “chronic periodontitis;” “aggressive periodontitis;” “periodontal diseases;” and “periodont*.”
Results:
a low number of studies evaluating the effectiveness of cranberry extract on periodontal disease were found, and no human studies were identified. In general, the eight studies included in the revision found that the compounds effectively inhibited the formation of a biofilm of Porphyromonas gingivalis and Fusobacterium nucleatum at concentrations equal or superior to 62.5 μg/ml, but did not significantly inhibit bacterial growth or promote the breakdown of preformed biofilm.
Conclusions:
while most of the studies presented certain methodological limitations, they did identify an inhibiting effect of cranberry on periodontal bacteria. These results serve as support for the development of further studies evaluating the most effective vehicle and ideal concentration that can be used without causing adverse effects on oral tissues.
Keywords: Biofilm, cranberry extract, Fusobacterium nucleatum, Porphyromonas gingivalis
INTRODUCTION
Over the past decade, cranberry extract and its molecular components have received increasing attention from researchers due to their general health benefits.[1] In particular, the properties of cranberry display potential for the prevention of microbial adhesion, especially with regard to controlling urinary tract infections.[2] Cranberry, which is present in a wide variety of products (sauce, jam, biscuits, and syrup), is consumed mainly in the form of juices and dried fruit.[3]
In terms of oral health, recent studies have indicated that cranberry extract, in various vehicles, has antimicrobial properties that can be used for the treatment of oral infections such as tooth decay. The inhibition of the production of organic acids produced by bacteria and the formation of dental biofilm justifies the use of such properties for the treatment and prevention of biofilm-dependent oral infections.[4]
For prevention/treatment of periodontal disease, the molecular components of cranberry also play an important role. The growth of two species of bacteria, Porphyromonas gingivalis and Fusobacterium nucleatum, associated with chronic periodontitis, is inhibited by cranberry extract.[5] Cranberry can also inhibit the adhesion of P. gingivalis to various proteins, including type I collagen, resulting in a further reduction of bacterial coaggregation in periodontopathogenic biofilms.[5]
Furthermore, Bodet et al. reported that cranberry components, specifically polyphenols, inhibited the proteolytic activity of red-complex bacteria. These bacteria, P. gingivalis, Treponema denticola, and Tannerella forsythia play an important role in the destruction of periodontal tissues.[6,7] These observations suggest that the polyphenols present in cranberry have the potential to suppress the proliferation of these species of bacteria in periodontal pockets by limiting the availability of amino acids and peptides, on which their growth depends.[1]
Considering that bacteria are the primary factors in the etiology of periodontal disease and that an uncontrolled immune response in the host can lead to the destruction of soft tissue and alveolar bone resorption, a study presenting scientific evidence supporting the effectiveness of cranberry extract in combating periodontal disease is of great significance. In addition, determining which components are associated with this action, as well as the main components of the extract that act on periodontopathogenic biofilms, is of paramount importance in understanding its clinical application. Therefore, the aim of the present study was to evaluate, through an integrative literature review, the effectiveness of cranberry extract on periodontopathogenic biofilm.
MATERIALS AND METHODS
An integrative review of all in vitro and in vivo laboratory and clinical studies published in literature was conducted to assess the effectiveness of cranberry extract against periodontal biofilm. The inclusion criteria were: in vitro and in vivo laboratory studies which evaluated the effect of cranberry extract on the growth, coaggregation and formation of periodontal biofilm, and which involved the application of cranberry extract and/or juice on planktonic and biofilm cultures of periodontal bacteria. No date or language restrictions were applied. Laboratory studies that evaluated the action of cranberry extract on pro-inflammatory chemical mediators such as interleukins and prostaglandins were excluded, along with studies that evaluated the effect of cranberry extract combined with another substance, herbal or otherwise, and studies that did not have a control group.
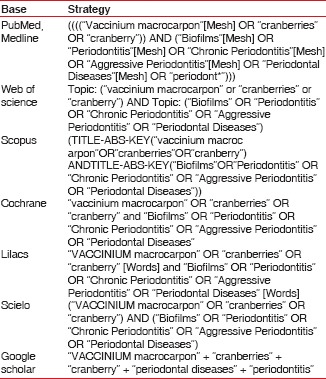
The electronic search strategies were performed independently by three researchers, from March to August 2015, on the following databases: Cochrane Library, MEDLINE, Web of Science, Scopus, LILACS, Scielo and Google Scholar, using the following descriptors and/or keywords: “Aggressive Periodontitis”[Mesh], “Biofilms”[Mesh], “Chronic Periodontitis”[Mesh], “cranberries”, “cranberry”, “periodont*”. The search strategies used for each data base are described in Chart 1, “Periodontal Diseases”[Mesh], “Periodontitis”[Mesh], “Vaccinium macrocarpon”[Mesh].
Chart 1.
Search strategies used for each database
After the database searches, the titles and abstracts were listed using a standardized form. The three researchers, using the same eligibility criteria, then selected the studies with the potential to be read in full and included in the review.
The data from the studies that were read in full and included in the review were recorded in a data extraction sheet by three authors who, independently and in a group of three, recorded the relevant data of the study (sample, country where the study was performed), methodological characteristics, details of extracts used, concentrations, and outcomes.
In the event of disagreement, the authors consulted the fourth author and through consensus, reached a joint decision.
RESULTS
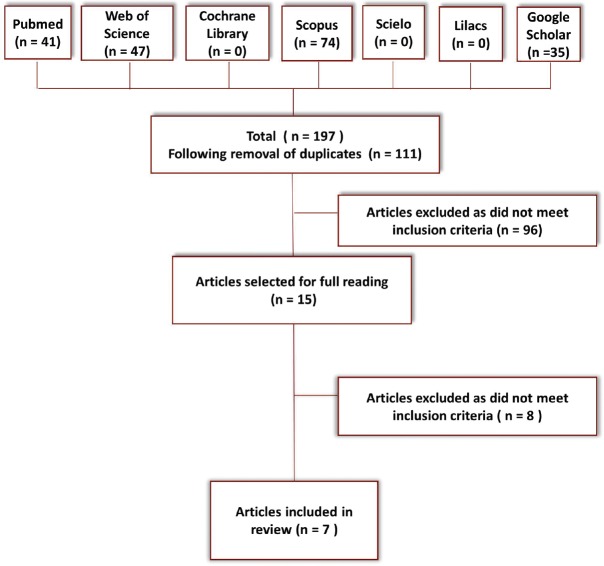
The electronic and manual search strategy found 197 titles and abstracts. Of these, 15 were selected in accordance with the inclusion and exclusion criteria and read in their entirety. Finally, eight studies were selected for inclusion in the review [Figure 1 and Chart 2].
Figure 1.
Flowchart of studies analyzed
Chart 2.
Articles excluded following complete reading and reasons for exclusion

The majority of studies selected featured in vitro analyses. In these, high molecular weight components of cranberry extract (nondialyzable materials [NDM]), polyphenol fractions, and A-type cranberry proanthocyanidins (AC-PAC) in different concentrations (from 1.56 μg/ml to 10 mg/ml) were used. Their effects were mostly analyzed on planktonic cultures and periodontopathogenic bacteria biofilms [Table 1].
Table 1.
Characteristics and summary of results of studies included in the review
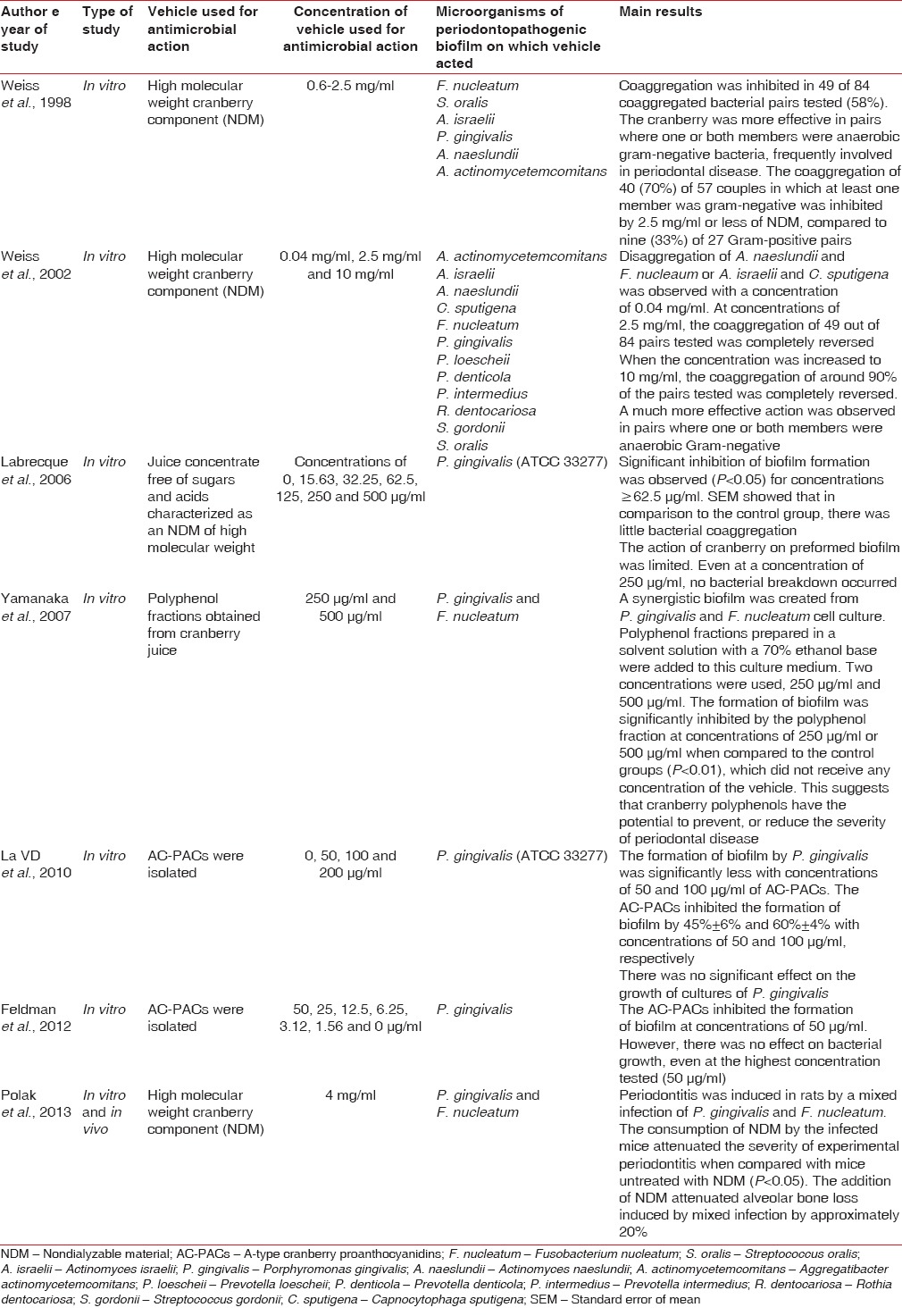
Weiss et al., 1998, performed in vitro analysis of the effect of a cranberry juice concentrate on bacterial coaggregation and disaggregation.[15] This was characterized as an NDM of high molecular weight derived from American cranberries. The authors added 0.05 ml of the concentrate (NDM) to preformed aggregates of two bacterial strains in mixtures of equal volume (0.05 ml). The results showed that coaggregation in 49 (58%) of the 84 pairs tested was completely reversed with NDM concentrations equal to or < 2.5 mg/ml. The study also showed that the NDM preferably acted on pairs where one or both members were Gram-Negative bacteria. In addition, it was found that the NDM was more effective at inhibiting coaggregation, than at disaggregating preformed coaggregates. For example, only 0.25 mg/ml of NDM was needed to completely inhibit coaggregation of a tested bacterial pair, while a higher concentration of 1 mg/ml NDM was needed to reverse the coaggregation of the same pair. In another study by the same authors, in 2002, the concentration of NDM was increased to 10 mg/ml.[16] In this study, around 90% of the coaggregation pairs tested were completely reversed, demonstrating that, in this case, a higher concentration resulted in greater inhibition of bacterial aggregation.[16]
Labrecque et al., in 2006, evaluated the effect of concentrated cranberry juice on the growth, adhesion and biofilm formation of P. gingivalis.[17] The juice concentrate was obtained from Ocean Spray Cranberries, Inc (Lakeville-Middleboro, MA, USA). It was dialyzed, lyophilized and dissolved in distilled water before use. Samples containing 50 μL of the solution were applied to the bacterial cultures. The effect of cranberry on bacterial growth was visually evaluated through comparison with the control group, which consisted of a saline solution of phosphate buffer and bacterial strains (pH 7.2). Biofilm formation was assessed by absorbance at 550 nm (A550). The results of the study revealed that cranberry was effective at inhibiting biofilm formation when compared to the control group when the concentration was < 62.5 μg/ml. However, the effect on preformed biofilm was limited, with even higher concentrations proving ineffective.
Yamanaka et al., 2007, in an in vitro study, obtained polyphenol fractions from cranberry juice, in concentrations of 250 and 500 μg/ml.[5] This resulted in a significant inhibition of biofilm formation when used on P. gingivalis and F. nucleatum, in concentrations of both 250 μg/mL and 500 μg/ml, when compared with the controls (P < 0.01), in which no concentration of the vehicle was used.
La et al., 2010 and Feldman, 2012 analyzed the effect of AC-PACs on the growth and biofilm formation of P. gingivalis (ATCC 33277).[18,19] Growth was assessed by a reading of the optical density with a microplate reader. Biofilm formation was assessed by absorbance at 550 nm (A550) after the cultures had been stained with crystal violet for 15 min. In both studies, biofilm formation was inhibited at concentrations equal to or >50 μg/ml. However, bacterial growth was not affected.
Polak et al., 2013, carried out in vitro and in vivo analysis of the action of high molecular weight components of cranberry extract (NDM) at a concentration of 4 mg/ml in rats with periodontitis induced by a mixed infection of P. gingivalis and F. nucleatum.[20] NDM consumption by the infected mice reduced the severity of experimental periodontitis in comparison to mice untreated with NDM (P < 0.05), and adding NDM to the mixed infection attenuated alveolar bone loss by approximately 20%.
DISCUSSION
The present study aimed to gather scientific evidence to assess the effectiveness of cranberry extract on periodontal disease, through an integrative review. All types of studies that met the established inclusion criteria were included in this study. It was not possible to perform a systematic review of randomized controlled trials, which have a high level of scientific evidence, due to the absence of such studies in existing literature, as research involving cranberry extract is still at the laboratory level.
The results of the search strategy identified a small number of studies that evaluated the effect of cranberry extract on periodontal disease. No human study has found, indicating that the level of scientific evidence, in terms of meeting the objective of this study, was low.
In recent years, the use of cranberry to treat urinary tract infections, especially in women, has shown satisfactory results in randomized controlled clinical trials.[21] This herbal medicine is responsible for inhibiting the adherence of Escherichia coli and other pathogens to the epithelial cells and for selecting bacteria with lower adhesion potential in the gastrointestinal tract, thereby reducing the formation of biofilms.[22] Its potential action on Gram-negative bacteria, therefore, generates possible discussions about the existence of inhibitory effects on periodontal bacteria, which are also associated with peri-implant disease.
The studies included in this review used different types of vehicles to verify the antibacterial action of cranberry extract on periodontopathogenic biofilm. Among the microorganisms studied, P. gingivalis and F. nucleatum were the most studied, as these are the anaerobic bacteria most related to the biofilm in question, one of the main etiological factors of chronic periodontitis.[23,24] The vehicles consisted mainly of polyphenol fractions, high molecular weight components of cranberry (NDM) and AC-PAC. The concentrations also varied, indicating that research in this area has not reached a consensus on the ideal vehicle for cranberry, or the optimal concentrations for the greatest antimicrobial action on periodontopathogenic biofilm.
In general, the studies included in the review identified one limitation of cranberry compounds: that they failed to significantly inhibit bacterial growth or promote the breakdown of preformed biofilm. They did, however, effectively inhibit the formation of P. gingivalis and F. nucleatum biofilm at concentrations equal to or >62.5 μg/ml.[13,15,16,17,18,19,20] The mechanism of action involved in this inhibition is still controversial. Some believe that cranberry causes a modification of the hydrophobicity of the molecular surface of bacteria.[13] Others, however, report that compounds derived from cranberry can cause the inhibition of cariogenic biofilm enzymes and proteases of periodontopathogenic biofilm.[7,18]
While this is not yet sufficiently clear, what is known is that irrespective of the fact that bacterial viability remains unchanged, if there is no biofilm formation, the chances of developing periodontal disease, and consequently peri-implant disease are lessened.
Although most of the studies presented certain methodological limitations (none compared the effectiveness of cranberry to an antimicrobial considered “gold standard” and there was no consensus on the optimal concentration of the vehicles used), the in vitro and in vivo laboratory studies performed until, nowadays, identify an inhibiting effect of cranberry on periodontal bacteria and serve as a support for the development of further studies assessing the most effective vehicle and the ideal concentration to be used, without causing adverse effects on oral tissues. From such studies, in the future, more accurate results will be obtained, which will, in turn, allow the application and clinical evaluation of cranberry extract on patients with the periodontal disease with greater safety. This is important as an alternative to the use of synthetic antibiotics, which are often associated with cases of bacterial resistance and numerous adverse effects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bonifait L, Grenier D. Cranberry polyphenols: Potential benefits for dental caries and periodontal disease. J Can Dent Assoc. 2010;76:a130. [PubMed] [Google Scholar]
- 2.LaPlante KL, Sarkisian SA, Woodmansee S, Rowley DC, Seeram NP. Effects of cranberry extracts on growth and biofilm production of Escherichia coli and Staphylococcus species. Phytother Res. 2012;26:1371–4. doi: 10.1002/ptr.4592. [DOI] [PubMed] [Google Scholar]
- 3.Girardot M, Guerineau A, Boudesocque L, Costa D, Bazinet L, Enguehard-Gueiffier C, et al. Promising results of cranberry in the prevention of oral candida biofilms. Pathog Dis. 2014;70:432–9. doi: 10.1111/2049-632X.12168. [DOI] [PubMed] [Google Scholar]
- 4.Feghali K, Feldman M, La VD, Santos J, Grenier D. Cranberry proanthocyanidins: Natural weapons against periodontal diseases. J Agric Food Chem. 2012;60:5728–35. doi: 10.1021/jf203304v. [DOI] [PubMed] [Google Scholar]
- 5.Yamanaka A, Kouchi T, Kasai K, Kato T, Ishihara K, Okuda K, et al. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J Periodontal Res. 2007;42:589–92. doi: 10.1111/j.1600-0765.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 6.Bodet C, Chandad F, Grenier D. Anti-inflammatory activity of a high-molecular-weight cranberry fraction on macrophages stimulated by lipopolysaccharides from periodontopathogens. J Dent Res. 2006;85:235–9. doi: 10.1177/154405910608500306. [DOI] [PubMed] [Google Scholar]
- 7.Bodet C, Piché M, Chandad F, Grenier D. Inhibition of periodontopathogen-derived proteolytic enzymes by a high-molecular-weight fraction isolated from cranberry. J Antimicrob Chemother. 2006;57:685–90. doi: 10.1093/jac/dkl031. [DOI] [PubMed] [Google Scholar]
- 8.Sethi R, Govila V. Inhibitory effect of cranberry juice on the colonization of streptococci species: An in vitro study. J Indian Soc Periodontol. 2011;15:46–50. doi: 10.4103/0972-124X.82271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'May C, Tufenkji N. The swarming motility of pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl Environ Microbiol. 2011;77:3061–7. doi: 10.1128/AEM.02677-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palaska I, Papathanasiou E, Theoharides TC. Use of polyphenols in periodontal inflammation. Eur J Pharmacol. 2013;720:77–83. doi: 10.1016/j.ejphar.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 11.Babu J, Blair C, Jacob S, Itzhak O. Inhibition of Streptococcus gordonii metabolic activity in biofilm by cranberry juice high-molecular-weight component. J Biomed Biotechnol. 2012;2012:590384. doi: 10.1155/2012/590384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg D, Feldman M, Ofek I, Weiss EI. Effect of a high-molecular-weight component of cranberry on constituents of dental biofilm. J Antimicrob Chemother. 2004;54:86–9. doi: 10.1093/jac/dkh254. [DOI] [PubMed] [Google Scholar]
- 13.Yamanaka A, Kimizuka R, Kato T, Okuda K. Inhibitory effects of cranberry juice on attachment of oral streptococci and biofilm formation. Oral Microbiol Immunol. 2004;19:150–4. doi: 10.1111/j.0902-0055.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 14.Signoretto C, Canepari P, Stauder M, Vezzulli L, Pruzzo C. Functional foods and strategies contrasting bacterial adhesion. Curr Opin Biotechnol. 2012;23:160–7. doi: 10.1016/j.copbio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Weiss EI, Lev-Dor R, Kashamn Y, Goldhar J, Sharon N, Ofek I, et al. Inhibiting interspecies coaggregation of plaque bacteria with a cranberry juice constituent [published erratam appear in J Am Dent Assoc 1999 Jan; 130(1):36 and 1999 Mar; 130 (3):332] J Am Dent Assoc. 1998;129:1719–23. doi: 10.14219/jada.archive.1998.0141. [DOI] [PubMed] [Google Scholar]
- 16.Weiss EL, Lev-Dor R, Sharon N, Ofek I. Inhibitory effect of a high-molecular-weight constituent of cranberry on adhesion of oral bacteria. Crit Rev Food Sci Nutr. 2002;42:285–92. doi: 10.1080/10408390209351917. [DOI] [PubMed] [Google Scholar]
- 17.Labrecque J, Bodet C, Chandad F, Grenier D. Effects of a high-molecular-weight cranberry fraction on growth, biofilm formation and adherence of Porphyromonas gingivalis. J Antimicrob Chemother. 2006;58:439–43. doi: 10.1093/jac/dkl220. [DOI] [PubMed] [Google Scholar]
- 18.La VD, Howell AB, Grenier D. Anti-Porphyromonas gingivalis and anti-inflammatory activities of A-type cranberry proanthocyanidins. Antimicrob Agents Chemother. 2010;54:1778–84. doi: 10.1128/AAC.01432-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman M, Grenier D. Cranberry proanthocyanidins act in synergy with licochalcone A to reduce Porphyromonas gingivalis growth and virulence properties, and to suppress cytokine secretion by macrophages. J Appl Microbiol. 2012;113:438–47. doi: 10.1111/j.1365-2672.2012.05329.x. [DOI] [PubMed] [Google Scholar]
- 20.Polak D, Naddaf R, Shapira L, Weiss EI, Houri-Haddad Y. Protective potential of non-dialyzable material fraction of cranberry juice on the virulence of P. Gingivalis and F. Nucleatum mixed infection. J Periodontol. 2013;84:1019–25. doi: 10.1902/jop.2012.120331. [DOI] [PubMed] [Google Scholar]
- 21.Micali S, Isgro G, Bianchi G, Miceli N, Calapai G, Navarra M, et al. Cranberry and recurrent cystitis: More than marketing? Crit Rev Food Sci Nutr. 2014;54:1063–75. doi: 10.1080/10408398.2011.625574. [DOI] [PubMed] [Google Scholar]
- 22.Vostalova J, Vidlar A, Simanek V, Galandakova A, Kosina P, Vacek J, et al. Are high proanthocyanidins key to cranberry efficacy in the prevention of recurrent urinary tract infection? Phytother Res. 2015;29:1559–67. doi: 10.1002/ptr.5427. [DOI] [PubMed] [Google Scholar]
- 23.Lamont RJ, Jenkinson HF. Life below the gum line: Pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–63. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamont RJ, Jenkinson HF. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol Immunol. 2000;15:341–9. doi: 10.1034/j.1399-302x.2000.150601.x. [DOI] [PubMed] [Google Scholar]