Abstract
Objective:
To investigate the relationship between tumor-associated macrophages (TAMs), neovascularization, and tumor cell migration in oral squamous cell carcinoma (OSCC) of an African subpopulation.
Materials and Methods:
Twenty OSCC paraffin blocks underwent immunohistochemistry to TAM1 (CCR7), TAM2 (CD206), Twist, E-cadherin, N-cadherin, and CD34. The relative percentage of CCR7 + and CD206 + cells to overall immune cell population was calculated for three high power fields and an average was taken. TAM-related microvessel density (MVD) was determined as the mean of the three recorded values. Cases that had no CD34 + vessels adjacent to the TAMs region were regarded as having an MVD score of 0.
Results:
Ten cases (50%) expressed greater CCR7 activity than CD206, seven cases (35%) expressed approximately equal activity of CCR7 and CD206, while three cases (15%) expressed greater activity of CD206 than CCR7. Twist expression was strong in some cases with strong N-cadherin and weak E-cadherin, but the expression of Twist was not consistently high in all cases that expressed strong N-cadherin and weak E-cadherin.
Conclusions:
TAMs distribution suggested antitumor activity and the potential for tumor metastasis was only partly due to Twist-mediated epithelial–mesenchymal transition.
Keywords: Angiogenesis, E-cadherin, N-cadherin, oral squamous cell carcinoma, tumor-associated macrophages, Twist, Angiogenèse, E-cadhérine, Le carcinome épidermoïde oral, Les macrophages associés aux tumeurs, N-cadhérine, Twist
Résumé
Objectif:
Étudier la relation entre les macrophages associés à la tumeur (TAM), la néovascularisation et la migration des cellules tumorales dans le carcinome épidermoïde oral (OSCC) d’une sous-population africaine.
Matériaux et méthodes:
Vingt blocs de paraffines OSCC ont subi une immunohistochimie à TAM1 (CCR7), TAM2 (CD206), Twist, E-cadhérine, N-cadhérine et CD34. Le pourcentage relatif de cellules CCR7+ et CD206+ dans la population globale de cellules immunitaires a été calculé pour 3 champs de puissance élevée et une moyenne prise. La densité de microvaisseaux (MVD) liée aux TAM a été déterminée comme la moyenne des trois valeurs enregistrées. Les cas qui n’avaient pas de navires CD34+ adjacents à la région des TAM étaient considérés comme ayant un score MVD de 0.
Résultats:
Dix cas (50%) ont exprimé une plus grande activité CCR7 que CD206, 7 cases (35%) ont exprimé une activité approximativement égale de CCR7 et CD206, tandis que 3 cas (15%) ont exprimé une plus grande activité de CD206 que CCR7. L’expression de la Twist était forte dans certains cas avec une N-cadhérine forte et une E-cadhérine faible, mais l’expression de la Twist n’était pas toujours élevée dans tous les cas, ce qui exprimait une N-cadhérine forte et une E-cadhérine faible.
Conclusions:
La distribution des TAM a suggéré une activité antitumorale et le potentiel de métastases tumorales était seulement dû à l’EMT à effet de Twist.
INTRODUCTION
The tumor microenvironment has such a great impact on the status of the tumor cells that it has been reported that a normal microenvironment can prevent malignant cells from committing to neoplasia and even reverse the malignant phenotype though the cancer cells retain their mutations.[1] The progression of tumors is characterized by the recruitment of innate and adaptive immune cells through cytokines and chemokines to the tumor site, and macrophages are especially abundant during tumor evolution.[2] Macrophages are hematopoietic cells in the innate immune system, which phagocytose cellular debris and foreign particles, and present antigens to T-cells during normal immune responses. They are influenced by their microenvironment during normal function and are differently activated depending on the tissue and the microenvironment. Unlike macrophages in a normal, healthy tissue or wound-healing environment, tumor-associated macrophages (TAMs) are modified in the context of the tumor microenvironment and lose the ability to phagocytize cancer cells or present tumor antigens to T-cell, thus becoming active co-conspirators in cancer progression.[3]
The role of TAMs is controversial because there is evidence for their participation in protumor as well as antitumor activities.[4] TAMs release several potent pro-angiogenic cytokines and growth factors such as vascular endothelial growth factor, tumor necrosis factor alpha, and interleukin-8. They also express some angiogenesis-modulating enzymes including matrix metalloproteinase-2 (MMP-2) and MMP-9.[5] Therefore, TAMs also support tumor growth by stimulating neovascularization that is essential for tumor progression.
It is also reported that TAMs appear to play roles in the release of metastatic cells from the primary tumor as well as the establishment of secondary tumors at distant sites.[6] TAMs actually exist in two distinctive states: one is the classically activated (M1) state and the other is the otherwise activated (M2) state. M1 macrophages possess antitumor activity, whereas M2 macrophages support tumor invasion and metastasis.[7] As these epithelial cells prepare for migration or invasion, they tend to go through the phase of epithelial–mesenchymal transition (EMT). The relative amount of change in this phase can be measured by E-cadherin (epithelial marker) and N-cadherin (mesenchymal marker).
We will investigate the relationship between TAMs, neovascularization, and tumor cell migration in oral squamous cell carcinoma (OSCC) with specific reference to an African subpopulation, to understand more of its biology. This is because OSCC in Sub-Saharan Africa occurs in younger individuals as compared to the Caucasian population, and only has weak association to established carcinogens.[8]
MATERIALS AND METHODS
Twenty formalin-fixed paraffin-embedded (FFPE) blocks of OSCC cases from the Oral Pathology Department of the University College Hospital, University of Ibadan, Nigeria, were sectioned and stained with hematoxylin and eosin for reevaluation and inclusion. At the Frankfurt Orofacial Regenerative Medicine Laboratory, Department for Oral, Cranio-Maxillofacial and Facial Plastic Surgery, Medical Center of the Goethe University Frankfurt, Frankfurt am Main, Germany, sections were prepared for immunohistochemistry to TAM1, TAM2, Twist, E-cadherin, N-cadherin, and anti-CD34 antibody staining using the manufacturer's specification. All the FFPE blocks were each cut into six sections, deparaffinized using xylene, and hydrated with alcohol. The tissue was immersed in heat-induced epitope retrieval 10 mMol citrate buffer pH 6.0 (TA-250-PM1X), diluted 1:100 with distilled water, and incubated at 95°C for 20 min. They were cooled in the buffer for 20 min and then rinsed in phosphate buffered saline for 5 min. Positive and negative controls were employed for each antibody. Thermo-Scientific peroxidase blocking reagent was added to each section for 15 min, and the sections were rinsed in 0.1% TBST for 5 min. The specimens were incubated for 60 min with the antibodies; Abcam mouse monoclonal anti-CCR7 antibody (Y59) (ab32527) (dilution 1:1000), Abcam rabbit polyclonal anti-CD206 antibody (anti-mannose receptor antibody) (ab64693) (dilution 1:1000), Dako mouse monoclonal anti-CD34 antibody QBEnd-10 (M7165) (dilution 1:25), Abcam mouse monoclonal anti-Twist antibody (10E4E6) (ab175430) (dilution 1:100), Abcam mouse monoclonal anti-E-cadherin antibody (HECD-1) (ab1416) (dilution 1:200), and Abcam mouse monoclonal anti-N-cadherin antibody (5D5) (ab98952) (dilution 1:200). They were then rinsed with TBST, followed by incubation with prediluted (ready-to-use) UltraVision Quanto Detection System/Horseradish peroxidase for 15 min. An appropriate volume of 1 ml of diaminobenzidine substrate with one drop of diaminobenzidine chromogen was added to cover the slides, followed by incubation in a humidity chamber for 5 min. The sections were then immersed in aqueous Gill's hematoxylin for 10 s and rinsed in distilled water for 5 min. The tissue was dehydrated and subsequently rinsed with xylene. DPX was applied, and a cover slip was placed. Cytoplasmic/membrane staining was taken as positive for E-cadherin, N-cadherin, CCR7, CD206, and CD34. Nuclear brown staining was considered as positive for Twist.
Quantification and scoring of CCR7 and CD206
Stained tissue sections were analyzed using a semiquantitative method with a light microscope at ×400 magnification. Three representative high power fields (hpf) showing maximum immunopositivity adjacent to tumor cells was selected for scoring by cell counting. The relative percentage of CCR7 + and CD206 + cells in relation to overall cellularity was calculated for each hpf, and the final score was taken as an average of the three hpf. Final scores for CCR7 and CD206 were classified as: <5%, 5%–25%, >25%–50%, and >50% (Steidl et al., 2010).
Quantification and scoring of Twist, N-cadherin, and E-cadherin
The Sinicrope scoring method was used to evaluate both the intensity of the immunohistochemical staining and the proportion of the stained epithelial cells. The staining intensity was classified as weak, moderate, or strong. The positive cells were quantified as a percentage of the total number of epithelial cells and assigned to one of the five categories (0, <5%; 1, 5%–25%; 2, 26%–50%; 3, 51%–75%; 4, >75%). The percentage of positivity of the tumor cells and the staining intensities were then multiplied to generate an immunoreactive score. The product of the proportion and intensity scores were calculated such that a final score of 0 indicated no expression, 0–4 indicated weak expression, 5–8 indicated moderate expression, and 9–12 indicated strong expression.
Quantification and scoring of CD34
TAM-related microvessel density (MVD) was evaluated by choosing three fields, with the highest number of vessels at ×10, adjacent to the TAMs to assess association of the vessels to the macrophages. Then, counting the CD34-positive vessels at ×40 magnification, the MVD was calculated as the mean of the three recorded values. Cases that had no CD34+ vessels adjacent to the TAMs region were regarded as having an MVD score of 0.
All slides were viewed with a Nikon ECLIPSE 80i microscope (Nikon, Tokyo, Japan), and microphotographs of the stained sections were recorded with a connected digital camera DS-Fi1 together with a Nikon digital sight control unit (Nikon, Tokyo, Japan).
RESULTS
Twenty OSCC cases were evaluated comprising 4 (20%) well-differentiated squamous cell carcinomas, 15 (75%) moderately differentiated carcinomas, and 1 (5%) poorly differentiated carcinoma. Case-by-case review for percentage expression of CD206 and CCR7 was done, and 10 cases (50%) expressed greater CCR7 activity than CD206, 7 cases (35%) expressed approximately equal activity of CCR7 and CD206, while 3 cases (15%) expressed greater activity of CD206 [Figure 1] than CCR7 [Table 1].
Figure 1.
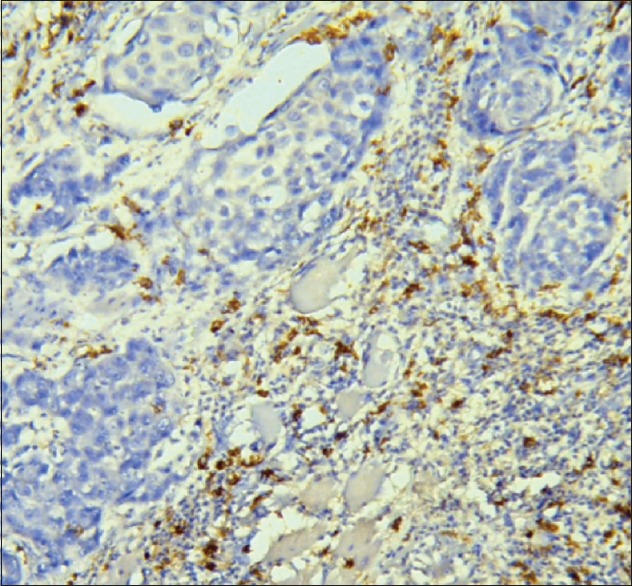
CD206-positive cells (25%–50%) adjacent to malignant epithelial nest of oral squamous cell carcinoma
Table 1.
Oral squamous cell carcinoma expression of CD206, CCR7, Twist, N-cadherin, E-cadherin, and CD34
| Grade of cancer | CD206 (%) | CCR7 (%) | Twist | N-cadherin | E-cadherin | CD34 (MVD) |
|---|---|---|---|---|---|---|
| WDSCC | 5-25 | <5 | None | Weak | None | 0 |
| 5-25 | >50 | Weak | Strong | None | 0 | |
| 5-25 | >50 | Weak | Moderate | Weak | 14 | |
| <5 | >50 | Weak | Moderate | Weak | 8 | |
| MDSCC | <5 | <5 | None | Weak | Weak | 0 |
| >50 | >50 | Weak | Strong | Moderate | 22 | |
| 25-50 | >50 | Strong | Strong | Weak | 4 | |
| 25-50 | >50 | None | Strong | Weak | 16 | |
| <5 | >50 | Moderate | Strong | Moderate | 8 | |
| <5 | >50 | None | Weak | Weak | 0 | |
| >50 | >50 | Moderate | Moderate | None | 0 | |
| 25-50 | 25-50 | Weak | Moderate | Weak | 0 | |
| >50 | >50 | None | Weak | Moderate | 17 | |
| >50 | 5-25 | Strong | Strong | Weak | 8 | |
| >50 | >50 | Strong | Moderate | Moderate | 15 | |
| >50 | 5-25 | None | Strong | Moderate | 8 | |
| <5 | >50 | Strong | Strong | Moderate | 19 | |
| <5 | 5–25 | Strong | Strong | Moderate | 8 | |
| >50 | >50 | Moderate | Moderate | Weak | 5 | |
| PDSCC | <5 | >50 | Moderate | Strong | Weak | 24 |
WDSCC=Well-differentiated squamous cell carcinoma, MDSCC=Moderately differentiated squamous cell carcinoma, PDSCC=Poorly differentiated squamous cell carcinoma, MVD=Microvessel density
For the same cases, every time, that Twist was strongly expressed (5 cases) [Figure 2], N-cadherin was also strongly expressed [Figure 3] (4 cases strong and 1 case moderate), and E-cadherin was weakly/moderately expressed (2 cases weak and 3 cases moderate). Contrariwise every time, that Twist was weakly expressed (5 cases), N-cadherin was strongly/moderately expressed (2 cases strong and 3 cases moderate), and E-cadherin was weak (3 cases), moderate (1 case), and not expressed at all (1 case) [Table 1].
Figure 2.
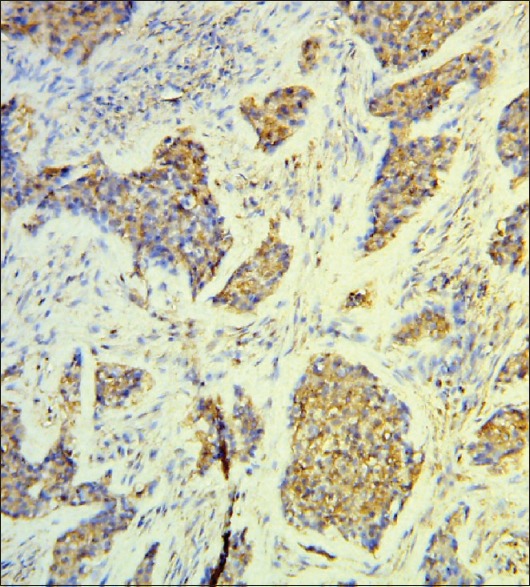
Twist ++ positive malignant epithelial cells
Figure 3.
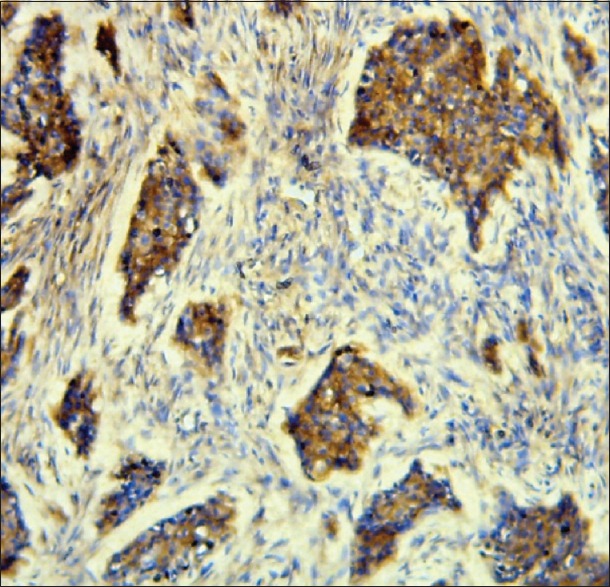
N-cadherin +++ positive malignant epithelial cells
The average MVD for three categories of N-cadherin was calculated. For weak N-cadherin expression, MVD = 4.3; for moderate N-cadherin expression, MVD = 7.0; and for strong N-cadherin expression, MVD = 10.3.
DISCUSSION
Most of our cases expressed higher CCR7 than CD206 on a case-by-case evaluation, signifying a higher activity of TAM1 as compared to TAM2 and hence suggesting good prognosis. This describes a tumor microenvironment that is mostly antitumor, unlike the submission by Lewis and Pollard,[3] where the microenvironment is a co-conspirator to promote tumor progression. Although most cases could be said to have good prognosis in this study based on the microenvironment biology, the neoplastic cells displayed relatively strong N-cadherin expression, which promotes metastasis and angiogenesis and is associated with poor survival.[9] Consequently, we can conclude that the patients’ outlook is still not so good. Our cases also showed a higher MVD average when N-cadherin expression was strong, which is in agreement with the report by Mariotti et al.[9]
The EMT is a biological event in which epithelial cells lose many of their phenotypic features and gain extra properties typical of mesenchymal cells.[10] However, abnormal expression of cadherins, or cadherin switching, is a key event in the EMT of some cancers, including OSCC.[11] In our study, most cases expressed N-cadherin while E-cadherin was decreased, this is a mark of EMT. On the contrary, in a study by Costa et al.,[10] none of their OSCC cases expressed N-cadherin but a reduction of E-cadherin, especially on the invasive front, and they interpreted this also as EMT. This is because not only the loss of E-cadherin but also the expression of N-cadherin increases the mobility of epithelial cells and its capacity for invasion.[10]
Twist expression was strong in some cases with strong N-cadherin and weak E-cadherin, supporting the concept of Twist-mediated EMT. However, we noted that the expression of Twist was not consistently high in all cases that expressed strong N-cadherin and weak E-cadherin. This suggests that EMT was being mediated through some other means, and actually, it has been reported that the presence of Twist, although weak, can stimulate Slug (a member of Snail family of zinc finger transcription factors) and induce an E- to N-cadherin switch.[12] This advises the decision that in treatment planning targeting EMT mediators, both Twist and Slug could form therapeutic foci.
CONCLUSION
We found that TAMs were mostly antitumor and that the potential for tumor metastasis was partly due to Twist-mediated EMT. For OSCC in this cohort, management should include analysis of both the existing immune competence of the tumor microenvironment and the dynamics affecting metastasis of the neoplastic cells.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We wish to thank Mrs. Verena Hoffmann and Mr. Babajide Okedere for all the technical assistance provided during this study.
REFERENCES
- 1.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noy R, Pollard JW. Tumor-associated macrophages: From mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 4.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: Implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 5.Lewis CE, Leek R, Harris A, McGee JO. Cytokine regulation of angiogenesis in breast cancer: The role of tumor-associated macrophages. J Leukoc Biol. 1995;57:747–51. doi: 10.1002/jlb.57.5.747. [DOI] [PubMed] [Google Scholar]
- 6.Koide N, Nishio A, Sato T, Sugiyama A, Miyagawa S. Significance of macrophage chemoattractant protein-1 expression and macrophage infiltration in squamous cell carcinoma of the esophagus. Am J Gastroenterol. 2004;99:1667–74. doi: 10.1111/j.1572-0241.2004.30733.x. [DOI] [PubMed] [Google Scholar]
- 7.Richards DM, Hettinger J, Feuerer M. Monocytes and macrophages in cancer: Development and functions. Cancer Microenviron. 2013;6:179–91. doi: 10.1007/s12307-012-0123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faggons CE, Mabedi C, Shores CG, Gopal S. Review: Head and neck squamous cell carcinoma in sub-Saharan Africa. Malawi Med J. 2015;27:79–87. doi: 10.4314/mmj.v27i3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mariotti A, Perotti A, Sessa C, Rüegg C. N-cadherin as a therapeutic target in cancer. Expert Opin Investig Drugs. 2007;16:451–65. doi: 10.1517/13543784.16.4.451. [DOI] [PubMed] [Google Scholar]
- 10.Pyo SW, Hashimoto M, Kim YS, Kim CH, Lee SH, Johnson KR, et al. Expression of E-cadherin, P-cadherin and N-cadherin in oral squamous cell carcinoma: correlation with the clinicopathologic features and patient outcome. J Craniomaxillofac Surg. 2007;35:1–9. doi: 10.1016/j.jcms.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121(Pt 6):727–35. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Cheng Q, Zhou Y, Wang Y, Chen X. Slug is a key mediator of hypoxia induced cadherin switch in HNSCC: Correlations with poor prognosis. Oral Oncol. 2013;49:1043–50. doi: 10.1016/j.oraloncology.2013.08.003. [DOI] [PubMed] [Google Scholar]


