Abstract
The evidence is scarce on the association between age at puberty and semen quality. A cohort of 320 Danish men aged 18–21 years enrolled in the “Healthy Habits for Two” birth cohort provided self-reported data on pubertal indicators and delivered semen and blood samples. The results indicated an association between older age at pubertal development and lower semen quality and altered reproductive hormones concentrations as measured in young adult life. Men who had their first nocturnal emission, start of pubic hair growth and first voice break episode when older than 15 years had 37.0%, 45.0% and 32.7% lower sperm concentration; 37.8%, 44.2% and 29.1% lower total sperm count; 7.4%, 13.4% and 15.3% lower testosterone concentration; and 21.3%, 1.5% and 3.7% lower inhibin B concentration, respectively, compared with the men who were younger than 13 years at their first pubertal indicators. Only few of the results were statistically significant, but similar tendencies were seen in several of the reproductive parameters suggesting an association between the timing of pubertal development and reproductive health later in life.
Keywords: pubertal development, puberty, reproductive health, reproductive hormones, semen quality
INTRODUCTION
Poor semen quality is the main cause of reduced male fecundity.1 In some countries, the proportion of men with a suboptimal semen quality is high,1,2 and a recent Danish study found that up to 77% had a suboptimal semen quality.3 Despite this, only relatively few potential risk factors of poor semen quality have been identified, e.g., genetics,4 obesity,5 older age,6 prenatal and adult smoking,7,8 hormonal exposure and pollution.9,10 Factors that affect the male fecundity are important to identify in order to prevent poor semen quality.
During puberty, boys attain adult secondary sexual characteristics and reproductive capability.11 The levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) are elevated before physical signs of puberty, with pronounced pulsation frequency and amplitude,12 resulting in an increased release of testosterone and onset of the spermatogenesis. The rise in testosterone levels induces testicular growth, followed by growth of the penis and pubic hair.13 Thus, testosterone plays a fundamental role in male sexual and reproductive function,14 and a relation between pubertal development, semen quality and reproductive hormones in adulthood through a modification of the hormonal balance is plausible.
Currently, only two studies have investigated the association between pubertal development and male reproductive health. The studies indicated a relation between delayed puberty and lower male reproductive health in young adult life.15,16
MATERIALS AND METHODS
Study population
The participants were sons of mothers included in the “Healthy Habits for Two” cohort,17 established from April 1984 to April 1987 in Denmark. A total of 11 980 pregnant women (87% of all invited) participated and provided information on lifestyle and health-related factors before and during pregnancy. Among these, 11 144 delivered live-born singletons, of which 5716 were boys. Those alive and living in Denmark by December 2004 were identified in the Danish Civil Registration System (n = 5109).18 In 2005, a follow-up study, primarily designed to examine the association between prenatal smoking exposure and adult semen quality, was established.8 A total of 716 young men aged 18–21 years were invited from February 2005 to January 2006. Among these, 100 (14%) men declined participation and 251 (35%) men did not respond. A total of 18 (3%) men were excluded because they failed to appear at three appointments, were unable to be reached by phone, inclusion period had ended, or they had moved.
A total of 347 (49%) men agreed to participate in the study and delivered semen and blood samples. Further, 27 (4%) men did not answer any question on age at pubertal indicators and were excluded from the study; thus, 320 (45%) men constituted the final study population in this study. All participants were financially compensated (≈ €75) for participating in the study.
In 2005, all children of mothers from the “Healthy Habits for Two” cohort were invited to complete a web-based questionnaire on health and lifestyle factors, including information on pubertal development.19 A total of 2810 (55%) men responded. Among the 320 men in the present study population, 235 (73%) men participated in both data collections. In case of missing data on pubertal indicators from the questionnaire filled in when delivering semen and blood samples, we used information from the web-based questionnaire (nocturnal emission, n = 22; pubic hair, n = 0; and voice break, n = 62). If their answers differed between the two questionnaires, the answers from the questionnaire filled in when delivering semen and blood samples were used (nocturnal emission, n = 114; pubic hair, n = 0; and voice break, n = 55), as we believe that the participants in the semen sample study were more inclined to answer the questions and they might have thought more thoroughly before answering than in the web-based questionnaire.
The study was approved by the Regional Ethics Committee (registered number 20040174) and the Danish Data Protection Agency (record number 2014-41-3048).
Questionnaires and assessment of indicators of pubertal development
In both data collections, participants completed the same questionnaires with information on health-related, reproductive and lifestyle factors. In the present study, they also provided information related to their semen sample.
To assess age at pubertal development, participants were asked “Have you had your first nocturnal emission?,” “Do you have pubic hair?,” and “Has your voice broken?” If they answered “Yes,” they were asked to state the age in years and months at which the event first occurred. We converted the month into a fraction of a year and added years to create a continuous outcome variable for each of the three indicators. Four groups were defined for each of the pubertal indicators: <13 years (reference), 13–14 years, >14–15 years and >15 years. There were no men who stated a pubertal age below the age of 9 years; therefore, there were no cases of precocious puberty in the cohort (defined as development of secondary sexual characteristics before the age of 9 years in boys).20
Collection and analyses of semen and blood samples
Before collecting semen, the participants were instructed to be sexually abstinent for at least 48 h. Semen samples were collected by masturbating into a plastic container at home. The containers were to be kept close to the body during transportation to avoid a large temperature drop and brought to a mobile laboratory where a trained medical laboratory technologist performed the initial semen analysis blind to the age at pubertal development. Using an improved Neubauer hemocytometer (Paul Marienfeld, Bad Mergentheim, Germany), the sperm concentration was assessed using an appropriative dilution. Semen volume was estimated by the sample's weight (1 g = 1 ml). Total sperm count was calculated as the sperm concentration multiplied by semen volume. After liquefaction in a warmed chamber at 37°C, sperm motility was assessed by classifying 200 sperm cells within each of two fresh drops of semen as either motile (Grades A and B) or immotile (Grade C).21 Examination of 83.1% of the samples was initiated within the 1st h, where motility is most stable,22 and examination of 99.7% was initiated within 2 h. Further, sperm DNA integrity was analyzed by sperm chromatin structure assay (SCSA), which measures DNA damage by DNA fragmentation index (DFI).23 Smears were air dried, fixed in 95% (v/v) ethanol and stained with a modification of Papanicolaou stain. Sperm morphology was determined by strict categorization.24 The analysis of sperm motility and sperm concentration was performed in accordance with the World Health Organization (WHO) Laboratory Manual for Examination of Human Semen-Cervical Mucus Interactions.21 The laboratory took part in the European Society for Human Reproduction and Embryology (ESHRE) external quality control program, and all control tests were in agreement with their criteria.
Blood samples were drawn from the participants, and serum was stored at −80°C until analyzed for testosterone, estradiol, FSH, LH, sex hormone binding globulin (SHBG) and inhibin B by the trained medical laboratory technologists unaware of sample status. The concentrations of testosterone, estradiol, FSH and LH were analyzed by Avida Centaur (Bayer Healthcare, Leverkusen, Germany). SHBG concentrations were determined by the use of IMMULITE (DPC, Koge, Denmark). Inhibin B concentrations were measured by enzyme-linked immunosorbent assay (Oxford Bio-Innovation Ltd., Oxford, UK). In two samples, the concentration of inhibin B was below the detection limit for the specific assay (15.0 pg ml−1). Thus, the concentrations of the two samples were set to 14.0 pg ml−1 before the statistical analyses. The inhibin B samples were analyzed at the Laboratory of Reproductive Biology, University Hospital of Copenhagen, Denmark, and all other samples were analyzed at the Department of Clinical Chemistry, Aarhus University Hospital, Denmark.
Covariates
A priori, we identified potential confounders using direct acyclic graphs (DAGs).25
From the questionnaires completed during pregnancy, we had information on factors related to maternal lifestyle and health. The following covariates were included in all analyses: maternal smoking during pregnancy (nonsmoker [reference], past smoker, 1–4 cigarettes day−1, 5–10 cigarettes day−1, 11–15 cigarettes day−1, 16–20 cigarettes day−1, and >20 cigarettes day−1); maternal drinking during pregnancy (0 drinks week−1 [reference], 0.5–2 drinks week−1 and >2 drinks week−1); maternal prepregnancy body mass index (BMI) (<18.5, 18.5–24.9 [reference] and >24.9 kg m−2); and socioeconomic status based on the highest ranking of job description or academic background between the mother and father at the time of pregnancy (white-collar workers [reference], blue-collar workers, unemployed and students).
Prepubertal BMI was based on height and weight around 5–8 years of age collected from school nurse registers and was available for 240 (75%) men. We divided the prepubertal BMI into tertiles with the middle tertile as reference, and included it in all analyses.
From the men's questionnaires that were turned in when delivering the semen and blood samples, we had information on factors related to their lifestyle and health as well as information related to collection of the semen sample. Analyses on semen characteristics were adjusted for diseases in the reproductive organs (cryptorchidism, mumps, operations in penis or scrotum, epididymitis, gonorrhea, or chlamydia [no diseases as reference]) and abstinence time from the last ejaculation to semen sample (<48 h, 48 h–5 days [reference] and >5 days). Further, analyses on sperm concentration were adjusted for spillage when providing the semen sample (spillage vs no spillage [reference]). Sperm motility analyses were adjusted for time interval between ejaculation and semen sample analysis as a continuous variable. In the analyses of semen volume and total sperm count, we excluded men who reported spillage (n = 82). Reproductive hormones’ analyses were adjusted for the time of day that the blood sample was collected (<9 am [reference], 9–12 am or >12 am).
Statistical analysis
Missing data
The number (%) of young men who provided an age at first nocturnal emission, start of pubic hair growth and first voice break episode in years was 196 (61%), 162 (51%) and 210 (67%), respectively. Only 17 (5.3%) and 41 (12.8%) reported age in both years and months at first nocturnal emission and first voice break episode, respectively. None had answered age at start of pubic hair growth in both years and months. To replace missing data, we used multiple imputations, which provide unbiased estimates if data are missing at random.26
We generated 100 complete data sets. We included the exposure and outcome variables, the covariates used in the linear regression (see above), as well as age at first regular shaving, age at first acne episode, adult BMI, current coffee consumption, current alcohol intake, current smoking habits and age in the imputation model.
Data analyses
Semen and reproductive outcome variables were log-transformed before statistical analyses. Median (25th-75th centile) of the semen and reproductive hormone characteristics were reported for each of the four exposure groups as descriptive measures. We performed multivariate linear regression analyses with the three pubertal indicators as the independent variable separately, and estimated the adjusted percentage mean difference (95% confidence interval [CI]) in semen quality and reproductive hormones parameters with the youngest puberty group (<13 years) as reference. In addition, we estimated how the average semen quality and reproductive hormone levels were associated with boys’ age at onset at each of the three pubertal indicators (difference per year), i.e., we used the three recorded ages as continuous covariates in a linear regression with semen quality and reproductive hormone levels, respectively, as outcomes. Complete case analyses as well as sensitivity analyses were performed in addition to multiple imputations to allow comparison.
The statistical analyses were performed using Stata 13 software (Stata Corporation, College Station, TX, USA).
RESULTS
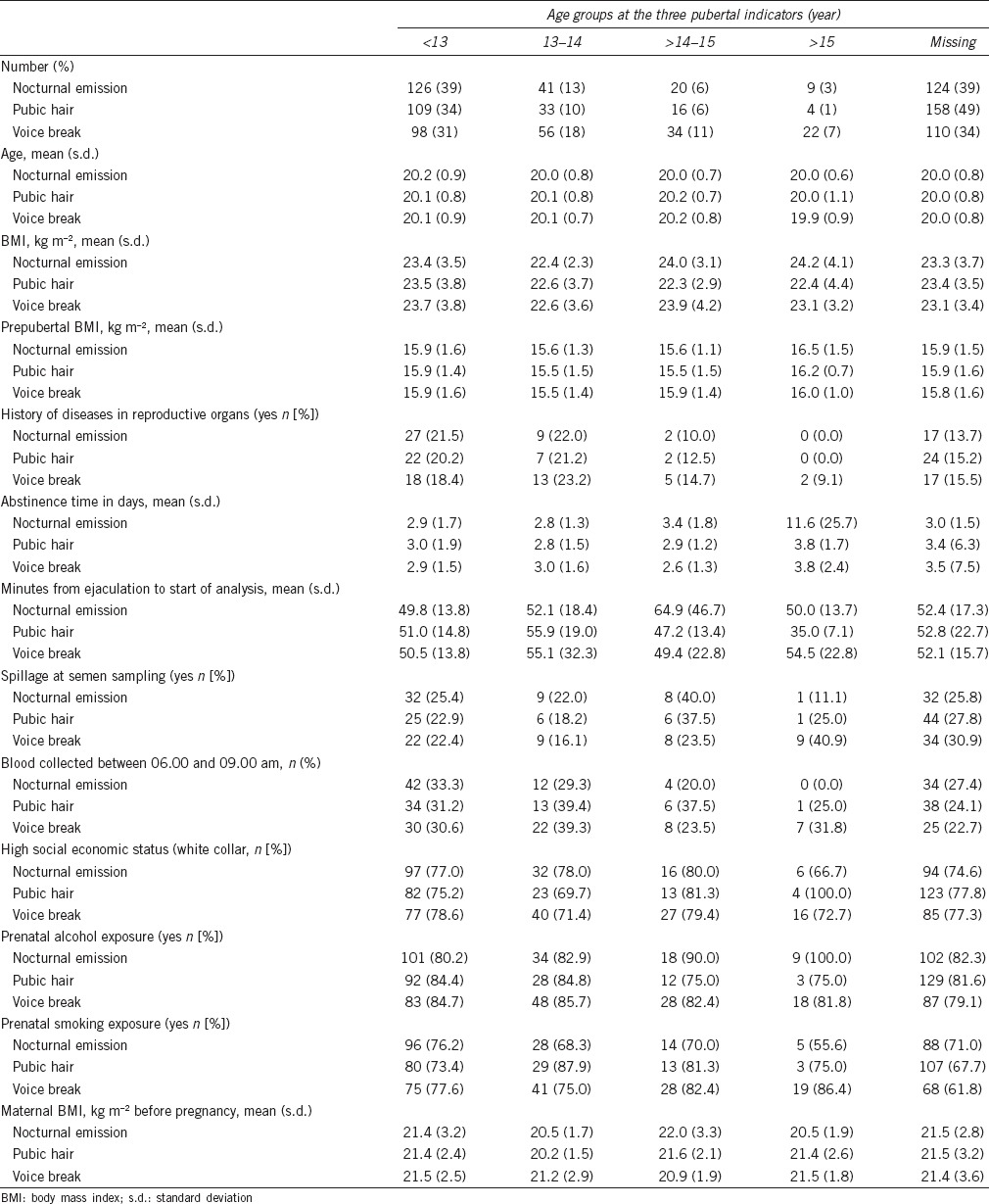
The characteristics of 320 participants according to age groups of the three indicators of pubertal development are listed in Table 1. Men with missing information on pubertal events had no systematic differences in any of the investigated variables compared with the men who provided the information. The median (25–75th centile) age of the 320 participants was 13.6 (12.8–14.6) years at first nocturnal emission, 13.6 (12.8–14.5) years at start of pubic hair growth and 14.2 (13.3–15.2) years at first voice break episode.
Table 1.
Characteristics of 320 males stratified by age at pubertal development, nonimputed data
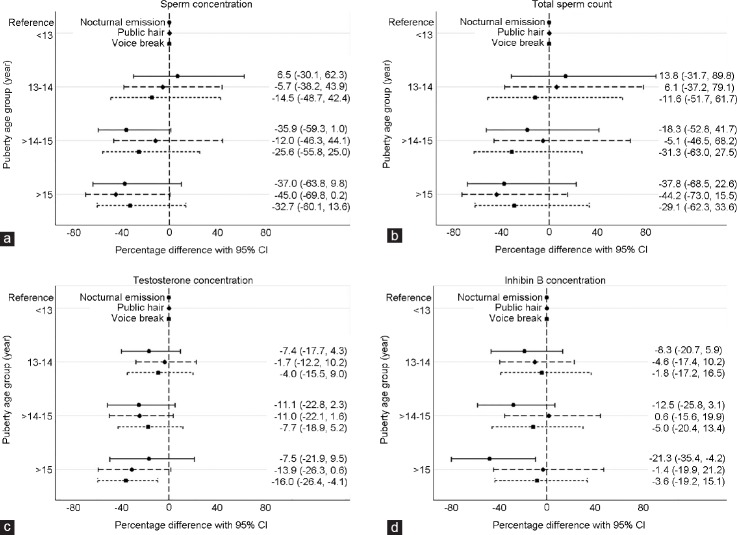
For all three indicators, the estimated difference per year ranged from 0.86 (95% CI: 0.74, 0.99) to 0.88 (95% CI: 0.78, 1.00) (Table 2), corresponding to a 12%–14% lower sperm concentration for each year of later onset of the pubertal indicators. Men who had experienced pubic hair growth after 15 years of age had a sperm concentration of −45.0% (95% CI: −69.8%, 0.2%) compared with men who started pubic hair growth before 13 years of age. Men in the oldest age group of first nocturnal emission and voice break had a sperm concentration of −37.0% (95% CI: −63.8%, 9.8%) and −32.7% (95% CI: −60.1%, 13.6%) compared with the youngest age group, respectively (Table 2 and Figure 1a).
Table 2.
Adjusted percentage difference (95% CI) from reference group (<13 years) for semen quality parameters according to age at indicators of pubertal development, imputed dataset (n=320)
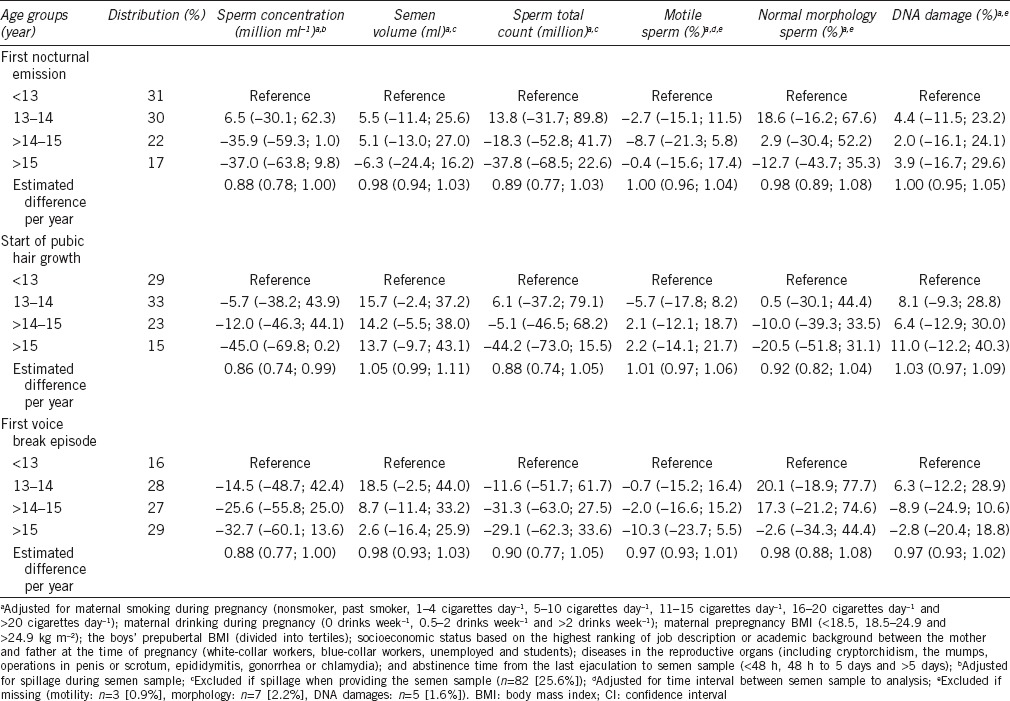
Figure 1.
The percentage difference (95% CI) in sperm concentration, total sperm count, testosterone concentration and inhibin B concentration compared to the reference group (<13 years at pubertal indicators). Nocturnal emission: —, pubic hair: - -, voice break: •••. (a) Sperm concentration (n = 320) – adjusted for maternal body mass index (BMI), smoking and drinking during pregnancy, socioeconomic status, abstinence time, diseases in reproductive organs, spillage and prepubertal BMI. (b) Total sperm count (n = 238) – adjusted for maternal BMI, smoking and drinking during pregnancy, socioeconomic status, abstinence time, diseases in reproductive organs and prepubertal BMI. Excluded if spillage when providing the semen sample (n = 82). (c) Testosterone concentration (n = 320) – adjusted for maternal BMI, smoking and drinking during pregnancy, socioeconomic status, time of blood sample and prepubertal BMI. (d) Inhibin B concentration (n = 320) – adjusted for maternal BMI, smoking and drinking during pregnancy, socioeconomic status, time of blood sample and prepubertal BMI.
With regard to total sperm count, the estimated difference per year varied between 0.88 (95% CI: 0.74, 1.05) and 0.90 (95% CI: 0.77, 1.05) (Table 2), equivalent to 10%–12% lower total sperm count for each year of later onset of puberty. Compared with men in the youngest age group, total sperm count for those having had pubic hair growth after 15 years of age was −44.2% (95% CI: −73.0%, 15.5%). For nocturnal emission and voice break, the estimates were in the same direction (Table 2 and Figure 1b).
We found no significant associations between age at pubertal development and semen volume, sperm motility, sperm cell morphology or DNA damage.
The estimated difference per year in testosterone concentration for all three indicators ranged from 0.95 (95% CI: 0.92, 0.99) to 0.98 (95% CI: 0.94, 1.02) (Table 3), corresponding to a 2%–5% lower testosterone concentration for each year of later pubertal development. Men who had experienced their first voice break episode after 15 years of age had a testosterone concentration of −16.0% (95% CI: −26.4%, −4.1%) compared with men aged younger than 13 years at their first voice break episode. As shown in Figure 1c, the results for first nocturnal emission and pubic hair were in the same direction and magnitude, but none of the estimates were statistically significant.
Table 3.
Adjusted percentage difference (95% CI) from reference group (<13 years) for reproductive hormones according to age at indicators of pubertal development, imputed dataset (n=320)
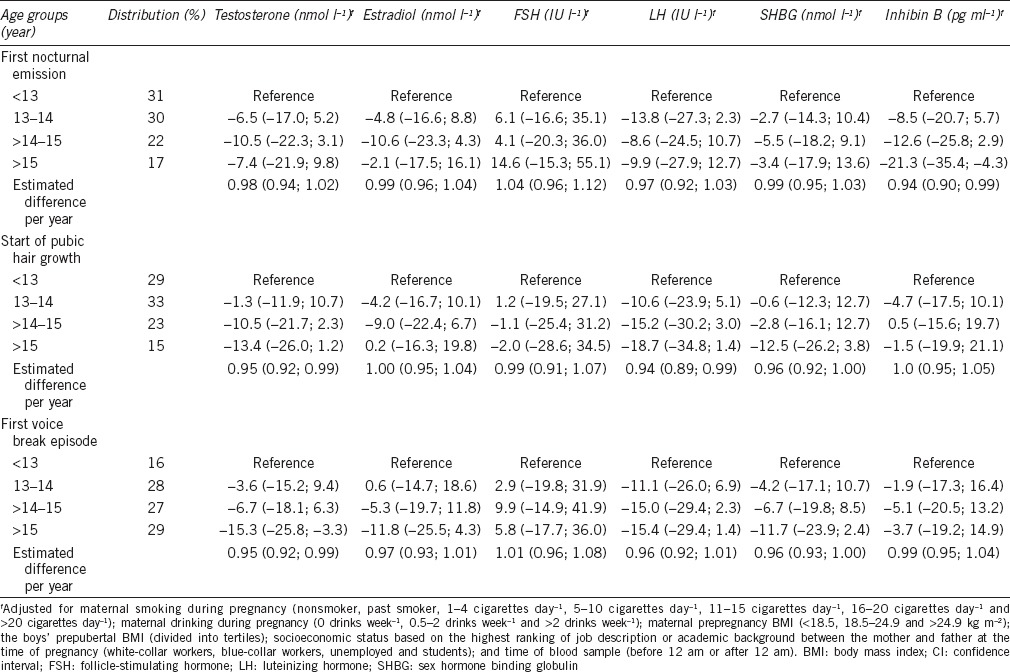
We found similar trends for inhibin B for men in the oldest age group of all three pubertal indicators. When comparing men younger than 13 years with those experiencing first nocturnal emission after 15 years of age, the inhibin B concentration was −21.3% (95% CI: −35.4%, −4.2%). The associations with age at public hair growth and voice break were weaker, but in the same direction (Table 3 and Figure 1d).
For LH and SHBG, the same trends of lower concentrations with older age at pubertal development were found (Table 3). No significant associations were found between age at pubertal development and estradiol and FSH.
The crude median semen quality and reproductive hormone parameters according to age at indicators of pubertal development are presented in Supplementary Table 1 (36.6KB, pdf) .
Crude median semen quality and reproductive hormone parameters according to age at indicators of pubertal development, imputed dataset (n=320)
DISCUSSION
The results indicate a trend toward an association between delay in pubertal development and lower semen quality in later life and altered reproductive hormones, supporting the results from the two previous studies on the subject.15,16
The present study had a large study population, and our participants were all healthy, young men with no known major diseases. A total of 48.5% of all invited provided a semen and blood sample, which is high for a semen quality study. Direct selection bias is unlikely owing to a lack of reproductive experience among the young men. Further, the participants were not aware of the hypothesis when entering the study.
We were able to conduct a thorough analysis of the men's reproductive status through both semen and hormonal parameters, which were performed by technicians blind to information on pubertal development or any other relevant characteristics. We had some ability to adjust for several potential confounders, including prenatal exposures, but we cannot rule out confounding by unknown factors. All participants were Danish, and the ethnicity was relatively homogeneous, which to some extent can limit bias based on race or genetics.
Nevertheless, there are limitations worth considering. We used retrospective, self-reported information on indicators of pubertal development with mean recall time of 5.8–6.4 years for the three pubertal indicators. Using questionnaires to assess the age at pubertal development might provide less accurate information on puberty onset compared with clinical examinations. However, questionnaires allow for a larger study population and have been used in several large-scale studies previously, e.g., in the ALSPAC cohort.27
Age at first nocturnal emission has been shown to be an accurate estimate of the onset of pubertal development in boys, compared to menarche in girls.28,29 Nevertheless, retrospective determination of the time at pubertal development in boys is complicated. Any misclassification will probably be of random nature, because of the sequence of reporting, which would often bias the association toward the null. Besides this, we observed tendencies between timing of pubertal development and reproductive health. Moreover, there were relatively high correlations between the three pubertal indicators ranging from 0.42 to 0.63, and our data on onset of pubertal indicators are comparable to those in previous studies with regard to the age span.27,29,30,31,32,33,34
The retrospectively collected data led to missing data, and we addressed this using multiple imputation. In complete case analyses, a substantial proportion of the study population is excluded, which can lead to selection bias and loss of precision and power.26 We reasoned that information on age at puberty was missing at random. Further, we assessed the robustness of this approach by performing complete case analyses and sensitivity analyses, in which the same associations were observed.
As men in this study were young, one could question their sexual maturity, when providing semen samples. Nonetheless, in a Danish study from 2005, 158 Danish men (mean age of 19.1 years at entry) were followed for 4 years and no significant changes in semen quality were observed.35 Likewise, Janczewski and Bablok found that the majority of boys had normozoospermia 21 months after the first ejaculation.36 This indicates that our participants were expected to be sexually mature which adds to the validity of our findings.
Although the possible decline in semen quality in Western countries37 has led to an extensive research focus on risk factors for poor semen quality, only relatively few have been established. Altered pubertal timing has been associated with impaired health in adult life38 and a number of serious adult diseases, e.g., testicular cancer,39 breast cancer,40 insulin resistance, and obesity,41 indicating that fetal programming of adult disorders may be mediated by puberty. If this association is true, it has public health interest.
The biological mechanism of the association remains unknown. In boys with delayed puberty, the testosterone peaks later, and it may never reach the same levels as boys with earlier timed pubertal onset. Consequently, lower testosterone levels could, in turn, lead to impaired sperm production in adulthood. This hypothesis is in line with our findings of both lower sperm concentration and lower testosterone levels among men with later pubertal development. This could indicate that the association is related to the pituitary or central nervous system level, but this is only speculative and there is very limited literature on this subject. To the best of our knowledge, the association has not been studied in animals.
One might also speculate whether the association could be a result of an underlying genetic profile or unknown environmental exposures, causing both later pubertal onset and poorer reproductive health.
CONCLUSION
Our results point toward an association between later age at pubertal development and lower semen quality in young adult life. Future studies with prospectively collected information on puberty are warranted to conclude on the importance of timing of puberty with regard to male reproductive health later in life.
AUTHORS CONTRIBUTIONS
LLBL performed the statistical analysis and interpretation, drafted the article, and gave the final approval of the version to be published. LHA contributed to the statistical analyses and interpretation of results, revised the article for important intellectual content, and gave the final approval of the version to be published. HS contributed with statistical advisement and interpretation of results, revised the article for important intellectual content, and gave the final approval of the version to be published. JO helped with interpretation of results, critically revising the article for important intellectual content and gave the final approval of the version to be published. CHRH gave substantial contributions to conception and design, interpretation of results, critically revising the article for important intellectual content and final approval of the version to be published. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This work was funded by the Danish Council for Independent Research (No. 12-127342).
Supplementary information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
- 1.Bonde JP, Ernst E, Jensen TK, Hjollund NH, Kolstad H, et al. Relation between semen quality and fertility: a population-based study of 430 first-pregnancy planners. Lancet. 1998;352:1172–7. doi: 10.1016/S0140-6736(97)10514-1. [DOI] [PubMed] [Google Scholar]
- 2.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joergensen N, Joensen UN, Jensen TK, Jensen MB, Almstrup K, et al. Human semen quality in the new millennium: a prospective cross-sectional population-based study of 4867 men. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-000990. pii: e000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aston KI. Genetic susceptibility to male infertility: news from genome-wide association studies. Andrology. 2014;2:315–21. doi: 10.1111/j.2047-2927.2014.00188.x. [DOI] [PubMed] [Google Scholar]
- 5.Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221–31. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartorius GA, Nieschlag E. Paternal age and reproduction. Hum Reprod Update. 2010;16:65–79. doi: 10.1093/humupd/dmp027. [DOI] [PubMed] [Google Scholar]
- 7.Vine MF. Smoking and male reproduction: a review. Int J Androl. 1996;19:323–37. doi: 10.1111/j.1365-2605.1996.tb00523.x. [DOI] [PubMed] [Google Scholar]
- 8.Ramlau-Hansen CH, Thulstrup AM, Storgaard L, Toft G, Olsen J, et al. Is prenatal exposure to tobacco smoking a cause of poor semen quality? A follow-up study. Am J Epidemiol. 2007;165:1372–9. doi: 10.1093/aje/kwm032. [DOI] [PubMed] [Google Scholar]
- 9.Toppari J, Juul A. Trends in puberty timing in humans and environmental modifiers. Mol Cell Endocrinol. 2010;324:39–44. doi: 10.1016/j.mce.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 10.Vested A, Giwercman A, Bonde JP, Toft G. Persistent organic pollutants and male reproductive health. Asian J Androl. 2014;16:71–80. doi: 10.4103/1008-682X.122345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunkel L, Quinton R. Induction of puberty. Eur J Endocrinol. 2014;170:229–39. doi: 10.1530/EJE-13-0894. [DOI] [PubMed] [Google Scholar]
- 12.Wu FC, Butler GE, Kelnar CJ, Stirling HF, Huhtaniemi I. Patterns of pulsatile luteinizing hormone and follicle-stimulating hormone secretion in prepubertal (midchildhood) boys and girls and patients with idiopathic hypogonadotropic hypogonadism (Kallmann's syndrome): a study using an ultrasensitive time-resolved immunofluorometric assay. J Clin Endocrinol Metab. 1991;72:1229–37. doi: 10.1210/jcem-72-6-1229. [DOI] [PubMed] [Google Scholar]
- 13.Tanner JM. Growth at adolescence: with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. Oxford, UK: Blackwell Scientific Publications; 1962. p. 325. [Google Scholar]
- 14.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8:1–28. doi: 10.1210/edrv-8-1-1. [DOI] [PubMed] [Google Scholar]
- 15.Bablok L, Janczewski Z. [Development of biological value of sperm in delayed puberty] Pol Tyg Lek. 1992;47:537–9. [Article in Polish] [PubMed] [Google Scholar]
- 16.Jensen TK, Finne KF, Skakkebaek NE, Andersson AM, Olesen IA, et al. Self-reported onset of puberty and subsequent semen quality and reproductive hormones in healthy young men. Hum Reprod. 2016;31:1886–94. doi: 10.1093/humrep/dew122. [DOI] [PubMed] [Google Scholar]
- 17.Olsen J, Frische G, Poulsen AO, Kirchheiner H. Changing smoking, drinking, eating behaviour among pregnant women in Denmark. Evaluation of a health campaign in a local region. Scand J Soc Med. 1989;17:277–80. doi: 10.1177/140349488901700404. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–5. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 19.Zhu JL, Basso O, Obel C, Bech BH, Nohr EA, et al. Parental infertility and sexual maturation in children. Hum Reprod. 2009;24:445–50. doi: 10.1093/humrep/den366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, et al. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocr Rev. 2003;24:668–93. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 21.Aitken RJ, Baker HW, Barratt LR, Behre HM, Comhaire F, et al. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Ann Ist Super Sanità. 1999;37:1–123. [PubMed] [Google Scholar]
- 22.Makler A, Zaidise I, Paldi E, Brandes JM. Factors affecting sperm motility. I. In vitro change in motility with time after ejaculation. Fertil Steril. 1979;31:147–54. doi: 10.1016/s0015-0282(16)43815-x. [DOI] [PubMed] [Google Scholar]
- 23.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;14:1039–49. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 24.Kruger TF, Acosta AA, Simmons KF, Swanson RJ, Matta JF, et al. Predictive value of abnormal sperm morphology in in vitro fertilization. Fertil Steril. 1988;49:112–7. doi: 10.1016/s0015-0282(16)59660-5. [DOI] [PubMed] [Google Scholar]
- 25.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008;8:70. doi: 10.1186/1471-2288-8-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;388:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteilh C, Kieszak S, Flanders WD, Maisonet M, Rubin C, et al. Timing of maturation and predictors of Tanner stage transitions in boys enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol. 2011;25:75–87. doi: 10.1111/j.1365-3016.2010.01168.x. [DOI] [PubMed] [Google Scholar]
- 28.Laron Z, Arad J, Gurewitz R, Grunebaum M, Dickerman Z. Age at first conscious ejaculation: a milestone in male puberty. Helvetica Paediatr Acta. 1980;35:13–20. [PubMed] [Google Scholar]
- 29.Carlier JG, Steeno OP. Oigarche: the age at first ejaculation. Andrologia. 1985;17:104–6. doi: 10.1111/j.1439-0272.1985.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen CT, Skakkebaek NE, Richardson DW, Darling JA, Hunter WM, et al. Onset of the release of spermatozoa (spermarche) in boys in relation to age, testicular growth, pubic hair, and height. J Clin Endocrinol Metab. 1986;62:532–5. doi: 10.1210/jcem-62-3-532. [DOI] [PubMed] [Google Scholar]
- 31.Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, et al. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110:911–9. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- 32.Mouritsen A, Aksglaede L, Soerensen K, Hagen CP, Petersen JH, et al. The pubertal transition in 179 healthy Danish children: associations between pubarche, adrenarche, gonadarche, and body composition. Eur J Endocrinol. 2013;168:129–36. doi: 10.1530/EJE-12-0191. [DOI] [PubMed] [Google Scholar]
- 33.Sorensen K, Aksglaede L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. J Clin Endocrinol Metab. 2010;95:263–70. doi: 10.1210/jc.2009-1478. [DOI] [PubMed] [Google Scholar]
- 34.Juul A, Magnusdottir S, Scheike T, Prytz S, Skakkebaek NE. Age at voice break in Danish boys: effects of pre-pubertal body mass index and secular trend. Int J Androl. 2007;30:537–42. doi: 10.1111/j.1365-2605.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 35.Carlsen E, Swan SH, Petersen JH, Skakkebaek NE. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod. 2005;20:942–9. doi: 10.1093/humrep/deh704. [DOI] [PubMed] [Google Scholar]
- 36.Janczewski Z, Bablok L. Semen characteristics in pubertal boys. I. Semen quality after first ejaculation. Arch Androl. 1985;15:199–205. doi: 10.3109/01485018508986912. [DOI] [PubMed] [Google Scholar]
- 37.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect. 2000;108:961–6. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep. 2015;5:11208. doi: 10.1038/srep11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss AR, Osmond D, Bacchetti P, Torti FM, Gurgin V. Hormonal risk factors in testicular cancer. A case-control study. Am J Epidemiol. 1986;124:39–52. doi: 10.1093/oxfordjournals.aje.a114369. [DOI] [PubMed] [Google Scholar]
- 40.Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer. 1999;85:2400–9. doi: 10.1002/(sici)1097-0142(19990601)85:11<2400::aid-cncr15>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 41.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003;27:1398–404. doi: 10.1038/sj.ijo.0802422. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crude median semen quality and reproductive hormone parameters according to age at indicators of pubertal development, imputed dataset (n=320)