Abstract
We aimed to explore the associations between different lipid profiles and semen quality in a large-scale general male population. Sperm concentration, total sperm motility, progressive motility, and normal sperm morphology of total 7601 participants were recorded. The association of these semen parameters with the triglyceride, total cholesterol, high-density lipoprotein, low-density lipoprotein, and very low-density lipoprotein of serum lipid profiles was analyzed. Sperm concentration was statistically positively correlated with triglyceride and very low-density lipoprotein (adjusted P = 0.001 and P = 0.005, respectively). Total sperm motility and progressive motility were statistically increased with increasing low-density lipoprotein and cholesterol levels (both adjusted P = 0.008 and P < 0.001, respectively). The similar J-shaped associations (high-low-low-high) were noted between individual lipid profile and normal sperm morphology, especially low-density lipoprotein and cholesterol with statistical significance (adjusted P = 0.017 and P = 0.021, respectively). The prevalence of abnormal total sperm motility and progressive motility was decreased in participants with high levels of cholesterol (P = 0.008 and P = 0.019, respectively), and the reverse J-shaped associations (low-high-high-low) were noted between high-density lipoprotein, triglyceride, very low-density lipoprotein, and the prevalence of abnormal normal sperm morphology (P = 0.010, P = 0.037, and P = 0.025, respectively). A high cholesterol level was associated with better sperm motility. Similar J-shaped associations were noted between all lipid profiles and normal sperm morphology; meanwhile, the reverse J-shaped trends were identified between them and abnormal normal sperm morphology prevalence.
Keywords: cholesterol, general population, lipoprotein, semen quality, triglyceride
INTRODUCTION
Evidence has indicated that decreasing semen quality is associated with increased obesity. A population study performed in France with a large sample size of 10 665 males showed that a higher body mass index (BMI) is not only associated with deleterious effects on sperm concentration but also sperm motility.1 Our recent study of 7630 Asian individuals indicated that a lower sperm concentration and lower normal sperm morphology are associated with increasing body adiposity.2 Higher intakes of saturated fat and processed meat were found to be negatively related to semen quality in studies performed in the USA and Spain,3,4 as well as in Asian countries.5 Besides, hyperlipidemia may play an important role in semen quality in addition to other environmental or lifestyle factors.6,7 The recent famous Longitudinal Investigation of Fertility and the Environment (LIFE) study indicated that serum lipid levels may affect semen parameters, specifically sperm head morphology, highlighting the importance of cholesterol and lipid homeostasis for male fecundity.8
A potential link between serum lipid and human fertility is feasible, on account of cholesterol being the main source for steroid synthesis, and playing a determinant role in steroidogenesis and spermatogenesis.9 Many animal studies have provided evidence of links between cholesterolemia, steroidogenesis, and male fertility.10,11,12 In addition, cholesterol-fed rats and rabbits showed reduced spermatid cell numbers, reduced seminiferous tubules’ diameters, and smaller Leydig cell nuclear dimensions.13 Even cholesterol-fed rats with mild hyperlipidemia exhibited significantly reduced sperm motility and density in the cauda epididymides and testis.14,15 A small number of human population studies have focused on lipid concentrations and semen quality of men in couples with infertility.16,17,18 However, no study has focused on the associations between lipid profiles and semen quality in the general population. This study intended to assess the relationships between lipid profiles and sperm parameters in a large cohort of males representing the general population of Taiwan, China.
MATERIALS AND METHODS
Design and subjects population
Initially, a total of 7920 healthy Taiwanese males aged 18 years or old who had participated in a standard medical screening program run by a private firm (MJ Health Management Institution, Taipei, Taiwan, China) between January 2008 and December 2014 were included in this study. The firm attracted paying participants all over Asia especially Taiwan because of its reputation for quality service, operational efficiency, and key facilities that were easily accessible. Membership of the program was required, with discounts in examination fee offered for people with a large-sized family or related individuals, and for regular members who came back for repeat examinations in subsequent years. These incentives succeeded in attracting and sustaining a large number of customers. Each participant signed a consent form authorizing MJ Health Management Institution to process the data generated from medical screening. Ethical reviews (Institutional Review Boards) were processed and approved by the MJ Health Management Institution and Tri-Service General Hospital in Taiwan, China. Data that could identify individuals were removed, and the participants remained anonymous during the entire study.
Semen collections and quality analyses
Semen samples were collected via masturbation following at least 3 days of abstinence using home-collection kits with a sterile plastic container, and the samples were sent to the laboratory for analysis within 1 h. Four dependent semen parameters, including sperm concentration (SC), total sperm motility (TSM), progressive motility (PRM), and normal sperm morphology (NSM), were recorded. Sperm concentration was evaluated by hemocytometer (Improved Neubauer; Hauser Scientific, Inc., Horsham, PA, USA). Samples were diluted in a solution of 0.6 mol l−1 NaHCO3 and 0.4% (v/v) formaldehyde in distilled water. Sperm motility was classified to progressive motility and total sperm motility (progressive and nonprogressive motility) according to the WHO 2010 classification.19 Briefly, 10 μl of well-mixed semen was placed on a clean glass slide that had been kept at 37°C and covered with a 22 mm × 22 mm coverslip. The slide was placed on the heating stage of a microscope at 37°C and immediately examined at ×400 magnification.
Measurements of anthropometric indexes and lipid profiles
Anthropometric indexes including BMI, waist circumference (WC), hip circumference (HC), body fat percentage, and biochemical data were recorded. While the semen samples were being collected, venous blood punctures were processed to collect blood samples after a minimum of an 8-h fast in the same day for the measurement of serum triglycerides, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and very low-density lipoprotein (VLDL) cholesterols.
Statistical analyses of the relationship
We used quartiles to evaluate the relationships between the lipid profiles and semen quality. Differences in the sperm parameters in individuals with varying degrees of lipid, as assessed by the different lipid profiles, were compared by one-way analysis of variance (ANOVA). The P values were calculated by the linear regression models after adjusting for confounding factors.
Abnormalities of sperm parameters in this study were defined as SC <15 × 106 ml−1, TSM <40%, and PRM <32% according to the WHO 2010 criteria.19 Otherwise, we adopted the criterion of abnormal NSM as <30% after consulting the WHO 1999 classification20 and the LIFE study definition21 on account of the latest WHO 2010 criteria (<4%) being too low to analyze the trend with different lipid profiles.
The frequencies of abnormal sperm parameters in each quartile were analyzed by Chi-square tests. We assessed the relationships between lipid profiles and semen parameters by first conducting logistic trend analyses, and then estimating the odds ratios (ORs). We also applied questionnaires to assess the relationship between smoking, dietary patterns, and semen quality, as described in detail previously.2,5
To avoid the risk of false positive results from the potential correlated factors, the four groups of triglyceride, total cholesterol, HDL, LDL, and VLDL, and smoking duration divided by quartile were assessed using one-way ANOVA with adjusting age, BMI, WC, HC, body fat (%), and smoking, and P < 0.05 was considered statistically significant. All analyses were conducted using SPSS statistical software (version 13.0, SPSS Inc., Chicago, IL, USA). BMI was calculated as weight in kilograms divided by height in meters squared. WC was obtained from the mid-point between the iliac crest and the costal margin, and HC was measured at the widest point around the greater trochanter. Measurements of percentage body fat were performed by the bioelectrical impedance analysis (BIA) technique using a body-composition analyzer Tanita TBF-410 (Tanita Corporation, Tokyo, Japan).
RESULTS
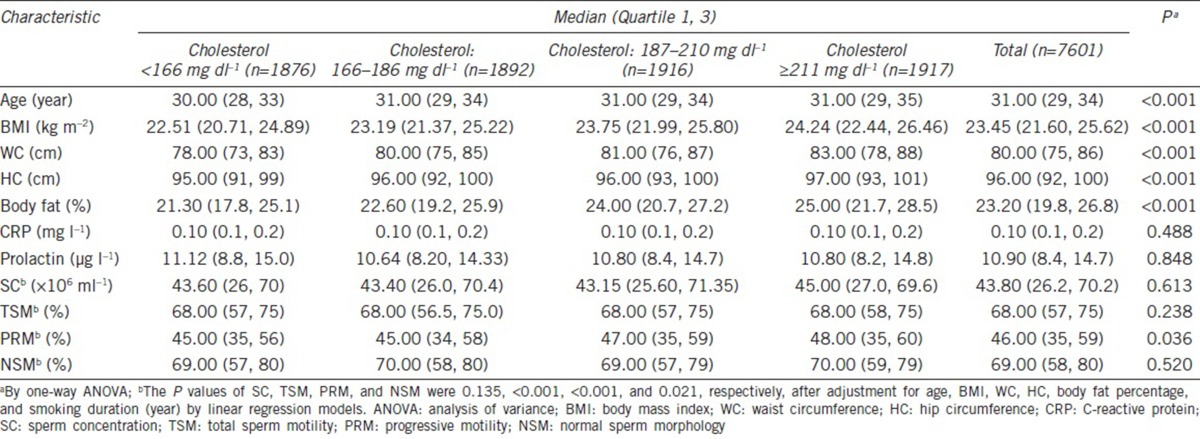
A total of 7601 men were enrolled in the study after excluding individuals with incomplete records and individuals with a history of major systemic disease or reproductive organ disorder. The median age of the participants in this cross-sectional study was 31 years (range: 18–70 years), with a median height of 172 cm (range: 151.4–193.5 cm) and a median body weight of 69.40 kg (range: 39.2–160.5 kg). The median BMI of whole population was 23.43 kg m−2 (range: 13.53–54.24 kg m−2), which including 68.5% of participants within a normal weight distribution and 31.4% were obese or overweight. The median values of the lipid parameters were triglyceride (96 mg dl−1 [range: 19–1684 mg dl−1]), total cholesterol (187.0 mg dl−1 [range: 77–379 mg dl−1]), HDL (49.0 mg dl−1 [range: 18–134 mg dl−1]), LDL (114.0 mg dl−1 [range: 22–277 mg dl−1]), and VLDL (19.0 mg dl−1 [range: 4–153 mg dl−1]). The median sperm parameters were SC (44.6 × 106 ml−1 [range: 0.01 × 106–700.00 × 106 ml−1]), TSM (68.0% [range: 0.01%–98.00%]), PRM (45.0% [range: 0.1%–94.0%]), and NSM (69.0% [range: 0.1%–98.0%]) (Table 1), and the prevalences of abnormal semen quality were 9.6%, 4.8%, 19.8%, and 0.8%, respectively.
Table 1.
Characteristics of the study participants (n=7601)
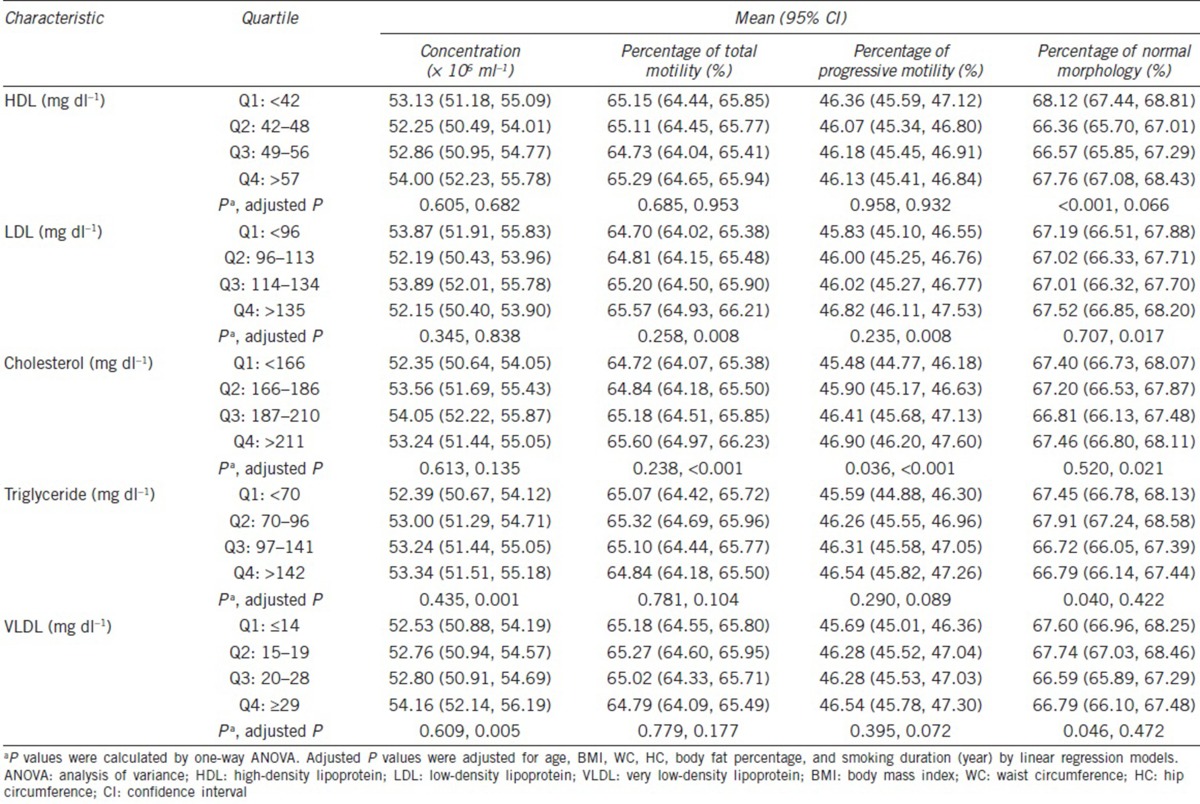
By analyzing the correlations between lipid profiles and semen parameters, we found that SC was statistically positively associated with triglyceride (adjusted P = 0.001) and VLDL (adjusted P = 0.005) (Table 2). The total cholesterol level was correlated with increasing TSM (adjusted P < 0.001) and PRM (adjusted P < 0.001) (Table 2). Similarly, the LDL level had positive associations with TSM and PRM, with statistical significance (both adjusted P = 0.008). Even though similar J-shaped correlations were noted between all lipid profiles and NSM, LDL and cholesterol levels with statistical significance (adjusted P = 0.017, P = 0.021, respectively), even for the lowest NSMs, were found in the third quartile.
Table 2.
Lipid profiles by semen parameters
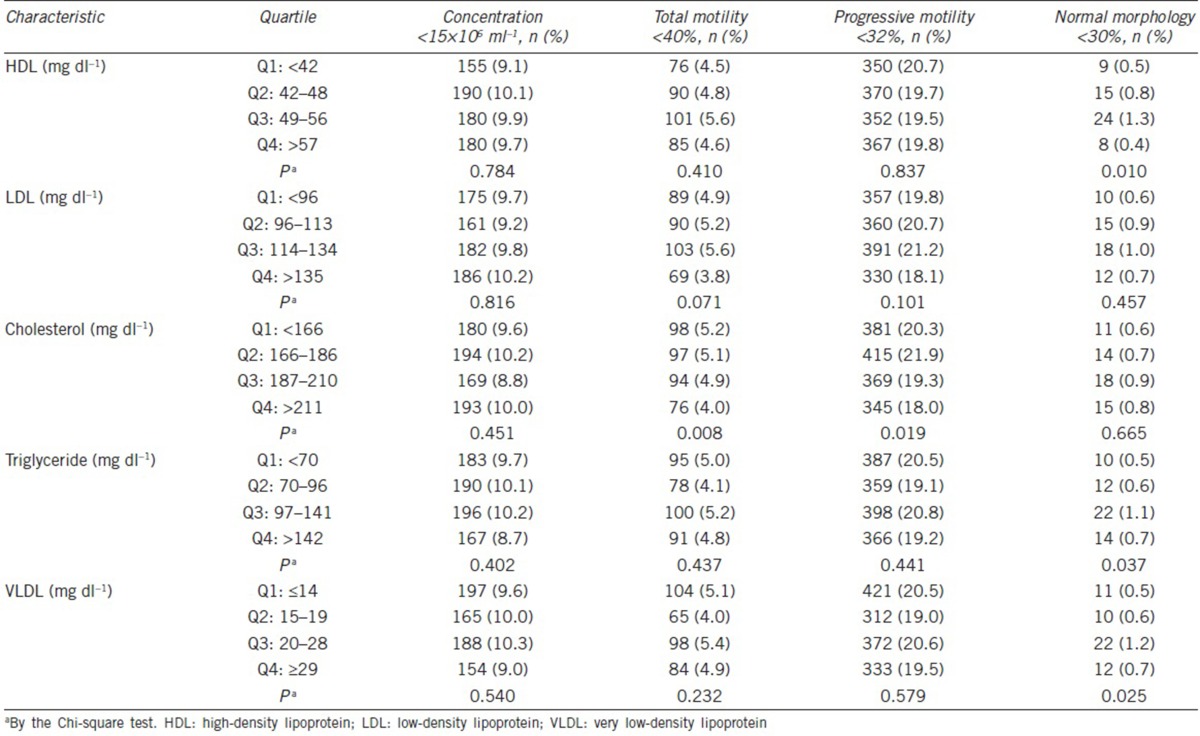
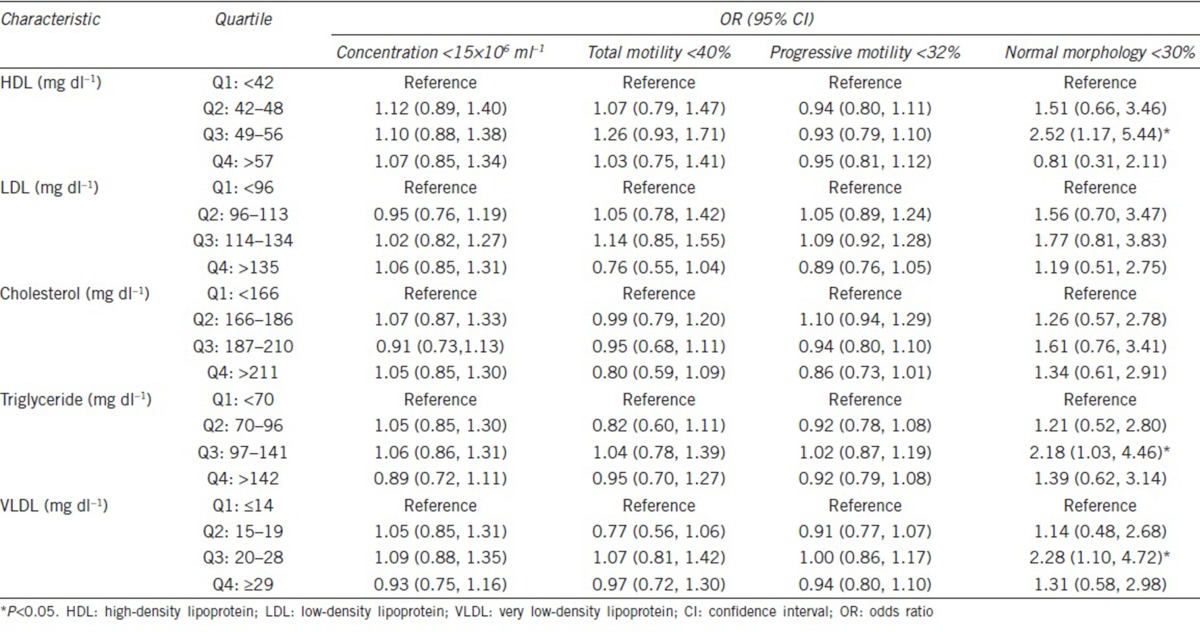
In order to illustrate the complex relationships between lipid profiles and semen quality, semen quality as assessed by the prevalence of abnormal sperm parameters was examined in the quartiles of all lipid profiles (triglyceride, total cholesterol, HDL, LDL, and VLDL cholesterols). The reverse associations were found between the prevalence of abnormal TSM, PRM, and cholesterol levels with statistical significance (TSM <40%, P = 0.008; PRM <32%, P = 0.019) (Table 3). In addition, the significant reverse J-shaped correlations were identified between HDL, triglyceride, VLDL, and abnormal NSM prevalence (NSM <30%, P = 0.010, P = 0.037, P = 0.025, respectively), with the highest abnormal NSM prevalence found in the third quartile (Table 3). Similar reverse J-shaped trends were also found between LDL, cholesterol, and abnormal NSM. Interestingly, according to quartile comparisons, the men within the third HDL quartile had 2.52-fold higher odds ratio of abnormal NSM (95% confidence interval [CI]: 1.17–5.44) as compared to those within the lowest HDL quartile (Table 4). In addition, in the individuals within the third quartile, triglyceride and VLDL odds ratios of an abnormal NSM were 2.18-fold higher (95% CI: 1.03–4.46) and 2.28-fold higher (95% CI: 1.10–4.72), respectively, than that in individuals within the lowest lipid profile quartile.
Table 3.
Associations between abnormal semen parameters and lipid profiles
Table 4.
The odds ratios and 95% confidence intervals of abnormal semen parameter associations with lipid profiles
DISCUSSION
Previously, we have discussed the importance of obesity and different dietary patterns in terms of their effects on semen quality.2,5 As expected, and similar to previous reports, all semen parameters showed an inverse correlation with age. In terms of an association with cigarette smoking, SC had a nominally significant inverse association with smoking duration. Other semen parameters, including TSM, PRM, and NSM, had a reverse trend with each smoking duration quartile, without statistical significance; data were reported in detail previously.2,5 In this study, we examined this topic again and extended our original study duration to collect and analyze more lipid profile data.
Various studies have focused on the associations between lipid profiles and semen quality. However, studies in this field have reported inconsistent conclusions and results. For example, Ergun et al.18 declared that high serum VLDL and total triglyceride levels were statistically correlated with low sperm motility in a study of 18 infertile men. However, a study conducted by Hagiuda et al.17 concluded that the serum triglyceride level is positively associated with sperm morphological traits and has no significant relationship with sperm concentration or motility. The LIFE study found that high serum levels of total cholesterol, free cholesterol, and phospholipids were statistically associated with a low percentage of spermatozoa with intact acrosomes and a smaller sperm head area and perimeter.8
The results of these studies were not wholly consistent with the conclusions of our study. One of the reasons for this may be due to the bias inherent in selecting study participants in the previous studies. As those studies explored the lipid profiles and semen quality not only in men of infertile couples but also in those seeking pregnancy, reproductive problems were often present in those individuals. On the other hand, the participants in our study were a large sample of the general male population who underwent an annual health examination, and we excluded those with a history of major systemic disease or reproductive organ disorder.
Our study demonstrated that the total cholesterol level was positively correlated with total sperm motility and progressive motility with statistical significance. A similar J-shaped correlation was observed between the total cholesterol, LDL level, and NSM with statistical significance. The triglyceride and VLDL lipid profiles showed statistically positive associations with SC.
As per the results of the LIFE study, which enrolled 501 male partners of couples seeking pregnancy,8 they found that triglyceride levels tended to have a positive association with SC (β = 0.014). The free cholesterol level was negatively associated with the percentage of sperm heads with acrosomes (β = −0.043, P < 0.05), sperm head area (β = −0.008, P < 0.05), and sperm head perimeter (β = −0.005, P < 0.05). Phospholipids were also found to be negatively associated with the sperm head area (β = −0.002, P < 0.05) and the percentage of sperm heads with acrosomes (β = −0.014, P < 0.05) in the LIFE study, which is in partial accordance with our results, in which statistical J-shaped associations were noted between cholesterol, LDL level, and NSM, and statistically positive correlations were identified between triglyceride, VLDL, and SC.
However, the previous studies just focused on the associations between lipids and semen parameters, not described the detailed relationships between lipids and semen abnormality. Our study was designed to evaluate main cholesterol fractions, not only cholesterol and triglyceride levels. The LIFE study concluded that the lack of an association between cholesterol concentration and sperm motility may be a result of analyzing the semen the other days after blood collection, while in our study, semen and blood samples were collected and sent for analysis immediately.
The etiology of the relationship between lipids and sperm production is complex and unclear. In humans, the amount of cholesterol in sperm varies considerably, even among ejaculates;22 meanwhile, the proportion of cholesterol present within sperm membranes is directly related to the sperm morphology23 and fertility potential.22 However, studies exploring the detailed associations between serum cholesterol or other lipid profiles and semen quality are limited.
There were some limitations of this cross-sectional study, similar to our previous studies.2,5 These included the lack of semen volume and total sperm count data in the initially designed protocol of the program, absence of detailed records of endocrine levels and lipid contents of semen, and lack of abstinence duration records for evaluation in the study. Moreover, the lipid-related pathological conditions, such as familial hypercholesterolemia or ApoE genotype, were not exploring in our study on account of the initially designed data requirement. The semen samples of our study were collected using home-collection kits; the quality may not be equal to that resulting from on-site collection. Further studies, including those assessing the lipid content of semen and performing measurement of lipid-related nuclear receptors such as liver X receptors (LXRs),24,25 peroxisome proliferator-activated receptors (PPARs),26 small heterodimer partner (SHP),27 and retinoid X receptors (RXRs),28 should be designed to further explore the relationships between lipids and semen quality.
CONCLUSIONS
Our study results showed that the men with increased total cholesterol were positively correlated with sperm motility. The reverse J-shaped correlations were identified between all lipid profiles and sperm morphology, especially the highest prevalence of abnormal NSM was identified in the third quartile of HDL, triglyceride, and VLDL with statistical significance in a large-scale study of a general male population.
AUTHOR CONTRIBUTIONS
CYL carried out the conception and wrote the manuscript. CWT participated in the design of the study and helped draft the manuscript. YCC participated in the statistical analysis and interpretation. SHL, STW, TLC, and HIC participated in the coordination of its design and helped draft the revision. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors would like to thank the MJ Health Management Institution for making their large dataset available for this study. Readers interested in the dataset can contact the MJ Health Management Institution directly (http://www.mjhrf.org) for access to or use of the original data.
REFERENCES
- 1.Belloc S, Cohen-Bacrie M, Amar E, Izard V, Benkhalifa M, et al. High body mass index has a deleterious effect on semen parameters except morphology: results from a large cohort study. Fertil Steril. 2014;102:1268–73. doi: 10.1016/j.fertnstert.2014.07.1212. [DOI] [PubMed] [Google Scholar]
- 2.Tsao CW, Liu CY, Chou YC, Cha TL, Chen SC, et al. Exploration of the association between obesity and semen quality in a 7630 male population. PLoS One. 2015;10:e0119458. doi: 10.1371/journal.pone.0119458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attaman JA, Toth TL, Furtado J, Campos H, Hauser R, et al. Dietary fat and semen quality among men attending a fertility clinic. Hum Reprod. 2012;27:1466–74. doi: 10.1093/humrep/des065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, et al. Food intake and its relationship with semen quality: a case-control study. Fertil Steril. 2009;91:812–8. doi: 10.1016/j.fertnstert.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Liu CY, Chou YC, Chao JC, Hsu CY, Cha TL, et al. The association between dietary patterns and semen quality in a general Asian population of 7282 males. PLoS One. 2015;10:e0134224. doi: 10.1371/journal.pone.0134224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford AG, Cote C, Couto J, Daskiran M, Gunnarsson C, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE Centricity electronic medical record database. Popul Health Manag. 2010;13:151–61. doi: 10.1089/pop.2009.0039. [DOI] [PubMed] [Google Scholar]
- 7.Charlton M. Obesity, hyperlipidemia, and metabolic syndrome. Liver Transpl. 2009;15(Suppl 2):S83–9. doi: 10.1002/lt.21914. [DOI] [PubMed] [Google Scholar]
- 8.Schisterman EF, Mumford SL, Chen Z, Browne RW, Boyd Barr D, et al. Lipid concentrations and semen quality: the LIFE study. Andrology. 2014;2:408–15. doi: 10.1111/j.2047-2927.2014.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gwynne JT, Strauss JF., 3rd The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982;3:299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- 10.Whitfield M, Pollet-Villard X, Levy R, Drevet JR, Saez F. Posttesticular sperm maturation, infertility, and hypercholesterolemia. Asian J Androl. 2015;17:742–8. doi: 10.4103/1008-682X.155536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Lin HY, Yeh SD, Yu IC, Wang RS, et al. Infertility with defective spermatogenesis and steroidogenesis in male mice lacking androgen receptor in Leydig cells. Endocrine. 2007;32:96–106. doi: 10.1007/s12020-007-9015-0. [DOI] [PubMed] [Google Scholar]
- 12.Rulli SB, Ahtiainen P, Makela S, Toppari J, Poutanen M, et al. Elevated steroidogenesis, defective reproductive organs, and infertility in transgenic male mice overexpressing human chorionic gonadotropin. Endocrinology. 2003;144:4980–90. doi: 10.1210/en.2003-0403. [DOI] [PubMed] [Google Scholar]
- 13.Gupta RS, Dixit VP. Effect of dietary cholesterol on spermatogenesis. Z Ernahrungswiss. 1988;27:236–43. doi: 10.1007/BF02019512. [DOI] [PubMed] [Google Scholar]
- 14.Purohit A, Daradka HM. Effect of mild hyperlipidaemia on testicular cell population dynamics in albino rats. Indian J Exp Biol. 1999;37:396–8. [PubMed] [Google Scholar]
- 15.Bataineh HN, Nusier MK. Effect of cholesterol diet on reproductive function in male albino rats. Saudi Med J. 2005;26:398–404. [PubMed] [Google Scholar]
- 16.Oborna I, Wojewodka G, De Sanctis JB, Fingerova H, Svobodova M, et al. Increased lipid peroxidation and abnormal fatty acid profiles in seminal and blood plasma of normozoospermic males from infertile couples. Hum Reprod. 2010;25:308–16. doi: 10.1093/humrep/dep416. [DOI] [PubMed] [Google Scholar]
- 17.Hagiuda J, Ishikawa H, Furuuchi T, Hanawa Y, Marumo K. Relationship between dyslipidaemia and semen quality and serum sex hormone levels: an infertility study of 167 Japanese patients. Andrologia. 2014;46:131–5. doi: 10.1111/and.12057. [DOI] [PubMed] [Google Scholar]
- 18.Ergun A, Kose SK, Aydos K, Ata A, Avci A. Correlation of seminal parameters with serum lipid profile and sex hormones. Arch Androl. 2007;53:21–3. doi: 10.1080/01485010600888961. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization Press; 2010. [Google Scholar]
- 20.World Health Organization. WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 21.Eisenberg ML, Kim S, Chen Z, Sundaram R, Schisterman EF, et al. The relationship between male BMI and waist circumference on semen quality: data from the LIFE study. Hum Reprod. 2014;29:193–200. doi: 10.1093/humrep/det428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugkraroek P, Kates M, Leader A, Tanphaichitr N. Levels of cholesterol and phospholipids in freshly ejaculated sperm and percoll-gradient-pelletted sperm from fertile and unexplained infertile men. Fertil Steril. 1991;55:820–7. [PubMed] [Google Scholar]
- 23.Meseguer M, Garrido N, Martinez-Conejero JA, Simon C, Pellicer A, et al. Relationship between standard semen parameters, calcium, cholesterol contents, and mitochondrial activity in ejaculated spermatozoa from fertile and infertile males. J Assist Reprod Genet. 2004;21:445–51. doi: 10.1007/s10815-004-8761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volle DH, Lobaccaro JM. Role of the nuclear receptors for oxysterols LXRs in steroidogenic tissues: beyond the “foie gras”, the steroids and sex? Mol Cell Endocrinol. 2007;265-266:183–9. doi: 10.1016/j.mce.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Volle DH, Mouzat K, Duggavathi R, Siddeek B, Dechelotte P, et al. Multiple roles of the nuclear receptors for oxysterols liver X receptor to maintain male fertility. Mol Endocrinol. 2007;21:1014–27. doi: 10.1210/me.2006-0277. [DOI] [PubMed] [Google Scholar]
- 26.Aquila S, Bonofiglio D, Gentile M, Middea E, Gabriele S, et al. Peroxisome proliferator-activated receptor (PPAR) gamma is expressed by human spermatozoa: its potential role on the sperm physiology. J Cell Physiol. 2006;209:977–86. doi: 10.1002/jcp.20807. [DOI] [PubMed] [Google Scholar]
- 27.Bowles J, Knight D, Smith C, Wilhelm D, Richman J, et al. Retinoid signaling determines germ cell fate in mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- 28.Dufour JM, Kim KH. Cellular and subcellular localization of six retinoid receptors in rat testis during postnatal development: identification of potential heterodimeric receptors. Biol Reprod. 1999;61:1300–8. doi: 10.1095/biolreprod61.5.1300. [DOI] [PubMed] [Google Scholar]