Abstract
Diabetes mellitus significantly affects the male reproduction and sexual function. In the present study, we investigated the diabetes-induced dysfunction of seminal vesicles (SVs) in the diabetes-rat model and the role of antioxidants. Streptozotocin-induced diabetes after 4 weeks caused smaller size of the organs, hypercontractility, histological abnormalities, increased concentrations of malondialdehyde in the serum and tissue, overexpression of oxidative stress markers, and cleaved caspase-3 as identified by immunohistochemistry in the SVs. In addition, diabetes resulted in deceased levels of serum testosterone and no newborns after the mating studies. Antioxidants significantly normalized all the above parameters, except for the severely decreased serum testosterone levels and the negative outcome of the mating studies. The present study gives evidence for the important role of diabetes-induced oxidative stress in the function and structure of these androgen-dependent organs. Antioxidants may be a promising supplementary therapy for diabetic male patients to alleviate ejaculatory disorders but alone is not efficient treatment for the mitigation of infertility.
Keywords: diabetes mellitus, male infertility, oxidative stress, seminal vesicles, seminiferous epithelium, smooth muscle cell contractions
INTRODUCTION
According to the International Diabetes Federation Guideline Development Group (2014), in 2011, there were 336 million diabetic patients around the world.1 This number is expected to reach 552 million by 2030.1,2 Furthermore, diabetes is one of the leading causes of death in the developed societies.2 Moreover, diabetes mellitus (DM) affects an increasing number of men who are at their reproductive age. Diabetes may affect the male reproduction by acting on the endocrine glands, and further causing sexual dysfunction or by disrupting the male accessory gland function.3 According to La Vignera et al.,3 lately, the average age for diabetes diagnosis is decreasing, especially for type 1 DM. In general, a percentage more than 90% of these patients is diagnosed before the age of 30 years.3 Neuropathy, one of the usual complications of DM, has a negative effect on the function of the seminal vesicles. This functional impairment of the seminal vesicles further affects the sexual function and the fertility potential of the male.4 Previously, La Vignera et al.5 have demonstrated that diabetic infertile patients with neuropathy had erectile dysfunction and also abnormalities in their seminal vesicles as indicated by ultrasound examination.5
We have demonstrated that diabetic rats have lower male reproductive potential.6 This detrimental effect in our previous communication was attributed to the hypospermatogenesis in the diabetic rats as supported by the lower Johnsen score and lower testicular weight. This detrimental effect on the testicular exocrine function may be mediated by the lower testicular testosterone secretion and the excessive oxidative stress.6 On the diabetes model in our previous study, we have performed mating studies to evaluate the reproductive potential of the diabetic male rats. At that time, a consistent postmating observation was noticed that there were very small copulatory plugs from the diabetic male rats in the vulva/vagina orifice of the female rats.
An attractive question that raised was whether the smaller size of the copulatory plugs serve as a secondary contributing factor to the diminished male reproductive potential considering that: (1) the lower copulatory plug further means fewer sperm entrance to the uterus, and (2) seminal plasma contains secretions with detrimental effects on the sperm physiology but also necessary for fertilization.
To further investigate whether this secretory deficiency contributed to the lower male reproductive ability, we performed organ bath studies to investigate the function of the seminal vesicles. Furthermore, we used the antioxidants edaravone and taurine to evaluate whether there is a role of DM-induced oxidative stress on the function of these organs.
MATERIALS AND METHODS
The experimental protocol was approved by the Tottori University Committee for Animal Experimentation (protocol approval number: 10-Y-49). The animal experiments were performed according to the Tottori University Committee for Animal Experimentation guidelines for the care and handling of laboratory animals, which conformed to the “Guidelines for Proper Conduct of Animal Experiments” developed by the Science Council of Japan. All of the studies including animals are reported according to “Animals in Research: reporting In Vivo Experiments” guidelines as introduced by McGrath et al.7 All efforts were made to minimize animal suffering and the number of animals needed to obtain reliable results.
Six-week-old male Wistar rats (180–210 g; JSLC, Shizuoka, Japan) were used for the purpose of the present study. The animals were housed in a room with a 12 h day/night cycle, temperature of 21 ± 2°C, and humidity of 45%–65%. Initially, the animals were divided randomly into four age-matched groups. The control group (Control) was consisted of ten animals, and at the beginning of the experimental period, they were administered an injection of a vehicle dose of 0.1 mol l−1 citrate–phosphate buffer (pH 4.2) intraperitoneally (i.p.) once. The remaining three groups were administered a single dose of streptozotocin (50 mg kg−1) i.p. dissolved into 0.1 mol l−1 citrate–phosphate to induce diabetes.6 The induction of diabetes was confirmed by measuring the urinary glucose with Pretest 3a II (Wako Pure Chemical, Osaka, Japan) 1 day after the streptozotocin injection, as previously described.6 The animals that demonstrated more than (+++) of urinary glucose levels were included in the study. The induction of the diabetes was successful in all animals. One diabetic group (DM group, n = 20) was treated with 0.5 ml saline i.p. Another group of diabetic animals (DM/Eda, n = 10) was treated with edaravone i.p. 10 mg kg−1 and the third diabetic group (DM/Tau, n = 10) was administered taurine i.p. 500 mg kg−1. The treatment started 2 days after the induction of diabetes, and it was administered once every day for 4 weeks. The doses of edaravone and taurine were based on previous published work from our laboratory.6 At the completion of the treatment period, the male rats were expected to have reached the age of sexual maturation (10-week-old). The animal groups were kept under identical conditions and had access to food and fresh drinking water ad libitum.
Five days before the completion of the treatment, mating studies were performed. After the separation from the female rats, all male rats were sacrificed with an overdose of pentobarbital (60 mg kg−1 i.p.). Blood samples were collected from the vena cava. Body weights and bilateral seminal vesicles weights were recorded. A sample of the seminal vesicles was prepared for the in vitro bath functional studies, another part of the tissue was fixed in 10% formalin solution, whereas another sample from seminal vesicles was immediately frozen and stored at −80°C until used. The stored blood samples and seminal vesicles tissues were used for biochemical and molecular studies.
In vitro organ bath studies
The contraction functional studies were performed according to our previous report.8 The seminal vesicles were cut into approximately 3-mm-long segments. Each section was suspended on a wire hook in an organ bath (25 ml) containing Krebs–Henseleit solution, and bubbled with 5% CO2 and 95% O2 (37°C). One hook was suspended from a transducer (type 45196A, San-ei Instruments, Tokyo, Japan) and the lower hook was fixed to a plastic support leg to a micrometer (Mitutoyo, Tokyo, Japan). Each segment was equilibrated unstretched for 30 min. A load of 1.0 g was applied to each segment by micrometer adjustment, and the load was readjusted to this level 30 min later. Changes in the tone were recorded by a force transducer on a personal computer (Macintosh G3; Apple Computer, Cupertino, CA, USA) by the use of Chart version 3.6.9 software and a PowerLab/16sp data acquisition system (AD Instruments, Castle Hill, Australia). Following a 30-min period of equilibration, the segments were exposed to 100 mmol l−1 KCl. In the seminal vesicle sections, the contractile response to norepinephrine (1 × 10−7–3 × 10−4 mol l−1) was determined cumulatively. After a 60-min washout period, the same procedure was followed for contractile responses induced by carbachol (1 × 10−7–3 × 10−4 mol l−1) and they were also determined cumulatively. The data for the contractions induced by norepinephrine or carbachol were normalized by the area of the seminal vesicular sections.
Measurement of serum glucose concentrations
The hexokinase method was used to measure glucose concentrations in the serum of the experimental animals (Glucose CI, Wako Pure Chemical, Osaka, Japan). The method was carried out according to the manufacturer's instructions. Twenty-four hours before sacrificing the animals, the food was removed.
Measurements of oxidative damage in the serum and seminal vesicles
To investigate the levels of diabetes-induced oxidative damage in the serum and seminal vesicles, the concentrations of malondialdehyde (MDA) were measured using a commercially available kit (NWLSSTM Malondialdehyde Assay, Northwest Life Science Specialties, LLC, Vancouver, WA, USA) according to the manufacturer's instructions. MDA was used as a reliable marker for evaluation of lipid peroxidation. The method that was performed is described in detail in our previous study.9
Evaluation of peripheral serum testosterone levels
The testosterone levels were measured in the serum, and they were assessed using an enzyme immunoassay kit for testosterone (Oxford Biomedical Research®; Enzyme Immunoassay for testosterone Product No. EA 78; Oxford Biomedical Research, Rochester Hills, MI, USA) following the manufacturer's instructions. The blood samples were always taken at the same time of the day as testosterone secretion is pulsatile and it can change significantly during the day.
Hematoxylin eosin staining and Masson's trichrome staining
After fixation, the seminal vesicles were embedded in paraffin. Tissue sections (5 μm) were cut from the paraffin blocks. Sections were deparaffinized, gradually hydrated, and examined by (a) hematoxylin and eosin (H and E) staining and (b) Masson's trichrome staining. Each section was viewed under light microscope at ×400 magnification. Histological examinations were done by a pathologist blinded to the experiment. Seminal vesicles were evaluated histologically for alterations in both muscular layer and epithelium.
Immunohistochemistry for oxidative stress parameters and apoptosis
First, the samples were deparaffinized in xylene (×3 times) and rehydrated in graded alcohols (100% ×2, 95% ×2, 70% ×1, 60% ×1). Then, the sections were subjected to antigen retrieval using a microwave in a 10 mmol l−1 citrate buffer (pH 6.0) for 10 min (this step was skipped for MDA). Triton X-100 incubation followed only for the 8-hydroxy-2’-deoxyguanosine (8-OHdG) samples for 10 min and afterward washing with PBS (×3 times). Accordingly, the samples were incubated in 0.3% H2 O2 for 15 min. After washing with PBS, blocking was performed using 1.5% normal horse serum (Vectastain, Peroxidase mouse IgG, PK-4002; Elite Vectastain Rabbit IgG PK-6101, Vector Laboratories, CA, USA) for 30 min in a humidified chamber in room temperature. The diluted first antibody was applied and the samples were incubated overnight at 4°C in a humidified chamber as well. The primary antibodies that we used were a mouse monoclonal antibody against 4-hydroxy-2-nonenal (4-HNE; 1:5, Japan Institute for the Control of Aging, Shizuoka, Japan), a mouse monoclonal antibody against 8-OHdG (1:10, Japan Institute for the Control of Aging, Shizuoka, Japan), a mouse antibody against MDA (1:50, NOF Corporation, Tokyo, Japan), and a rabbit monoclonal antibody against cleaved caspase-3 (1:2000, #9664, Cell Signaling Technology, Inc., Danvers, MA, USA). The next day, after washing with PBS 3 times for 5 min, biotinylated horse anti-mouse or anti-rabbit IgG (1:200) was applied onto the tissue sections, and incubated for 30 min at room temperature in humidified chamber. Immunoreaction was performed with an avidin–biotin complex alkaline phosphatase kit (Vectastain, Vector Laboratories, Burlingame, CA, USA). The sections were counterstained with hematoxylin. Negative control sections, which were incubated in the absence of the primary antibody, were also processed and evaluated for specificity or background staining levels.
Mating studies
Each male rat was placed for 5 days in the same cage with two female rats. Five days is the length of one complete estrous cycle in the rat.10 The female rats were of proven fertility, having regular cycles as determined by the vaginal smears. Afterward, female rats were separated and placed in individual cages. Twenty-one to twenty-three days after the mating studies, the number and weight of the newborns were recorded.
Data analysis
The values of ED50 and Emax were obtained by a Macintosh computer (G3) loaded with Chart version 3.6.9 software and a PowerLab/16sp data acquisition system. The data of the contractions induced by norepinephrine or carbachol were normalized by the cross-sectional area in square millimeters.8 The cross-sectional area was calculated according to the following equation: cross-sectional area = weight/(length × 1.05), where 1.05 is the supposed density of the muscle.8 Data are shown as means ± s.e.m. of nine separate determination from each group. A statistical comparison of differences between groups was performed using analysis of variance and Fisher's multiple comparison tests. P < 0.05 was considered statistically significant.
Drugs and chemicals
Edaravone was kindly provided by Mitsubishi Tanabe Pharma Corporation (Osaka, Japan). Streptozotocin and taurine were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals not otherwise mentioned were reagent grade and available commercially.
RESULTS
Diabetes-induced smaller size of the animal body and seminal vesicles
At the end of the experimental period, 12 of 20 male rats had survived from DM group, 9 of 10 animals from DM/Eda group, and 9 of 10 animals from DM/Tau group. All 10 animals from Control group were alive. Therefore, nine animals were used per group for evaluation. The survival rate for the Control group was 100%, for the DM group was 60%, and for the treatment groups was the same at 90%. Animals in the DM group had significantly lower body weights and seminal vesicular weights compared to the Control group (P < 0.0001 and P < 0.0001, respectively). Treatment with edaravone and taurine significantly increased body weight (BW) (P = 0.0015 and P = 0.0009, respectively), seminal vesicular weight (SVW) (P = 0.0132 and P = 0.0115, respectively), as well as the ratio of SVW to BW (P = 0.0248 and P = 0.0127, respectively) compared to the DM group (Table 1).
Table 1.
General features of the animals and biochemical parameters
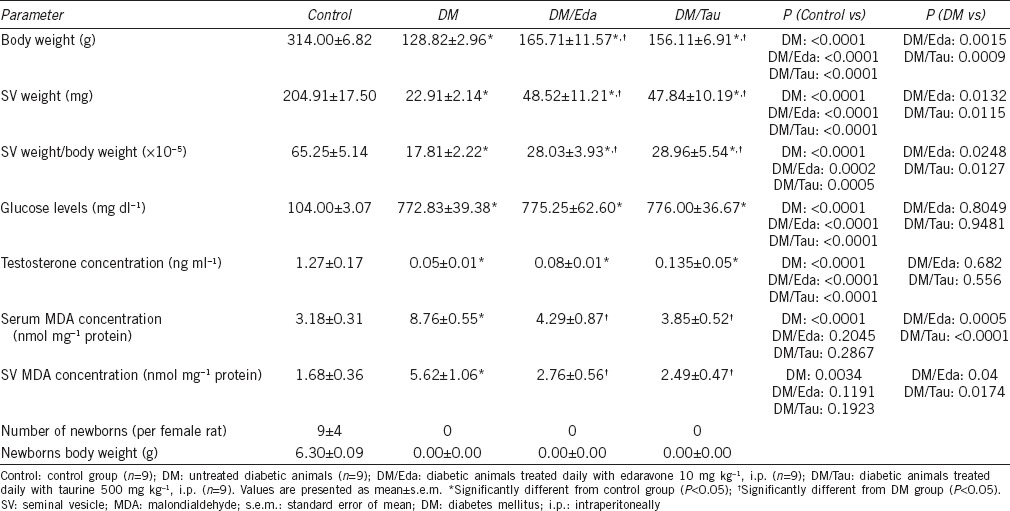
Treatment with antioxidants did not improve hyperglycemia or testosterone concentrations in the serum
In Table 1, the serum glucose levels and the serum testosterone levels are presented. All three diabetic groups had significantly increased concentrations of glucose in the serum compared to the Control (for all three diabetic groups P < 0.0001 compared to the Control). On the other hand, testosterone concentrations were extremely decreased in all diabetic groups compared to the Control group (for all three diabetic groups P < 0.0001 compared to the Control). Neither edaravone nor taurine increased the testosterone concentrations in the serum (Table 1).
Functional studies revealed hypercontractility of the tissue induced by diabetes
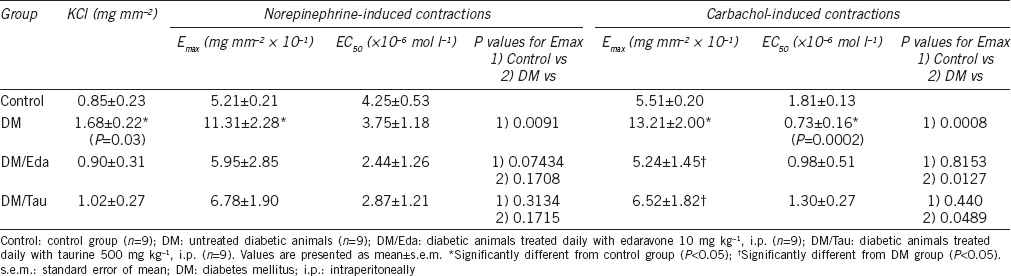
The functional data obtained by the in vitro organ bath studies are presented in Table 2. In both NE-induced and carbachol-induced contractions in the DM group, the maximum contractions (Emax) of the seminal vesicles were significantly increased, demonstrating hypercontractility of the tissue, compared to the Control group (P = 0.0091 for NE-induced contractions and P = 0.0008 for carbachol-induced contractions). Although treatment with edaravone and taurine decreased the Emax at NE-induced contractions, the differences were not statistically significant compared to the DM group (P = 0.3170 and P = 0.3068, respectively). At carbachol-induced contractions, treatment with edaravone and taurine significantly corrected the hypercontractility observed in the DM group (P = 0.0127 and P = 0.0489, respectively).
Table 2.
Data from functional in vitro organ bath studies in the seminal vesicles
Diabetes-induced significant elevation of lipid peroxidation both in serum and seminal vesicles
The induction of diabetes significantly increased the MDA levels in the serum as well as in the tissue of seminal vesicles in the diabetic animals of DM (serum MDA: P < 0.0001 and tissue MDA: P = 0.0034) group compared to the Control. Both edaravone and taurine treatment significantly decreased the MDA concentrations in the serum (P = 0.0005 and P < 0.0001, respectively) and the seminal vesicles (P = 0.0400 and P = 0.0174, respectively), as well, compared with the DM group (Table 1).
Severe histological alterations were generated by diabetes
Severe histological damage was induced by diabetes in the seminal vesicles both in the muscle layer and in the epithelium (Figure 1). More specifically, we observed a highly condensed thicker internal circular muscular layer and slight disorganization of the thin external longitudinal muscular layer. In addition, the cytoplasm of the muscle cells in the internal muscular layer appeared to have a comparative shrinking compared to the Control. Moreover, small vacuoles appeared in the smooth muscle cells, while the nuclei of the muscle cells appeared to be hyperchromatic, probably as a result of a vigorous response of the cells to the diabetes-induced damage.
Figure 1.
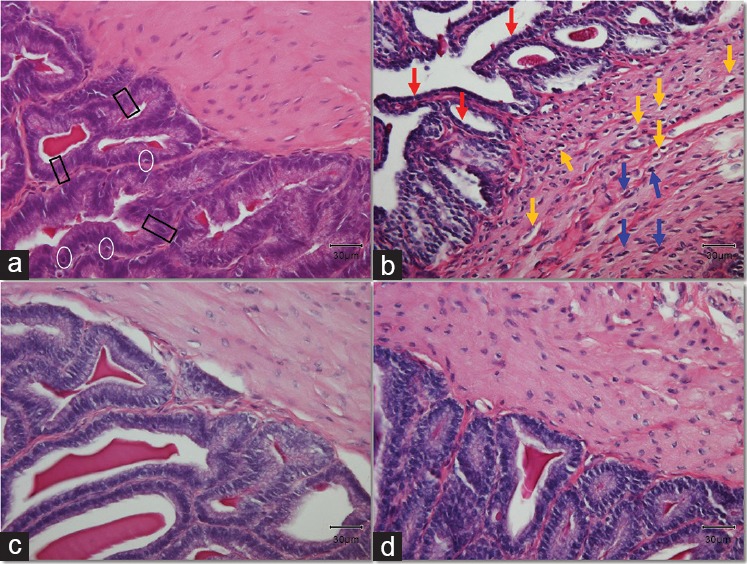
Histological alterations in the tissue of the seminal vesicles. Black rectangles mark typical pseudostratified tall columnar epithelial cells. White circles surround typical basal cells in the epithelium. Yellow arrows point at the vacuolation observed in the cytoplasm of inner circular muscle cells. Blue arrows indicate typical hyperchromatic nuclei of muscle cells. Red arrows show the shrinking of the epithelial cells. Original magnification: ×400. The scale bar is 30 μm. (a) Control group; (b) DM group, untreated diabetic animals; (c) DM/Eda group, diabetic animals treated daily with edaravone 10 mg kg−1, i.p.; (d) DM/Tau group, diabetic animals treated daily with taurine 500 mg kg−1, i.p.
The epithelium of the seminal vesicles consists of basal cells and nonciliated, pseudostratified tall columnar epithelial cells. The epithelium in the DM group had severe atrophy compared to the Control. The structure of the cells was completely disorganized while the pseudostratified columnar epithelial cells appeared to have a shrinking cytoplasm with decreased height. The complex papillary folds surrounding the lumen were observed in samples from all groups. Interestingly, within these folds (lamina propria), which were surrounded with epithelial cells, we observed a stronger staining with magenta color in samples from the DM group. The Masson's trichrome staining revealed fibrotic characteristics in the DM group stained with a light silver/grey color (Figure 2b). There was a thick layer of connective tissue between epithelium and the internal circular muscle area, as well as between the papillary folds, while in many cases, the collagen fibers were extending within the circular muscle layer in the samples from DM group, demonstrating a histological image a lot different from the Control group (Figure 2).
Figure 2.
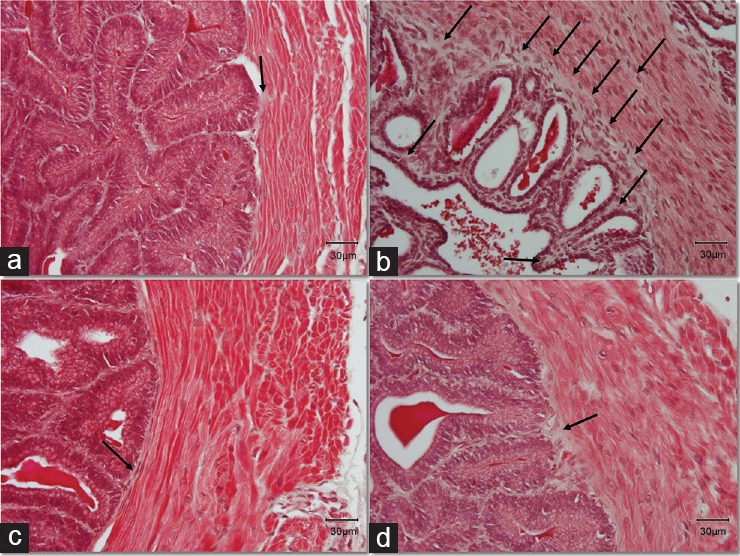
Masson's trichrome stain indicates increase of connective tissue in the diabetic animals. Black arrows indicate the layer of connective tissue (collagen), stained with a light silver/grey color. Bright red color stain is representing the muscle fibers and the nuclei are stained dark red/purple. Original magnification: ×400. The scale bar is 30 μm. (a) Control group; (b) DM group, untreated diabetic animals; (c) DM/Eda group, diabetic animals treated daily with edaravone 10 mg kg−1, i.p.; (d) DM/Tau group, diabetic animals treated daily with taurine 500 mg kg−1, i.p.
Treatment with edaravone and taurine resulted in a better histological profile compared to the DM group. In both treatment groups, the circular muscle cells, as well as the epithelial cells, appeared to have normal cytoplasm without atrophic characteristics. In addition, treatment with edaravone or taurine alleviated the fibrosis (Figure 2c and 2d).
Increase of apoptosis and oxidative stress markers expression in the seminal vesicles by the induction of diabetes
The immunohistochemistry demonstrated strong positive staining for DM samples for cleaved caspase-3 (Figure 3a–3d), 4-HNE (Figure 3e–3h), MDA (Figure 4a–4d), and 8-OHdG (Figure 4e–4h). More specifically, cleaved caspase-3 was mainly localized and expressed in the epithelial cells and secondarily in the inner circular muscle layer of the DM group (Figure 3b). In the Control group, only a small number of cells demonstrating weak expression were detected, mainly in the muscular layer (Figure 3a). The expression of cleaved caspase-3 in the treatment groups was considerably weaker compared to the DM group. A small number of epithelial cells appeared to be positive (basal or pseudostratified columnar epithelial cells; Figure 3c and 3d). Lipid peroxidation markers 4-HNE and MDA demonstrated strong staining both in the muscular area and in the epithelium of the seminal vesicular tissue in the DM group compared to the Control (Figure 3e and 3f, and Figure 4a and 4b, respectively). Treatment with edaravone or taurine resulted in a staining pattern of weak positivity for both 4-HNE and MDA (Figure 3g and 3h, and Figure 4c and 4d, respectively). Finally, the marker for DNA oxidative damage, 8-OHdG, demonstrated positive staining in the samples from DM group. More specifically, 8-OHdG was expressed strongly and was detected in the epithelium and in a more weak fashion in the muscular layer of the DM samples (Figure 4f). The Control samples demonstrated negative staining for the 8-OHdG (Figure 4e). The 8-OHdG expression in the epithelium of DM/Eda group samples was clearly decreased compared to the DM group (Figure 4g). In the DM/Tau group samples, the 8-OHdG was expressed and localized both in the muscle and epithelial cells but in a weaker fashion compared to the DM group (Figure 4h). Treatment with edaravone proved to be more beneficial for this marker compared with taurine.
Figure 3.
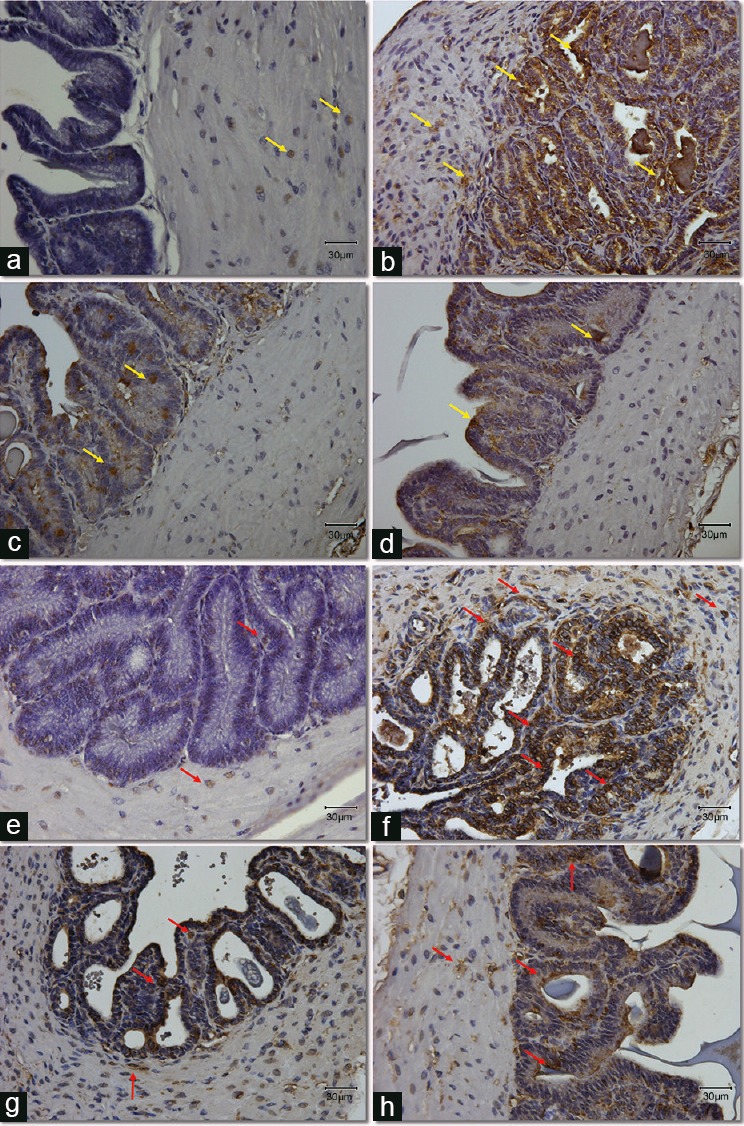
Expression and localization of apoptotic protein, cleaved caspase-3 (a–d) and lipid peroxidation marker, 4-HNE (e–h) in seminal vesicles section from all groups. (a–d) Yellow arrows point at the positive cells stained with brown color for the cleavedcaspase-3 antibody. (e–h) Red arrows point at the positive cells stained with brown color for the 4-HNE antibody. Original magnification: ×400. The scale bar is 30 μm. (a/e) Control group; (b/f) DM group, untreated diabetic animals; (c/g) DM/Eda group, diabetic animals treated daily with edaravone 10 mg kg−1, i.p.; (d/h) DM/Tau group, diabetic animals treated daily with taurine 500 mg kg−1, i.p. 4-HNE: 4-hydroxy-2-nonenal.
Figure 4.
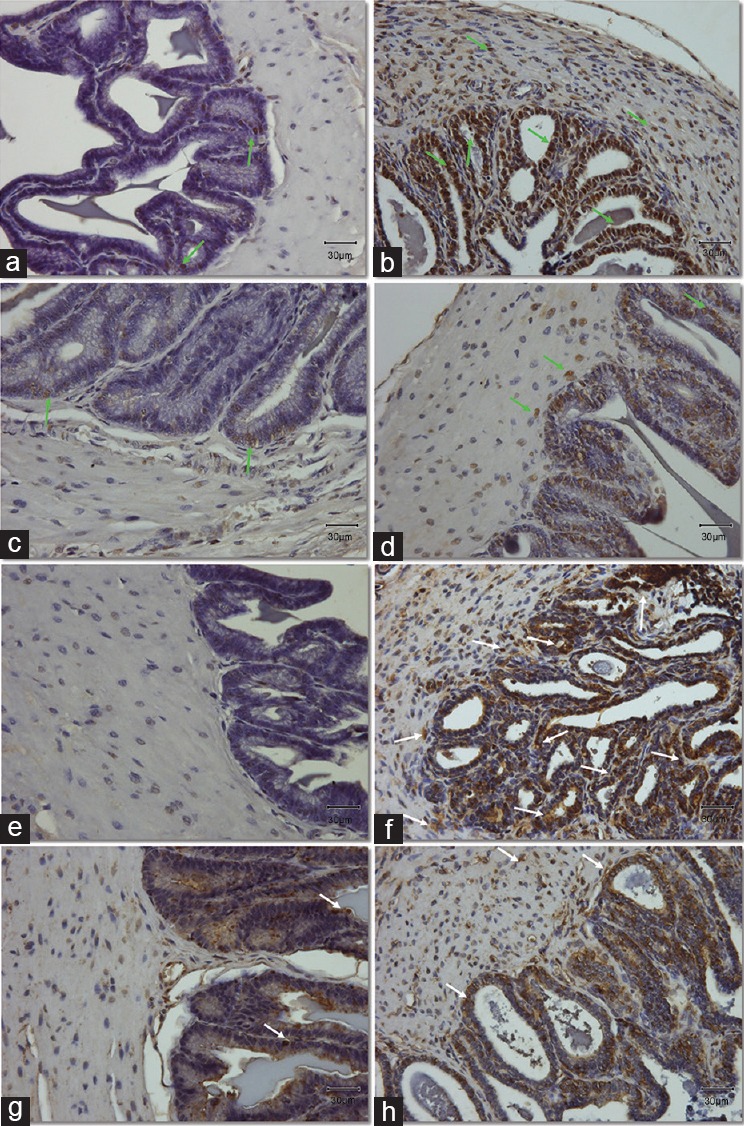
(a and b) Expression and localization of lipid peroxidation marker, MDA in the muscle area and epithelium of seminal vesicles section. (e–h) Expression and localization of DNA oxidative stress marker, 8-OHdG in seminal vesicles sections. (a–d) Light green arrows indicate the positive cells stained with brown color for the MDA antibody. (e–h) White arrows point at the positive cells stained with brown color for the 8-OHdG antibody. Original magnification: ×400. The scale bar is 30 μm. (a/e) Control group; (b/f) DM group, untreated diabetic animals; (c/g) DM/Eda group, diabetic animals treated daily with edaravone 10 mg kg−1, i.p.; (d/h) DM/Tau group, diabetic animals treated daily with taurine 500 mg kg−1, i.p. MDA: malondialdehyde; 8-OHdG: 8-hydroxy-2’-deoxyguanosine.
Negative controls for all markers are presented in Figure 5.
Figure 5.
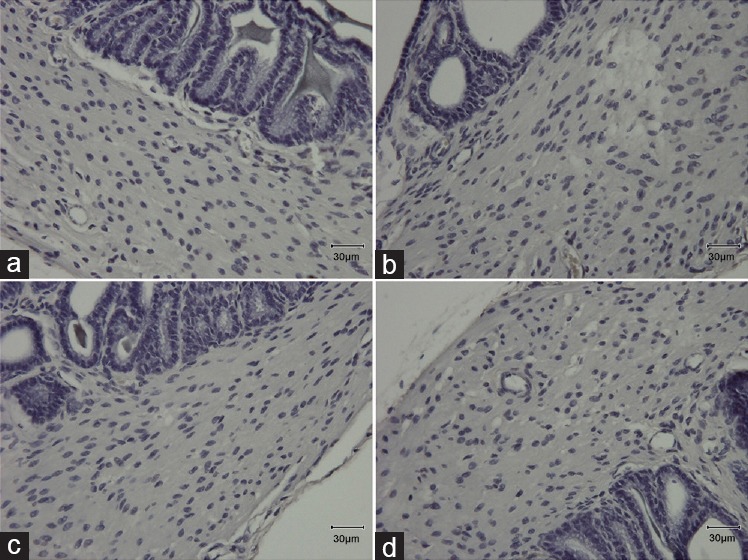
Negative controls for immunohistochemistry. (a) Negative control for cleaved caspase-3; (b) negative control for 4-HNE; (c) negative control for MDA; (d) negative control for 8-OHdG. 4-HNE: 4-hydroxy-2-nonenal; MDA: malondialdehyde; 8-OHdG: 8-hydroxy-2’-deoxyguanosine.
No delivery recorded in any of the diabetic animals
The mating studies resulted in no newborns for any of the diabetic animal groups (Table 1). At the completion of the mating studies, the copulatory plugs found in the female rats that mated with DM rats were very small, while those generated by each treatment group appeared to be larger compared to the respective from the DM group, but still smaller compared to the respective from the Control group.
DISCUSSION
The male accessory sex organs are mainly dependent on androgen hormones for their normal growth, maintenance, and secretory function.11 The present study demonstrated the deleterious effect of diabetes mellitus in the growth and function of the seminal vesicles. Diabetes induced a severe atrophy of the seminal vesicles which was demonstrated by the significantly decreased weight of the organ and the alterations in the histological evaluation. This atrophy resulted in an inability of the organs to function normally to contract and secrete appropriate volume of seminal vesicular fluid with the necessary components.
The diabetes-induced abnormal contractions of the seminal vesicular tissue can be attributed to: (a) the cytoplasmic atrophy of the muscle cells and the overall condensation of the muscle layer, (b) the decreased levels of testosterone, and (c) the excessive oxidative stress. According to our findings, treatment with edaravone and taurine could alleviate the hypercontractility of the seminal vesicles, by restoring the histology of the tissue, significantly decreasing the oxidative stress parameters but without having any positive effect on the testosterone concentrations in the serum. This gives evidence for the crucial role of diabetes-induced oxidative stress in the dysfunction of seminal vesicles. We do not exclude the important role of testosterone in the diabetes-induced dysfunction of the seminal vesicles, but we aim to raise concern on an alternative nonhormonal mechanism which can affect the function of these organs.
In accordance to our results, La Vignera et al.12 in an attempt to evaluate seminal vesicles by ultrasonography in a sample of infertile diabetic patients with neuropathy concluded that these patients have peculiar seminal vesicles ultrasound features.12 Such characteristics include the presence of parietal inflammatory lesions which are induced by the vesicular fluid stasis, regressive-reparative and destructive lesions of the muscular and fibroelastic fibers.12 Furthermore, the authors described changes in the epithelial cells, such as the loss of the main columnar cells cilia or metaplasia of the same cells which further advances the stasis.12 Morrison et al.13 by investigating the effect of streptozotocin-induced diabetes in the seminal vesicles suggested that the changes observed in the organ may be related to the remodeling and regrowth of sympathetic nerve endings damaged in the early stages of hyperglycemia.13 These changes may also contribute to disorders of ejaculation in diabetes.
The pseudostratified tall columnar epithelial cells are predominantly secretory, containing microvesicular lipid droplets and characteristic lipofuscin pigment granules. The severe atrophy of the epithelium, that we observed, induced by the diabetes can explain the extreme low volume of the seminal vesicular fluid in the DM group and, further, the very small copulatory plugs in the vulva/vagina orifice of the female rats after the mattings. Treatment with edaravone and taurine abrogated the histological alterations in the epithelial cells which further resulted in larger volume of the seminal fluid, and consequentially of the copulatory plugs as observed.
Immunohistochemistry revealed the expression and extensive localization of apoptosis marker, cleaved caspase-3 in the epithelium of DM group. Previously, Tanji et al.14 demonstrated the castration-induced apoptosis of the epithelium in the seminal vesicles of the mouse.14 The decreased androgen concentration resulted in upregulation of the apoptotic cells in the epithelium of the seminal vesicles.14 In our study, we demonstrated a profound decrease of the intensity of caspase-3 staining at the treatment groups, compared to the DM group, although testosterone levels were not altered. This observation adds evidence to our hypothesis that oxidative stress may have a role in the induction of apoptosis in the epithelium of the seminal vesicles of diabetic animals.
Yonezawa et al.15 had previously demonstrated that in diabetic rats, the insulin replacement at the onset of early stage diabetes could prevent ejaculatory dysfunction of these animals.15 On the contrary, when the dysfunction takes place, insulin treatment alone cannot restore the ejaculatory capacity back to normal levels. Finally, by measuring the seminal emissions and coagulated seminal materials, they suggested that the loss of seminal emissions happens because of decreased seminal vesicular fluid, which is probably involved in the mechanism of ejaculatory dysfunction in diabetic rats.15 In our study, we found abnormal hypercontractility of the seminal vesicles in nontreated diabetic rats, but after mating, we observed copulatory plugs in the vagina of the female rats. Even though these copulatory plugs were small, they give evidence to the fact that the animals could perform intercourse and ejaculate, even with the given abnormalities. This further means that the absence of newborns is a result of the sperm inability to fertilize the oocyte. This, among other reasons, may happen because of (1) diabetes-induced hypospermatogenesis, (2) diabetes-induced decrease in testosterone production, or (3) decreased seminal vesicular fluid production, which has an impact in the composition of the seminal plasma reflecting an insufficiency of necessary components.
It has been demonstrated that hyperglycemia induces oxidative stress, and it has been characterized as one of the major connections between diabetes and diabetic complications.16 Hyperglycemia results into production of free radicals due to autoxidation of glucose and glycosylation of proteins.17 Moreover, the oxidative stress seems to have some role in inducing nerve damage in the human, and several experimental animal models of diabetes.17,18,19,20,21,22 The mechanisms involved in oxidative stress-induced nerve dysfunctions include (a) generation of reactive oxygen species, (b) increased reactive nitrogen species, (c) lipid peroxidation, (d) DNA damage, and (e) reduction in cellular antioxidants.17 The normal ejaculation involves sympathetic neuronal input, release of the ductus ejaculatory closure resistance, and coordinated contraction of the seminal vesicles, ductus deferens, and vas deferens smooth muscle cells.23 Therefore, it appears that diabetes-induced oxidative stress also affects in a negative way the innervation of the seminal vesicles. From the findings of our study, we may suggest that diabetes-induced oxidative stress mediates probably a secondary mechanism to damage the seminal vesicles function.
For the investigation of diabetes-induced oxidative stress in the seminal vesicles, we employed both biochemical techniques and immunohistochemistry. All evaluated parameters for lipid peroxidation or DNA oxidative damage demonstrated an increased expression of these parameters in the DM group. Immunohistochemistry revealed that principally, the epithelium is negatively affected by diabetes.
Treatment with edaravone and taurine could alleviate the oxidative stress and apoptosis parameters. Both edaravone and taurine have proven antioxidant properties. More specifically, edaravone (3-methyl-l-phenyl-pyrazolin-5-1) is a neuroprotective drug with antioxidant action based on the inactivation of hydroxyl radicals (OH) and subsequently inhibits the OH-dependent and OH-independent lipid peroxidation.24 The mechanism through which edaravone managed to mitigate oxidative stress markers may be through the decrease of the lipid peroxidation both in the seminal vesicles and the serum. An additional mechanism may be through protection of the preganglionic neurons which innervate the seminal vesicles25 against the excessive hyperglycemia-induced oxidative stress. Taurine has both antioxidant and anti-inflammatory properties and it participates in various essential biological processes, such as membrane stabilization and immunity,26 and it protects many organs against toxicity and oxidative stress-induced by various toxic insults, such as diabetes.27 The seminal vesicles have been shown to secrete superoxide dismutase, glutathione peroxidase, glutathione reductase, catalase, and glutathione (GSH) into the seminal fluid,28 as an additional antioxidative support for the spermatozoa. The mechanism responsible for the protective effect of taurine on seminal vesicular anatomy and secretory function may involve the enhancement of intracellular GSH level and increase of catalase activity and glutathione peroxidase29 in the seminal vesicles. In this way, an enhancement of the antioxidant defense of the organ may counteract on the effects of the excessive oxidative stress.
Interestingly, the androgen-independent improvements in the seminal vesicular function and histology which were documented post-treatment did not increase the male fertilizing capacity. These results are consistent with a previous study in our laboratory demonstrating that diabetic rats treated with edaravone or taurine improved spermatogenesis as evaluated by the Johnsen score and decreased oxidative stress damage and DNA damage.6 However, the results were not accompanied by an improvement in the live birth rate.6 This result was attributed to the excessive production of ROS induced by the severe hyperglycemia which further impairs the late stages of spermatogenesis or spermiogenesis.6 Future studies are necessary to elucidate the role of oxidative stress-induced dysfunction in the male genitalia and investigate in more detail about the alterations in the sperm maturation process in the epididymis in the diabetes model.
Seminal vesicles are responsible for the secretion of almost 50%–80% of the ejaculated seminal plasma.30 Bromfield et al.31 recently showed the importance of components of the seminal fluid for the growth and general health of the male offspring.31 In addition, Poiani32 emphasizes in the components of the seminal vesicular fluid of the mammals contribute to: (1) sperm defense, (2) stimulation of sperm capacitation, (3) seminal clot formation, (4) inhibition of neutrophils, (5) regulation of the acrosome reaction, (6) modulation of immune activity in female reproductive tract, (7) sperm transport by stimulation contractions in both male and female reproductive tract, (8) antimicrobial activity, and (9) liquefaction of the seminal fluid affecting sperm speed.32 Furthermore, McGraw et al.33 point out that the large number of proteins present in the seminal plasma is indicative of the substantial role of the seminal plasma in fertilization that happens naturally in the female.33 It is apparent from the above studies that there is an important role of seminal vesicular fluid and its components in the fertility of the male and the spermatozoon ability for fertilization. Although the inhibition of oxidative stress, alone, in diabetic animals by antioxidants can restore the contractility of the seminal vesicles and the histology, it seems to be inadequate to restore the fertility potential of the animals.
The current findings suggest vividly that diabetes-induced oxidative stress mediates probably a secondary mechanism to damage the seminal vesicles function. The alleviation of this damage via antioxidant treatments alone may provide a more optimal ejaculation to the male by normalizing the contractions of the seminal vesicles. On the other hand, antioxidant treatments do not appear to be efficient to correct the reproductive potential of the diabetic male.
AUTHOR CONTRIBUTIONS
PT developed the concept of the study, designed the experimental protocol, conducted experiments, performed statistical analysis of the data, wrote the paper, and critically revised the manuscript for important intellectual content. MH developed the concept of the study, designed the experimental protocol, and critically revised the manuscript for important intellectual content. FD developed the concept of the study, designed the experimental protocol, conducted experiments, and critically revised the manuscript for important intellectual content. BK performed statistical analysis of the data and critically revised the manuscript for important intellectual content. KH critically revised the manuscript for important intellectual content. KM critically revised the manuscript for important intellectual content. MS developed the concept of the study, designed the experimental protocol and critically revised the manuscript for important intellectual content. NS developed the concept of the study, designed the experimental protocol, and critically revised the manuscript for important intellectual content. AT developed the concept of the study, designed the experimental protocol, and critically revised the manuscript for important intellectual content. All authors contributed to the conceptualization of the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declared that they have no competing financial interests.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Yukako Kinoshita, a lot for her valuable advices, Mr. Fumiya Ohmasa, and Mr. Itaru Satoh for their technical support. This study was partially supported by the Grant-In-Aid (KAKENHI) by the Japan Society for the Promotion of Science (25-03102).
REFERENCES
- 1.International Diabetes Federation Guideline Development Group. Guideline for postmeal glucose in diabetes. Diabetes Res Clin Pract. 2014;103:256–68. doi: 10.1016/j.diabres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 3.La Vignera S, Condorelli RA, Di Mauro M, Lo Presti D, Mongioì LM, et al. Reproductive function in male patients with type 1 diabetes mellitus. Andrology. 2015;3:1082–7. doi: 10.1111/andr.12097. [DOI] [PubMed] [Google Scholar]
- 4.Ali ST, Shaikh RN, Siddiqi NA, Siddiqi PQ. Semen analysis in insulin-dependent/non-insulin-dependent diabetic men with/without neuropathy. Arch Androl. 1993;30:47–54. doi: 10.3109/01485019308988368. [DOI] [PubMed] [Google Scholar]
- 5.La Vignera S, Condorelli RA, Vicari E, Lotti F, Favilla V, et al. Seminal vesicles and diabetic neuropathy: ultrasound evaluation after prolonged treatment with a selective phosphodiesterase-5 inhibitor. Andrology. 2013;1:245–50. doi: 10.1111/j.2047-2927.2012.00025.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsounapi P, Saito M, Dimitriadis F, Koukos S, Shimizu S, et al. Antioxidant treatment with edaravone or taurine ameliorates diabetes-induced testicular dysfunction in the rat. Mol Cell Biochem. 2012;369:195–204. doi: 10.1007/s11010-012-1382-z. [DOI] [PubMed] [Google Scholar]
- 7.McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–6. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito M, Ohmasa F, Dimitriadis F, Tsounapi P, Sejima T, et al. Hydroxyfasudil ameliorates penile dysfunction in the male spontaneously hypertensive rat. Pharmacol Res. 2012;66:325–31. doi: 10.1016/j.phrs.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Tsounapi P, Honda M, Dimitriadis F, Shimizu S, Hikita K, et al. Post-fertilization effect of bilateral primary testicular damage induced by unilateral cryptorchidism in the rat model. Andrology. 2016;4:297–305. doi: 10.1111/andr.12154. [DOI] [PubMed] [Google Scholar]
- 10.Sofikitis N, Takahashi C, Kadowaki H, Okazaki T, Shimamoto T, et al. The role of seminal vesicles and coagulating glands in fertilization in the rat. Int J Androl. 1992;15:54–61. doi: 10.1111/j.1365-2605.1992.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 11.Kochakian CD. Hypotaurine: regulation of production in seminal vesicles and prostate of guinea-pig by testosterone. Nature. 1973;241:202–3. doi: 10.1038/241202a0. [DOI] [PubMed] [Google Scholar]
- 12.La Vignera S, Condorelli RA, Di Mauro M, D’Agata R, Vicari E, et al. Seminal vesicles and diabetic neuropathy: ultrasound evaluation. J Androl. 2011;32:478–83. doi: 10.2164/jandrol.110.011676. [DOI] [PubMed] [Google Scholar]
- 13.Morrison JF, Dhanasekaran S, Sheen R, Frampton CM, Mensah-Brown E. The effect of streptozotocin-induced diabetes on the rat seminal vesicle: a possible pathophysiological basis for disorders of ejaculation. Ann N Y Acad Sci. 2006;1084:267–79. doi: 10.1196/annals.1372.013. [DOI] [PubMed] [Google Scholar]
- 14.Tanji N, Satoh H, Takagi-Morishita Y, Sugihara A, Terada N, et al. Induction of apoptosis by castration in epithelium of the mouse seminal vesicles. Arch Androl. 2003;49:409–15. doi: 10.1080/01485010390236369. [DOI] [PubMed] [Google Scholar]
- 15.Yonezawa A, Ebiko M, Yoshizumi M, Ise S, Watanabe C, et al. Effects of insulin replacement on ejaculatory dysfunction in streptozotocin-induced diabetic rats. Int J Urol. 2009;16:208–11. doi: 10.1111/j.1442-2042.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- 16.Oyenihi AB, Ayeleso AO, Mukwevho E, Masola B. Antioxidant strategies in the management of diabetic neuropathy. Biomed Res Int 2015. 2015:515042. doi: 10.1155/2015/515042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Faris NA, Al-Sawadi AD, Alokail MS. Effect of samh seeds supplementation (Mesembryanthemum forsskalei Hochst) on liver enzymes and lipid profiles of streptozotocin (STZ)-induced diabetic Wistar rats. Saudi J Biol Sci. 2010;17:23–8. doi: 10.1016/j.sjbs.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, et al. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001;50:1927–37. doi: 10.2337/diabetes.50.8.1927. [DOI] [PubMed] [Google Scholar]
- 19.Obrosova IG, van Huysen C, Fathallah L, Cao XC, Greene DA, et al. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB. 2002;16:123–5. doi: 10.1096/fj.01-0603fje. [DOI] [PubMed] [Google Scholar]
- 20.Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, et al. High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB. 2002;16:1738–48. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- 21.Yorek MA. The role of oxidative stress in diabetic vascular and neural disease. Free Radic Res. 2003;37:471–80. doi: 10.1080/1071576031000083161. [DOI] [PubMed] [Google Scholar]
- 22.Feldman EL, Vincent A. The prevalence, impact, and multifactorial pathogenesis of diabetic peripheral neuropathy. Adv Stud Med. 2004;4:S642–9. [Google Scholar]
- 23.Birowo P, Uckert S, Kedia GT, Scheller F, Meyer M, et al. Evaluating the role of the serotoninergic system in the control of human seminal vesicle smooth muscle - An in vitro approach. J Sex Med. 2009;6:2672–9. doi: 10.1111/j.1743-6109.2009.01423.x. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe K, Morinaka Y, Iseki K, Watanabe T, Yuki S, et al. Structure-activity relationship of 3-methyl-1-phenyl-2- pyrazolin-5-one (edaravone) Redox Rep. 2003;8:151–5. doi: 10.1179/135100003225001520. [DOI] [PubMed] [Google Scholar]
- 25.Sun XQ, Xu C, Leclerc P, Benoît G, Giuliano F, et al. Spinal neurons involved in the control of the seminal vesicles: a transsynaptic labeling study using pseudorabies virus in rats. Neuroscience. 2009;158:786–97. doi: 10.1016/j.neuroscience.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 26.Schuller-Levis GB, Park E. Taurine and its chloramine: modulators of immunity. Neurochem Res. 2004;29:117–26. doi: 10.1023/b:nere.0000010440.37629.17. [DOI] [PubMed] [Google Scholar]
- 27.Anuradha CV. Aminoacid support in the prevention of diabetes and diabetic complications. Curr Protein Pept Sci. 2009;10:8–17. doi: 10.2174/138920309787315194. [DOI] [PubMed] [Google Scholar]
- 28.Tramer F, Rocco F, Micali F, Sandri G, Panfili E. Antioxidant systems in rat epididymal spermatozoa. Biol Reprod. 1998;59:753–8. doi: 10.1095/biolreprod59.4.753. [DOI] [PubMed] [Google Scholar]
- 29.Schaffer SW, Azuma J, Mozaffari M. Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol. 2009;87:91–9. doi: 10.1139/Y08-110. [DOI] [PubMed] [Google Scholar]
- 30.King BF, Hattery RR, Lieber MM, Berquist TH, Williamson B, Jr, et al. Congenital cystic disease of the seminal vesicle. Radiology. 1991;178:207–11. doi: 10.1148/radiology.178.1.1984306. [DOI] [PubMed] [Google Scholar]
- 31.Bromfield JJ, Schjenken JE, Chin PY, Care AS, Jasper MJ, et al. Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc Natl Acad Sci U S A. 2014;111:2200–5. doi: 10.1073/pnas.1305609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poiani A. Complexity of seminal fluid: a review. Behav Ecol Sociobiol. 2006;60:289–310. [Google Scholar]
- 33.McGraw LA, Suarez SS, Wolfner MF. On a matter of seminal importance. Bioessays. 2015;37:142–7. doi: 10.1002/bies.201400117. [DOI] [PMC free article] [PubMed] [Google Scholar]


