Abstract
This study examined the effects of melatonin on leptin-induced changes in sperm parameters in adult rats. Five groups of Sprague-Dawley rats were treated with either leptin or leptin and melatonin or melatonin for 6 weeks. Leptin was given daily via the intraperitoneal route (60 μg kg−1 body weight) and melatonin was given in drinking water (10 mg kg−1 or 20 mg kg−1 body weight per day). Upon completion, sperm count, sperm morphology, 8-hydroxy-2-deoxyguanosine, Comet assay, TUNEL assay, gene expression profiles of antioxidant enzymes, respiratory chain reaction enzymes, DNA damage, and apoptosis genes were estimated. Data were analyzed using ANOVA. Sperm count was significantly lower whereas the fraction of sperm with abnormal morphology, the level of 8-hydroxy-2-deoxyguanosine, and sperm DNA fragmentation were significantly higher in rats treated with leptin only. Microarray analysis revealed significant upregulation of apoptosis-inducing factor, histone acetyl transferase, respiratory chain reaction enzyme, cell necrosis and DNA repair genes, and downregulation of antioxidant enzyme genes in leptin-treated rats. Real-time polymerase chain reaction showed significant decreases in glutathione peroxidase 1 expression with increases in the expression of apoptosis-inducing factor and histone acetyl transferase in leptin-treated rats. There was no change in the gene expression of caspase-3 (CASP-3). In conclusion, the adverse effects of leptin on sperm can be prevented by concurrent melatonin administration.
Keywords: 8-hydroxy-2-deoxyguanosine, DNA fragmentation, leptin, melatonin, sperm
INTRODUCTION
Leptin, a 16-kDa protein that is produced and secreted mainly by the adipose tissue, is a hormone that has been shown to have roles in diverse physiological processes including regulation of body weight and food intake, immune function, hematopoiesis, inflammation, sexual maturation, and normal reproduction.1,2,3,4 However, a number of recent reports have indicated some adverse effects of leptin on sperm count and morphology. Exogenous leptin administration to normal rats for 6 weeks was found to decrease sperm concentration while increasing the fraction of sperm with abnormal morphology.5,6,7,8 Although the precise mechanism for this remains unclear, leptin-induced oxidative stress has been implicated, as leptin administration has been shown to increase free radical production.8,9 Reactive oxygen species and their metabolites cause damage to the membrane lipids, DNA, and cellular proteins, and oxidative stress is now hypothesized as a possible cause of male infertility.10,11,12,13 While increased free radical production might be responsible for the leptin-induced decreases in sperm count and abnormal sperm morphology, it, however, remains unknown if concurrent antioxidant supplementation to leptin treated rats would prevent the adverse effects of leptin on some of these sperm parameters. Melatonin interacts with various reactive oxygen and reactive nitrogen species and also upregulates antioxidant enzymes and downregulates pro-oxidant enzymes. Therefore, diverse beneficial effects of melatonin have been claimed to protect against various degenerative conditions caused by oxidative stress. This study examined the effects of concurrent leptin treatment and melatonin supplementation on sperm count, sperm morphology, apoptosis, and DNA damage in normal adult rats.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats, aged 12 weeks, were obtained from the Laboratory Animal Care Unit (LACU), Universiti Teknologi MARA. Rats were housed in standard rat cages with commercial wood chip bedding at room temperature and with a 12/12 light/dark cycle. Rats had access ad libitum to commercial rat feed (Specialty Feeds Pty Ltd., Perth, Australia) and tap water throughout the experimental period. The experimental protocol used in this study was approved by the Animal Care and Use Committee (ACUC), Universiti Teknologi MARA. Rats were randomized into five groups consisting of control, leptin, leptin-melatonin-10 (LM10), leptin-melatonin-20 (LM20), and melatonin-10 (M10) treated groups with 6 rats per group. All leptin-treated groups received intraperitoneal injections (i.p.) of leptin once daily for 42 days (60 μg kg−1 body weight; recombinant rat leptin, purity >95%, from BioVision Inc., Milpitas, California, USA). Rats in the leptin- and melatonin-treated groups, in addition to leptin, were given melatonin either 10 mg kg−1 or 20 mg kg−1 body weight per day (Sigma-Aldrich, St. Louis, MO, USA,) in drinking water for 42 days. Control rats received 0.1 ml of normal saline i.p. for 42 days. Body weights of control and experimental animals were monitored weekly. The duration and dose of leptin treatment were based on our previous studies in the rat where a more pronounced effect of leptin was evident following 42 days rather than after 7 days or 21 days of treatment.5,6 Although the dose of leptin used in these studies ranged from 5 μg kg-1 to 30 μg kg−1, to ensure a more pronounced effect, a dose of 60 μg kg−1 body weight was used in this study. The study design and the use of animals were approved by the ACUC of Universiti Teknologi MARA.
Sperm collection
Upon completion of treatment, the animals were euthanized and the testes and epididymis were removed. The epididymis was minced in 2 ml normal saline and then filtered through a nylon mesh to prepare an epididymal suspension for analysis.5 The left testis was immediately snap-frozen in liquid nitrogen and stored at −80°C for gene analysis at a later date. The right testis was immediately kept in 10% (v/v) buffered formalin for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay.
Sperm count and morphology
The epididymal suspension was first mixed well and a small drop was placed in the center of the Makler chamber (Sefi Medical Instruments Ltd., Haifa, Israel) and covered with a glass cover. The droplet was allowed to spread on the entire area of the disc. The number of total and abnormal sperm in 10 squares was counted. This number represented the concentration of sperm in million per ml. This was repeated and the average of the two was determined and the concentration was expressed in million per ml. The number of abnormal sperm in the same 10 squares was recorded. The sperm was classified into normal and abnormal types.7 In brief, the sperm with the following characteristics was considered abnormal: (i) headless, (ii) hookless, (iii) cephalo-cauda (head pointing toward the tail), (iv) double-headed, (v) broken tail, (vi) microcephalic, (vii) coiled tail, and (viii) double-tailed.7
Quantitative measurement of 8-hydroxy-2-deoxyguanosine (8-OHdG)
Genomic DNA was isolated from the sperm sample of each animal using Genomic DNA Isolation Kit (BioVision Inc., Milpitas, California, USA). The DNA was hydrolyzed into nucleotides with 100 U ml−1 nuclease P1 (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for 60 min in 20 mmol l−1 sodium acetate buffer (pH 4.5).14 Then, 1 unit of alkaline phosphatase (Sigma-Aldrich, St. Louis, MO, USA) was added to 100 μg of DNA and incubated at 37°C for 30 min.14 The DNA lysate was boiled for 10 min, following which it was placed on ice at room temperature. 8-OHdG EIA Kit (Cayman Chemical, Ann Arbor, MI, USA) was used to estimate 8-OHdG.
Sperm Comet assay
Comet assay was performed on sperm to assess its DNA integrity.15 Frosted slides were covered with 100 μl of 1% (w/v) normal-melting agarose (NMA) with a cover slip and allowed to solidify at 4°C. After 5 min, the cover slips were gently removed. Following this, 10 μl of the epididymal sperm sample from each rat containing 1 × 105 sperm was added to 90 μl of 1% (w/v) low-melting agarose (LMA), and 85 μl of the suspension from each rat was then gently pipetted onto the frosted slides with solidified NMA to form the second layer. Fresh cover slips were placed and the agarose was allowed to set at 4°C for 5–10 min. After this, the cover slips were removed and the slides were then placed in Coplin jars containing lysis buffer (2.5 mol l-1 NaCl, 100 mmol l-1 Na2 EDTA, 10 mmol l-1 Tris base containing 1% (v/v) Triton X-100, and 40 mmol l-1 DL-dithiothreitol (Sigma-Aldrich St. Louis, MO, USA) and proteinase K 0.5 mg ml−1 (Affymetrix, Santa Clara, California, USA) at 37°C for 24 h. Following cell lysis, all slides were washed three times with deionized water for 10 min to remove salt and detergent from the gels. Slides were then coded and placed in a horizontal electrophoresis tank (Model Sub-Cell GT Basic, Bio-Rad, Hercules, CA, USA; max. volts 200 V, max. current 400 mA) filled with fresh alkaline electrophoresis buffer (300 mmol l-1 NaOH and 1 mmol l-1 EDTA, pH 13) for 20 min to allow the DNA from the cells to unwind. Electrophoresis was performed at room temperature and conducted at 25 V, 300 mA for 20 min.16 The slides were then washed and neutralized with neutralization buffer (0.4 mol l-1 Tris-Base, pH 7.5) in 3 Coplin jars for 5 min each. After neutralization, slides were dried and stained with DNA SYBR Green 1 (Invitrogen-Molecular Probes, Foster, CA, USA; 1:10 000 dilution) for 30 min. The slides were then rinsed briefly with double-distilled water and cover slips were placed for image analysis. The fluorescent-labeled DNA was visualized (200×) using BX 61 Imager fluorescence microscope (Olympus Co., Tokyo, Japan) and the resulting images were captured on a computer. Comet Assay Software Project (CASP) lab Version 1.2.3b1(Free Software Foundation Inc., Boston, MA, USA) was used to analyze the Comet images. Samples were run in duplicate and 100 cells were randomly analyzed per slide for a total of 200 cells per sample and scored for tail length (TL), tail moment (TM), olive tail moment (OTM), and % tail DNA.
TUNEL assay
Testicular tissues were fixed in 10% (v/v) buffered formalin, embedded in paraffin, and cut into thin sections with a microtome. TUNEL assay was used to study DNA fragmentation according to the manufacturer's instructions (BioVision Inc., Milpitas, California, USA). TUNEL-positive cells were observed under fluorescent microscopy and images were acquired. The cells were quantified in each tissue section by counting the number of TUNEL-positive cells in the seminiferous tubule using Image Processing and Analysis Software (ImageJ 1.48v; NIH, Bethesda, Maryland, USA). Ten high-vision fields on each slide were examined and for each vision field, TUNEL-positive cells labeled in green were counted in a total of 100 cells. At least, a total of 1000 cells were evaluated in each section and the apoptotic index (AI) was calculated.
Microarray analysis
The left testis was thawed and then total cellular RNA was extracted using innuPREP RNA Mini Kit (Analytik Jena, Jena, Germany) according to the manufacturer's protocol, followed by treatment with DNase (Thermo Scientific, Foster, CA, USA).17 The RNA quality and concentration were ascertained.17,18 About 200 ng of total RNA was used to prepare amplified cDNA with reagents provided in the Applause WT-Amp plus ST System Kit (Nugen Technology, San Carlos, CA, USA). The cDNA was purified with QIAGEN's MinElute reaction Cleanup Kit (QIAGEN, Hilden, Germany) and its concentration and purity were determined.17 The Encore Biotin Module (Part No. 4200) was used to label the cDNA and then hybridized using GeneChip Hybridization kit (Affymetrix Rat GeneChip St 2) for 18 h in the GeneChip Hybridization Oven 640 (Affymetrix, Santa Clara, CA, USA). Immediately following hybridization, the array was washed and stained with streptavidin phycoerythrin conjugate on the GeneChip Fluidic Station 450 (Affymetrix, Santa Clara, CA, USA), followed by scanning on a GeneChip Scanner. Data from microarray were displayed as CEL, DAT, and JPG files. These output data from microarray were analyzed as gene-level differential expression by Affymetrix software (Expression Console-1-3-1-64 bit and Transcriptome Analysis Console-2-0-64 bit). Gene expressions in leptin- and melatonin-treated rats that were either 2 fold greater or 2-fold lesser than those in the control were considered significant (P < 0.05). Upregulated and downregulated genes were then grouped according to their functions using software (TIBCO® Spotfire® software Version 1.0.0, Palo Alto, CA, USA) Server: https://spotfire.cloud.tibco.com/.
Real-time PCR
Gene expressions of caspase-3 (CASP-3), apoptosis-inducing factor (AIF), glutathione peroxidase 1 (GPX1), and histone acetyltransferases (HAT) that were found altered in the cDNA microarray analysis were further confirmed by real-time polymerase chain reaction (PCR). Briefly, total RNA was extracted from testicular tissue with the aid of innuPREP RNA Mini Kit (Analytik Jena, Jena, Germany) according to the manufacturer's protocol. Then, 2 μl RNA (30 ng) was used in a 20 μl cDNA reaction using 5x iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) according to the manufacturer's protocol. The cDNA was further PCR-amplified by specific primers in 10 μl PCR mixture (SYBR Green Supermix and 25 μmol l-1 forward and reverse primers template) with iQ™ 5 Real-Time PCR detection system (Bio-Rad, Hercules, CA, USA). The paired primer sets included forward CASP-3 (5’-TGAAGGGGTCATTTATGGGACA3’) and CASP-3 reverse (5’-TCCCATAAATGACCCCTTCATCA3’), forward AIF (5’-CCACAGCAGGAGACTGTGTGTATC3’) and reverse AIF (5’-GATCCGGCGTGTACTTCCATC3’), GPX1 forward (5’-GTGCAATCAGTTCGGACACCA3’) reverse GPX1 (5’- GAGACGCGACATTCTCAATGA3’) and HAT forward (5’-ACAATGTTCCGTGTTGAATATGC3’) and HAT reverse (5’-AGGTATGAAGTAAGGTTCCGAATG3’), Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) forward (5’- CAACTCCCTCAAGATTGTCAGCAA3’) and GAPDH reverse (5’- GGCATGGACTGTGGTCATGA3’) and TATA box binding protein (TBP) forward (5’-TGGGATTGTACCACAGCTCCA3’), and TBP reverse (5’-CTCATGATGACTGCAGCAAACC3’). Normalization of gene expression was done with GAPDH and TBP housekeeping genes. The iQ™ 5 Real-Time PCR software (Bio-Rad, Hercules, CA, USA) was used to calculate the gene expression for all samples.
Statistical analysis
ANOVA with Tukey's post hoc test contained in SPSS version 21 (SPSS Inc., Chicago, Illinois, USA) was used to analyze the data. The data are expressed as mean ± standard deviation. A significant difference was accepted when P < 0.05.
RESULTS
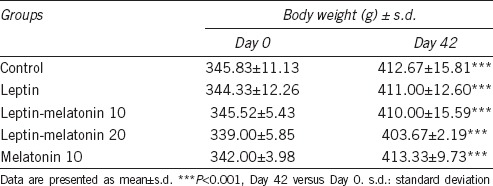
Body weight increased in all rats over the 6-week study period (Table 1). However, no differences were evident in body weight between leptin, LM10, LM20, melatonin-only treated groups, and that of the control group.
Table 1.
Body weight in control and leptin-treated rats
Total sperm count was lower in leptin-only and LM10-treated rats than that in the control (P < 0.001 and P < 0.01, respectively, Figure 1a). No difference was evident in sperm count between control and LM20- and M10-treated rats.
Figure 1.
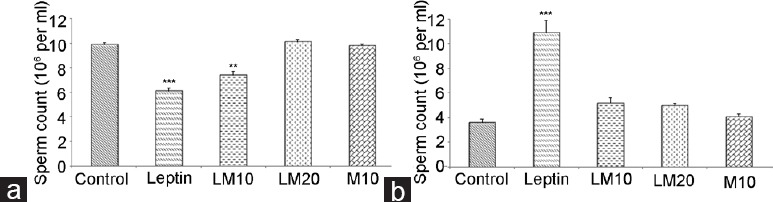
Sperm count (a) and sperm morphology (b) in leptin- and melatonin-treated rats compared to control. **P < 0.01; ***P < 0.001.
The fraction of sperm with abnormal morphology was higher in leptin only-treated rats compared to that in the control (P < 0.001; Figure 1b). No differences were evident in the fraction of sperm with abnormal morphology between M10, LM10, LM20, and that of saline-treated control group. The major morphologically abnormal sperm noticeable was the headless and coiled tail types.
TUNEL assay revealed evidence of higher DNA fragmentation and apoptosis in the seminiferous tubules of leptin-treated rats than that in the control rats (Figure 2). In addition, the AI in leptin-treated rats was higher than that in the control rats (Figure 2f). There was no difference in the AI between controls and rats given melatonin at a dose of 20 mg kg−1 body weight.
Figure 2.
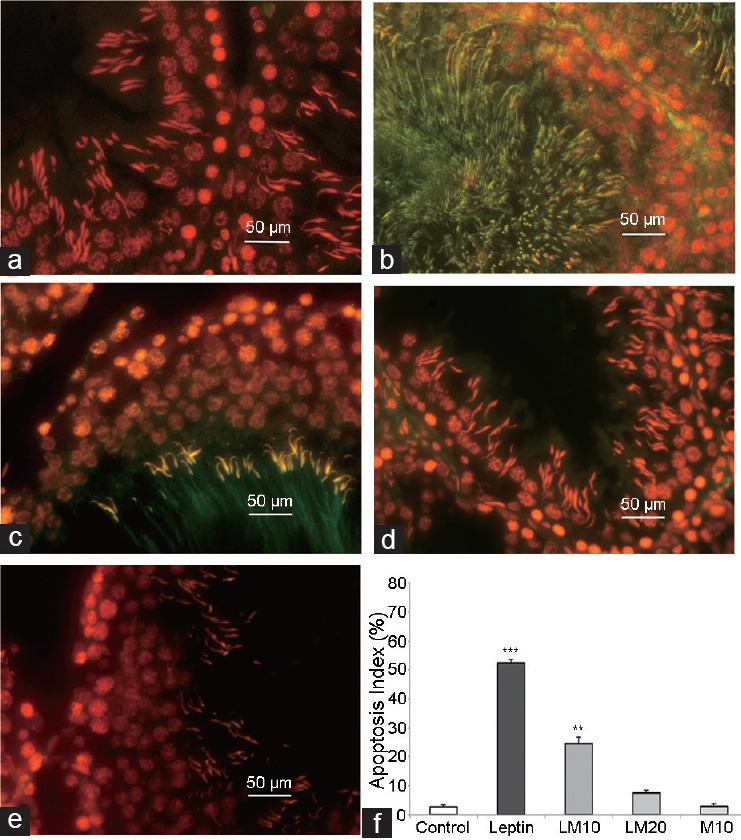
Results of TUNEL assay in control (a), leptin-treated (b), leptin-melatonin-10 (LM10) treated (c), leptin-melatonin-20 (LM20) treated (d), melatonin-10 (M10) treated (e) rats, and apoptosis index (f). **P < 0.01; ***P < 0.001; leptin, LM10, LM20, and M20 versus control.
No difference was evident in the level of sperm 8-OHdG between control and LM20- and M10-treated rats. However, 8-OHdG level was higher in leptin-only and LM10-treated rats than that in controls (P < 0.001 and P < 0.05, respectively, Figure 3).
Figure 3.
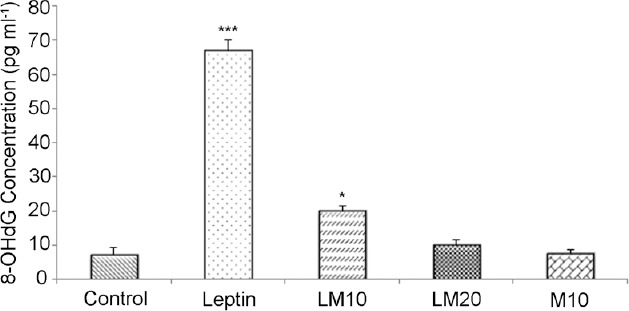
Sperm 8-hydroxy-2-deoxyguanosine (8-OHdG) levels in control, leptin, leptin-melatonin-10 (LM10), leptin-melatonin-20 (LM20), and melatonin-10 (M10) rats. *P < 0.05; ***P < 0.001.
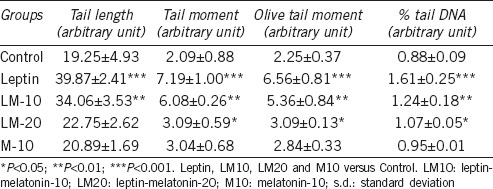
Tail length, tail moment, olive tail moment, and % tail DNA were higher in leptin-only, LM10-, and LM20-treated rats than that in the controls (Table 2). These four parameters were higher in leptin- and LM10-treated rats than that in the LM20-treated rats.
Table 2.
Sperm with ssDNA break in rat of experimental groups shown by Comet assay (mean±s.d.)
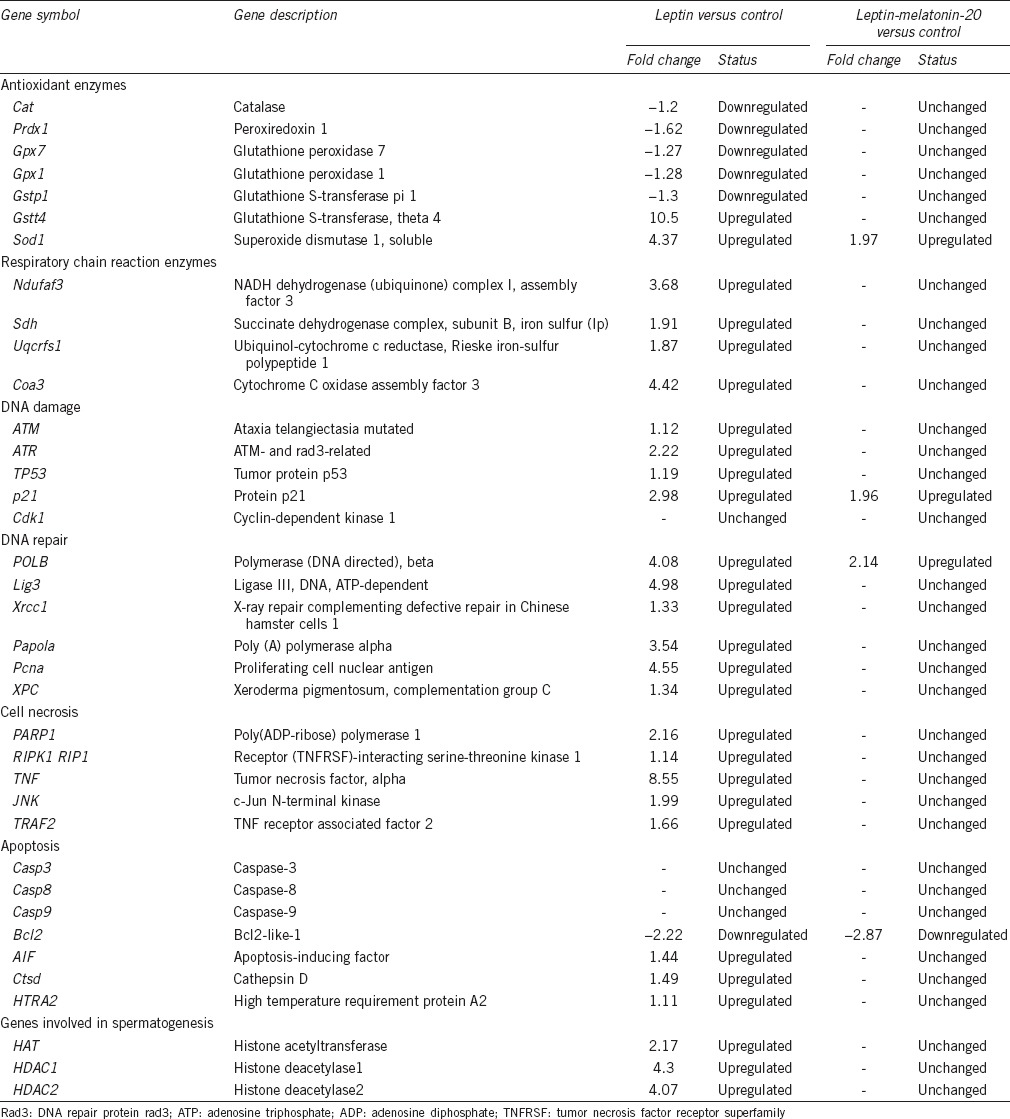
Microarray analysis revealed 1893 and 1119 genes that were upregulated in leptin-treated rats and LM20-treated rats, respectively. A total of 3800 and 898 genes were downregulated in leptin- and LM20-treated rats, respectively, when compared with the expressions in the controls. Of these, some of the genes regulating apoptosis, DNA damage, DNA repair, cell necrosis, antioxidant enzymes, and respiratory chain reaction enzymes are shown in Table 3.
Table 3.
Detailed gene profile analysis by microarray analysis
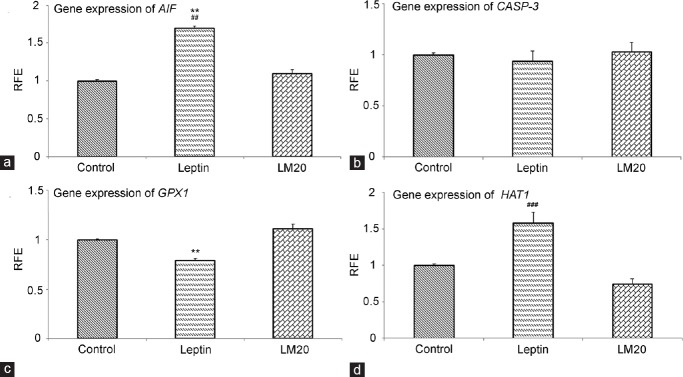
Real-time PCR revealed a higher mRNA expression of AIF in the testes of leptin-treated rats than that in LM20-treated and control rats (P < 0.01; Figure 4a). No difference was evident in CASP-3 mRNA expression between leptin-treated, LM20-treated, and control rats (Figure 4b). The mRNA expression of GPX1 was lower in leptin-treated rats than those in LM20-treated and control rats (P < 0.01; Figure 4c). The HAT mRNA expression was higher in leptin- and LM20-treated rats than that in control rats (P < 0.01; Figure 4d).
Figure 4.
Relative fold expression (RFE) mRNA of AIF (a), CASP-3 (b), GPX1 (c), and HAT (d) in control, leptin-treated, and leptin-melatonin-20 treated (LM20) rats. *P < 0.05, ***P < 0.01, leptin versus control; ##P < 0.01, ###P < 0.001, leptin versus LM20.
DISCUSSION
The major findings of this study include: (a) lower total sperm count in leptin-treated rats when compared with that in saline-treated controls, (b) higher fraction of sperm with abnormal morphology in leptin-treated rats, (c) higher apoptotic activity and DNA fragmentation in leptin-treated rats, (d) higher levels of 8-OHdG in leptin-treated rats, (e) differential expression of a number of genes following leptin and melatonin treatment, and (f) no significant differences in sperm count, abnormal sperm morphology, apoptotic activity, DNA fragmentation, and 8-OHdG levels between controls and leptin + melatonin-treated rats.
The precise mechanism for the lower total sperm count and higher fraction of sperm with abnormal morphology following leptin treatment is still unclear. Decreases in sperm count and increased fraction of sperm with abnormal morphology following leptin treatment have been reported before.5,6,8 Increased sperm 8-OHdG, a marker of DNA damage due to oxidative stress in rats, and increased intracellular levels of reactive oxygen species (ROS) following leptin treatment have been reported recently.7,8 The main sources of ROS are seminal leukocytes and spermatozoa, and low levels of ROS are necessary for normal sperm functions including capacitation, hyperactivation, and acrosome reaction. However, when produced in large amounts, it is detrimental to sperm and germinal cells. Leptin has also been shown to increase superoxide anion production (O2•−) in vascular smooth muscle cells in culture9 and in aortic endothelial cells via protein kinase-A-mediated fatty acid oxidation.19 It also causes peroxynitrite-mediated oxidative stress in steatohepatic lesions.20 Leptin-induced ROS production in mouse hepatocytes is inhibited by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, MAP kinase/ERK kinase 1 (MEK1), and Janus kinase 2 (JAK2) inhibitors, suggesting the possible involvement of these pathways in leptin-induced ROS production.21
The expression of HAT and histone deacetylase (HDAC) was significantly higher in leptin-treated rats than that in control and LM20-treated rats. Leptin has been shown to increase the involvement of histone acetyl transferases in growth-stimulating activity of breast cancer cells.22 Histone acetylation plays an important role in spermatogenesis.23 Histone acetylation of lysine residues, clustered at the amino-terminal end of core histones, is regulated by HAT that facilitate acetylation, and HDAC that decrease acetylation.23,24 A recent study demonstrated a significant positive relationship between HAT activity and sperm DNA fragmentation index.23 When histones are acetylated, their affinity to DNA is decreased by the loss of the positive charge. This then loosens the DNA chromatin structure or makes it less compact for active transcription.25 However, if histones bound to DNA are hyperacetylated, the DNA chromatin becomes further less compact and becomes more susceptible to fragmentation.
The impact of leptin on DNA damage possibly occurs through leptin-induced increase in production of ROS. In this regard, microarray analysis results revealed a significant upregulation of the electron transfer chain enzymes in mitochondria and downregulation of the expression of antioxidant enzymes, catalase (CAT), glutathione peroxidase 1 (GPX1), peroxiredoxin 1 (Prdx1), and glutathione S-transferase pi 1 (Gstp1) following leptin treatment. This might result in increased production of ROS and oxidative stress, as has been reported recently following leptin treatment.8 The upregulation of HAT gene at the same time might make the DNA less compact and more susceptible to ROS attack, and thereby leading to an increase in DNA fragmentation.
Microarray analysis of the DNA damage genes also revealed an upregulation in the expression of ataxia telangiectasia mutated (ATM), ATM and DNA repair protein rad3-related (ATR), and TP53 and p21 genes in leptin-treated rats. It has been reported that the early molecules that respond to DNA damage consist of ATM and ATR genes.26 The ATM and ATR proteins belong to the phosphatidylinositol 3-kinase-like (PIKK) family of serine/threonine protein kinases and these phosphorylate the DNA damage mediator protein TP53. The target of the transcription factor TP53 is p21, which, in turn, inhibits cyclin-dependent kinase 1 (CDK1) activity, causing arrest of the cell cycle. When the cell cycle arrests, the DNA repair machinery becomes effective.26 If the DNA repair is successful, the cell cycle arrest is lifted, but if the repair is unsuccessful, then programed cell death follows. DNA repair genes, polymerase beta (POLB), ligase III, (Lig3), X-ray repair complementing defective repair (Xrcc1), and poly (A) polymerase alpha (Papola) were significantly upregulated following leptin treatment. The increased cell death, as evident from the TUNEL assay, despite the increased expression of DNA repair genes, suggests that the repair was inadequate to sustain the cell cycle in leptin-treated rats. TUNEL assay and Comet assay revealed higher apoptotic activity and DNA fragmentation in rats treated with leptin alone than that in the control and melatonin-treated rats, particularly at a dose of 20 mg kg−1 body weight.
To understand the mechanism of leptin-induced apoptosis, the expression of caspase-dependent and caspase-independent cell death-related genes in the testis was determined. Of the genes involved in caspase-dependent apoptosis, caspase-3, caspase-8, and caspase-9 were not different between control and leptin-treated rats. Interestingly, the expression of AIF, high temperature requirement protein A2 (HTRA2), and cathepsin D (Ctsd) genes involved in caspase-independent cell death showed a significant upregulation, suggesting that leptin-induced cell death via a caspase-independent pathway. Pro-survival and pro-death signals are generated in response to multiple intracellular stress conditions and these signals converge to a mitochondrion-centered control mechanism. When lethal signals prevail, mitochondrial outer membrane permeabilization (MOMP) occurs and leads to mitochondrial trans-membrane potential dissipation,27 leading to ROS overgeneration and release of proteins into the cytosol that are normally confined within the mitochondrial intermembrane space (IMS). These proteins include apoptosis-inducing factor and HTRA2. Apoptosis-inducing factor is a mitochondrial protein that plays a pivotal role in programmed cell death. Apoptosis-inducing factor becomes an active cell killer when it is released from mitochondria to the cytosol, from where it then translocates to the nucleus and triggers peripheral chromatin condensation and DNA fragmentation and DNA loss.28,29 In addition, the serine protease HTRA2 also contributes to caspase-independent apoptosis by cleaving a wide array of cellular substrates including cytoskeletal proteins.30 The lysosomal protease cathepsin D has been reported to trigger apoptosis-inducing factor release independent of the caspase cascade.29,30 In addition, the death cell receptors including tumor necrosis factor (TNF) have been reported to induce a necrotic-like cell death when caspases are inhibited and this type of cell death is mediated by ROS generation.31 Tumor necrosis factor initiates necrosis of the cell through sustained activation of c-Jun N-terminal kinase (JNK), which is mediated by the central initiator of necrosis receptor (tumor necrosis factor receptor superfamily)-interacting serine-threonine kinase 1 (RIP1) and TNF receptor-associated factor 2 (TRAF2). Activated c-Jun N-terminal kinase impairs mitochondrial membrane integrity causing release of mitochondrial inter-membrane space proteins and consequent necrosis.32 Our results show that TNF, RIP1, TARF2, and JNK genes are significantly upregulated in leptin-treated rat testes. This finding suggests that leptin induced a necrotic-like cell death through the activation of TNF and JNK pathways.
Concurrent daily administration of melatonin prevented the adverse effects of leptin, particularly when given at a dose of 20 mg kg−1 body weight per day. Melatonin has been reported to prevent gentamycin-induced testicular toxicity in rats.33 In addition, it has also been shown to have potent protective effects against anticancer drug-induced testicular toxicity, including reduced sperm count and lowered sperm motility.34,35 Recent studies have also demonstrated that melatonin prevents oxidative damage and testicular toxicity induced by ochratoxin A, cyclophosphamide, electromagnetic radiation, testicular ischemia-reperfusion, and hypoxia, and it also supports the antioxidant redox system in the testis.36,37,38,39,40 More interestingly, leptin-induced increases in O2•− in primary cultured vascular smooth muscle cells were prevented by melatonin.9 It is, therefore, possible that melatonin reduced the adverse effects of leptin by reducing the level of oxidative stress. Besides functioning as a synchronizer of the biological clock, melatonin is a powerful antioxidant,41 particularly protecting nuclear and mitochondrial DNA.42 It scavenges hydroxyl radical (OH•), peroxynitrite anion (ONOO−), O2•−, nitric oxide radical (NO•), and peroxy radicals.43 It is also known to enhance the antioxidant activities of superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase, and glutathione reductase (GR).44,45,46 Besides this, melatonin has been shown to increase GPx protein in the kidney of spontaneously hypertensive rats (SHR),47 and increase GPx-1 mRNA expression in human chorion, rat liver, and rat brain cortex.48,49,50 Moreover, it also enhances the ability of cells to resist oxidative damage by inhibiting the pro-oxidant nitric oxide synthase.51 Reproductive systems of different species have binding sites for melatonin, so it seems reasonable to assume that melatonin also exerts its actions through direct interaction with the cells of the reproductive organs.52,53
CONCLUSION
It appears that leptin administration increases the levels of oxidative stress in the testes causing necrotic-like cell death through TNF and JNK pathways. Melatonin prevents these leptin-induced adverse effects on sperm count, sperm morphology, DNA fragmentation, and apoptosis, which might be due to its antioxidant activity. These findings suggest that melatonin might be a useful protective agent against leptin-induced damage to the male reproductive organs and infertility.
AUTHOR CONTRIBUTIONS
FAA was involved in the conduct of the study, collection and analysis of laboratory data, preparation and revision of the manuscript. KO was involved in the planning of the project and training in the use of Comet and TUNEL assays, preparation of the manuscript. SFI was involved in the planning of the project and training in the use of Comet and TUNEL assays, preparation of the manuscript. SG was involved in the planning and training in sperm collection, microscopic examination, preparation and revision of the manuscript. JG was involved in the training in the use of microarray analysis and rtPCR, preparation of the manuscript. EI was involved in the training in the use of microarray analysis and rtPCR, preparation of the manuscript. HJS was involved in the overall planning of the project, submission for funding, supervision of the study, analysis of laboratory data, preparation and revision of the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was funded under the Fundamental Research Grant Scheme from the Ministry of Higher Education (600-RMI/ST/FRGS 5/3Fst) (60/2010).
REFERENCES
- 1.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol. 2000;68:437–46. [PubMed] [Google Scholar]
- 2.Mantzoros CS. Role of leptin in reproduction. Ann N Y Acad Sci. 2000;900:174–83. doi: 10.1111/j.1749-6632.2000.tb06228.x. [DOI] [PubMed] [Google Scholar]
- 3.Waelput W, Brouckaert P, Broekaert D, Tavernier J. A role for leptin in the systemic inflammatory response syndrome (SIRS) and in immune response, an update. Curr Med Chem. 2006;13:465–75. doi: 10.2174/092986706775527929. [DOI] [PubMed] [Google Scholar]
- 4.Sayed-Ahmed A, Abd-Elmaksoud A, Elnasharty M, El-Magd MA. In situ hybridization and immunohistochemical localization of leptin hormone and leptin receptor in the seminal vesicle and prostate gland of adult rat. Acta Histochem. 2012;114:185–91. doi: 10.1016/j.acthis.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 5.Haron MN, D'souza UJ, Jaafar H, Zakaria R, Singh HJ. Exogenous leptin administration decreases sperm count and increases the fraction of abnormal sperm in adult rats. Fertil Steril. 2010;93:322–4. doi: 10.1016/j.fertnstert.2009.07.995. [DOI] [PubMed] [Google Scholar]
- 6.Haron MN, Singh HJ, D'souza UJ, Jaafar H, Zakaria R. Leptin administration decreases sperm count in Sprague-Dawley rats. Novus Sci J. 2013;2:1–8. doi: 10.1016/j.fertnstert.2009.07.995. [DOI] [PubMed] [Google Scholar]
- 7.Almabhouh FA, Osman K, Siti Fatimah I, Sergey G, Gnanou J, et al. Effects of leptin on sperm count and morphology in Sprague–Dawley rats and their reversibility following a 6-week recovery period. Andrologia. 2015;47:751–8. doi: 10.1111/and.12325. [DOI] [PubMed] [Google Scholar]
- 8.Abbasihormozi S, Shahverdi A, Kouhkan A, Cheraghi J, Akhlaghi AA, et al. Relationship of leptin administration with production of reactive oxygen species, sperm DNA fragmentation, sperm parameters and hormone profile in the adult rat. Arch Gynecol Obstet. 2013;287:1241–9. doi: 10.1007/s00404-012-2675-x. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Martínez E, Miana M, Jurado-López R, Bartolomé MV, Souza Neto FV, et al. The potential role of leptin in the vascular remodeling associated with obesity. Int J Obes. 2014;38:1565–72. doi: 10.1038/ijo.2014.37. [DOI] [PubMed] [Google Scholar]
- 10.Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res. 2009;129:357–67. [PubMed] [Google Scholar]
- 11.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32:1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014;28:684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Durairajanayagam D, Halabi J, Peng J, Vazquez-Levin M. Proteomics, oxidative stress and male infertility. Reprod Biomed Online. 2014;29:32–58. doi: 10.1016/j.rbmo.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Sakai Y, Aminaka M, Takata A, Kudou Y, Yamauchi H, et al. Oxidative stress in mature rat testis and its developmental changes. Dev Growth Differ. 2010;52:657–63. doi: 10.1111/j.1440-169X.2010.01201.x. [DOI] [PubMed] [Google Scholar]
- 15.Trivedi PP, Kushwaha S, Tripathi DN, Jena GB. Evaluation of male germ cell toxicity in rats: correlation between sperm head morphology and sperm comet assay. Mutat Res. 2010;703:115–21. doi: 10.1016/j.mrgentox.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Hughes CM, Lewis SE, McKelvey-Martin VJ, Thompson W. Reproducibility of human sperm DNA measurements using the alkaline single cell gel electrophoresis assay. Mutat Res. 1997;374:261–8. doi: 10.1016/s0027-5107(96)00241-2. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi H, Tainaka H, Umezawa M, Takeda K, Tanaka H, et al. Evaluation of testicular toxicology of doxorubicin based on microarray analysis of testicular specific gene expression. J Toxicol Sci. 2011;36:559–67. doi: 10.2131/jts.36.559. [DOI] [PubMed] [Google Scholar]
- 18.Kijima K, Toyosawa K, Yasuba M, Matsuoka N, Adachi T, et al. Gene expression analysis of the rat testis after treatment with di (2-ethylhexyl) phthalate using cDNA microarray and real-time RT-PCR. Toxicol Appl Pharmacol. 2004;200:103–10. doi: 10.1016/j.taap.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 19.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, et al. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–100. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee S, Ganini D, Tokar EJ, Kumar A, Das S, et al. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J Hepatol. 2013;58:778–84. doi: 10.1016/j.jhep.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schroyen B, Guimarães EL, Dollé L, Coulon S, Empsen C, et al. Leptin-mediated reactive oxygen species production does not significantly affect primary mouse hepatocyte functions in vitro. Eur J Gastroenterol Hepatol. 2012;24:1370–80. doi: 10.1097/MEG.0b013e328357ce1c. [DOI] [PubMed] [Google Scholar]
- 22.Saxena NK, Vertino PM, Anania FA, Sharma D. Leptin-induced growth stimulation of breast cancer cells involves recruitment of histone acetyltransferases and mediator complex to CYCLIN D1 promoter via activation of Stat3. J Biol Chem. 2007;282:13316–25. doi: 10.1074/jbc.M609798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim JH, Jee BC, Lee JM, Suh CS, Kim SH. Histone acetylation level and histone acetyltransferase/deacetylase activity in ejaculated sperm from normozoospermic men. Yonsei Med J. 2014;55:1333–40. doi: 10.3349/ymj.2014.55.5.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davie JR. Covalent modifications of histones: expression from chromatin templates. Curr Opin Genet Dev. 1998;8:173–8. doi: 10.1016/s0959-437x(98)80138-x. [DOI] [PubMed] [Google Scholar]
- 25.Turner BM. Histone acetylation and control of gene expression. J Cell Sci. 1991;99:13–20. doi: 10.1242/jcs.99.1.13. [DOI] [PubMed] [Google Scholar]
- 26.Houtgraaf JH, Versmissen J, van der Giessen WJ. A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovasc Revasc Med. 2006;7:165–72. doi: 10.1016/j.carrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, et al. Molecular definitions of cell death subroutines: recommendations of the nomenclature committee on cell death. Cell Death Differ. 2012;19:107–20. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, et al. Mediation of poly (ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–63. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 29.Bröker LE, Kruyt FA, Giaccone G. Cell death independent of caspases. Clin Cancer Res. 2005;11:3155–62. doi: 10.1158/1078-0432.CCR-04-2223. [DOI] [PubMed] [Google Scholar]
- 30.Ravagnan L, Gurbuxani S, Susin SA, Maisse C, Daugas E, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–43. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 31.Morgan MJ, Kim YS, Liu ZG. TNFα and reactive oxygen species in necrotic cell death. Cell Res. 2008;18:343–9. doi: 10.1038/cr.2008.31. [DOI] [PubMed] [Google Scholar]
- 32.Festjens N, Vanden Berghe T, Vandenabeele P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim Biophys Acta. 2006;1757:1371–87. doi: 10.1016/j.bbabio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Kim SH, Lee IC, Baek HS, Shin IS, Moon C, et al. Melatonin prevents gentamicin-induced testicular toxicity and oxidative stress in rats. Andrologia. 2014;46:1032–40. doi: 10.1111/and.12191. [DOI] [PubMed] [Google Scholar]
- 34.Ateşşahin A, Sahna E, Türk G, Ceribaşi AO, Yilmaz S, et al. Chemoprotective effect of melatonin against cisplatin-induced testicular toxicity in rats. J Pineal Res. 2006;41:21–7. doi: 10.1111/j.1600-079X.2006.00327.x. [DOI] [PubMed] [Google Scholar]
- 35.Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, et al. Potential chemoprotective effect of melatonin in cyclophosphamide-and cisplatin-induced testicular damage in rats. Fertil Steril. 2009;92:1124–32. doi: 10.1016/j.fertnstert.2008.07.1758. [DOI] [PubMed] [Google Scholar]
- 36.Malekinejad H, Mirzakhani N, Razi M, Cheraghi H, Alizadeh A, et al. Protective effects of melatonin and Glycyrrhiza glabra extract on ochratoxin A - Induced damages on testes in mature rats. Hum Exp Toxicol. 2011;30:110–23. doi: 10.1177/0960327110368416. [DOI] [PubMed] [Google Scholar]
- 37.Koksal M, Oğuz E, Baba F, Eren MA, Ciftci H, et al. Effects of melatonin on testis histology, oxidative stress and spermatogenesis after experimental testis ischemia-reperfusion in rats. Eur Rev Med Pharmacol Sci. 2012;16:582–8. [PubMed] [Google Scholar]
- 38.Zepeda A, Aguayo LG, Fuentealba J, Figueroa C, Acevedo A, et al. Blueberry extracts protect testis from hypobaric hypoxia induced oxidative stress in rats. Oxid Med Cell Longev. 2012;2012:975870. doi: 10.1155/2012/975870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chabra A, Shokrzadeh M, Naghshvar F, Salehi F, Ahmadi A. Melatonin ameliorates oxidative stress and reproductive toxicity induced by cyclophosphamide in male mice. Hum Exp Toxicol. 2014;33:185–95. doi: 10.1177/0960327113489052. [DOI] [PubMed] [Google Scholar]
- 40.Oksay T, Naziroğlu M, Doğan S, Güzel A, Gümral N, et al. Protective effects of melatonin against oxidative injury in rat testis induced by wireless (2.45 GHz) devices. Andrologia. 2012;46:65–72. doi: 10.1111/and.12044. [DOI] [PubMed] [Google Scholar]
- 41.Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine. 2005;27:119–30. doi: 10.1385/endo:27:2:119. [DOI] [PubMed] [Google Scholar]
- 42.Reiter RJ, Acuña-Castroviejo D, Tan DX, Burkhardt S. Free radical-mediated molecular damage. Mechanisms for the protective actions of melatonin in the central nervous system. Ann N Y Acad Sci. 2001;939:200–15. [PubMed] [Google Scholar]
- 43.Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2:181–97. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 44.Reiter RJ, Tan DX, Osuna C, Gitto E. Actions of melatonin in the reduction of oxidative stress. J Biomed Sci. 2000;7:444–58. doi: 10.1007/BF02253360. [DOI] [PubMed] [Google Scholar]
- 45.Mayo JC, Sainz RM, Antoli I, Herrera F, Martin V, et al. Melatonin regulation of antioxidant enzyme gene expression. Cell Mol Life Sci. 2002;59:1706–13. doi: 10.1007/PL00012498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez C, Mayo JC, Sainz RM, Antolín I, Herrera F, et al. Regulation of antioxidant enzymes: a significant role for melatonin. J Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee SK, Sirajudeen KN, Sundaram A, Zakaria R, Singh HJ. Effects of antenatal, postpartum and post-weaning melatonin supplementation on blood pressure and renal antioxidant enzyme activities in spontaneously hypertensive rats. J Physiol Biochem. 2011;67:249–57. doi: 10.1007/s13105-010-0070-2. [DOI] [PubMed] [Google Scholar]
- 48.Kotler M, Rodríguez C, Sáinz RM, Antolín I, Menéndez-Peláez A. Melatonin increases gene expression for antioxidant enzymes in rat brain cortex. J Pineal Res. 1998;24:83–9. doi: 10.1111/j.1600-079x.1998.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 49.Ohta Y, Kongo M, Kishikawa T. Effect of melatonin on changes in hepatic antioxidant enzyme activities in rats treated with alpha-naphthylisothiocyanate. J Pineal Res. 2001;31:370–7. doi: 10.1034/j.1600-079x.2001.310413.x. [DOI] [PubMed] [Google Scholar]
- 50.Okatani Y, Wakatsuki A, Shinohara K, Kaneda C, Fukaya T. Melatonin stimulates glutathione peroxidase activity in human chorion. J Pineal Res. 2001;30:199–205. doi: 10.1034/j.1600-079x.2001.300402.x. [DOI] [PubMed] [Google Scholar]
- 51.Pozo D, Reiter RJ, Calvo JR, Guerrero JM. Inhibition of cerebellar nitric oxide synthase and cyclic GMP production by melatonin via complex formation with calmodulin. J Cell Biochem. 1997;65:430–42. doi: 10.1002/(sici)1097-4644(19970601)65:3<430::aid-jcb12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 52.Oner-Iyidoğan Y, Gürdöl F, Oner P. The effects of acute melatonin and ethanol treatment on antioxidant enzyme activities in rat testes. Pharmacol Res. 2001;44:89–93. doi: 10.1006/phrs.2001.0828. [DOI] [PubMed] [Google Scholar]
- 53.Köylü H, Mollaoglu H, Ozguner F, Naziroglu M, Delibas N. Melatonin modulates 900 MHz microwave-induced lipid peroxidation changes in rat brain. Toxicol Ind Health. 2006;22:211–6. doi: 10.1191/0748233706th263oa. [DOI] [PubMed] [Google Scholar]