Abstract
This study was conducted to clarify the toxic effects of sertraline (SRT) on the reproductive system of male rats and to elucidate the underlying mechanisms. Rats were treated orally with SRT at doses of 5, 10, and 20 mg kg−1 for 28 consecutive days. At the end of the treatment period, sperm concentration, sperm motility, and sperm morphology were investigated by computer-assisted sperm analysis system whereas sperm DNA damage was detected by comet assay. The oxidative status of the testes was investigated, and a histopathological examination was conducted. Serum testosterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH) levels were measured to determine the effects of SRT on the spermatogenesis process. One-way ANOVA, post-hoc Dunnett's T3 test for the sperm comet assay, and post-hoc Tukey's test for the others were performed for statistical analysis. The results showed that SRT caused an increase in sperm DNA damage and induced histopathological lesions in all groups treated with SRT. There was abnormal sperm morphology and increased malondialdehyde (MDA) in the 10 mg kg−1 treatment group. More dramatic changes were observed in the 20 mg kg−1 treatment group. Decreased sperm count was accompanied by a significant increase in abnormal sperm morphology, DNA damage, and degeneration in cellular-tubular structures. Serum LH and testosterone levels were elevated in the 20 mg kg−1 treatment group. Decreased glutathione (GSH) and increased MDA were signs of enhanced oxidative stress (OS). In conclusion, SRT induced testicular toxicity in a dose-dependent manner and OS is suggested as a crucial mechanism.
Keywords: DNA damage, oxidative stress, reproductive toxicity, sertraline
INTRODUCTION
Infertility is defined as unsuccessful pregnancy resulting from regular and unprotected intercourse between couples during a 1-year period, and infertility incidence among couples has been calculated to be 15%.1 Infertility etiology between couples comprises 40%–50% of women-only induced factors, 30% of men-only induced factors, and 20% of both men- and women-induced factors. Direct or indirect men-induced factors compose approximately 30%–50% of infertility cases.2,3 Varicocele, congenital anomalies, urogenital infections, endocrine illnesses, and immunological factors can be counted among the etiologic factors causing infertility in men. Obesity, radiation, and weather also affect fertility in men.3 Repeated doses of exposed drugs may also cause infertility in men either directly by affecting the gonads or indirectly by affecting pituitary gonadotropins and causing changes in sperm parameters such as sperm count, mortality, and morphology.4,5
Selective serotonin reuptake inhibitors (SSRIs) are drugs that are used for short- and long-term treatment of mental illnesses. Most prevalent psychiatric illnesses are anxiety disorders, and SSRIs are the first-line drugs in the treatment of these disorders.6 Sertraline (SRT), one of the three commonly prescribed SSRIs,7 is used in the treatment of obsessive-compulsive disease, anxiety, and depression.8,9 During SSRI treatment, various central and peripheral nervous system-relatedly hormonal and neurochemical changes occur, which are responsible for the adverse effects on the reproductive system.10 SSRIs are known to cause adverse sexual effects such as anorgasmia, erectile dysfunction, and decreased libido.10,11
Previous studies have assessed sperm quality as a result of SSRI toxicity. Normal sperm morphology,12,13,14 sperm concentration,12,13,14 and sperm motility13,14 were found decreasing, and abnormal sperm DNA fragmentation was induced12,13,15 in male patients under SSRI treatment including citalopram, escitalopram, fluoxetine, paroxetine, and SRT. However, the effects of SRT on the male reproductive system remain unclear. The present study investigated sperm parameters such as sperm morphology, motility, and concentration simultaneously with the affected hormone levels, oxidative stress (OS) parameters, DNA damage, and pathological conditions to provide more detailed information on the underlying mechanisms of SRT toxicity in the male reproductive system.
MATERIALS AND METHODS
Materials
SRT hydrochloride (C17H18Cl3N) was donated by the IE Ulagay-Menarini Group (Istanbul, Turkey). Testosterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), malondialdehyde (MDA), and glutathione (GSH) levels were determined using ELISA kits from Cusabio Biotech Co. Ltd. (Hubei, China).
Animals
Male Wistar rats weighing 200–250 g and aged 8 to 10-week-old were obtained from Anadolu University Research Center for Animal Experiments. The rats were housed in a room at a controlled temperature (24°C) under a 12-h light/12-h dark cycle (lights on at 08:00 am) with access to food and water ad libitum. Animals were acclimatized to the laboratory environment for at least 48 h before the experimental session. The experimental protocol was approved by the Local Ethical Committee on Animal Experimentation of Anadolu University, Eskisehir, Turkey (certificate number: 2014-33), in adherence with the National Institutes of Health Guidelines for the Use of Laboratory Animals.16
Experimental groups
The rats were assigned randomly into the following treatment groups – control group: animals received distilled water by oral gavage per day (7 days per week) for 4 weeks (n = 8). 5 mg kg−1 SRT-treated group: animals received 5 mg kg−1 dose of SRT by oral gavage per day (7 days per week) for 4 weeks (n = 8). 10 mg kg−1 SRT-treated group: animals received 10 mg kg−1 dose of SRT by oral gavage per day (7 days per week) for 4 weeks (n = 8). 20 mg kg−1 SRT-treated group: animals received 20 mg kg−1 dose of SRT by oral gavage per day (7 days per week) for 4 weeks (n = 8). In previous studies, the reproductive toxicity of SRT has not been investigated within a dose-response relationship in rats. For this reason, the doses of SRT were determined according to the previous studies17,18,19 investigating SRT-induced antidepressant effects to obtain a dose-response relationship in therapeutic concentrations of SRT in rats, and these doses were in accordance with the guidelines extrapolating human doses to animal doses.20 All drugs were administered at a volume of 1 ml per 100 g body weight by dissolving in distilled water. The treatment period was in accordance with the guideline.21
At the end of 4 weeks, the animals were anesthetized by intraperitoneal injection of 1.5 g kg−1 urethane.22 Blood samples for hormonal analysis (FSH, LH, and testosterone) were collected from the right ventricle of the animals via a syringe. The animals were euthanized via withdrawal of large amounts of blood from the heart.
Testicular and epididymis tissues were removed. The left testis and epididymis were cleaned of blood in a phosphate buffer solution (PBS) (composition: NaCl: 8 g l−1, KCl: 0.2 g l−1, KH2PO4: 0.2 g l−1 and Na2HPO4: 1.14 g l−1 at pH 7.4) and weighed. The left epididymis was used to determine the levels of GSH and MDA. The right testis was cleaned of blood and other contaminants in PBS and fixed for histological examination. The cauda of the right epididymis was used for sperm parameters.
Collection and evaluation of sperm samples
Spermatozoa obtained from the right epididymis immediately after euthanizing rats were placed in a Petri dish containing DMEM/Hams F-12 at 37°C. The cauda epididymis was transferred to a new Petri dish with 1 ml of the same medium, and blood vessels and fat tissue were removed. Approximately 0.5 cm of the cauda epididymis was removed and placed in another Petri dish containing 1 ml of the same medium, and spermatozoa were allowed to swim out of the tissue for 1 min.23,24
Assessment of sperm concentration and motility
Five microliters of a concentrated spermatozoa cloud was collected and placed on a Leja slide (Leja Products BV, Nieuw Vennep, The Netherlands). The Leja slide was placed onto a temperature controlled stage of the Nikon E200 microscope (set at 37°C). A negative phase contrast objective (4×) in conjunction with a phase contrast condenser was used to determine sperm motility and concentration via a motility/concentration module of the Sperm Class Analyzer® (SCA), version 5.4.0.1 (Microptic Automatic Diagnstic System, Barcelona, Spain), at 50 frames s−1. The data were collected by capturing images with a digital camera (Basler AG, Ahrensburg, Germany). For the sperm motility evaluation, a total of eight fields were captured with the SCA system, and 200 motile spermatozoa were counted25 and analyzed as recommended by the World Health Organization.26
Assessment of sperm morphology
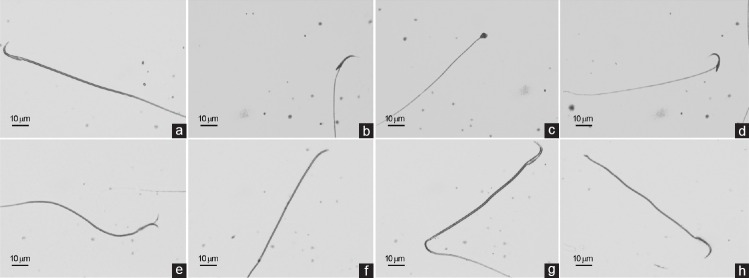
Fresh sperm smears were prepared for morphometric analysis by placing 5 μl of the fresh semen on the clear end of a frosted slide by dragging the drop across the slide. The smears were allowed to air dry before staining. Three semen smears were prepared and stained with Spermblue; (Microptic Automatic Diagnostic System, Barcelona, Spain) according to Van der Horst and Maree (2009).27 Stained slides were used to perform a morphology evaluation using a morphometry module of SCA version 5.4.0.1 software. The machine was equipped with a Nikon Eclipse model 50i (Nikon Corporation, Tokyo, Japan) microscope with a 60× bright-field objective and a video camera (Basler, AG, Ahrensburg, Germany). A total of 200 sperms/animal were analyzed randomly. The morphometric parameters of the head and tail were determined, and abnormal sperms were detected according to previous criteria.28,29,30,31,32 Sperms with a banana-shaped head, amorphous head, and bent neck and two-headed and headless sperms were counted for head abnormalities whereas sperms with a bent tail and broken tail were counted for tail abnormalities (Figure 1).
Figure 1.
Effects of sertraline treatment on sperm morphology in rats. (a) Normal sperm; (b) banana-shaped head; (c) amorphous head; (d) bent neck; (e) two headed; (f) headless; (g) bent tail; and (h) broken tail. Scale bars = 10 μm.
Sperm comet assay
Frosted microscope slides were covered with 1% normal melting point agarose in Ca2+ - and Mg2+ -free PBS. The sperm sample (10 μl) containing 1 × 105 sperm ml−1 was suspended in 75 μl of 1% (w/v) low melting point agarose. Then, 85 μl of this suspension was applied to the surface of a microscope slide (precoated with 1% normal melting point agarose) to form a microgel and allowed to set at 4°C for 5 min. Slides were dipped in cell lysis buffer (2.5 mol l−1 NaCl, 100 mmol l−1 EDTA, 10 mmol l−1 Tris-HCl, pH 10.0, containing 1% Triton X-100 added just before use, and 40 mmol l−1 dithiothreitol) for 24 h at room temperature. Following the initial lysis, proteinase K was added to the lysis solution (0.5 mg ml−1), and an additional lysis was performed at 37°C for 24 h. After cell lysis, all slides were washed three times with deionized water at 10 min intervals to remove salt and detergent from the microgels. Slides were placed in a horizontal electrophoresis unit and were allowed to equilibrate for 20 min with running buffer (500 mmol l−1 NaCl, 100 mmol l−1 Tris-HCl and 1 mmol l−1 EDTA, pH 9.0) before electrophoresis (0.60 V cm−1, 250 mA) for 30 min. After electrophoresis, slides were then neutralized with 0.4 mol l−1 Tris (pH 7.5), stained with SYBR Green I (1:10.000) (Sigma-Aldrich, Taufkirchen, Germany) for 1 h and covered with cover slips.33
Biochemical analysis
Determination of serum FSH, LH, and testosterone levels
After drawing blood for 30 min to allow clotting, blood samples from rats were centrifuged for 15 min (at 4°C and at 1000 g), and serum was separated. The hormonal analyses were performed using commercially available kits according to the manufacturer's instructions.
Determination of GSH and MDA levels in testes
The left testis was divided into equal parts and stored at -20°C after freezing in liquid nitrogen. The GSH and MDA levels in the testis were determined using commercially available kits and according to the instructions of the manufacturer.
Histological analysis
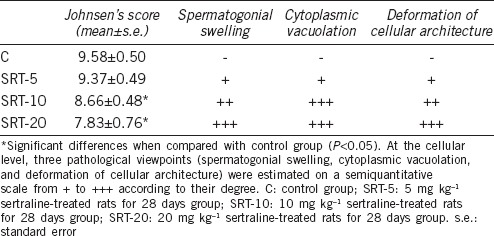
The right testis tissues were sliced into small pieces (approximately 2 mm3) and then fixed in paraformaldehyde (4%) in phosphate buffer pH 7.2. They were dehydrated in a graded series of alcohols. To improve infiltration, the samples were treated with a mixture of LR White (Electron Microscopy Sciences, Ft. Washington, PA, USA) and ethanol (2:1) (v/v) for 1 h at room temperature. The samples were then embedded in LR White and sectioned at 700 nm (0.7 μm) thickness using the Leica EM UC7 ultramicrotome. Semithin sections were stained with 1% toluidine blue/borax (pH 8.4) for 2 min and observed under a Leica DM 750 microscope equipped with a DFC camera.34 Observations were conducted on every 24 sections of the testis (three sections per animal). Five representative seminiferous tubules of stage XI and XII were selected and examined from each section in random order under blinded conditions. Testicular injury and spermatogenesis were evaluated using Johnsen's mean testicular biopsy score criteria.35 A score of 1–10 was assigned to each tubule cross-section according to the range from no cells to complete spermatogenesis. At the cellular level, three pathological viewpoints (spermatogonial swelling, cytoplasmic vacuolation, and deformation of cellular architecture) were estimated on a semiquantitative scale from + to +++ according to their degrees.36
Statistical analysis
All data are expressed as the mean ± standard error. Statistical analyses of the groups were performed using the SigmaPlot Version 10 package program (Systat Software, San Jose, California, USA). All values were verified to be normally distributed. In the sperm comet assay, one-way analysis of variance (ANOVA) followed by Dunnett's T3 test as a post-hoc test was performed. In the other experiments, one-way ANOVA followed by Tukey's test as a post-hoc test was performed. P < 0.05 was considered statistically significant.
RESULTS
Effects of SRT treatment on testis and epididymis weights in rats
In comparing the relative testicle and epididymis weights among the groups, the relative testicle and epididymis weights obtained from the SRT-administered groups were indistinguishable from the control group. Among the SRT-administered groups, no significant differences were observed (Table 1).
Table 1.
Effects of sertraline on relative organ weights and sperm parameters of male rats
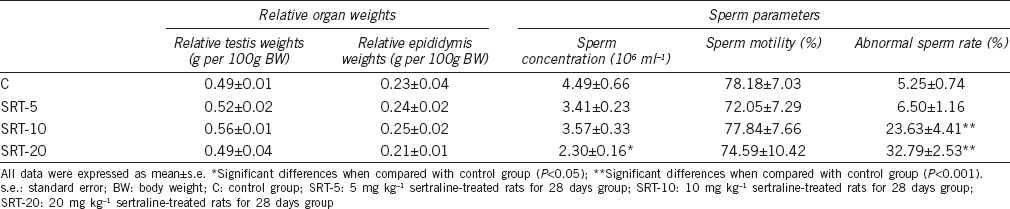
Effects of SRT treatment on sperm concentration and motility in rats
According to sperm concentration data, one-way ANOVA revealed a difference among the groups (F3,28 = 3.232, P = 0.044). Pairwise post-hoc comparisons (Tukey's test) indicated a decreased sperm concentration in the 20 mg kg−1 SRT-administered group in comparison with the control group (P = 0.027). Sperm concentrations determined in other SRT-administered groups were indistinguishable among groups (Table 1).
Although the sperm motility percentage in SRT-administered groups decreased in comparison with the control group, this difference was not significant. In addition, no significant difference was observed among the SRT-administered groups (Table 1).
Effects of SRT treatment on sperm morphology in rats
One-way ANOVA showed a difference (F3,28 = 26.128; P < 0.001) in sperm morphology data among groups. Tukey's test revealed an increase in the percentage of the sperm abnormalities observed in the 10 (P < 0.001) and 20 mg kg−1 SRT-administered groups (P < 0.001) compared to the control. Among the SRT-administered groups, the percentages of the sperm abnormalities were increased in the 10 (P = 0.002) and 20 mg kg−1 SRT-administered groups (P < 0.001) compared to the 5 mg kg−1 SRT-administered group (Table 1).
These abnormalities were found to occur more often in the tail, represented by bent tail and broken tail (Figure 1) in the 10 and 20 mg kg−1 SRT-administered groups at 12.79% and 19.69%, respectively. In addition, the percentages of sperm head abnormalities including banana-shaped head, amorphous head, bent neck, and two-headed and headless sperms (Figure 1) were determined as 10.44% and 13.19% in the 10 and 20 mg kg−1 SRT-administered groups, respectively.
Sperm DNA damage
The results expressed as tail moment (Extent tail moment: tail length × tail% DNA/100) for sperms exposed to various doses of SRT or in the control group are shown in Figure 2. One-way ANOVA revealed a difference among the groups (F3,196 = 131.862; P < 0.05). All the experimental groups showed an increase in sperm DNA damage. Exposure to 5 and 10 mg kg−1 of SRT increased the tail moment by approximately 49.70% and 86.35%, respectively. There was no significant difference between these groups in tail moment. However, according to post-hoc comparisons (Dunnett's T3 test), the 20 mg kg−1 treatment resulted in a dramatic increase in tail moment (over 5-fold compared to control, over 3-fold compared to the 5 mg kg−1 SRT-administered group, and over 2-fold compared to the 10 mg kg−1 SRT-administered group) (P < 0.05).
Figure 2.
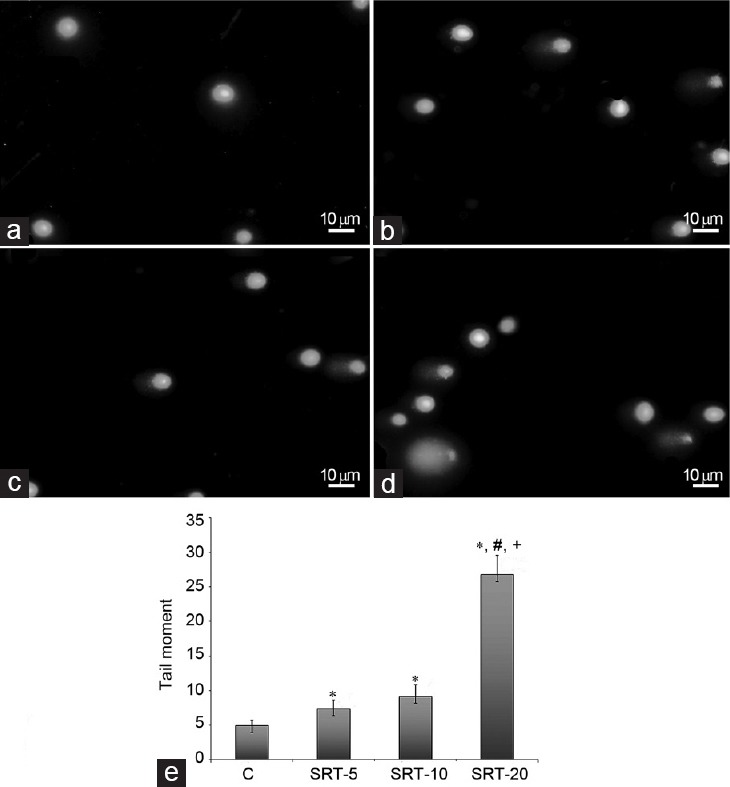
Effect of sertraline on the sperm DNA damage. (a) Sperm comet assay photo of control group; (b) sperm comet assay photo of SRT-5; (c) sperm comet assay photo of SRT-10; (d) sperm comet assay photo of SRT-20; (e) tail moment graph: C: control group; SRT-5: 5 mg kg−1 sertraline-treated rats for 28 days group; SRT-10: 10 mg kg−1 sertraline-treated rats for 28 days group; SRT-20: 20 mg kg−1 sertraline-treated rats for 28 days group. *Significant differences when compared with control group (P < 0.05); #Significant differences when compared with SRT-5 (P < 0.05); +Significant differences when compared with SRT-10 (P < 0.05). Scale bars = 10 μm in a–d.
Effects of SRT treatment on the serum hormone levels in rats
In comparing serum FSH levels of the groups, no significant differences were observed among the groups (Table 2).
Table 2.
Effects of sertraline on circulating hormone levels and oxidative stress markers of male rats
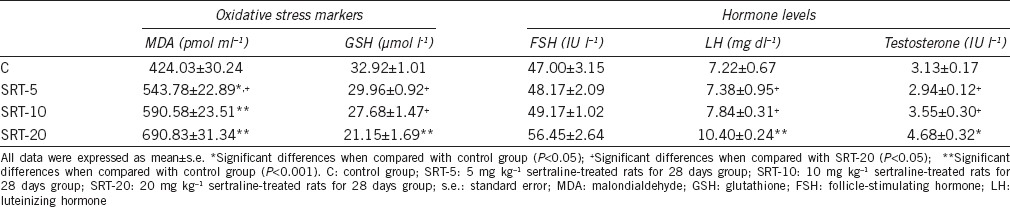
According to LH levels, one-way ANOVA indicated a difference among groups (F3,28 = 36.643; P < 0.001). Serum LH levels showed an increase in the 20 mg kg−1 SRT-administered group in comparison with the control, 5 mg kg−1 and 10 mg kg−1 SRT-administered groups (P < 0.001) (Table 2).
In comparing serum testosterone levels of the groups, a difference was obtained (F3,28 = 5.826, P = 0.005). Serum testosterone levels showed an increase in the 20 mg kg−1 SRT-administered group in comparison with the control (P = 0.023), 5 mg kg−1 (P = 0.005) and 10 mg kg−1 SRT-administered groups (P = 0.045) (Table 2).
Effects of SRT treatment on GSH and MDA levels in rats
There was a difference in GSH levels compared among groups (F3,28 = 10.838; P < 0.001). The testicular GSH levels in the 20 mg kg−1 SRT-administered group decreased in comparison with the control group (P < 0.001). In addition, it was shown that GSH levels of the 20 mg kg−1 SRT-administered group were also decreased compared to those of 5 mg kg−1 (P = 0.002) and 10 mg kg−1 SRT-administered groups (P = 0.027) (Table 2).
One-way ANOVA revealed a difference according to the MDA levels of the groups (F3,28 = 18.121; P < 0.001). MDA levels increased in the 5 mg kg−1 (P = 0.003), 10 mg kg−1 (P < 0.001), and 20 mg kg−1 SRT-administered groups (P < 0.001) compared to the control group. The MDA levels in the 5 mg kg−1 SRT-administered group were also found to be lower compared to those of the 20 mg kg−1 SRT-administered groups (P = 0.014) (Table 2).
Histological analysis
Figure 3 illustrates the histopathological changes in the seminiferous tubules and spermatogenic series of animals that were treated with different doses of SRT. The testicular structure of the control rats was normal. The seminiferous tubules appeared uniform in size and shape with regularly arranged rows of spermatogenic series and usual structure of the germ cells (Figure 3a and 3e). There was a slight degeneration in the seminiferous tubules of the 5 mg kg−1 SRT-administered group. Vacuolization of Sertoli cells, hypocellularity, and widened gaps between cells of the spermatogenic series were observed (Figure 3b and 3f). Swelling of the cells with an increased number of vacuoles was characterized in the 10 mg kg−1 SRT-administered group. There was a deformation in the cellular and tubular architecture. Seminiferous tubules showed a single layer of basal spermatogonia as a result of a reduction of cells in the spermatogenic series (Figure 3c and 3g). The 20 mg kg−1 SRT-administered group showed tubular and cellular atrophy, loss of intact germinal layer, and spermatogenesis as a result of dramatic degeneration in germ cells. Pyknotic nuclei were observed along with karyolysis and cellular lysis. Necrosis, vacuolization, and disruption of the seminiferous epithelium were also observed (Figure 3d, 3h, and 3i). Leydig cells also showed a damaged nucleus, pyknotic nuclei, and apoptotic bodies (Figure 3j). Johnsen's scores and semiquantitative comparison of pathology at the cellular level are shown in Table 3.
Figure 3.
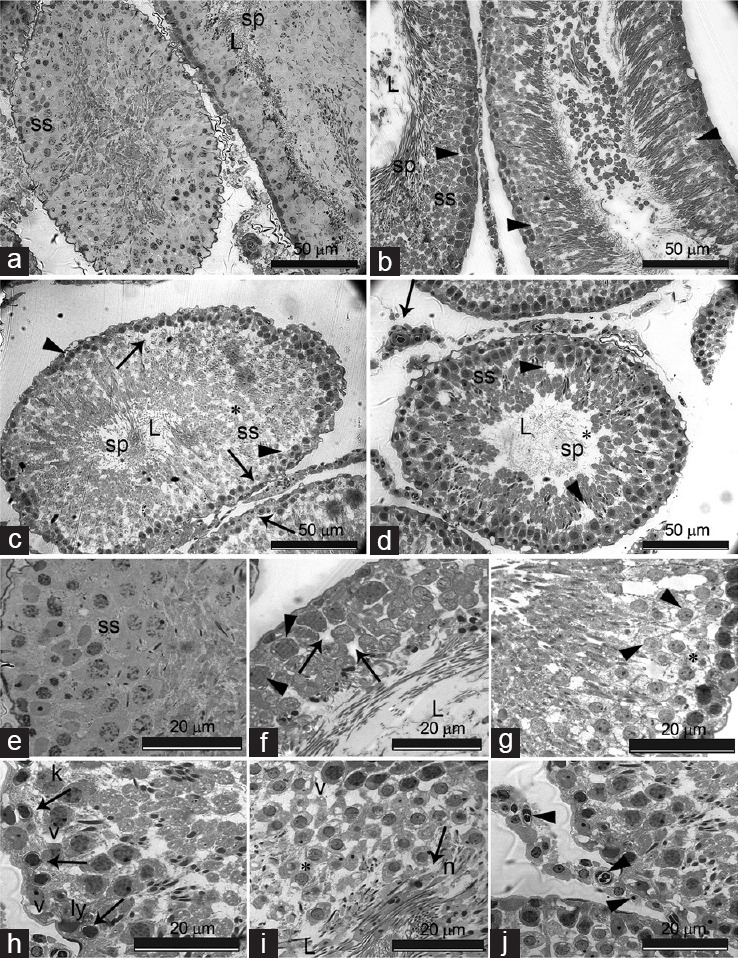
(a–d) Cross section of the testis of control and experimental group rats (L: lumen; ss: spermatogenic series; sp: spermatozoa). (a) Control; normal aspect of the seminiferous tubules containing cells of the spermatogenic series and spermatozoa in the lumen; (b) SRT-5 (5 mg kg−1 sertraline-treated rats for 28 days group); seminiferous tubule showing slight degeneration with wide gaps between neighboring cells (arrowheads); (c) SRT-10 (10 mg kg−1 sertraline-treated rats for 28 days group); degeneration of seminiferous epithelium (*), vacuolation and reduction of cells in the spermatogenic series (arrowheads), seminiferious tubules showing a single layer of basal spermatogonia (arrows); (d) SRT-20 (20 mg kg−1 sertraline-treated rats for 28 days group); Seminiferous tubules with atrophy, loss of intact germinal layer (arrowheads) and spermatogenesis (*). Leydig cells with damaged nucleus (arrow); (e–j) high magnification of cells of the spermatogenic series in control and experimental group rats; (e) control; spermatogenic series (ss) with normal appearance of cells; (f) SRT-5; slight vacuolation of sertoli cells (arrowheads), expanded intercellular spaces (arrows); (g) SRT-10; Swelling of cells with increased number of small vacuoles (arrowheads), deformation of cellular architecture (*); (h and i) SRT-20; deformation of basal lamina (arrowhead), degenerating germ cells showing nuclear pyknosis (arrows), karyolysis (k) and cellular lysis (ly), multiple extracellular vacuoles (v), necrotic (n) germ cells sloughed out into the lumen (L) of seminiferous tubules (arrow), disintegrated epithelium (*); (j) SRT-20; Leydig cells with damaged nucleus (long arrow), pyknotic nuclei (arrowheads), and apoptotic bodies (arrow). Scale bars = 50 μm for (a–d) and 20 μm for (e–j).
Table 3.
Johnsen's scores and semiquantitative comparison of pathology at the cellular level
DISCUSSION
In the present study, we only evaluated the reproductive toxic effect of SRT in male rats independent of other risk factors related to infertility and in repeated pharmacological doses. The results have shown that SRT decreased the sperm concentration and normal sperm morphology, increased sperm DNA damage, and induced histopathological changes in testicular tissue. These toxic effects induced by SRT have been accompanied by decreased tissue GSH level, increased MDA, and the changes in serum LH and testosterone levels, which play a role in the spermatogenesis process.
Male factor infertility is usually diagnosed through the evaluation of such parameters as sperm concentration, sperm motility, and sperm morphology.37 In terms of sperm concentration, motility, and morphology, which we evaluated in our study, sperm concentration and normal sperm morphology decreased in the 20 mg kg-1 SRT-administered group. Case reports that support our results draw attention to decreases in sperm concentration, motility, and normal sperm morphology in patients with SRT treatment.12,13,38
The endocrine balance between the hypothalamic–pituitary–testicular axis controls spermatogenesis.39,40 Gonadotropin-releasing hormone (GnRH) released into the hypothalamic-hypophyseal portal circulation stimulates the release of FSH and LH gonadotropins into the blood circulation. FSH mediates spermatogenesis by binding to its localized receptors, and LH mediates spermatogenesis-stimulating testosterone release by affecting Sertoli and peritubular cells of the seminiferous tubules by binding to its receptors in Leydig cells.39,40,41 In our study, the increased serum LH and testosterone levels were observed in the high-dose SRT-administered group. There were very limited data related to the effects of SRT treatment in rats on the hormones mediating spermatogenesis, which showed an increase in FSH and decreased testosterone levels without any change in LH levels.3 SSRIs were also shown to affect these hormones but not in a particular manner.3,42,43 GnRH secretion regulates several neurotransmitters, such as serotonin, noradrenaline, and dopamine.42,43 In immortalized GnRH neural cells, serotonin stimulates GnRH release.43 Hence, treatment with SSRIs could affect GnRH secretion. However, studies have emphasized that the effect of serotonin could be minor compared to other neurotransmitters on the hypothalamic/hypophyseal pathway.42,43 Otherwise, SSRIs, citalopram, fluoxetine, fluvoxamine, paroxetine, and SRT were able to inhibit the aromatase enzyme and thus potentially were able to change the balance between androgens and estrogens as shown in an in vitro study.44 In addition, in our study, the altered serum hormone levels that accompany the decreased sperm concentration can be attributed to the compensatory mechanism against the decrease in sperm concentration via inducing spermatogenesis by these hormones. Human studies have shown that serum FSH and LH levels have an inverse/negative correlation with sperm concentration.45,46
Male infertility is associated with increased DNA damage in spermatozoa.47,48 DNA damage can occur independently of standard semen parameters, and increased sperm DNA fragmentation is correlated with infertility.10 For this reason, measurement of DNA damage in spermatozoa is very important to evaluate the reproductive toxic effects of chemicals.49 The comet assay is a sensitive method used in the measurement of DNA strand breaks.50,51 In this assay, sperm with high DNA strand breaks show intense comet tail and increased comet tail length due to the increased DNA fragmentation.40 According to the results of our study, sperm DNA damage increased with SRT treatment, depending on dose. Consistent with our results, it has been observed that SRT treatment increases sperm DNA damage in patients with depression13 and in patients with premature ejaculation.12 Moreover, in various experimental models in which the genotoxic effects of SRT were investigated, different results have been obtained. SRT was found to increase DNA damage in lymphocyte comet and micronucleus assays52 in patients and sister chromatid exchange frequencies53 in rats, which indicates a possible genotoxicity of the drug and is also described as nongenotoxic in in vitro test batteries.54
Abnormal spermatozoa percentage and structural abnormalities (head-tail attachments, incomplete acrosomal development, and sperm cytoskeleton abnormalities) are important indicators of a defective spermatozoa production, maturation and infertility.28,55,56 It is known that even if sperm concentration or motility is normal, a morphological abnormality may be the one and only factor reflecting the fertilization potential.55 Male subjects are considered to be usually infertile when sperm cell abnormalities were determined as >10%.57 According to our morphological evaluation, abnormal sperm cells were increased resulting in both head and tail abnormalities in the 10 and 20 mg kg−1 SRT-administered groups (which occurred in a dose-dependent manner). It is important to emphasize that tail abnormalities, which are known to have a positive correlation with infertility,30 were significantly increased with SRT treatment. Furthermore, abnormal spermatozoa morphology is also associated with increases in DNA fragmentation and reactive oxygen species (ROS) overproduction.55,58,59 Trivedi et al. (2010)60 suggested that sperm DNA damage may lead to abnormalities in the sperm head, and the performance of the sperm comet assay along with the sperm head morphological evaluation is essential to identify and evaluate germ cell toxicants. Therefore, our study, in which the sperm comet assay was performed along with the sperm morphological evaluation, indicated the potential of SRT as a possible germ cell toxicant.
OS is considered one of the most important factors in the etiology of male infertility.61,62 Testicular tissue and spermatozoa are very sensitive to ROS and lipid peroxidation. The vulnerability of testicular tissue to OS is associated with sperm membrane, which contains high levels of polyunsaturated fatty acids3,61,62,63 and has a restricted antioxidant defense system.64 Herein, it is possible to associate the decreased sperm concentration and normal sperm morphology obtained from our study with OS in the testicles induced by SRT. Clinical research has shown that ROS cause a reduction in sperm count, sperm motility, and normal sperm morphology.42,65,66,67,68 In addition, it is possible to associate the dose-dependent sperm DNA damage observed in the SRT groups with the burst of free radicals. It is also known that lipid peroxides may interact with DNA and can cause strand breaks and DNA fragmentation.50 In the present study, MDA was increased depending on the dose in the 10 and 20 mg kg−1 treatment groups. The GSH dependent antioxidant defense system was of major importance at this point. A decreased GSH level in the 20 mg kg−1 treatment group was a sign of effort to overcome the oxidative imbalance.
Prolonged OS in testicles may cause important histopathological changes in the testis structure.69,70,71 Free radicals disrupt the membrane integrity in mammalian testicles, which may result in testicular atrophy and seminiferous tubule degeneration.72 Histopathology of the testes showed degeneration with an increasing severity particularly at the doses of 10 and 20 mg kg−1. Damage, including germinal cell degeneration, seminiferous tubules with atrophy, extracellular vacuoles, and reduction of cells in spermatogenic series, was observed in the testicular tissue and was especially apparent in our high-dose SRT group. Pyknosis, membrane bound vesicles (apoptotic bodies), and necrosis were observed in spermatogenic series and in Leydig cells in 20 mg kg−1 SRT-treated animals. In a previous study, SRT-associated toxicity was attributed to the mitochondrial dysfunction in the liver of rats.73 This dysfunction disrupts ATP generation and results in structural disorganization, apoptotic, necroptotic, and necrotic cell death.74 Our findings revealed that the testes of the SRT-treated rats are likely to be found within similar conditions. Together with the OS induced by SRT in the testicle, it is possible to associate degenerative findings detected in testicular tissue with the elevated serum LH levels. Some previous studies suggest that an increase in the serum LH level may cause degeneration of the germinal cells in rats and mice.75,76
Another important point that must be noted is that the excess levels or absence of serotonin may cause disorders in sperm parameters.3 Injecting serotonin to adult rats systemically has been reported to cause testicular atrophy, damage to spermatogenesis, and suppression of steroidogenesis.77 Serotonergic receptors, which are located at the hypothalamic-pituitary-testicular axis, mediate the negative effects of SSRIs on sperm parameters.11,13 It is alleged that increasing serotonin in the hypothalamus can cause dysregulation of this axis.11 SRT-induced reproductive effects can also be attributed to the increased serotonin levels.
According to the results of our study, induced OS and altered LH and testosterone levels with SRT administration adversely affected the sperm parameters by reducing sperm concentration and increasing abnormal sperm morphology and sperm DNA damage and caused damage in normal testicular morphology in rats. Hence, it can be suggested that the mechanism of SRT toxicity in the male reproductive system arises because of enhanced OS and altered hormonal status. In the future, testicular toxicity and the potential fertility level in patients treated with SRT should be examined in clinical studies. Further studies are needed to identify the effects of other SSRI drugs, which are frequently prescribed, on male fertility and on the conditions comprising these stress conditions. It is also necessary to clarify how SSRI drugs affect spermatogenesis in future research. In addition, health-care providers should give consideration to the potential adverse effects of SSRI drugs in patients of reproductive age.
AUTHOR CONTRIBUTIONS
OA conceived of the study, carried out the experimental design, drafted the manuscript, participated in animal handling and dissection, collection and evaluation of sperm samples, assessment of sperm motility, sperm morphology, biochemical assays, evaluation of the results, and statistical analysis. MB participated in animal handling and dissection, collection of sperm samples, assessment of sperm motility, sperm morphology, and biochemical assays. GAK participated in sperm comet assay, histological analysis, evaluation of results and helped draft the manuscript. VK participated in animal handling and dissection, sperm comet assay, histopathological analysis, statistical analysis, and evaluation of the results. SU participated in animal handling and dissection, sperm comet assay, and histopathological analysis. BK participated in animal handling, dissection, and biochemical assays. SI carried out the experimental design and coordination, participated in animal handling and dissection, collection and evaluation of sperm samples, assessment of sperm motility, sperm morphology, biochemical assays, evaluation of the results and helped draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by the Anadolu University Scientific Research Projects Commission under the grant no. 1404S128.
REFERENCES
- 1.Mehraban F, Jafari M, Akbartabar Toori M, Sadeghi H, Joodi B, et al. Effects of date palm pollen (Phoenix dactylifera L.) and Astragalus ovinus on sperm parameters and sex hormones in adult male rats. Iran J Reprod Med. 2014;12:705–12. [PMC free article] [PubMed] [Google Scholar]
- 2.Kaya Z, Sogut E, Cayli S, Suren M, Arici S, et al. Evaluation of effects of repeated sevoflurane exposure on rat testicular tissue and reproductive hormones. Inhal Toxicol. 2013;25:192–8. doi: 10.3109/08958378.2013.773109. [DOI] [PubMed] [Google Scholar]
- 3.Erdemir F, Atilgan D, Firat F, Markoc F, Parlaktas BS, et al. The effect of sertraline, paroxetine, fluoxetine and escitalopram on testicular tissue and oxidative stress parameters in rats. Int Braz J Urol. 2014;40:100–8. doi: 10.1590/S1677-5538.IBJU.2014.01.15. [DOI] [PubMed] [Google Scholar]
- 4.Alonso V, Linares V, Bellés M, Albina ML, Sirvent JJ, et al. Sulfasalazine induced oxidative stress: a possible mechanism of male infertility. Reprod Toxicol. 2009;27:35–40. doi: 10.1016/j.reprotox.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Olayemi FO. A review on some causes of male infertility. Afr J Biotechnol. 2010;9:2834–42. [Google Scholar]
- 6.Bystritsky A, Khalsa SS, Cameron ME, Schiffman J. Current diagnosis and treatment of anxiety disorders. Pharm Ther. 2013;38:30–57. [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez C, Reines EH, Montgomery SA. A comparative review of escitalopram, paroxetine, and sertraline: are they all alike? Int Clin Psychopharmacol. 2014;29:185–96. doi: 10.1097/YIC.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies SJ, Mulsant BH, Flint AJ, Meyers BS, Rothschild AJ, et al. SSRI-antipsychotic combination in psychotic depression: sertraline pharmacokinetics in the presence of olanzapine, a brief report from the STOP-PD study. Hum Psychopharmacol. 2016;31:252–5. doi: 10.1002/hup.2532. [DOI] [PubMed] [Google Scholar]
- 9.Lu S, Zhao Y, Hu J, Li X, Zhang H, et al. Combined use of phosphodiesterase-5 inhibitors and selective serotonin reuptake inhibitors for temporary ejaculation failure in couple undergoing assisted reproductive technologies. Fertil Steril. 2009;91:1806–8. doi: 10.1016/j.fertnstert.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Attia SM, Bakheet SA. Citalopram at the recommended human doses after long-term treatment is genotoxic for male germ cell. Food Chem Toxicol. 2013;53:281–5. doi: 10.1016/j.fct.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 11.Csoka AB, Shipko S. Persistent sexual side effects after SSRI discontinuation. Psychother Psychosom. 2006;75:187–8. doi: 10.1159/000091777. [DOI] [PubMed] [Google Scholar]
- 12.Akasheh G, Sirati L, Kamran AR, Sepehrmanesh Z. Comparison of the effect of sertraline with behavioral therapy on semen parameters in men with primary premature ejaculation. Urology. 2014;83:800–4. doi: 10.1016/j.urology.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Safarinejad MR. Sperm DNA damage and semen quality impairment after treatment with selective serotonin reuptake inhibitors detected using semen analysis and sperm chromatin structure assay. J Urol. 2008;180:2124–8. doi: 10.1016/j.juro.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Koyuncu H, Serefoglu EC, Yencilek E, Atalay H, Akbas NB, et al. Escitalopram treatment for premature ejaculation has a negative effect on semen parameters. Int J Impot Res. 2011;23:257–61. doi: 10.1038/ijir.2011.35. [DOI] [PubMed] [Google Scholar]
- 15.Tanrikut C, Feldman AS, Altemus M, Paduch DA, Schlegel PN. Adverse effect of paroxetine on sperm. Fertil Steril. 2010;94:1021–6. doi: 10.1016/j.fertnstert.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 16.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press, US; 2011. [PubMed] [Google Scholar]
- 17.Kaygisiz B, Ozatik FY, Erol K. Interaction of sertraline and nimodipine on some behavioural tests in rats. Adv Clin Exp Med. 2014;23:169–75. doi: 10.17219/acem/37043. [DOI] [PubMed] [Google Scholar]
- 18.Abdel Salam OM, Mohammed NA, Sleem AA, Farrag AR. The effect of antidepressant drugs on thioacetamide-induced oxidative stress. Eur Rev Med Pharmacol Sci. 2013;17:735–44. [PubMed] [Google Scholar]
- 19.Pereira-Figueiredo I, Sancho C, Carro J, Castellano O, López DE. The effects of sertraline administration from adolescence to adulthood on physiological and emotional development in prenatally stressed rats of both sexes. Front Behav Neurosci. 2014;8:260. doi: 10.3389/fnbeh.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER): Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Rockville, MD: 2005. [Google Scholar]
- 21.OECD. Test No.407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals. Sec. 4. Paris: OECD Publishing; 2008. [Google Scholar]
- 22.Takeuchi K, Takayama S, Hashimoto E, Itayama M, Amagase K, et al. Effect of rebamipide on gastric bleeding and ulcerogenic responses induced by aspirin plus clopidogrel under stimulation of acid secretion in rats. J Gastroenterol Hepatol. 2014;29:37–46. doi: 10.1111/jgh.12774. [DOI] [PubMed] [Google Scholar]
- 23.Mdhluli MC, van der Horst G. The effect of oleanolic acid on sperm motion characteristics and fertility of male Wistar rats. Lab Anim. 2002;36:432–7. doi: 10.1258/002367702320389107. [DOI] [PubMed] [Google Scholar]
- 24.Opuwari CS, Monsees TK. In vivo effects of Aspalathus linearis (rooibos) on male rat reproductive functions. Andrologia. 2014;46:867–77. doi: 10.1111/and.12158. [DOI] [PubMed] [Google Scholar]
- 25.Maree L, van der Horst G. Quantification and identification of sperm subpopulations using computer-aided sperm analysis and species-specific cut-off values for swimming speed. Biotech Histochem. 2013;88:181–93. doi: 10.3109/10520295.2012.757366. [DOI] [PubMed] [Google Scholar]
- 26.WHO. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 27.Van der Horst G, Maree L. SpermBlue: a new universal stain for human and animal sperm, which is also amenable to automated sperm morphology analysis. Biotech Histochem. 2009;84:299–308. doi: 10.3109/10520290902984274. [DOI] [PubMed] [Google Scholar]
- 28.Mori K, Kaido M, Fujishiro K, Inoue N, Koide O, et al. Dose dependent effects of inhaled ethylene oxide on spermatogenesis in rats. Br J Ind Med. 1991;48:270–4. doi: 10.1136/oem.48.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filler R. Methods for evaluation of rat's epididymal sperm morphology. In: Chapin RE, Heindel JJ, editors. Male Reproductive Toxicology. California: Academic Press; 1993. pp. 334–43. [Google Scholar]
- 30.Narayana K, D’souza UJ, Seetharama Rao KP. Ribavirin-induced sperm shape abnormalities in Wistar rat. Mutat Res. 2002;513:193–6. doi: 10.1016/s1383-5718(01)00308-4. [DOI] [PubMed] [Google Scholar]
- 31.Gromadzka-Ostrowska J, Dziendzikowska K, Lankoff A, Dobrzyñska M, Instanes C, et al. Silver nanoparticles effects on epididymal sperm in rats. Toxicol Lett. 2012;214:251–8. doi: 10.1016/j.toxlet.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Martinez CS, Torres JG, Peçanha FM, Anselmo-Franci JA, Vassallo DV, et al. 60-Day chronic exposure to low concentrations of HgCl 2 impairs sperm quality: hormonal imbalance and oxidative stress as potential routes for reproductive dysfunction in rats. PLoS One. 2014;9:e111202. doi: 10.1371/journal.pone.0111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kushwaha S, Jena GB. Telmisartan ameliorates germ cell toxicity in the STZ-induced diabetic rat: studies on possible molecular mechanisms. Mutat Res. 2013;755:11–23. doi: 10.1016/j.mrgentox.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. Edinburgh: Churchill Livingstone; 2002. pp. 673–4. [Google Scholar]
- 35.Johnsen SG. Testicular biopsy score count - A method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970;1:2–25. doi: 10.1159/000178170. [DOI] [PubMed] [Google Scholar]
- 36.Ozkaya AK, Dilber E, Gurgen SG, Kutlu O, Cansu A, et al. Effects of chronic amiodarone treatment on rat testis. Acta Histochem. 2016;118:271–7. doi: 10.1016/j.acthis.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Brezina PR, Yunus FN, Zhao Y. Effects of Pharmaceutical medications on male fertility. J Reprod Infertil. 2012;13:3–11. [PMC free article] [PubMed] [Google Scholar]
- 38.Koyuncu H, Serefoglu EC, Ozdemir AT, Hellstrom WJ. Deleterious effects of selective serotonin reuptake inhibitor treatment on semen parameters in patients with lifelong premature ejaculation. Int J Impot Res. 2012;24:171–3. doi: 10.1038/ijir.2012.12. [DOI] [PubMed] [Google Scholar]
- 39.Babu SR, Sadhnani MD, Swarna M, Padmavathi P, Reddy PP. Evaluation of FSH, LH and testosterone levels in different subgroups of infertile males. Indian J Clin Biochem. 2004;19:45–9. doi: 10.1007/BF02872388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramaswamy S, Weinbauer GF. Endocrine control of spermatogenesis: role of FSH and LH/testosterone. Spermatogenesis. 2015;4:e996025. doi: 10.1080/21565562.2014.996025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmed M, Ali D, Harrath AH, Hussain T, Al-Daghri N, et al. Ultrastructural and hormonal changes in rat cauda epididymal spermatozoa induced by Boswellia papyrifera and Boswellia carterii. C R Biol. 2014;337:250–7. doi: 10.1016/j.crvi.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Safarinejad MR. Evaluation of endocrine profile and hypothalamic-pituitary-testis axis in selective serotonin reuptake inhibitor-induced male sexual dysfunction. J Clin Psychopharmacol. 2008;28:418–23. doi: 10.1097/JCP.0b013e31817e6f80. [DOI] [PubMed] [Google Scholar]
- 43.Prasad P, Ogawa S, Parhar IS. Serotonin reuptake inhibitor citalopram inhibits GnRH synthesis and spermatogenesis in the male zebrafish. Biol Reprod. 2015;93:102. doi: 10.1095/biolreprod.115.129965. [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen NW, Hansen CH, Nellemann C, Styrishave B, Halling-Sørensen B. Effects of selective serotonin reuptake inhibitors on three sex steroids in two versions of the aromatase enzyme inhibition assay and in the H295R cell assay. Toxicol In Vitro. 2015;29:1729–35. doi: 10.1016/j.tiv.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 45.Fakhrildin MB. Correlation between seminal fluid analysis and levels of gonadotropins in serum and seminal plasma of normozoospermic men and infertile patients. IJFS. 2007;1:55–62. [Google Scholar]
- 46.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- 47.Chen XL, Gong LZ, Xu JX. Antioxidative activity and protective effect of probiotics against high-fat diet-induced sperm damage in rats. Animal. 2013;7:287–92. doi: 10.1017/S1751731112001528. [DOI] [PubMed] [Google Scholar]
- 48.Kumar S, Behari J, Sisodia R. Influence of electromagnetic fields on reproductive system of male rats. Int J Radiat Biol. 2013;89:147–54. doi: 10.3109/09553002.2013.741282. [DOI] [PubMed] [Google Scholar]
- 49.Lu Z, Wang L, Zhou R, Qiu Y, Yang L, et al. Evaluation of the spermicidal and contraceptive activity of platycodin D, a saponin from Platycodon grandiflorum. PLoS One. 2013;8:e82068. doi: 10.1371/journal.pone.0082068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmed MA. Amelioration of nandrolone decanoate-induced testicular and sperm toxicity in rats by taurine: effects on steroidogenesis, redox and inflammatory cascades, and intrinsic apoptotic pathway. Toxicol Appl Pharmacol. 2015;282:285–96. doi: 10.1016/j.taap.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 51.Namoju RC, Khan S, Patel RS, Shera FY, Trivedi PP, et al. Pre-pubertal exposure of cytarabine-induced testicular atrophy, impaired spermatogenesis and germ cell DNA damage in SD rats. Toxicol Mech Methods. 2014;24:703–12. doi: 10.3109/15376516.2014.970679. [DOI] [PubMed] [Google Scholar]
- 52.Bozkurt G, Abay E, Ates I, Karabogaz G, Ture M, et al. Clastogenicity of selective serotonin-reuptake inhibitors. Mutat Res. 2004;558:137–44. doi: 10.1016/j.mrgentox.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Battal D, Aktas A, Sungur MA, Kadioglu E, Eker ED, et al. In vivo genotoxicity assessment of sertraline by using alkaline comet assay and the cytokinesis-block micronucleus assay. Basic Clin Pharmacol Toxicol. 2013;11:339–46. doi: 10.1111/bcpt.12095. [DOI] [PubMed] [Google Scholar]
- 54.Davies TS, Kluwe WM. Preclinical toxicological evaluation of sertraline hydrochloride. Drug Chem Toxicol. 1998;21:521–37. doi: 10.3109/01480549809002220. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal A, Tvrda E, Sharma R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol. 2014;27:12–45. doi: 10.1186/1477-7827-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Menkveld R, Holleboom CA, Rhemrev JP. Measurement and significance of sperm morphology. Asian J Androl. 2011;13:59–68. doi: 10.1038/aja.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saba AB, Oridupa OA, Oyeyemi MO, Osanyigbe OD. Spermatozoa morphology and characteristics of male Wistar rats administered with ethanolic extract of Lagenaria breviflora Roberts. Afr J Biotechnol. 2009;8:1170–5. [Google Scholar]
- 58.Agarwal A, Said TM. Oxidative stress, DNA damage and apoptosis in male infertility: a clinical approach. BJU Int. 2005;95:503–7. doi: 10.1111/j.1464-410X.2005.05328.x. [DOI] [PubMed] [Google Scholar]
- 59.Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014;28:684–703. doi: 10.1016/j.rbmo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 60.Trivedi PP, Kushwaha S, Tripathi DN, Jena GB. Evaluation of male germ cell toxicity in rats: correlation between sperm head morphology and sperm comet assay. Mutat Res. 2010;703:115–21. doi: 10.1016/j.mrgentox.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 61.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aktan G, Doğru-Abbasoğlu S, Küçükgergin C, Kadıoğlu A, Ozdemirler-Erata G, et al. Mystery of idiopathic male infertility: is oxidative stress an actual risk? Fertil Steril. 2013;99:1211–5. doi: 10.1016/j.fertnstert.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 63.Sharma P, Singh R, Jan M. Dose-dependent effect of deltamethrin in testis, liver, and kidney of Wistar rats. Toxicol Int. 2014;21:131–9. doi: 10.4103/0971-6580.139789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sarıozkan S, Bucak MN, Canturk F, Özdamar S, Yay A, et al. The effects of different sugars on motility, morphology and DNA damage during the liquid storage of rat epididymal sperm at 4°C. Cryobiology. 2012;65:93–7. doi: 10.1016/j.cryobiol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Saleh RA, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002;23:737–52. [PubMed] [Google Scholar]
- 66.Sikka SC. Role of oxidative stress and antioxidants in andrology and assisted reproductive technology. J Androl. 2004;25:5–18. doi: 10.1002/j.1939-4640.2004.tb02751.x. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal A, Gupta S, Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 2006;18:325–32. doi: 10.1097/01.gco.0000193003.58158.4e. [DOI] [PubMed] [Google Scholar]
- 68.Tremellen K. Oxidative stress and male infertility - A clinical perspective. Hum Reprod Update. 2008;14:243–58. doi: 10.1093/humupd/dmn004. [DOI] [PubMed] [Google Scholar]
- 69.Ayinde OC, Ogunnowo S, Ogedegbe RA. Influence of Vitamin C and Vitamin E on testicular zinc content and testicular toxicity in lead exposed albino rats. BMC Pharmacol Toxicol. 2012;14:13–7. doi: 10.1186/2050-6511-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitra S, Srivastava A, Khandelwal S. Tributyltin chloride induced testicular toxicity by JNK and p38 activation, redox imbalance and cell death in sertoli-germ cell co-culture. Toxicology. 2013;314:39–50. doi: 10.1016/j.tox.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 71.El-Neweshy MS, El-Maddawy ZK, El-Sayed YS. Therapeutic effects of date palm (Phoenix dactylifera L.) pollen extract on cadmium-induced testicular toxicity. Andrologia. 2013;45:369–78. doi: 10.1111/and.12025. [DOI] [PubMed] [Google Scholar]
- 72.Mandal TK, Das NS. Correlation of testicular toxicity and oxidative stress induced by chlorpyrifos in rats. Hum Exp Toxicol. 2011;30:1529–39. doi: 10.1177/0960327110392400. [DOI] [PubMed] [Google Scholar]
- 73.Li Y, Couch L, Higuchi M, Fang JL, Guo L. Mitochondrial dysfunction induced by sertraline, an antidepressant agent. Toxicol Sci. 2012;127:582–91. doi: 10.1093/toxsci/kfs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalogeris T, Bao Y, Korthuis RJ. Mitochondrial reactive oxygen species: a double edged sword in ischemia/reperfusion vs. preconditioning. Redox Biol. 2014;2:702–14. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarkar R, Mohanakumar KP, Chowdhury M. Effects of an organophosphate pesticide, quinalphos, on the hypothalamo-pituitary-gonadal axis in adult male rats. J Reprod Fertil. 2000;118:29–38. [PubMed] [Google Scholar]
- 76.Fattahi E, Parivar K, Jorsaraei SG, Moghadamnia AA. The effects of diazinon on testosterone, FSH and LH levels and testicular tissue in mice. Iran J Reprod Med. 2009;7:59–64. [Google Scholar]
- 77.Csaba Z, Csernus V, Gerendai I. Intratesticular serotonin affects steroidogenesis in the rat testis. J Neuroendocrinol. 1998;10:371–6. doi: 10.1046/j.1365-2826.1998.00217.x. [DOI] [PubMed] [Google Scholar]