Abstract
Both pulsatile gonadotropin-releasing hormone (GnRH) infusion and combined gonadotropin therapy (human chorionic gonadotropin and human menopausal gonadotropin [HCG/HMG]) are effective to induce spermatogenesis in male patients with congenital hypogonadotropic hypogonadism (CHH). However, evidence is lacking as to which treatment strategy is better. This retrospective cohort study included 202 patients with CHH: twenty had received pulsatile GnRH and 182 had received HCG/HMG. Patients had received therapy for at least 12 months. The total follow-up time was 15.6 ± 5.0 months (range: 12–27 months) for the GnRH group and 28.7 ± 13.0 months (range: 12–66 months) for the HCG/HMG group. The median time to first sperm appearance was 6 months (95% confidence interval [CI]: 1.6–10.4) in the GnRH group versus 18 months (95% CI: 16.4–20.0) in the HCG/HMG group (P < 0.001). The median time to achieve sperm concentrations ≥5 × 106 ml−1 was 14 months (95% CI: 5.8–22.2) in the GnRH group versus 27 months (95% CI: 18.9–35.1) in the HCG/HMG group (P < 0.001), and the median time to concentrations ≥10 × 106 ml−1 was 18 months (95% CI: 10.0–26.0) in the GnRH group versus 39 months (95% CI unknown) in the HCG/HMG group. Compared to the GnRH group, the HCG/HMG group required longer treatment periods to achieve testicular sizes of ≥4 ml, ≥8 ml, ≥12 ml, and ≥16 ml. Sperm motility (a + b + c percentage) evaluated in semen samples with concentrations >1 × 106 ml−1 was 43.7% ± 20.4% (16 samples) in the GnRH group versus 43.2% ± 18.1% (153 samples) in the HCG/HMG group (P = 0.921). Notably, during follow-up, the GnRH group had lower serum testosterone levels than the HCG/HMG group (8.3 ± 4.6 vs 16.2 ± 8.2 nmol l−1, P < 0.001). Our study found that pulsatile GnRH therapy was associated with earlier spermatogenesis and larger testicular size compared to combined gonadotropin therapy. Additional prospective randomized studies would be required to confirm these findings.
Keywords: combined gonadotropin therapy, congenital hypogonadotropic hypogonadism, pulsatile GnRH therapy, spermatogenesis
INTRODUCTION
Congenital hypogonadotropic hypogonadism (CHH) is a rare disease caused by a deficiency or dysfunction of gonadotropin-releasing hormone (GnRH). In male patients, it presents with absent or diminished development during puberty and infertility. CHH is categorized into anosmic (Kallmann syndrome [KS]) and normosmic CHH (nCHH) based on olfactory status.1 To restore fertility, pulsatile GnRH infusion2 or combined gonadotropin therapy (human chorionic gonadotropin and human menopausal gonadotropin [HCG/HMG]) may be effective to induce spermatogenesis.3 In China, pulsatile GnRH administration via a portable mini-pump has been approved for induction of spermatogenesis for approximately 4 years.
Although pulsatile GnRH therapy more closely mimics physiological conditions, it remains inconclusive whether it is superior to HCG/HMG therapy. Several studies have shown that pulsatile GnRH results in greater testicular growth than HCG/HMG therapy, but no differences in spermatogenesis induction were reported in these studies.4,5,6 However, these conclusions were based on very limited sample sizes. Importantly, one study that included 36 patients with CHH found that pulsatile GnRH induced earlier spermatogenesis and promoted larger testicular size than HCG/HMG therapy;7 however, the study did not report on the effects of the different therapies on sperm concentration and motility. In this study, we investigated the efficacy of pulsatile GnRH and HCG/HMG therapy on spermatogenesis in CHH patients.
MATERIALS AND METHODS
Patients
A diagnosis of CHH was made if a male patient met all of the following criteria:8 (1) absence of pubertal development before the age of 18 years; (2) serum testosterone level below 100 ng dl−1 (3.5 nmol l−1) with low or inappropriately normal levels of gonadotropins prior to any treatment; (3) normal levels of other pituitary hormones; (4) normal sellar magnetic resonance imaging (MRI); and (5) exclusion of other pathological conditions for secondary hypogonadotropic hypogonadism.
Clinical presentation, presence of cryptorchidism, medical history, and family history were recorded on the patient's first visit to the hospital. Plasma gonadotropins and testosterone were measured. MRI of the pituitary gland and olfactory bulb and tract was performed. The study protocol was reviewed and approved by the Ethics Committee of the Peking Union Medical College Hospital.
A total of 202 Chinese patients with CHH with more than 12 months follow-up were eligible for inclusion in this retrospective cohort study. All patients had been treated with pulsatile GnRH or HCG/HMG between January 2008 and December 2014 at Peking Union Medical College Hospital. Patients were categorized based on what treatment type they had received into either the GnRH group (n = 20) or the HCG/HMG group (n = 182). Prior to January 2012, HCG/HMG therapy was the only treatment available in China for induction of spermatogenesis in CHH patients. In January 2012, portable GnRH pumps became commercially available in China and patients subsequently had a choice of either gonadotropins or pulsatile GnRH therapy.
Hormone assay
Morning fasting blood samples were taken. Follicular-stimulating hormone (FSH), luteinizing hormone (LH), and total testosterone were measured with commercial kits by the chemiluminescent method (ACS: 180 Automatic Chemiluminescence Systems, Bayer, Germany). The intra- and inter-assay coefficients of variation were 3.9% and 4.5% for FSH, 2.3% and 2.8% for LH, and 5.6% and 6.6% for total testosterone, respectively. The lowest measurable limits were 0.65 IU l−1 for FSH, 0.07 IU l−1 for LH, and 0.2 nmol l−1 for total testosterone. FSH, LH, and total testosterone were measured every 2–3 months during the follow-up period. In the HCG/HMG group, testosterone levels were determined 48 h after HCG injection. In the GnRH group, LH, FSH, and total testosterone were measured 30 min after the last pulsatile GnRH infusion. Post-treatment parameters collected for data analysis included the mean values of LH, FSH, and testosterone at each patient's most recent three visits.
Treatment and follow-up
Testosterone therapy was discontinued for at least 3 months before pulsatile GnRH or HCG/HMG therapy was initiated.
In the HCG/HMG group, standard therapy was initiated. Intramuscular HCG (2000 U, Livzon Pharmaceutical Co., Guangdong, China) was administered twice weekly for 3 months followed by the co-administration of HMG (75 U, Livzon Pharmaceutical Co., Guangdong, China) and HCG (2000 U) twice weekly. Regular follow-up was conducted at intervals of 2–3 months. HCG dosages were adjusted to maintain plasma testosterone level in the normal range (10–15 nmol l−1). HMG dosage was increased to 150 U if testicular size was smaller than 6 ml after 6 months of combined gonadotropin therapy. Testicular size, plasma gonadotropins, plasma testosterone, and sperm count were measured at each visit. Testosterone levels were determined 48 h after HCG injection. Testicular sizes were measured using a Prader orchidometer; the mean value of bilateral testicular volumes was used in data analysis. Semen samples were produced by masturbation and analyzed according to the standard World Health Organization method.9
In the pulsatile GnRH treatment group, patients were started on pulsatile gonadorelin (10 μg per 90 min,10,11 Ma’anshan Fengyuan Pharmaceutical Co., Anhui, China) delivered using a mini portable GnRH pump (Shanghai Micro Invasive Life Technology Ltd Co., Shanghai, China). Regular follow-up was conducted at intervals of 2–3 months. Dosages were adjusted to attain LH and FSH levels between 5–10 IU l−1. Testicular sizes, plasma gonadotropins, plasma testosterone, and sperm count were measured on each visit.
Outcomes
Successful spermatogenesis was defined as the appearance of at least one sperm under microscopy after centrifugation of the semen sample. Treatment time to achieve sperm concentrations of >0 × 106 ml−1 (any sperm seen by microscopy), ≥5 × 106 ml−1, ≥10 × 106 ml−1 and ≥15 × 106 ml−1, and to achieve four testicular size thresholds of ≥4 ml, ≥8 ml, ≥12 ml and ≥16 ml were recorded. Sperm motility parameters, a + b and a + b + c percentage (a: rapid progressive motility; b: slow progressive motility; c: nonprogressive motility and d: immotility) were calculated only for semen samples with sperm concentrations >1 × 106 ml−1.
Statistical analysis
SPSS version 17.0 (IBM Corporation, Armonk, New York, NY, USA) was used for data analysis. Normally distributed data were expressed as mean ± standard deviation (s.d.). Nonnormally distributed data were reported as median (quartiles). Kaplan–Meier analyses were used to estimate the median time to achieve the various sperm and testicular size thresholds. A nonpaired t-test was used to compare differences in the age, testicular size, plasma testosterone, and other parameters between the two groups. Differences in the rates of cryptorchidism, Kallmann syndrome, and family history of CHH between the two groups were compared by Chi-square test (χ2 test). Statistical significance was set at P < 0.05.
RESULTS
Clinical characteristics of CHH patients
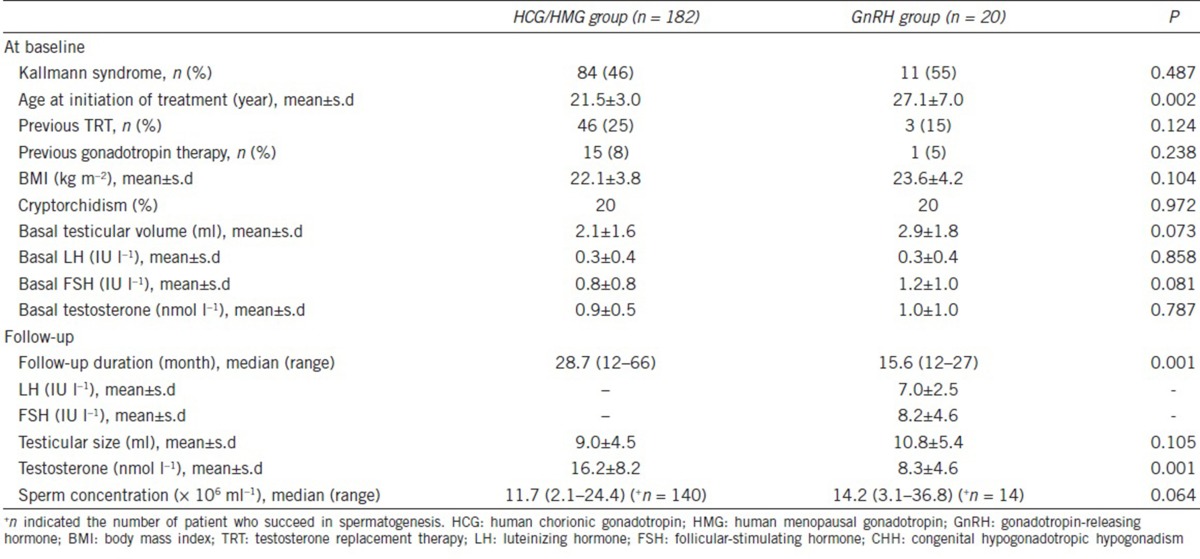
A total of 202 patients with CHH receiving either pulsatile GnRH or HCG/HMG therapy for at least 12 months were included in the analysis. The mean follow-up time was 15.6 ± 5.0 months (range: 12–27 months) and 28.7 ± 13.0 months (range: 12–66 months) for the GnRH group (n = 20) and HCG/HMG group (n = 182), respectively. All patients were generally in good health with normal routine blood and urine test results and normal liver and renal function. Their thyroid hormone, cortisol, and IGF-1 levels were all within the normal range.
The prevalence of Kallmann syndrome was 55% in the GnRH group and 46% in the HCG/HMG group (P = 0.487) (Table 1). The rate of cryptorchidism was 20% in both groups. Five percent of patients in the GnRH groups had a history of previous gonadotropin therapy compared with 8% of patients in the HCG/HMG group (P = 0.238) with treatment periods between 1 and 3 months (1.6 ± 0.4 months). The mean testicular volume was 2.9 ± 1.8 ml in the GnRH group versus 2.1 ± 1.6 ml in the HCG/HMG group (P = 0.073). At baseline, plasma hormone levels were not significantly different between the two treatment groups (Table 1).
Table 1.
Features of patients with CHH by treatment group at baseline and during follow-up
There were 5% and 8% of patients in the GnRH group and HCG/HMG group, respectively, who had a history of previous gonadotropin therapy (P = 0.238). The treatment periods ranged from 1 to 3 months (1.6 ± 0.4 months). For the present analysis, it was presumed that such a short period of gonadotropin therapy would not significantly influence the results of spermatogenesis.
In the GnRH group, pulsatile gonadorelin treatment was initiated at a dose of 10 μg per 90 min, administered subcutaneously. Throughout treatment, the dose was adjusted to maintain FSH and LH between 5 and 10 IU l−1. The mean dose used was 8.8 ± 1.2 μg per 90 min. The dose was upregulated in two patients, downregulated in eight patients, and unchanged in ten patients. Patients in the GnRH group were recruited after January 2012 when pulsatile GnRH therapy became commercially available in China.
In the HCG/HMG group, the dose of HCG was gradually adjusted to 2857 ± 253 U per injection in order to maintain a targeting testosterone level between 10 and 15 nmol l−1. The average dosage of HMG was 97 ± 28 U per injection; 129 patients received 75 U per injection and 53 patients received 150 U per injection. In the HCG/HMG group, 117 patients started therapy prior to January 2012; the remaining 65 patients in the HCG/HMG chose HCG/HMG over GnRH therapy after January 2012.
Changes in gonadotropins and testosterone during treatment
In the GnRH group (n = 20), LH increased from 0.3 ± 0.4 to 7.0 ± 2.5 IU l−1 (P < 0.001), FSH increased from 1.2 ± 1.0 to 8.2 ± 4.6 IU l−1 (P < 0.001), and total testosterone increased from 1.0 ± 1.0 to 8.3 ± 4.6 nmol l−1 (P < 0.001) after treatment for 12 months. In the HCG/HMG group, plasma testosterone increased from 0.9 ± 0.5 to 16.2 ± 8.2 nmol l−1 after treatment for 12 months (P < 0.001). The GnRH group had a lower mean level of total testosterone at the end of treatment compared to the HCG/HMG group (8.3 ± 4.6 vs 16.2 ± 8.2 nmol l−1, P < 0.001) (Table 1).
Induction of spermatogenesis
The rates of successful spermatogenesis induction in the GnRH and HCG/HMG groups were 70.0% (14/20) and 76.9% (140/182) (P = 0.580), respectively. The median time until the first sperm detection was 6 months (95% CI: 1.6–10.4) in the GnRH group versus 18 months (95% CI: 16.4–20.0) in the HCG/HMG group (P < 0.001) (Figure 1a). The median time to achieve sperm concentrations ≥5 × 106 ml−1 was 14 months (95% CI: 5.8–22.2) versus 27 months (95% CI: 18.9–35.1) (P < 0.001) in the GnRH and HCG/HMG groups, respectively (Figure 1b); the median time to achieve sperm concentrations ≥10 × 106 ml−1 was 18 months (95% CI: 10.0–26.0) in the GnRH group versus 39 months (95% CI unknown) in the HCG/HMG group (Figure 1c). The median time to achieve sperm concentrations ≥15 × 106 ml−1 was 24 months (95% CI: 15.0–33.0) in the GnRH group but could not be calculated in the HCG/HMG group due to a limited number of eligible cases (Figure 1d).
Figure 1.
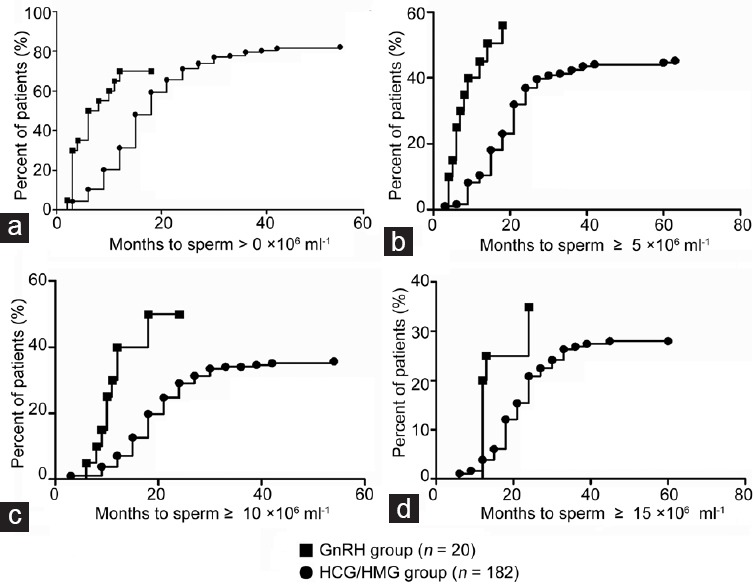
Time to achieve sperm concentration thresholds >0, ≥5, ≥10 and ≥15 × 106 ml−1 (Kaplan–Meier analysis). (a) Percentage of patients in each group (GnRH group, n = 20; and HCG/HMG group, n = 182) to achieve sperm concentration >0 (P < 0.001). (b) Percentage of patients in each group to achieve sperm concentration ≥5 × 106 ml−1 (P < 0.001). (c) Percentage of patients in each group to achieve sperm concentration ≥10 × 106 ml−1. (d) Percentage of patients in each group to achieve sperm concentration ≥15 × 106 ml−1. GnRH: gonadotropin-releasing hormone; HCG/HMG: human chorionic gonadotropin/human menopausal gonadotropin.
Testicular size
The GnRH group achieved target testicular sizes earlier than the HCG/HMG group during treatment. Compared to the GnRH group, the HCG/HMG group required a longer median treatment time to achieve the various levels of testicular size: ≥4 ml, ≥8 ml, ≥12 ml, and ≥16 ml. It took a median of 2 months (95% CI: 0.9–3.1) in the GnRH group versus 6 months (95% CI: 5.0–7.0) in the HCG/HMG group to reach the testicular size ≥4 ml (P < 0.001) (Figure 2a). Similarly, it took 4 months (95% CI: 2.2–5.8) in the GnRH group versus 18 months (95% CI: 15.8–20.2) in the HCG/HMG group to achieve testicular size ≥8 ml (P < 0.001) (Figure 2b). The GnRH group took 18 months (95% CI: 11.9–24.1) to achieve testicular size ≥12 ml compared to 42 months (95% CI: 34.9–49.1) in the HCG/HMG group (P < 0.001) (Figure 2c). In the HCG/HMG group, an average of 60 months (95% CI: 48.3–71.7) were required to achieve testicular size ≥ 16 ml; data regarding time to testicular size ≥16 ml could not be determined for the GnRH group due to the shorter treatment and follow-up time in this group (Figure 2d).
Figure 2.
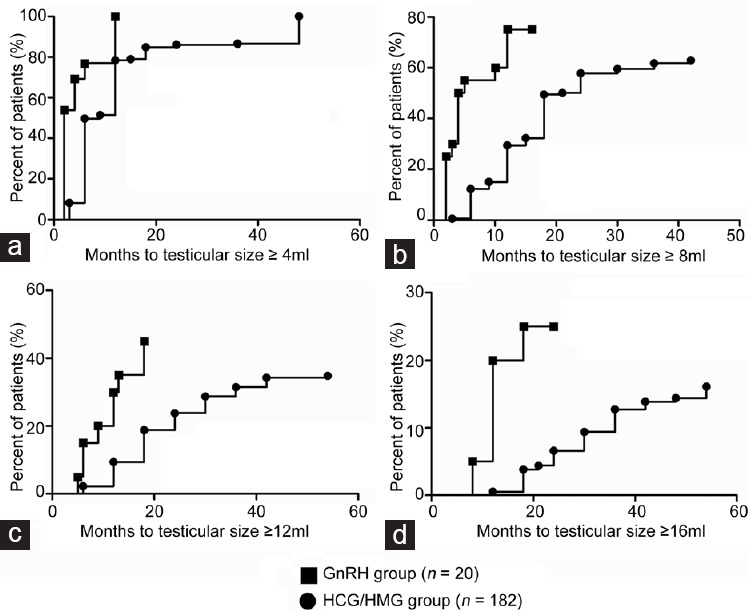
Time to achieve testicular size thresholds ≥4, ≥8, ≥12 and ≥16 ml (Kaplan–Meier analysis). (a) Percentage of patients in each group (GnRH group, n = 20; and HCG/HMG group, n = 182) to achieve testicular size ≥4 ml (P < 0.001). (b) Percentage of patients in each group to achieve testicular size ≥8 ml (P < 0.001). (c) Percentage of patients in each group to achieve testicular size ≥12 ml (P < 0.001). (d) Percentage of patients in each group to achieve testicular size ≥16 ml. GnRH: gonadotropin-releasing hormone; HCG/HMG: human chorionic gonadotropin/human menopausal gonadotropin.
Sperm motility
Sperm motility was similar between the GnRH and HCG/HMG groups. Sperm motility (a + b + c percentage) was only evaluated in semen samples that had sperm concentration >1 × 106 ml−1. In total, 16 samples (from seven patients) in the GnRH group and 153 samples (from 65 patients) in the HCG/HMG group were assessed. The sperm progressive motility (a + b) was 38.2% ± 18.0% in the GnRH group versus 37.2% ± 18.0% in the HCG/HMG group (P = 0.839), and the total motility (a + b + c) was 43.2% ± 18.1% versus 43.7% ± 20.4% (P = 0.921) in the GnRH group and the HCG/HMG group, respectively.
Fertility
Twenty pregnancies occurred in 18/32 (56.3%) couples in the HCG/HMG group, including 19 natural conceptions (two women had two pregnancies) and one conceived by in vitro fertilization (IVF). Sixteen infants, including nine girls and seven boys, were delivered with normal external genitalia. Four pregnancy losses occurred, including two spontaneous miscarriages and two induced abortions. In the GnRH group, five natural conceptions occurred in 14 couples (5/14, 35.7%) attempting pregnancy. Three babies were delivered uneventfully, and two women remain pregnant at the time of this manuscript's submission.
Spermatogenesis in patients with a history of cryptorchidism
The incidence of cryptorchidism was the same between the two groups (20% vs 20%). In the HCG/HMG group, the success rate of spermatogenesis in the subgroup of patients with cryptorchidism (n = 36) was lower than that of the subgroup without cryptorchidism (n = 146) (50% vs 83%, P = 0.032). The cryptorchidism subgroup consistently had a longer median time to first sperm detection in semen (24 vs 15 months, P < 0.001) and a lower median sperm concentration (1.9 [0.5, 8.6] × 106 vs 11.1 [1.0, 25.0] × 106 ml−1, P = 0.006) during treatment compared to the HCG/HMG sub-group without cryptorchidism. In the GnRH group, the success rates of spermatogenesis in the subgroups with (n = 4) and without (n = 16) cryptorchidism were 25% versus 81%, respectively (P = 0.043).
Of the 36 patients with CHH in the HCG/HMG group with a history of cryptorchidism, 18 had had unilateral cryptorchidism and 18 had had bilateral cryptorchidism. Orchidopexy was conducted in 23 patients at a mean age of 7.3 ± 4.0 years (range: 1–16 years). After treatment with HCG/HMG, those with a history of cryptorchidism experienced an increase in serum testosterone from 0.8 ± 0.4 nmol l−1 to 14.1 ± 7.2 nmol l−1 (P < 0.001) and in testicular volume from 1.6 ± 1.1 ml to 6.3 ± 4.1 ml (P < 0.001).
Eighteen patients with a history of cryptorchidism (18/36, 50%) and received HCG/HMG had successful spermatogenesis; ten of the 18 had had unilateral cryptorchidism while the remaining eight had had bilateral cryptorchidism. Among these 18 individuals, the mean time to achieve first sperm was 19 months.
There were four patients with a history of cryptorchidism in the GnRH group; two had had unilateral and two had had bilateral cryptorchidism. Orchidopexy was conducted in two patients at the ages of 6- and 8-year-old, respectively. By the end of the follow-up, the serum testosterone in these individuals had increased from 0.7 ± 0.7 nmol l−1 to 7.9 ± 5.3 nmol l−1 (P = 0.017) and testicular volume had increased from 2.6 ± 1.9 ml to 6.9 ± 3.4 ml (P = 0.012). Sperm was detected in one patient (unilateral cryptorchidism) after 9 months of treatment.
Safety evaluation
During the study period, gynecomastia occurred in 16 of 182 subjects (8.8%) in the HGC/HMG group and in one of twenty subjects (5.0%) in the GnRH group (P = 0.083). Acne occurred in 11/182 (6.0%) patients in the HCG/HMG group and in 0/20 (0%) patients in the GnRH group (P = 0.021). Seven (35%) patients in the GnRH group had mild to moderate dermatologic allergic reactions. No hepatorenal impairment was identified.
DISCUSSION
Although the rates of successful spermatogenesis were similar between the group treated with pulsatile GnRH and the group treated with HCG/HMG, pulsatile GnRH treatment was associated with earlier spermatogenesis and a shorter time to achieve higher sperm concentrations and greater testicular size.
The abilities of these two methods to restore fertility in CHH patients have been investigated in several previous studies. Most studies concluded that the efficacy of pulsatile GnRH did not differ from that of HCG/HMG therapy.4,5,6 For example, one study with a large sample of CHH patients (n = 90) receiving pulsatile GnRH treatment showed an 83% success rate of spermatogenesis induction,10 similar to the 80%–84% success rate seen with HCG/HMG therapy.12,13 Some smaller studies have suggested that pulsatile GnRH might be superior to HCG/HMG therapy. One study of 36 CHH patients estimated that pulsatile GnRH therapy induced a greater increase in testicular size (8.1 vs 4.8 ml) and required a shorter time to induce spermatogenesis (12 vs 20 months).7 A recent meta-analysis of 48 studies of HCG/HMG therapy and 16 studies of pulsatile GnRH therapy showed that the rate of successful spermatogenesis induction in hypogonadotropic hypogonadism patients was 68% (95% CI: 58%–77%) in HCG/HMG treatment; this success rate was lower than the 77% (95% CI: 63%–87%) success rate estimated in pulsatile GnRH therapy.3 However, this meta-analysis only evaluated appearance of sperm and did not include any other sperm thresholds or the time it took to reach any particular endpoint. Our current findings, together with the prior studies, indicate that pulsatile GnRH therapy may promote earlier spermatogenesis and larger testicular size compared to HCG/HMG therapy.
Our study found that the median time to achieve first sperm appearance in the semen in the GnRH group was 6 months; four patients (4/20, 20.0%) had their first sperm appear after treatment for 3 months. The period of sperm induction in the GnRH group was significantly shorter than the 18 months required in the HCG/HMG group for sperm to first appear. Notably, other studies found the median time to induce spermatogenesis in HCG/HMG-treated individuals to be 7–9 months.12,13,14,15,16 Furthermore, the GnRH group was associated with shorter time to achieve sperm concentrations at predetermined various thresholds. If confirmed by future prospective studies, the shorter time to induce spermatogenesis and to reach higher sperm concentrations in those taking pulsatile GnRH groups may benefit patients with CHH who desire fertility. Several factors may contribute to the advantage of pulsatile GnRH over HCG/HMG therapy. First, the pulsatile gonadotropins induced by GnRH are more physiological17 and may have higher efficacy in sperm induction compared to the more variable HCG and HMG levels achieved by twice weekly injections.18 Second, in some patients, the presence of autoantibodies against HCG may cause Leydig cells to fail to respond to exogenous HCG.19 Exogenous GnRH may also induce autoantibodies and cause chronic intestinal pseudo-obstruction.20 However, the effect of these antibodies on the function of the pituitary-gonad-axis has not been recognized.
Our study found that the median time for the first sperm detection was 6 months, which was consistent with previous results.21 The interval of time between initiation of GnRH therapy and the appearance of sperm in ejaculate was variable from 2 to 24 months.21 Patients with baseline testicular sizes over 4 ml had 100% success rate in initiating spermatogenesis by 6 months, while in patients with testicular sizes smaller than 4 ml, less than 50% of them had spermatogenesis by 6 months.21 Previous studies reported an 82% success rate of spermatogenesis, a value higher than the 70% success rate seen in our study. The difference is possibly due to a longer therapeutic duration in previous studies (15.6 vs 24 months).21
Seminiferous tubules account for 90% of testicular volume; larger testicular size is associated with higher sperm concentration.22 Therefore, an increase in testicular size during treatment may be an important indicator for the potential of spermatogenesis.12,16,23,24,25 In our study, the GnRH group achieved larger testicle size compared to the HCG/HMG group; this result is consistent with that found previously in another study.7 Another recent study focusing on adolescents with CHH also found that pulsatile GnRH can induce larger testicular sizes than HCG; however, this study did not directly evaluate the effects on spermatogenesis.11 Interestingly, long-term gonadotropin therapy followed by subsequent treatment with pulsatile GnRH was found to result in a further increase in testicular volumes.5 Pulsatile GnRH therapy may provide another chance for spermatogenesis induction in patients who have failed combined gonadotropin therapy.26,27
We hypothesized that the GnRH group would have higher sperm motility due to the larger testicular size and the more physiological gonadotropin stimulation. However, in our study, sperm motility was similar between the two groups. The underlying mechanism for these results is not clear. In addition, we had a limited number of patients and relatively fewer eligible semen samples to analyze from the GnRH group; these factors may prevent meaningful interpretation of the sperm motility results.
The GnRH group had a lower testosterone concentration than the HCG/HMG group. Previous studies have aimed to sustain testosterone levels between 10 and 15 nmol l−1 during HCG/HMG therapy.6,7 Our results suggest that a plasma (peripheral) testosterone level of 8–10 nmol l−1 may be sufficient to induce production of sperm in CHH patients. In fact, higher testosterone levels have not been shown to correlate with higher sperm concentrations28,29 in certain populations.
Our study showed that CHH patients with cryptorchidism require a much longer time for initiation of spermatogenesis in both the HCG/HMG and GnRH groups; the successful rates of spermatogenesis were 50% and 25%, respectively. These results are highly in line with other previous studies, indicating that cryptorchidism is a dominant, unfavorable predictor for spermatogenesis in CHH patients.12,21,30
Our study is not without limitations. First, the HCG/HMG group had a longer follow-up time and a larger sample size than the GnRH group. Despite this, an advantage of pulsatile GnRH over HCG/HMG therapy was still observed during the first 2 years of treatment. Second, this was a retrospective study, and the patients included in the present study were not randomized. Some patients were given a choice of treatment after pulsatile GnRH therapy became available in China, and thus selected which group to which they belonged. However, the two groups had similar features and hormonal profiles at baseline, including testicular size, rates of cryptorchidism, and LH, FSH and testosterone levels, suggesting that the two groups were comparable. Third, dosing for the GnRH group was performed based on FSH and LH levels, while dosing for the HCG/HMG group was done according to testosterone level; this may have direct implications for the different testosterone levels seen between the two groups. In addition, the difference in dosing regimens may compound differences seen in the spermatogenesis results. In future research, groups should be dosed to target to a similar testosterone level.
CONCLUSIONS
In summary, our study indicates that although the overall rates of success in inducing spermatogenesis were similar between individuals receiving pulsatile GnRH and those receiving HCG/HMG, satisfactory results may be obtained more slowly with HCG/HMG treatment. Pulsatile GnRH therapy may induce earlier sperm production, larger testicular size, and higher sperm concentrations. More prospective, randomized, and multicenter studies are needed to further verify this conclusion.
AUTHOR CONTRIBUTIONS
JFM, ZXL, XW, HLX, BKH, and JJZ collected clinical data. JFM wrote the article; MN performed patient follow-up and collected clinical data. LM, UBK, and XYW designed the study and edited the article. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Fraietta R, Zylberstejn DS, Esteves SC. Hypogonadotropic hypogonadism revisited. Clinics (Sao Paulo) 2013;68(Suppl 1):81–8. doi: 10.6061/clinics/2013(Sup01)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dwyer AA, Sykiotis GP, Hayes FJ, Boepple PA, Lee H, et al. Trial of recombinant follicle-stimulating hormone pretreatment for GnRH-induced fertility in patients with congenital hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2013;98:E1790–5. doi: 10.1210/jc.2013-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology. 2014;2:794–808. doi: 10.1111/andr.262. [DOI] [PubMed] [Google Scholar]
- 4.Buchter D, Behre HM, Kliesch S, Nieschlag E. Pulsatile GnRH or human chorionic gonadotropin/human menopausal gonadotropin as effective treatment for men with hypogonadotropic hypogonadism: a review of 42 cases. Eur J Endocrinol. 1998;139:298–303. doi: 10.1530/eje.0.1390298. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Chaudhari N, Corle D, Sherins RJ. Comparison of pulsatile subcutaneous gonadotropin-releasing hormone and exogenous gonadotropins in the treatment of men with isolated hypogonadotropic hypogonadism. Fertil Steril. 1988;49:302–8. doi: 10.1016/s0015-0282(16)59720-9. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Banks SM, Barnes KM, Sherins RJ. Two-year comparison of testicular responses to pulsatile gonadotropin-releasing hormone and exogenous gonadotropins from the inception of therapy in men with isolated hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 1988;67:1140–5. doi: 10.1210/jcem-67-6-1140. [DOI] [PubMed] [Google Scholar]
- 7.Schopohl J, Mehltretter G, von Zumbusch R, Eversmann T, von Werder K. Comparison of gonadotropin-releasing hormone and gonadotropin therapy in male patients with idiopathic hypothalamic hypogonadism. Fertil Steril. 1991;56:1143–50. [PubMed] [Google Scholar]
- 8.Raivio T, Falardeau J, Dwyer A, Quinton R, Hayes FJ, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357:863–73. doi: 10.1056/NEJMoa066494. [DOI] [PubMed] [Google Scholar]
- 9.WHO. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010. [Google Scholar]
- 10.Sykiotis GP, Hoang XH, Avbelj M, Hayes FJ, Thambundit A, et al. Congenital idiopathic hypogonadotropic hypogonadism: evidence of defects in the hypothalamus, pituitary, and testes. J Clin Endocrinol Metab. 2010;95:3019–27. doi: 10.1210/jc.2009-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong C, Liu Y, Qin M, Wu D, Wang X. Pulsatile GnRH is superior to hCG in therapeutic efficacy in adolescent boys with hypogonadotropic hypogonadodism. J Clin Endocrinol Metab. 2015;100:2793–9. doi: 10.1210/jc.2015-1343. [DOI] [PubMed] [Google Scholar]
- 12.Warne DW, Decosterd G, Okada H, Yano Y, Koide N, et al. A combined analysis of data to identify predictive factors for spermatogenesis in men with hypogonadotropic hypogonadism treated with recombinant human follicle-stimulating hormone and human chorionic gonadotropin. Fertil Steril. 2009;92:594–604. doi: 10.1016/j.fertnstert.2008.07.1720. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto AM, Snyder PJ, Bhasin S, Martin K, Weber T, et al. Stimulation of spermatogenesis with recombinant human follicle-stimulating hormone (follitropin alfa; GONAL-f): long-term treatment in azoospermic men with hypogonadotropic hypogonadism. Fertil Steril. 2009;92:979–90. doi: 10.1016/j.fertnstert.2008.07.1742. [DOI] [PubMed] [Google Scholar]
- 14.Efficacy and safety of highly purified urinary follicle-stimulating hormone with human chorionic gonadotropin for treating men with isolated hypogonadotropic hypogonadism. European Metrodin HP Study Group. Fertil Steril. 1998;70:256–62. doi: 10.1016/s0015-0282(98)00156-3. [DOI] [PubMed] [Google Scholar]
- 15.Bouloux P, Warne DW, Loumaye E. Efficacy and safety of recombinant human follicle-stimulating hormone in men with isolated hypogonadotropic hypogonadism. Fertil Steril. 2002;77:270–3. doi: 10.1016/s0015-0282(01)02973-9. [DOI] [PubMed] [Google Scholar]
- 16.Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, et al. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. J Clin Endocrinol Metab. 2009;94:801–8. doi: 10.1210/jc.2008-1648. [DOI] [PubMed] [Google Scholar]
- 17.Spratt DI, O’Dea LS, Schoenfeld D, Butler J, Rao PN, et al. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol. 1988;254:E658–66. doi: 10.1152/ajpendo.1988.254.5.E658. [DOI] [PubMed] [Google Scholar]
- 18.Ulloa-Aguirre A, Mendez JP, Diaz-Sanchez V, Altamirano A, Perez-Palacios G. Self-priming effect of luteinizing hormone-human chorionic gonadotropin (hCG) upon the biphasic testicular response to exogenous hCG. I. Serum testosterone profile. J Clin Endocrinol Metab. 1985;61:926–32. doi: 10.1210/jcem-61-5-926. [DOI] [PubMed] [Google Scholar]
- 19.Thau RB, Goldstein M, Yamamoto Y, Burrow GN, Phillips D, et al. Failure of gonadotropin therapy secondary to chorionic gonadotropin-induced antibodies. J Clin Endocrinol Metab. 1988;66:862–7. doi: 10.1210/jcem-66-4-862. [DOI] [PubMed] [Google Scholar]
- 20.Ohlsson B, Veress B, Janciauskiene S, Montgomery A, Haglund M, et al. Chronic intestinal pseudo-obstruction due to buserelin-induced formation of anti-GnRH antibodies. Gastroenterology. 2007;132:45–51. doi: 10.1053/j.gastro.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Pitteloud N, Hayes FJ, Dwyer A, Boepple PA, Lee H, et al. Predictors of outcome of long-term GnRH therapy in men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:4128–36. doi: 10.1210/jc.2002-020518. [DOI] [PubMed] [Google Scholar]
- 22.Fuse H, Akashi T, Kazama T, Katayama T. Gonadotropin therapy in males with hypogonadotropic hypogonadism: factors affecting induction of spermatogenesis after gonadotropin replacement. Int Urol Nephrol. 1996;28:367–74. doi: 10.1007/BF02550500. [DOI] [PubMed] [Google Scholar]
- 23.Farhat R, Al-zidjali F, Alzahrani AS. Outcome of gonadotropin therapy for male infertility due to hypogonadotrophic hypogonadism. Pituitary. 2010;13:105–10. doi: 10.1007/s11102-009-0203-1. [DOI] [PubMed] [Google Scholar]
- 24.Miyagawa Y, Tsujimura A, Matsumiya K, Takao T, Tohda A, et al. Outcome of gonadotropin therapy for male hypogonadotropic hypogonadism at university affiliated male infertility centers: a 30-year retrospective study. J Urol. 2005;173:2072–5. doi: 10.1097/01.ju.0000158133.09197.f4. [DOI] [PubMed] [Google Scholar]
- 25.Trsinar B, Muravec UR. Fertility potential after unilateral and bilateral orchidopexy for cryptorchidism. World J Urol. 2009;27:513–9. doi: 10.1007/s00345-009-0406-0. [DOI] [PubMed] [Google Scholar]
- 26.Blumenfeld Z, Makler A, Frisch L, Brandes JM. Induction of spermatogenesis and fertility in hypogonadotropic azoospermic men by intravenous pulsatile gonadotropin-releasing hormone (GnRH) Gynecol Endocrinol. 1988;2:151–64. doi: 10.3109/09513598809023623. [DOI] [PubMed] [Google Scholar]
- 27.Berezin M, Weissenberg R, Rabinovitch O, Lunenfeld B. Successful GnRH treatment in a patient with Kallmann's syndrome, who previously failed HMG/HCG treatment. Andologia. 1988;20:285–8. doi: 10.1111/j.1439-0272.1988.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 28.Jensen TK, Andersson AM, Jorgensen N, Andersen AG, Carlsen E, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–70. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 29.Qin DD, Yuan W, Zhou WJ, Cui YQ, Wu JQ, et al. Do reproductive hormones explain the association between body mass index and semen quality? Asian J Androl. 2007;9:827–34. doi: 10.1111/j.1745-7262.2007.00268.x. [DOI] [PubMed] [Google Scholar]
- 30.Dwyer AA, Raivio T, Pitteloud N. Gonadotrophin replacement for induction of fertility in hypogonadal men. Best Pract Res Clin Endocrinol Metab. 2015;29:91–103. doi: 10.1016/j.beem.2014.10.005. [DOI] [PubMed] [Google Scholar]