Abstract
The aim of this study is to identify optimal patients for adjuvant radiation therapy (ART) in pT3 prostate cancer. The role of ART for patients with adverse pathologic features after radical prostatectomy (RP) has been demonstrated, but over- or under-treatment remains a significant concern. Two-hundred and five patients with pT3N0M0 who underwent robot-assisted RP without ART were analyzed. Multivariate Cox proportional regression analyses were used to identify predictors of biochemical recurrence (BCR) and clinical progression (CP). The estimated 5-year BCR-free survival (BCRFS) and CP-free survival (CPFS) were 52.8% and 85.6%, respectively. Preoperative prostate-specifc antigen (PSA) ≥10 ng ml−1 (hazard ratio [HR]: 3.288–6.027; P = 0.003), pathologic Gleason score (pGS) ≥8 (HR: 4.146; P = 0.014), and lymphovascular invasion (LVI) (HR: 2.167; P = 0.026) were associated with BCR. Based on these factors, a risk stratification tool was developed. Patients with no risk factors (PSA <10 ng ml−1, pGS 6, and absent LVI) showed excellent BCRFS and CPFS at 5 years (91.9% and 100.0%, respectively), but those with two or more risk factors (PSA ≥10 ng ml−1, pGS ≥8, or present LVI) had poor BCRFS and CPFS (12.1% and 54.6%, respectively). In addition, the multivariate analysis revealed that pathologic stage pT3b (HR: 5.393; P = 0.025) was the only predictor of CP. Our study demonstrated the heterogeneity of oncologic outcomes in patients with pT3 prostate cancer. The proposed risk stratification can be used to identify patients who are at risk for disease progression and may aid in identifying the best patients for ART.
Keywords: prostatectomy, prostate-specific antigen, recurrence, survival
INTRODUCTION
Although radical prostatectomy (RP) is an effective treatment to manage clinically localized prostate cancer (PCa), up to 25% of patients ultimately succumb to biochemical recurrence (BCR) at 10 years.1 In particular, BCR rates range 40%–65% in men with adverse pathologic findings such as extraprostatic extension (EPE), seminal vesicle invasion (SVI), and/or positive surgical margin (PSM).2,3,4
Approximately 20%–30% of patients are found to have pathologic stage T3 (pT3) PCa despite the stage migration to less advanced disease in the prostate-specific antigen (PSA) era.5,6 Studies have demonstrated that the unfavorable prognostic features associated with pT3 may influence disease progression.7,8 To this end, the American Society for Therapeutic Radiology and Oncology/American Urological Association (ASTRO/AUA) guidelines have strongly recommended the use of adjuvant radiation therapy (ART) in all patients with pT3 disease.9 However, because patients with adverse pathologic features do not necessarily recur, adopting the AUA/ASTRO guidelines may result in significant overtreatment. Indeed, close examination of the randomized phase III trials that confirmed the clinical efficacy of ART suggested that some subgroups of patients are not likely to benefit from ART.2,3,4,10
In the present study, we aimed to identify the prognostic factors affecting the oncologic outcomes in patients with pT3N0M0 prostate cancer. Furthermore, a risk stratification model has been proposed to identify subgroups of patients who are likely to benefit from ART.
MATERIALS AND METHODS
Study population
A retrospective review of the prospectively maintained database was performed under the Institutional Review Board approval. Between January 2006 and December 2014, 1129 patients underwent robot-assisted RP (RARP) for clinically localized PCa by a single surgeon (IYK) at the Rutgers Cancer Institute of New Jersey. All patients had a metastatic workup with abdominal and pelvic computed tomography (CT) and bone scintigraphy that was negative. Clinical stage was assigned according to the 2002 American Joint Committee on Cancer (AJCC) staging classification.11 Two hundred and fifty-four men with pathologically confirmed EPE or SVI were initially selected to be included. Of these men, 205 patients were included in the study after excluding individuals with prior neoadjuvant androgen deprivation therapy (ADT) (n = 1), ART (n = 11), involvement of pelvic lymph nodes (n = 19), and follow-up period of less than 12 months (n = 18).
All RP specimens were processed according to the Stanford protocol, step-sectioned transversely at 5 mm intervals, and mounted as quarter sections for microscopic evaluation.12 Tumor volumes on RP specimens were calculated by visual estimates of tumor percentages. Each slide was recorded as the estimated percentage of tumor involvement. Percentages of tumor volume were calculated by summing up each slide and averaging the results of all slides analyzed.13,14,15 Lymphovascular invasion (LVI) was defined as the unequivocal presence of tumor cells within endothelial-lined space with no underlying muscular walls or the presence of tumor emboli in small intraprostatic vessels.16 The distinction between lymphatic or vascular invasion was not made in this study. Pathologic stage was defined according to the 2009 AJCC staging classification.17 Gleason grading of specimen was done as recommended by the International Society of Urological Pathology (ISUP) guidelines.18
Follow-up and outcome measures
After surgery, PSA values were measured quarterly in the first year, followed by biannual measurements in the second year, and annual measurements in the third year and thereafter. BCR was defined based on postoperative PSA rise on two consecutive measurements with the last value ≥0.2 ng ml−1.19 The time to BCR was taken as the time of the first evidence of PSA rising above or equal to 0.2 ng ml−1. Clinical progression (CP) was defined as evidence of local recurrence or distant metastasis by imaging studies, exclusive of BCR. Metastatic diseases such as extrapelvic nodal metastasis, bony metastasis and visceral metastasis were determined by abdominal and pelvic CT scan, bone scintigraphy, or spinal magnetic resonance imaging (MRI) scan. Men without evidence of BCR or CP were censored at the last follow-up visit.
Statistical analysis
Descriptive statistics were expressed as medians and interquartile ranges (IQRs) for continuous variables and frequencies and proportions for categorical variables. Comparisons between groups for categorical variables were conducted using Chi-square or Fisher's exact test when indicated. Univariate and multivariate Cox proportional hazards regression analyses were used to identify prognostic factors. The BCR-free survival (BCRFS) was estimated using the Kaplan–Meier method. Survival curves among groups were compared with the Log-rank test. The proposed model for BCRFS was built based on the combination of independent predictors identified in the multivariate analysis. All statistical analyses were performed using SPSS version 21 (IBM Corporation, Armonk, NY, USA) and R 3.2.3 (CRAN: the Comprehensive R Archive Network at http://cran.r-project.org) with two-sided P < 0.05 considered as statistically significant.
RESULTS
Patient characteristics
Baseline clinical and pathological variables are shown in Tables 1 and 2. Median age at surgery was 62.0 years (IQR: 57.0–67.0). Median preoperative PSA was 6.3 ng ml−1 (IQR: 4.5–8.9); 37 (18.0%) patients had PSA ≥10 ng ml−1. Pathologic stage T3a (pT3a) was seen in 173 (84.4%) patients. Pathologic Gleason score (pGS) ≥7 was seen in 171 (83.4%) patients. PSMs were noted in 111 (54.1%) patients, and 33 (16.1%) patients had LVI. Overall, 159 (77.6%) patients underwent bilateral pelvic lymph node (LN) dissection with a limited template and median number of retrieved LNs was 4 (IQR: 2–7). Three months after RP, the PSA level was undetectable (defined as <0.1 ng ml−1) in 187 (91.2%) men. The median follow-up was 32 months (IQR: 18.5–53.0).
Table 1.
Descriptive characteristic of patients (numerical variables)
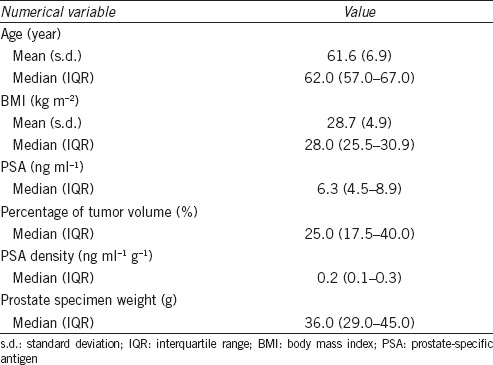
Table 2.
Descriptive characteristic of patient (categorical variables)
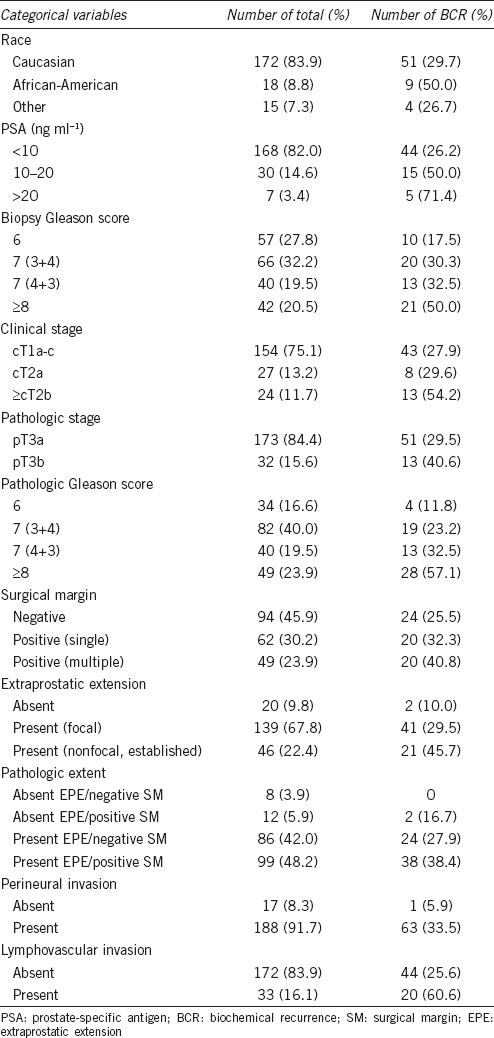
During a follow-up, BCR occurred in 64 (31.2%) patients and median time to BCR was 13 months (IQR: 4.3–28.5). Each frequency and proportion of BCR in the categorical variables is described in Table 2. After an occurrence of BCR, 56 (27.3%) men received salvage therapy; 52 (25.4%) men received salvage RT and 4 (1.9%) men had ADT. The remaining 8 (3.9%) patients were under close surveillance or lost to follow-up. The median PSA prior to salvage therapy was 0.30 ng ml−1 (IQR: 0.26–0.48). CP was identified in 13 patients (6.3%) and median time to CP was 35 months (IQR: 25.5–48.0). Among those with CP, distant metastases developed in nine patients (4.4%). The actuarial BCRFS and CP-free survival (CPFS), and metastasis-free survival at 5 years were 52.8% (95% confidence interval [CI]: 42.6–65.5), 85.6% (95% CI: 77.7–94.4), and 91.2% (95% CI: 85.2–97.6), respectively.
Predictors of BCR and CP
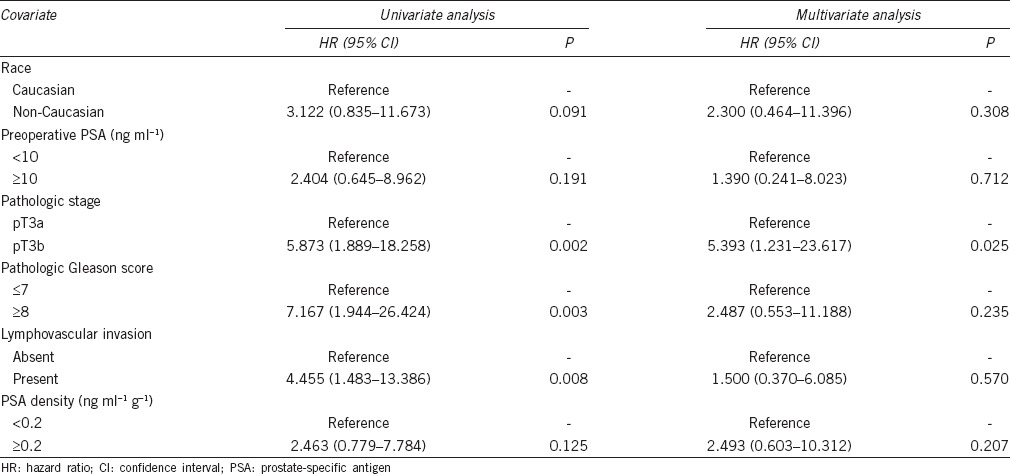
The univariate and multivariate analyses for the prediction of BCR are shown in Table 3. To determine the optimal cutoff values for percentage of tumor volume and PSA density (PSAD), Youden's index was calculated using the receiver operating curve. In univariate analysis, non-Caucasian, preoperative PSA, pGS, PSM, LVI, percentages of tumor volume, PSAD, and marginally pathologic stage were associated with BCR. However, preoperative PSA ≥10 ng ml−1 (vs <10 ng ml−1; hazard ratio [HR]: 3.288–6.027; 95% CI: 1.482–20.084; P = 0.003), pGS ≥ 8 (vs pGS 6; HR: 4.146; 95% CI: 1.339–12.835; P = 0.014), and presence of LVI (vs absence of LVI; HR: 2.167; 95% CI: 1.099–4.273; P = 0.026) remained as significant predictors of BCR in multivariate analysis.
Table 3.
Univariate and multivariate Cox proportional hazards regression analysis for predicting biochemical recurrence
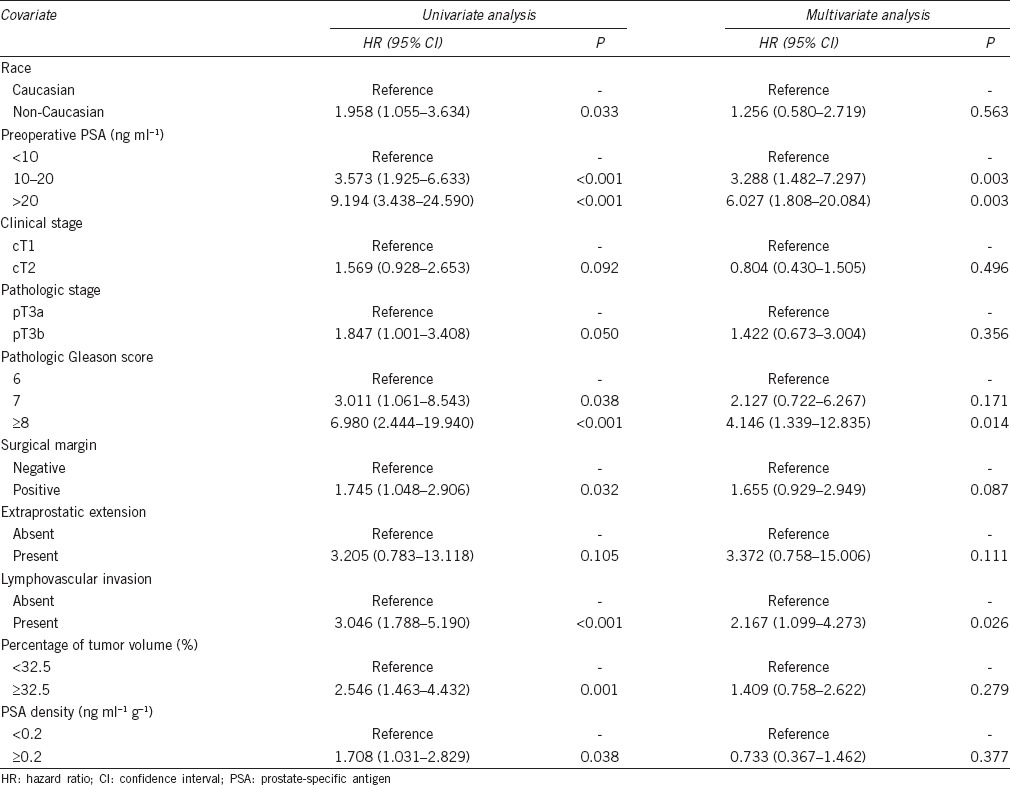
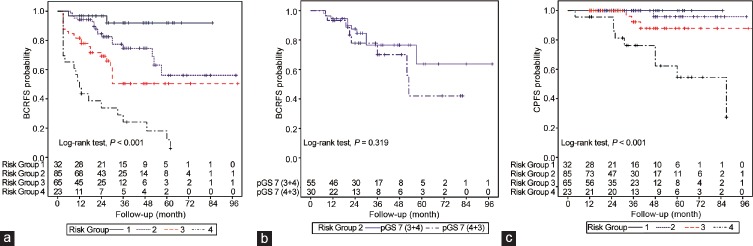
Figure 1a illustrates BCRFS according to the risk stratification by a combination of predictors obtained from the multivariate regression: Risk Group 1 (PSA <10 ng ml−1, pGS 6, and absence of LVI; n = 32), Risk Group 2 (PSA <10 ng ml−1, pGS 7, and absence of LVI; n = 85), Risk Group 3 (PSA ≥10 ng ml−1, pGS ≥8, or presence of LVI; one out of three predictors; n = 65), and Risk Group 4 (PSA ≥10 ng ml−1, pGS ≥8, or presence of LVI; two or more out of three predictors; n = 23). In the risk stratification, from Risk Group 1 to Risk Group 4, the actuarial 5-year BCRFS were 91.9%, 56.1%, 50.4%, and 12.1%, respectively (Log-rank test, P < 0.001). In addition, the pairwise comparison of BCRFS between each risk group showed a significant difference (P < 0.05 for all comparisons). Although the difference in actuarial 5-year BCRFS between pGS 7 (3 + 4) and pGS 7 (4 + 3) in Risk Group 2 appears to be substantial (63.8% vs 42.1%), no statistically significant difference was detected between pGS 7 (3 + 4) and pGS 7 (4 + 3) (Log-rank test, P = 0.319) (Figure 1b).
Figure 1.
Biochemical recurrence-free survival (BCRFS) and clinical progression-free survival (CPFS) according to the risk stratification. (a) BCRFS stratified by Risk Group. (b) BCRFS according to the Gleason score subclassification in Risk Group 2. pGS: pathologic Gleason score. (c) CPFS stratified by Risk Group.
Figure 1c shows CPFS according to the risk stratification. CP was not identified in Risk Group 1. Therefore, survival comparisons were limited to Risk Group 2, 3, and 4. The actuarial 5-year CPFS in Risk Group 2, 3, and 4 were 95.8%, 87.9%, and 54.6%, respectively (P < 0.001). There were significant differences of CPFS between Risk Group 4 and the other Risk Groups (Log-rank test, P < 0.01 for all comparisons). Descriptive analysis for postoperative variables in each risk group is described in Table 4. Those patients in Risk Group 4 were more likely to have detectable PSA postoperatively compared with the other Risk Groups (34.8% vs 3.1%–6.0%; P < 0.01). The time to BCR was shorter in the Risk Group 4 than the other Risk Groups, but it did not reach statistical significance.
Table 4.
Comparison of postoperative variables by the risk stratification
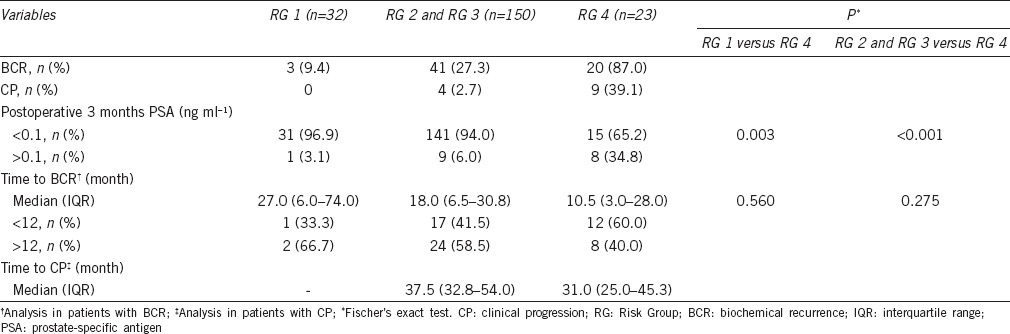
Table 5 presents the results of univariate and multivariate analysis for the prediction of CP. There were only 13 patients (6.3%) who developed CPs. Pathologic stage, pGS, and LVI were univariate predictors of CP; however, pathologic stage pT3b (vs pT3a; HR: 5.393; 95% CI: 1.231–23.617; P = 0.025) was the only significant predictor of CP on multivariate analysis. The predicted 5-year CPFS in pT3a and pT3b were 90.9% and 58.3%, respectively (Log-rank test, P = 0.001).
Table 5.
Univariate and multivariate Cox proportional hazards regression analysis for predicting clinical progression
DISCUSSION
Given the potential complications of radiotherapy, deciding whether ART is appropriate for all patients with extraprostatic disease after RP remains a difficult task. In this regard, further risk stratification of the at-risk patients may aid in determining the best candidates for ART. Recently, three randomized trials – SWOG 8794, EORTC 22911, and ARO 96-02– demonstrated that ART improves BCRFS at 10 years with HRs between 0.43 and 0.51.2,3,4 Based on the data from these trials, the ASTRO/AUA guideline has supported the use of ART in the setting of adverse pathologic features after RP. However, the impact of ART on subsequent metastases and overall survival is less clear. Only SWOG 8794 trial showed a modest reduction of metastasis risk between wait-and-see group and ART group (17.5% vs 9.3%; HR: 0.71), but two other studies failed to demonstrate a substantial benefit of metastasis-free or overall survival on subanalysis. In fact, despite the use of ART, 35%–46% of patients will have BCR at 10 years follow-up. Conversely, a third of patients without receiving ART had not developed BCR.2,3,4 Thus, some patients with adverse pathologic features following RP will experience suboptimal disease control irrespective of immediate ART while those with favorable characteristics can safely be followed up with observation.
In this study, the predicted 5-year BCRFS and CPFS were 52.8% and 85.6%, respectively. These rates are somewhat lower, but comparable to other series of high-risk PCa patients treated with RARP.20 The observed lower rates may have stemmed from our cohort selection exclusively based on pT3 disease. In multivariate analysis for predicting BCR, higher PSA, pGS, and presence of LVI were associated with an increased risk of BCR. LVI may be a prognostic factor in other genitourinary cancers including testis and urothelial cancers.21,22 The ISUP recommended a routine examination of LVI as a part of standard pathologic report at RP.16 Although the utility of LVI as a prognostic factor remains controversial,23,24,25 the magnitude of independent association of LVI (HR: 2.167; 95% CI: 1.099–4.273; P = 0.026) in our cohort suggested that it may be a relevant predictor of BCR. In subgroup analysis for patients with undetectable PSA postoperatively, preoperative PSA ≥10 ng ml−1 (vs <10 ng ml−1; HR: 3.192; 95% CI: 1.333–7.645; P = 0.009) and pGS ≥8 (vs pGS 6; HR: 9.310; 95% CI: 2.028–42.734; P = 0.004) were also independent predictors of BCR in multivariate analysis. However, the presence of LVI (vs absence of LVI; HR: 2.015; 95% CI: 0.996–4.079; P = 0.051) showed a marginal association with BCR on univariate analysis. LVI was more frequently observed in patients with detectable PSA level compared to those with undetectable value (55.6% vs 12.4%, P < 0.001).
To make a better informed treatment decision in patients with pT3 disease, a risk stratification model was created based on the BCR risk and corresponding independent variables. It was found that considerable differences exist with respect to BCRFS in each risk group. While the overall 5-year BCRFS in our cohort was 52.8%, it ranged from 12.1% to 91.9% depending on the patient risk stratification, suggesting heterogeneity within our study cohort. Similarly, the CPFS varied from 54.6% to 100.0% (Figure 1). However, before delving into our analysis, it must be noted that these calculated 5-year BCRFS and CPFS results have been derived from a small number of patients at 60 months of follow-up. At that time of follow-up, the remaining number of patients at risk were only 18 and 31, respectively. Although this study has relatively high censored data, our findings collectively suggest the following important points. First, patients who belong to Risk Group 1, which accounted for 15.6% of our cohort, would not need immediate ART. The 5-year BCRFS and CPFS rates in this group were excellent (91.9% and 100.0%, respectively). None of this group has received salvage therapy due to stable PSA levels below 0.25 ng ml−1 even in cases of BCR during follow-up. Second, the 5-year BCRFS in patients with Risk Group 2 was only 56.1%. Indeed, pathologic GS 7 appears to have an influence on the BCC risk as this is the only difference when Risk Group 1 and 2 were compared. In Risk Group 3, the 5-year BCRFS rate was similar as compared to that of Risk Group 2 (50.4% vs 56.1%). These similar results may be attributed to few patients on follow-up at 60 months in Risk Group 3. It is likely that the actual 5-year BCRFS in Risk Group 3 may have worse outcome than calculated 5-year BCRFS if more cases with long-term follow-up will be added. Therefore, an immediate ART should be given to patients in these subsets. Finally, patients in Risk Group 4, which included 11.2% of patients, were thought to be in a very high-risk category as reflected from the poor 5-year BCRFS and CPFS rates (12.1% and 54.6%, respectively). Although 18 (78.3%) patients received early salvage RT in this group, a half of patients ended up having CP later. Therefore, given this abysmal clinical course, adjunctive use of RT alone may not be sufficient in this group and a more aggressive therapy with close monitoring is required. Specifically, multimodal treatments such as combination of ART and ADT and/or early systemic therapy, if clinically indicated, should be considered.26 In short, our results suggest that immediate ART may be an unnecessary intervention for Risk Group 1. However, a larger sample size with a long-term follow-up period is needed to confirm this concept. In addition, it may be insufficient for Risk Group 4 when considering RT as an adjuvant monotherapy following RP.
Preoperative PSA, pGS, and LVI were identified as independent predictors of risk for BCR in this study. Notwithstanding, additional pathologic features associated with BCR after RP have been reported. PSM is known to be a predictor of disease recurrence after RP.27 In our cohort study, the PSM rate was up to 56.1% in pT3b. In multivariate regression analysis for BCR, PSM status did not show a statistical significance (P = 0.087). Preoperative PSA, pathologic stage, pGS, and surgical experience can affect the risk of PSM. Since PSM strongly correlates with these variables, one of these parameters may drop out and lose its prognostic significance on multivariate analysis. SVI is believed to be associated with earlier BCR, disease progression, and occult micrometastatic disease.7 Although higher incidence of BCR was found in patients with pT3b than those with pT3a (40.6% vs 29.5%), only a marginal significance was noted on univariate analysis for BCR (P = 0.050). This is likely explained by the fact that both pT3a and pT3b diseases have high BCR rates, and thus it is underpowered to discriminate between the two groups. In addition, small sample size may also have influenced the result.
Thirteen CPs including nine distant metastases developed during a follow-up period. The CP rate was much higher in pT3b (18.8%) than pT3a (4.0%). Multivariate analysis revealed that pathologic stage (pT3b) was the only independent variable of CPFS (vs pT3a; HR: 5.393; P = 0.025). The numbers of CP events are too rare to achieve sufficient statistical power for other variables examined in this study. Despite the well-known risk for disease recurrence, there is a debatable question regarding the role of ART in pT3b patients. The EORTC 22911 and ARO 96-02 studies failed to demonstrate a clear benefit for metastasis-free or overall survival in men with pT3b treated with ART alone.3,4 A high pGS ≥8 was the most consistent factor associated with metastasis or cancer-specific death in patients with pT3b.7,28 Therefore, multimodal therapies’ approaches should be considered in patients with pT3b and associated unfavorable histology.
Pelvic LN dissection (PLND) in PCa is the most effective staging procedure for assessing LN metastases. The median value of nodal yield at PLND during RARP ranged from 3.3 to 24.0 nodes depending on the extent of dissection.29 Bilateral PLND was performed with a limited fashion in our study, not an extended template. The median retrieved LNs of our cohort was approximately 4 (IQR: 2–7). The number of removed LNs has increased gradually over the study period from a median 2 (IQR: 1–4) in the first fifty cases to 7 (IQR: 5–11) in the subsequent cases. However, as seen in RP series including RARP, LN positivity rate correlates with the extent of PLND, thus it can be underestimation of the real nodal metastases by limited template dissection.29 In recent years, extended PLND has been conducted for patients with intermediate- and high-risk patients at our institution.
Limitations of this study include the retrospective design. The relatively small sample size and small number of CP events limited the power to detect differences among the variables that were analyzed. In addition, the median follow-up was limited, and additional events may not have been captured. A larger sample size with a longer follow-up is necessary to confirm our proposed risk stratification tool.
CONCLUSIONS
The ASTRO/AUA guideline recommended ART for patients with pT3 after RP. However, our results indicated that this at-risk group is not homogeneous and that preoperative PSA, pGS, and LVI may permit the identification of patients who will benefit from ART.
AUTHOR CONTRIBUTIONS
JHH participated in the design of the study, acquisition of data, statistical analysis, and drafted manuscript. YSK and IYK conceived of the study, participated in its design and statistical analysis, and helped draft the manuscript. IYK revised it critically for important intellectual content. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
The present research was conducted by the research fund of Dankook University in 2015.
REFERENCES
- 1.Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–65. doi: 10.1016/s0094-0143(05)70163-4. [DOI] [PubMed] [Google Scholar]
- 2.Thompson IM, Tangen CM, Paradelo J, Lucia MS, Miller G, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181:956–62. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolla M, van Poppel H, Tombal B, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: long-term results of a randomised controlled trial (EORTC trial 22911) Lancet. 2012;380:2018–27. doi: 10.1016/S0140-6736(12)61253-7. [DOI] [PubMed] [Google Scholar]
- 4.Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66:243–50. doi: 10.1016/j.eururo.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Broering JM, Litwin MS, Lubeck DP, Mehta SS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004;171:1393–401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 6.Schymura MJ, Sun L, Percy-Laurry A. Prostate cancer collaborative stage data items-their definitions, quality, usage, and clinical implications: a review of SEER data for 2004-2010. Cancer. 2014;120(Suppl 23):3758–70. doi: 10.1002/cncr.29052. [DOI] [PubMed] [Google Scholar]
- 7.Pierorazio PM, Ross AE, Schaeffer EM, Epstein JI, Han M, et al. A contemporary analysis of outcomes of adenocarcinoma of the prostate with seminal vesicle invasion (pT3b) after radical prostatectomy. J Urol. 2011;185:1691–7. doi: 10.1016/j.juro.2010.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong BC, Chalfin HJ, Lee SB, Feng Z, Epstein JI, et al. The relationship between the extent of extraprostatic extension and survival following radical prostatectomy. Eur Urol. 2015;67:342–6. doi: 10.1016/j.eururo.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Thompson IM, Valicenti RK, Albertsen P, Davis BJ, Goldenberg SL, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol. 2013;190:441–9. doi: 10.1016/j.juro.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Sineshaw HM, Gray PJ, Efstathiou JA, Jemal A. Declining use of radiotherapy for adverse features after radical prostatectomy: results from the national cancer data base. Eur Urol. 2015;68:768–74. doi: 10.1016/j.eururo.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Greene F, Page D, Fleming I, Fritz A, Balch CM. American Joint Committee on Cancer Staging Manual. 6th ed. New York: Springer; 2002. American Joint Committee on Cancer. Prostate; pp. 309–16. [Google Scholar]
- 12.Stamey TA, Yemoto CM, McNeal JE, Sigal BM, Johnstone IM. Prostate cancer is highly predictable: a prognostic equation based on all morphological variables in radical prostatectomy specimens. J Urol. 2000;163:1155–60. doi: 10.1016/s0022-5347(05)67713-0. [DOI] [PubMed] [Google Scholar]
- 13.Carvalhal GF, Humphrey PA, Thorson P, Yan Y, Ramos CG, et al. Visual estimate of the percentage of carcinoma is an independent predictor of prostate carcinoma recurrence after radical prostatectomy. Cancer. 2000;89:1308–14. doi: 10.1002/1097-0142(20000915)89:6<1308::aid-cncr16>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Manoharan M, Civantos F, Kim SS, Gomez P, Soloway MS. Visual estimate of percent of carcinoma predicts recurrence after radical prostatectomy. J Urol. 2003;170:1194–8. doi: 10.1097/01.ju.0000080402.72984.b3. [DOI] [PubMed] [Google Scholar]
- 15.Ramos CG, Roehl KA, Antenor JA, Humphrey PA, Catalona WJ. Percent carcinoma in prostatectomy specimen is associated with risk of recurrence after radical prostatectomy in patients with pathologically organ confined prostate cancer. J Urol. 2004;172:137–40. doi: 10.1097/01.ju.0000132139.40964.75. [DOI] [PubMed] [Google Scholar]
- 16.Magi-Galluzzi C, Evans AJ, Delahunt B, Epstein JI, Griffiths DF, et al. International Society of Urological Pathology (ISUP) consensus conference on handling and staging of radical prostatectomy specimens. Working group 3: extraprostatic extension, lymphovascular invasion and locally advanced disease. Mod Pathol. 2011;24:26–38. doi: 10.1038/modpathol.2010.158. [DOI] [PubMed] [Google Scholar]
- 17.Edge S, Byrd D, Compton C, Fritz A, Greene F. American Joint Committee on Cancer Staging Manual. 7th ed. New York: Springer-Verlag; 2010. American Joint Committee on Cancer. Prostate; pp. 457–68. [Google Scholar]
- 18.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 19.Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, et al. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. 2016;69:428–35. doi: 10.1016/j.eururo.2015.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdollah F, Sood A, Sammon JD, Hsu L, Beyer B, et al. Long-term cancer control outcomes in patients with clinically high-risk prostate cancer treated with robot-assisted radical prostatectomy: results from a multi-institutional study of 1100 patients. Eur Urol. 2015;68:497–505. doi: 10.1016/j.eururo.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, et al. EAU guidelines on testicular cancer: 2011 update. Eur Urol. 2011;60:304–19. doi: 10.1016/j.eururo.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 22.Tilki D, Shariat SF, Lotan Y, Rink M, Karakiewicz PI, et al. Lymphovascular invasion is independently associated with bladder cancer recurrence and survival in patients with final stage T1 disease and negative lymph nodes after radical cystectomy. BJU Int. 2013;111:1215–21. doi: 10.1111/j.1464-410X.2012.11455.x. [DOI] [PubMed] [Google Scholar]
- 23.Loeb S, Roehl KA, Yu X, Antenor JA, Han M, et al. Lymphovascular invasion in radical prostatectomy specimens: prediction of adverse pathologic features and biochemical progression. Urology. 2006;68:99–103. doi: 10.1016/j.urology.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Ng J, Mahmud A, Bass B, Brundage M. Prognostic significance of lymphovascular invasion in radical prostatectomy specimens. BJU Int. 2012;110:1507–14. doi: 10.1111/j.1464-410X.2012.11115.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Huang H, Pan XW, Xu DF, Cui XG, et al. The prognostic value of lymphovascular invasion in radical prostatectomy: a systematic review and meta-analysis. Asian J Androl. 2016;18:780–5. doi: 10.4103/1008-682X.156636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briganti A, Joniau S, Gandaglia G, Cozzarini C, Sun M, et al. Patterns and predictors of early biochemical recurrence after radical prostatectomy and adjuvant radiation therapy in men with pT3N0 prostate cancer: implications for multimodal therapies. Int J Radiat Oncol Biol Phys. 2013;87:960–7. doi: 10.1016/j.ijrobp.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Cookson MS, Chang SS. Margin control in open radical prostatectomy: what are the real outcomes? Urol Oncol. 2010;28:205–9. doi: 10.1016/j.urolonc.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Secin FP, Bianco FJ, Jr, Vickers AJ, Reuter V, Wheeler T, et al. Cancer-specific survival and predictors of prostate-specific antigen recurrence and survival in patients with seminal vesicle invasion after radical prostatectomy. Cancer. 2006;106:2369–75. doi: 10.1002/cncr.21895. [DOI] [PubMed] [Google Scholar]
- 29.Ploussard G, Briganti A, de la Taille A, Haese A, Heidenreich A, et al. Pelvic lymph node dissection during robot-assisted radical prostatectomy: efficacy, limitations, and complications – a systematic review of the literature. Eur Urol. 2014;65:7–16. doi: 10.1016/j.eururo.2013.03.057. [DOI] [PubMed] [Google Scholar]