Dear Editor,
Anejaculation is a rare ejaculatory disorder in men (1%–4% of sexually active men) that can cause male factor infertility.1 Although these patients are generally healthy individuals that may have erections and nocturnal emissions, they cannot ejaculate. As we all know, there is evidence that the etiology of ejaculatory dysfunction is partially genetic, especially related to serotonergic genes.2 Here, we report two polymorphic regions in serotonin transporter (5-HTT) gene for the first time in three Chinese males with anejaculation.
Case 1 was a married male suffering lifelong anejaculation. He presented to the Andrology Outpatient Clinic complaining of an inability to consciously ejaculate even after >50 min of masturbation. His sexual potency was normal, but no nocturnal emission was reported. Case 2 was a 32-year-old male referred from the reproductive medicine unit, where he and his wife had presented for evaluation of infertility after 3 years of marriage. He complained of an inability to ejaculate, despite no loss of libido or erectile function. He did not experience ejaculation during either coitus or masturbation, however, continued to experience erections and normal nocturnal emissions every few months. Case 3 was a college student. He presented initially to the clinic complaining of anejaculation, but otherwise normal sexual function, after the commencement of a relationship 2 years ago. He had anejaculation specific to penetrative sex, but could ejaculate normally during masturbation and had nocturnal emissions. Cases’ detailed information and clinical characteristics are shown in Table 1.
Table 1.
Information of cases and clinical characteristics
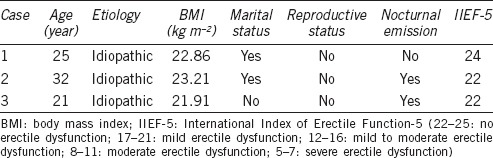
Three cases were from unrelated families and diagnosed as idiopathic anejaculation. Their past medical history was not significant. Careful gynecologic evaluation of female partners revealed no female factor contributing to anejaculation. Neurologic examination revealed normal sensory and motor systems and intact reflexes. Serum concentrations of follicle stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T), prolactin (PRL), and estradiol (E2) were in the normal range. In three cases, obstruction of the ejaculatory duct and retrograde ejaculation were ruled out by transrectal ultrasound and examination of urine after masturbation, respectively. Digital rectal examination and transrectal ultrasound to define prostate and seminal vesicles were normal.
The 5-HTT linked polymorphic region (5-HTTLPR) is a 43 bp insertion/deletion (L/S) polymorphism in the promoter region of SLC6A4, which encodes 5-HTT. Another polymorphism is a variable number of tandem repeat in the second intron of SLC6A4, which is called STin2 VNTR. The STin2 allelic variants were identified as 9-repeat, 10-repeat and 12-repeat alleles of a 16/17 bp element that have been identified. Informed consent to carry out molecular genetic analysis was obtained. The study was approved by Ethics Committee of the First Affiliated Hospital of Anhui Medical University (No. 20150047). We designed PCR primers (Table 2) by Primer3 Software Online Program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The 5-HTTLPR and STin2 VNTR polymorphisms were genotyped using a PCR-based technology (Supplemental Information (48.1KB, pdf) ). The PCR products were analyzed on a 3730XL Genetic Analyzer (Applied Biosystems, Carlsbad, California, USA). Genotypes were called by visual observation of peak sizes using GeneMapper 4.0 (Applied Biosystems, Carlsbad, California, USA).
Table 2.
Oligonucleotide primers used for genomic DNA amplification of human SLC6A4 gene 5-HTTLPR and STin2 VNTR polymorphisms

At the beginning of the scientific research on the mechanism of ejaculation, the hypothesis was formulated that this symptom could be considered a neurobiological disorder, with a major role played by a hypothetical genetic diathesis in the central serotoninergic pathway. Central 5-HT is the main drive controlling ejaculation.3 There is substantial evidence showing that the use of selective serotonin reuptake inhibitors (SSRIs), by increasing central serotoninergic tone, delays ejaculation.4 By determining magnitude and duration of 5-HT synaptic signal, 5-HTT plays a key role in the regulation of serotonergic neurotransmission,5 and is therefore considered to be an interesting candidate in ejaculatory association studies.
In the controls, the allelic frequencies distribution of 5-HTTLPR were 0.54 for S and 0.46 for L, and allelic distribution of STin2 VNTR were 0.75 for STin2.12, 0.23 for STin2.10 and 0.02 for STin2.9, respectively. However, the genotypes of three cases were S/S + STin2.10/12, S/S + STin2.10/10 and S/L + STin2.10/12, respectively. Obviously, S and STin2.10 alleles were more common in three patients with anejaculation. Replying the study in a bigger cohort of anejaculation patients is needed in further study.
The previous study had shown that 5-HTTLPR polymorphism contributes to the regulation of the expression of SLC6A4,6 and S allele could reduce the transcriptional activity of SLC6A4, hence decreasing transporter expression.7 As well, STin2 VNTR polymorphism acts as a transcriptional regulator, and the transcriptional regulatory activity is determined by the number of the repeat, with the STin2.12 allele having a higher expression than the STin2.10 allele.8 In this study, we found all the three cases carried lower expressing alleles, such as S and STin2.10 alleles, which could have less functioning 5-HTT and therefore lead to a higher 5-HT availability. As a result, at least in some degree, they experienced the trouble of anejaculation.
According to the theory of De Jong et al.9 it has been hypothesized that the ejaculatory threshold for men with low 5-HT levels and/or 5-HT2C receptor hyposensitivity may be genetically set at a lower point, resulting in a more rapid ejaculation. In contrast, men with a very high set point may experience delayed or even absence of ejaculation despite prolonged sexual stimulation and despite achieving a full erection. However, nocturnal emission is a type of spontaneous orgasm, involving ejaculation during sleep. This is an autonomous reflex mediated by the sympathetic nervous system, so it may also occur in patients with anejaculation.
The etiologies of the anejaculation can be categorized into three major groups: organic, idiopathic and drug related.10 The word “idiopathic” is used frequently when we cannot determine the accurate physiologic cause of a problem, but it is also used frequently when describing conditions that are functional. With the etiology of idiopathic anejaculation remaining largely unknown, the former use of the word seems appropriate. Furthermore, the current study showed evidence that there might be some genetic component of idiopathic anejaculation. Although anejaculation is uncommon, further research in this interesting field is needed to replicate our results in the adolescent population for a better understanding of male ejaculatory disorders.
AUTHOR CONTRIBUTIONS
YYH carried out the molecular genetic studies, participated in the sequence alignment and drafted the manuscript. XSZ and CZL participated in the design of the study and performed the statistical analysis. JJG and PG conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We thank all patients who provided the plasma and information necessary for this study. We also thank Shanghai Genesky Bio-Tech Co., Ltd., Shanghai, China (www.geneskybiotech.com) for help with the genotype testing. This study was funded by the National Natural Science Foundation of China (No. 81571429).
Supplementary information is linked to the online version of the study on the Asian Journal of Andrology website.
REFERENCES
- 1.Jannini EA, Simonelli C, Lenzi A. Sexological approach to ejaculatory dysfunction. Int J Androl. 2002;25:317–23. doi: 10.1046/j.1365-2605.2002.00371.x. [DOI] [PubMed] [Google Scholar]
- 2.Jern P, Santtila P, Johansson A, Varjonen M, Witting K, et al. Evidence for a genetic etiology to ejaculatory dysfunction. Int J Impot Res. 2009;21:62–7. doi: 10.1038/ijir.2008.61. [DOI] [PubMed] [Google Scholar]
- 3.Waldinger MD. The neurobiological approach to premature ejaculation. J Urol. 2002;168:2359–67. doi: 10.1016/S0022-5347(05)64146-8. [DOI] [PubMed] [Google Scholar]
- 4.Corona G, Ricca V, Bandini E, Mannucci E, Lotti F, et al. Selective serotonin reuptake inhibitor-induced sexual dysfunction. J Sex Med. 2009;6:1259–69. doi: 10.1111/j.1743-6109.2009.01248.x. [DOI] [PubMed] [Google Scholar]
- 5.Lesch KP, Mossner R. Genetically driven variation in serotonin uptake: is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biol Psychiatry. 1998;44:179–92. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 6.Smith GS, Lotrich FE, Malhotra AK, Lee AT, Ma Y, et al. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology. 2004;29:2226–34. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- 7.Heils A, Teufel A, Petri S, Stober G, Riederer P, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 8.MacKenzie A, Quinn J. A serotonin transporter gene intron 2 polymorphic region, correlated with affective disorders, has allele-dependent differential enhancer-like properties in the mouse embryo. Proc Natl Acad Sci USA. 1999;96:15251–5. doi: 10.1073/pnas.96.26.15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jong TR, Veening JG, Waldinger MD, Cools AR, Olivier B. Serotonin and the neurobiology of the ejaculatory threshold. Neurosci Biobehav Rev. 2006;30:893–907. doi: 10.1016/j.neubiorev.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.McMahon CG, Abdo C, Incrocci L, Perelman M, Rowland D, et al. Disorders of orgasm and ejaculation in men. J Sex Med. 2004;1:58–65. doi: 10.1111/j.1743-6109.2004.10109.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


