Abstract
Previous research has shown that rats reared in simple/impoverished environments demonstrate greater repetitive responding for sensory reinforcers (e.g., light onset). Moreover, the brains of these rats are abnormally developed, compared to brains of rats reared in more complex/enriched environments. Repetitive behaviors are commonly observed in individuals with developmental disorders. Some of these repetitive behaviors could be maintained by the reinforcing effects of the sensory stimulation that they produce. Therefore, rearing rats in impoverished conditions may provide an animal model for certain repetitive behaviors associated with developmental disorders. We hypothesize that in rats reared in simple/impoverished environments, the normal habituation process to sensory reinforcers is impaired, resulting in high levels of repetitive behaviors. We tested the hypothesis using an operant sensory reinforcement paradigm in rats reared in simple/impoverished (IC), standard laboratory (SC), and complex/enrichened conditions (EC, treatments including postnatal handling and environmental enrichment). Results show that the within-session habituation of the reinforcer effectiveness of light onset was slower in the IC and SC rats than in the EC rats. A dishabituation challenge indicated that within-session decline of responses was due to habituation and not motor fatigue or sensory adaptation. In conclusion, rearing rats in simple/impoverished environments, and comparing them to rats reared in more complex/enriched environments, may constitute a useful approach for studying certain repetitive behaviors associated with developmental disorders.
Keywords: stereotypic behavior, reinforcer effectiveness, sensory reinforcement, autism, environmental enrichment, operant
1. Introduction
Restricted, repetitive, and stereotyped patterns of behaviors are commonly observed in developmental disorders, such as autism spectrum disorder (ASD) and stereotypic movement disorder (SMD) [1, 2]. These behaviors are disruptive to learning and coping. It has been proposed that some of these behaviors could be maintained by sensory reinforcement – the sensory consequences produced by the behaviors [3]. Therefore, they are also called self-stimulatory behaviors in some literature [3–5], and the mechanism has been variously labeled as perceptual reinforcement [3], automatic reinforcement [6–8], or sensory reinforcement [9, 10]. Altering sensory consequences or providing alternative reinforcers has been shown to reduce repetitive behaviors in children with developmental disorders [8, 11, 12].
Environmental factors are known to play an important role in modulating repetitive behaviors. Animals and humans raised in simple/impoverished environments display more repetitive, stereotyped behaviors than those raised in more complex/enriched environments. For example, the repetitive pacing in zoo animals in their cages is attributed to the lack of normal environmental complexity [13]. Likewise, children raised in intuitional settings without environmental complexity show greater frequencies of repetitive behaviors [14]. In contrast, exposure to complex environments, or environmental enrichment, can effectively ameliorate or prevent repetitive behaviors in zoo and lab animals [15–21]. Similarly, relatively complex foster-care environments can significantly reduce stereotypies in children with a history of institutional care [22].
We have suggested that repetitive behaviors maintained by sensory reinforcement are due to impaired habituation of reinforcer effectiveness of the sensory stimuli [23]. Habituation is the simplest form of learning, which allows organisms to cease to respond to irrelevant stimuli [24]. For example, in normal individuals, the reinforcing effects of irrelevant sensory stimuli generated by body rocking rapidly habituate. For individuals with developmental disorders or delays, habituation of the reinforcing effects of sensory stimuli generated by rocking may occur more slowly or perhaps not at all. Slow/impaired habituation to sensory stimuli may underlie the enhanced repetitive behaviors, which are reinforced and maintained by the sensory consequences they produce.
The goal of this present study was to demonstrate that rearing rats in simple/impoverished environments increased repetitive behaviors maintained by sensory reinforcers (i.e., response-contingent light onset) in an operant paradigm. Furthermore, we hypothesize that the increase in repetitive behaviors is caused by impaired habituation of the reinforcer effectiveness of the sensory stimuli. Using an operant paradigm to investigate repetitive behaviors is different from other animal models of repetitive behaviors that assess general activity or utilize observational measures [21]. This approach allows us to specifically investigate how repetitive behaviors are maintained by sensory reinforcers. The reason why we used light onset as the sensory stimulus was that, unlike food or water, it is biologically unimportant. Repeated responding to such stimuli represents typical repetitive behaviors. The validity of this model is supported by the observation of an association between increases in sensitivity to sensory stimuli and repetitive behaviors in children with ASD and developmental delays [25–28].
Three different animal-rearing environments were used: impoverished (IC), standard laboratory (SC), and enriched conditions (EC). The EC condition was composed of two consecutive interventions, postnatal handling (pre-weaning) and environmental enrichment (post-weaning). They are widely used animal models to study prevention or intervention of various psychological disorders [21, 29–41], and have been shown to exert additive beneficial effects when applied together [42–44]. Moreover, a test of dishabituation was used to provide evidence that the within-session decline in responding in rats was due to impaired habituation, not sensory adaptation or motor fatigue.
2. Methods
2.1 Animals and rearing conditions
Male Sprague-Dawley rats were bred in house. Briefly, male and virgin female breeders (Envigo, Indianapolis, IN, USA) were housed in pairs in breeding cages until mating plugs were found. Then females were singly housed in standard plastic cages until giving birth. The colony rooms were on a 12h/12h reverse light/dark cycle with light on during 7:00 p.m. – 7:00 a.m. All procedures were approved by the Institutional Animal Care and Use Committee of University at Buffalo, The State University of New York.
After birth, pups were culled to 10 (with ≤ 8 males) per litter. Litters were randomly assigned to the IC, SC, and EC groups and used in different projects. On average, 3.6 rats per litter were used in this study. Littermates, however, were not allocated to different groups due to application of the pre-weaning treatment (i.e., postnatal handling).
Before weaning, each litter (pups and dam) in the IC and SC groups was housed in standard plastic cages (25×48×20 cm) and left undisturbed except for weekly cage changes. Pups in the EC group underwent a brief maternal separation (15 min) and handling procedure once daily during postnatal days (PDs) 2–20. The purpose of postnatal handling was to enhance maternal behavior, as a complemental enrichment procedure in the early developmental period [42, 45].
Pups were weaned on PD 21, and only male rats were kept for the experiments. After weaning, rats in the IC group were singly housed in small metal hanging cages (17×24×20 cm), which were facing a wall without disturbance (no cage change). Rats in the SC group were housed in pairs in standard plastic cages and not disturbed except for weekly cage changes. Rats in the EC group were group housed (10 per cage) in a large 4-level pet cage (64×92×160cm, Model: CG-71111, Drs. Foster & Smith, Rhinelander, WI, USA) with 30 small pet toys, including pods, hideouts, ropes, and wheels (Drs. Forrest and Smith). The toys were relocated or changed daily to create novelty. The EC rats were transferred to temporary cages during toy reconfiguration, which typically took 15 min per day. All of the housing conditions were maintained until the completion of the experiments.
2.2. Apparatus
Twenty-four locally built experimental chambers, previously described in detail [46], were used for the operant procedure. Briefly, the left and right side walls each had one snout poke aperture. The test chamber was located inside of a sound-and-light attenuating box, with a wall-mounted fan that provided ventilation and masking noise. The reinforcer light used in the experiments was located on the ceiling, midway between the two snout poke apertures. Snout poke could cause onset of the light, which produced an illuminance of 68 lx, as measured from the center of the test chamber. Snout pokes were monitored with infrared photo sensors located in the snout poke apertures. The chambers were connected to a computer using the MED Associates (Fairfax, VT, USA) interface. The MED PC® programming language was used for programming of the experimental contingencies.
2.3. Procedure
Eight-week-old rats (IC: n=16; SC: n=10; EC: n=10) underwent light-onset reinforcement training in the operant chambers. One poke aperture was randomly assigned as the “active” hole and the other the “inactive” hole. During the pre-exposure phase, rats underwent 10 continual daily 60-min test sessions in the unlit chambers. Snout pokes were recorded but had no programmed consequences. In the following light-onset phase, rats underwent sessions 11–20, in which snout poking into the active hole turned on the light for 5s under a variable-interval 1-min schedule (Fleshler and Hoffman, 1962), while snout poking into the inactive hole had no programmed consequences. Because the response rate was very low in the last 42 min of the 60-min sessions, only the first 18 min of each session were analyzed. The 18 min were divided into six 3-min epochs for data analysis. All of the testing procedures were conducted during the dark phase of the light-dark cycle.
2.4. Dishabituation challenge
After the light-onset phase, the rats underwent a dishabituation challenge, during which a continuous loud (~90 dB) warbling sound (noise) produced by a Sonalert (Model: SC110, Mallory, Indianapolis, IN, USA) was presented from 31st to 36th min of the test session. The 60-min dishabituation test session was divided into ten 6-min epochs, and responses in each epoch were expressed as percentage of the responses in the first 6 min of the baseline sessions. Averages from two regular 60-min light-onset sessions just before the dishabituation challenge were used as baseline.
2.5. Data Analysis
Responses were analyzed by averaging numbers of nose pokes to the active or inactive holes in the first 18 min of 2 consecutive sessions during both pre-exposure and light-onset reinforcement phases. The reinforcer effectiveness of light onset was measured by the proportional response to the active hole/total responses.
The within-session decline in responding (habituation) was quantified by two methods. The first method was area under the curve (AUC) measure. The procedure for computing AUC has been described in detail previously [47]. Briefly, nose pokes from each 3-min epoch were normalized to the maximal number of responses in an epoch (typically the first of the 6 epochs). Using the normalized values as y-coordinates, the six 3-min epochs that made up the 18-min observation period were partitioned into five trapezoid areas for computing the AUC measure. Smaller AUC values could indicate faster within-session decline in responding and more rapid habituation.
The second method was to fit an exponential decay curve to the within-session decline in responding, which was only applied in the light-onset phase, and averages from sessions 13–20 were used for curve fitting. Sessions 11–12 were excluded from this analysis, because a stable habituation pattern (within-session decline in responding) had not emerged during this initial behavioral acquisition period. The equation for curve fitting was
where A was the amplitude, and τ the decay time constant, with x0 = 0, y0 = 1. The parameter τ characterized the rate of response decline (habituation). The smaller this parameter, the faster the decline and the greater habituation. Larger values of A indicate higher rates of initial responding. Averaging across sessions, instead of using responses from individual sessions, helps smooth out the data and make curve fitting feasible.
One-way or 3-way mixed analysis of variance (ANOVA) was used to make group comparisons, with litter as a nested variable, so that potential litter effects could be controlled for. The post hoc Tukey test was used to detect specific pairwise differences following significant main or interaction effects. The Pearson product-moment correlation coefficient test was used to compute correlation coefficients. Before performing curve fitting, 19 outliers (including 7 from EC, 6 from SC, and 6 from IC groups) out of 432 datum points (responses within 3-min epochs) were identified, using boxplots [48]. They were all greater than the maximums set by boxplots, and thus the outliers were brought down to the next highest values within the same group in a given epoch. Statistical analyses were conducted via SAS 9.4 (SAS Institute Inc., Cary, NC, USA), and the alpha criterion was set at 0.05. Curve fitting was performed using Origin 2016 (OriginLab Co., Northampton, MA, USA).
3. Results
3.1. Rearing in impoverished and standard laboratory environments leads to high levels of repetitive responding and reinforcer effectiveness of light onset
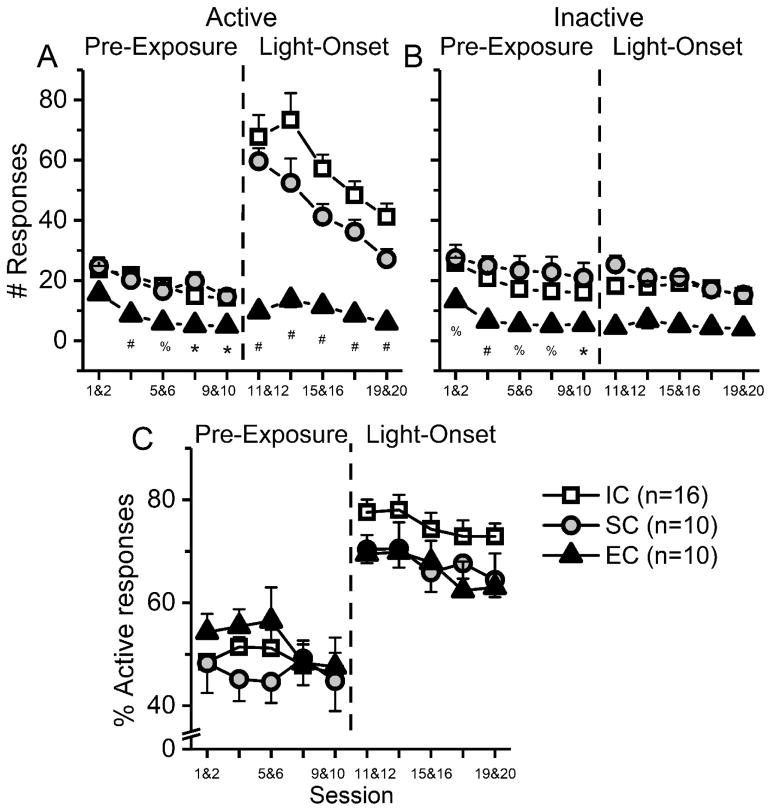
During the pre-exposure phase, there were a session main effect, F4,140=17.29, p<0.001, and a group X hole interaction effect, F2,33=4.09, p< 0.05 (Fig. 1A & 1B, left panel), based on a 3-way (group, active/inactive hole, and session) mixed ANOVA with litter as a nested variable (litter effect: F13,20=9.82, p<0.001). The post hoc Tukey test revealed differences between the IC and EC groups and between the SC and EC groups, for responses to the active (IC vs. EC: p< 0.001, SC vs. EC: p< 0.001) or inactive hole (IC vs. EC: p< 0.001, SC vs. EC: p< 0.001). The numbers of responses were the lowest in the EC group, and there were no differences between the IC and SC groups.
Fig. 1.
Rats reared in the impoverished (IC) and standard (SC) conditions responded more to the novel environment and contingent light-onset than rats reared in the enriched condition (EC). In each panel, the dashed line separates the pre-exposure (left, sessions 1–10) and light-onset (right, sessions 11–20) phases. (A) & (B) Rats in the IC and SC groups made more responses to the active & inactive holes respectively than rats in the EC group. (C) The proportion of responses to the active hole/total responses to both holes increased after introduction of the contingent light-onset in all 3 groups. Plotting is based on two-session blocks, and each symbol represents the average from two consecutive sessions. Data are expressed as Mean±SEM. *: p<0.05; %: p<0.01; #: p<0.001, post hoc Tukey test: IC vs. EC groups.
During the light-onset phase, there was a 3-way interaction effect for group, active/inactive hole, and session, F20,124=2.71, p<0.001 (Fig. 1A & 1B, right panel), revealed by a 3-way mixed ANOVA with litter as a nested variable (litter effect: F13,20=6.75, p<0.001). Rats in the IC group responded the most to the active hole, followed by rats in the SC group, while rats in the EC group responded the least (IC vs. SC, p<0.01, post hoc Tukey test; SC vs. EC, p<0.001). There were no differences in responding to the inactive hole between the IC and SC rats, while both the IC and SC rats responded more to the inactive hole than the EC rats (IC vs EC, p<0.01; SC vs EC, p<0.001).
The proportional response to the active hole/total responses to both holes reflects relative reinforcer effectiveness of light onset. There was a significant group X light-onset interaction effect, F2,33=6.45, p<0.01 (Fig. 1C; 3-way mixed ANOVA of group, pre-exposure/light-onset, and session, with litter as a nested variable, litter effect: F13,20=3.30, p<0.01). Rats in all 3 groups increased the proportion of responses to the active hole after contingent light-onset was introduced (averages across sessions 1–10 and sessions 11–20, IC: 49±2.3% to 75±2.6%, p<0.001, post hoc Tukey test; SC: 46±3.6% to 68±2.9%, p<0.001; EC: 52±3.1% to 67±3.2%, p<0.001). These results indicate that contingent light-onset was reinforcing to all animals.
3.2. Rearing in impoverished and standard laboratory environments leads to slow habituation of reinforcer effectiveness
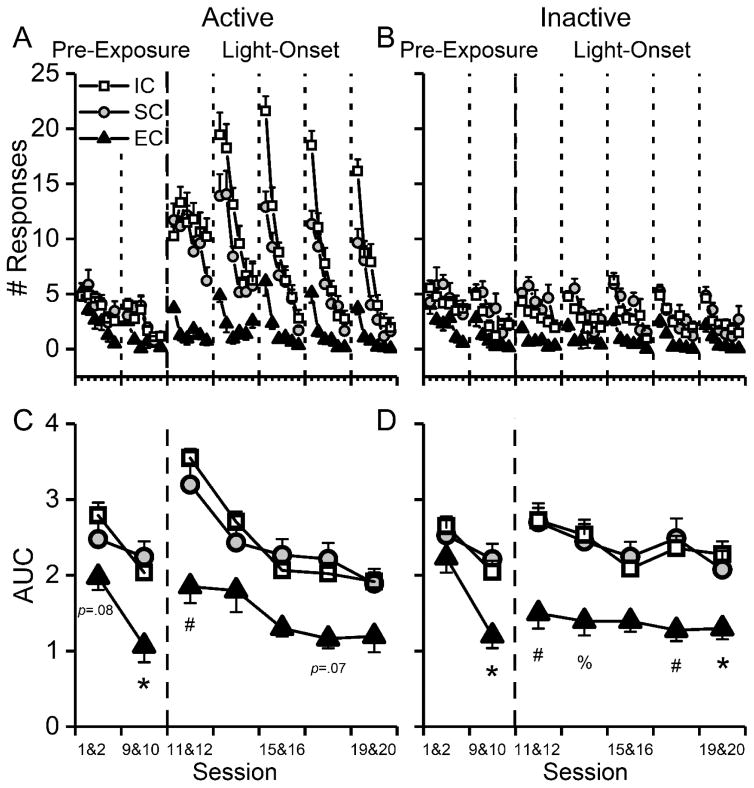
The general responding for both active (Fig 2A) and inactive (Fig 2B) holes clearly declined during the initial 18-min period of each session, suggesting within-session habituation. The decline was quantitatively characterized by the AUC measure (Fig 2C & 2D) and exponential decay curve fitting (Fig. 3).
Fig. 2.
Rats reared in the impoverished (IC) and standard (SC) conditions habituated slower than rats reared in the enriched condition (EC), as suggested by the measure of area under the curve (AUC). In each panel, the dashed line separates the pre-exposure (left, sessions 1–2, and 9–10) and light-onset (right, sessions 11–20) phases. In the pre-exposure phase, only the first two (1–2) and last two sessions (9–10) are displayed. (A) & (B) Rats’ responses to the active & inactive holes declined respectively, suggesting within-session habituation. Each data point depicts responses in a 3-min epoch during the first 18 min of the session. (C) & (D) Rats in the IC and SC groups habituated at a slower rate, indicated by larger AUCs, than rats in the EC group, for the active & inactive holes respectively. Plotting is based on two-session blocks, and each measure represents the average from two consecutive sessions. Data are expressed as Mean±SEM. *: p<0.05; %: p<0.01; #: p<0.001, post hoc Tukey test: IC vs. EC groups.
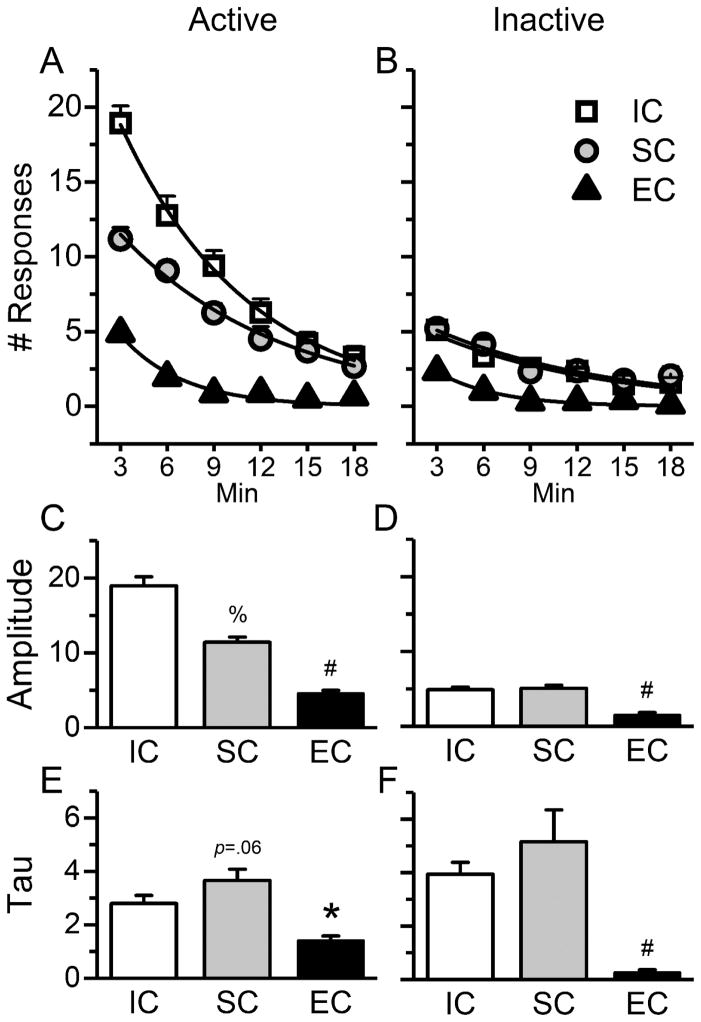
Fig. 3.
Rats reared in the impoverished (IC) and standard (SC) conditions habituated slower than rats reared in the enriched condition (EC), as suggested by exponential decay curve fitting. The fitting curves ( ,x0 = 0, y0 = 1) were constructed based on average responses in sessions 13–20 of the light-onset phase. (A) & (B) Exponential fitting curves of responses to the active & inactive holes respectively, in the IC, SC, and EC rats. (C) Rats in the IC group had the highest responses to the active hole at the beginning of each session (baseline), followed by rats in the SC and EC groups, as indicated by the magnitude of parameter A (amplitude) from curve fitting. (D) Rats in the IC and SC groups had higher responses to the inactive hole at the beginning of each session (baseline) than rats in the EC group, as indicated by the magnitude of parameter A from curve fitting. (E) & (F) Rats in the IC and SC groups habituated at a slower rate for the active & inactive holes respectively than rats in the EC group, indicated by parameter τ (decay time constant) from curve fitting. Data are expressed as Mean±SEM. *: p<0.05; %: p<0.01; #: p<0.001, post hoc Tukey test. % and the p-value above the SC bars in (C) & (E): IC vs. SC groups, and all the other symbols: IC vs. EC groups.
The results from the pre-exposure phase (Fig 2C & 2D, left panel) showed a main effect of group, F2,20=88.94, p<0.001, and a main effect of session, F4,140=11.60, p<0.001 (3-way mixed ANOVA of group, session, active/inactive holes, with litter as a nested variable, litter effect: F13,20=2.40, p<0.05). There was no difference between the active and inactive holes. The AUC measure was the lowest in the EC group (IC vs. EC: p<0.001, post hoc Tukey test; SC vs. EC, p<0.001), and there was no group difference in AUC between the IC and SC rats.
During the light-onset phase (Fig 2C & 2D, right panel), a 3-way mixed ANOVA revealed a group X hole X session interaction effect, F20,124=2.49, p<0.01 (litter was included as a nested variable, liter effect: F13,20=4.63, p<0.01). In addition, the AUC measure was the lowest in the EC group for both active (IC vs. EC: p<0.001, post hoc Tukey test; SC vs. EC, p<0.001) and inactive holes (IC vs. EC: p<0.001; SC vs. EC, p<0.001). There was no difference in AUC between the IC and SC groups.
Exponential curve fitting (Fig. 3A & 3B) was also used to quantify habituation (illustrated in Fig 2A & 2B), and parameters A (amplitude) and τ (time constant) were compared between groups. Significant group differences in A were detected for both the active, F2,20=35.57, p<0.001 (Fig. 3C; one-way ANOVA with litter as a nested variable, litter effect: non-significant) and inactive holes, F2,20=37.71, p<0.001 (Fig. 3D; one-way ANOVA with litter as a nested variable, litter effect: non-significant). The EC group had the lowest A, compared with the IC and SC groups, for either the active hole (IC vs. EC: p<0.001, post hoc Tukey test; SC vs. EC: p<0.001) or the inactive hole (IC vs. EC: p<0.001; SC vs. EC: p<0.001). There was also a difference between the IC and SC groups for the active hole (p<0.01).
Group differences were also revealed in the time constant τ for both the active, F2,20=12.15, p<0.001 (Fig. 3E; one-way ANOVA with litter as a nested variable, litter effect: non-significant) and inactive holes, F2,20=20.44, p<0.001 (Fig. 3F; one-way ANOVA with litter as a nested variable, litter effect: F13,20=3.36, p<0.01). The EC group had the lowest τ for both holes (for the active hole, IC vs. EC: p<0.05, post hoc Tukey test, SC vs. EC: p<0.001; for the inactive hole, IC vs. EC: p<0.001, SC vs. EC: p<0.001). There was a trend of difference in τ between the IC and SC groups for the active hole (p=0.06). Taken together, these results indicated that IC and SC rats had slower habituation of reinforcer effectiveness of the sensory stimuli, which is consistent with the AUC results.
We also observed significant correlation between time constant τ and AUC (averages across sessions 13–20) for either the active (r=0.88, p<0.001) or the inactive hole (r=0.83, p<0.001), indicative of satisfactory reliability of these two different measures.
3.3. The within-session decline in response to light-onset was due to habituation of reinforcer effectiveness
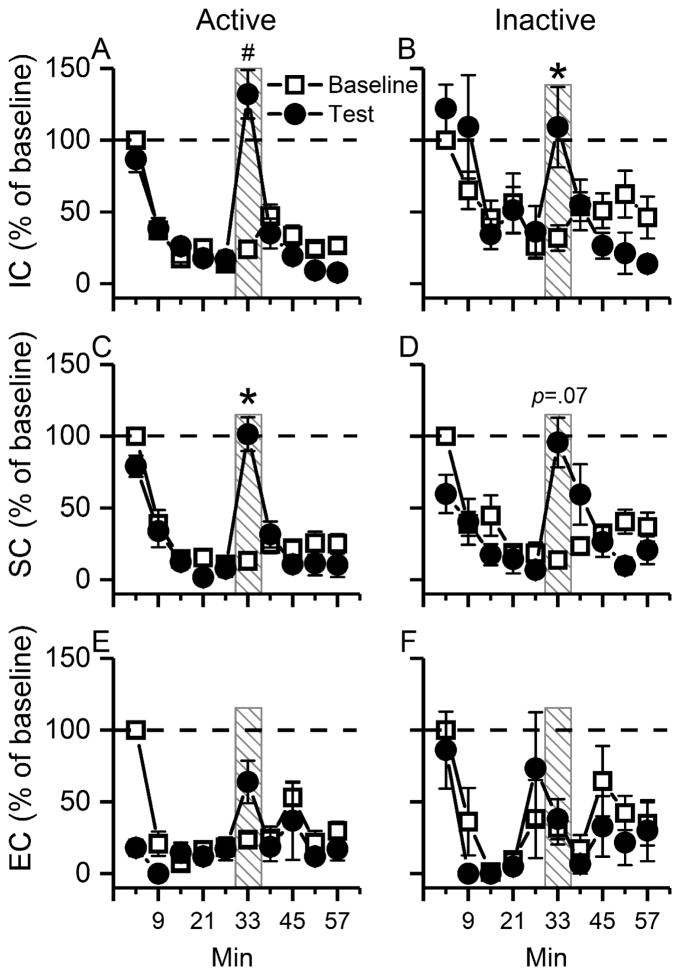
Dishabituation was tested by presenting an external noise. The total responses during sound (noise) presentation (6 min), normalized to the baseline, were compared with the same period during the previous two sessions without the noise. The results showed a significant group X hole X noise interaction, F5,31=2.70, p<0.05 (Fig. 4; 3-way mixed ANOVA of group, active/inactive hole, without noise/noise, with litter as a nested variable, litter effect: non-significant). Rats in the IC and SC groups increased responding to the active hole during noise presentation (IC: p<0.001, post hoc Tukey test; SC: p<0.05). The IC rats also increased responding to the inactive hole (p<0.05), and there was a trend of enhanced responses to the inactive hole in the SC rats (p=0.07) during noise presentation. In contrast, the noise presentation did not lead to a significant surge in responding to either active or inactive hole in the EC rats, although visual inspection of Fig. 4E reveals an increase in response to the active hole in the presence of the noise. The results showed a typical dishabituation pattern of responses, and supported that within-session decline in responses was due to habituation of reinforcer effectiveness, not sensory adaptation/fatigue or motor fatigue.
Fig 4.
The dishabituation test provides evidence that within-session decreases in responding were due to habituation, not sensory adaptation or motor fatigue. Data are expressed as a % of the first epoch of the baseline session. (A) & (C) A loud warbling sound (noise) presentation during 31st-36th min induced dishabituation, indicated by increased responses to the active hole, in rats reared in the impoverished (IC) and standard (SC) conditions respectively. (B) The noise presentation induced dishabituation, indicated by increased responses to the inactive hole in the IC rats. (D) There was a trend of increase in response to the inactive hole in the SC rats, in the presence of the noise. (E) & (F) The noise presentation induced no significant increase in response to the active (E) or the inactive hole (F) in the EC rats. Data are expressed as Mean±SEM. *: p<0.05; #: p<0.001, post hoc Tukey test: baseline vs. noise presentation.
4. Discussion
4.1. Summary
The results from the present study clearly demonstrate that light onset is a sensory reinforcer, which is consistent with previous studies [49, 50], including those from our own laboratories [46, 51]. The reinforcer effectiveness of light onset is indicated by the increase in the proportion of active responding during the light-onset phase. This index clearly shows that light onset is a reinforcer to all groups of animals and there are no group differences. We have also found that the overall responding to light onset in a given session is greater in IC and SC rats, compared to that in EC rats. Furthermore, the IC and SC rats also respond more during the pre-exposure phase. This is consistent with the hypothesis that IC and SC rats habituate more slowly to the unprogrammed consequences of snout poking as well as to the contingent light onset, and suggests that the rearing condition influences habituation to all sensory reinforcers (not just light onset) in a general way.
We have observed that IC and SC rats habituate more slowly than EC rats. This conclusion is drawn from both habituation measures: the AUC and time constant from curve fitting. Because of the fluctuation in the data of an animal from one single session, it is difficult to quantify habituation by using the slope of the response curve. Therefore, we applied the AUC approach to characterize the rapidity of response decline in each rat. Area under the curve is actually a more robust method than slope to measure the decline in responses/habituation, because AUC is computed by using percentage scores. The use of ratio/percentage scores eliminates the effects of differences in the rate of responding [52]. We also used time constant from curve fitting to measure habituation. Similar to the results obtained from the AUC measurement, the time constant is greater in the IC and SC rats than in the EC rats, indicating slower decline of responding. These results consistently support our hypothesis that less complex rearing environments lead to slower habituation. We also found that AUC and time constant were highly correlated. Therefore, we feel that either method is sufficient to measure within-session habituation. An additional advantage of AUC is that it can be calculated for individual test sessions, while the time constant analysis requires averaging data from a few sessions for better curve fitting.
An interesting finding is that the between-group differences of habituation is consistent for both the pre-exposure and the light-onset phases. Also, rats within a specific group show similar within-session habituation patterns, measured by AUC, in responding to the active and inactive holes. This consistency is not influenced by significantly increased responding in the light-onset phase, compared to the pre-exposure phase. It appears that nose-poking itself generates unprogrammed sensory reinforcers. This interpretation is consistent with our hypothesis that repetitive behavior is maintained by the sensory reinforcers it produces (e.g., rocking).
We perform a dishabituation challenge to determine if the within-session decrease in responding is due to the habituation process. We present a loud/novel tone in the middle of the test session. This causes an increase in active and inactive responding in both IC and SC rats, but not in EC rats. This observation along with rather stable between-session recovery in responding depicted in Fig. 2A & 2B strongly support that the within-session decline in responses is indeed caused by the habituation process, instead of sensory adaptation or motor fatigue [24]. The reason for a lack of increased responding to the loud noise in EC rats is not clear. We speculate that the presentation of a loud sound might have constituted a stressor. It is well documented that environmental enrichment facilitates stress coping [37, 53–55]. As such, EC rats are possibly not as stressed out as IC and SC rats by the loud noise in the dishabituation test, and therefore do not show obvious dishabituation.
In previous studies, we have described the importance of the pre-exposure phase in conducting light reinforcement experiments. When animals are allowed to habituate to the novel testing apparatus during the pre-exposure phase, they respond more to the light onset [46]. We suggest that pre-exposure phase allows the rats to habituate to the environmental stimuli in the testing apparatus before the response-contingent light onset is introduced. This process ensures that the light onset is a novel and effective sensory reinforcer, the effect of which can be measured without the interference of other novel sensory reinforcers during testing. The addition of the pre-exposure phase also reveals that within-session habituation pattern is consistent across the pre-exposure and light-onset phases. One possible confound of using the pre-exposure phase is that handling and the operant procedure during the 10-day pre-exposure phase could serve as “enrichment” on IC rats, countering the impoverishment effect. However, we do believe that this effect is limited, because the session time and the duration of the pre-exposure phase are short, in comparison to weeks of impoverished rearing. Future studies adding an extra group of animals without the pre-exposure phase will help understand the possible “enrichment” effect on IC rats, contributed by the pre-exposure phase.
It is worth noting that IC and SC rats have similar low habituation rates compared to the EC rats. This observation suggests that the environment of SC rats is so simple, barren, and restrictive that it impairs brain development. In support of this observation, others have reported that the behavioral phenotypes such as locomotor level, anxiety, and addictive behaviors are similar in IC and SC rats [56–58]. These results indicate that the standard housing condition with 2–3 rats in a cage with bedding, food and water ad lib – so-called “social environment,” should be reconsidered as a proper control rearing condition for behavioral experiments. Similar concerns have been expressed by other investigators [59–61].
4.2. Repetitive behaviors and impaired habituation
The findings of this study support the hypothesis that rearing animals in simple/impoverished environments produces increased repetitive behavior. Similar observations have been reported in animal studies by others, utilizing non-operant procedures [21, 62]. The operant light reinforcement paradigm described in this paper has several advantages over animal models of other repetitive behaviors that use general activity or observational measures. Operant responding for a light reinforcer measures the frequency of a topographically well-defined behavior that produces a precisely defined stimulus. In addition, the presentation of the response-contingent sensory stimulus is controlled by the experimenter. In contrast, it is difficult to precisely identify stimuli that proceed, accompany, or follow the occurrence of repetitive behaviors when observational and/or general activity procedures are used. Furthermore, these procedures do not allow for control of the frequency of response-contingent stimuli. On the contrary, experimental control provided by the light reinforcement procedure offers a way to more directly test the perceptual reinforcement hypothesis of repetitive behaviors in animals [3, 63].
The hypothesis that repetitive behaviors associated with certain developmental disorders may be due to impaired habituation of the reinforcer effectiveness of sensory stimuli is consistent with previous studies showing that rearing in a simple/impoverished environment impairs habituation of locomotor activity in a novel environment, which is maintained by sensory reinforcers in the environment [57, 64, 65], and habituation of operant responding using light onset as the reinforcing stimulus [66–68].
It is worth noting that there are different types of repetitive behaviors. The results generated from the present study should help better understand repetitive behaviors maintained by sensory reinforcement, such as repetitive behaviors reported in individuals with ASD and SMD. These repetitive behaviors are often associated with increased sensitivity to sensory stimuli [25–28]. Other types of repetitive behaviors are commonly observed in patients with obsessive-compulsive disorder and tic disorders [2], which are topographically different from the simpler forms of repetitive behaviors observed in ASD and SMD [69, 70].
4.3. Possible neural mechanism
At the present time, the neural mechanisms underlying habituation of the reinforcer effectiveness of sensory stimuli are not well understood. Existing evidence indicates that activity of midbrain dopamine (DA) system is likely to play a role in regulating repetitive, stereotypic behaviors. Enhancing DA release by psychostimulants increases repetitive behavior [1, 71]. We have shown that psychostimulants that augment DA transmission reduces habituation to sensory reinforcers [23, 46, 51, 72]. It has also been shown that in monkeys, the reinforcing properties of novel light stimuli are mediated by the phasic activity of DA neurons [73]. The phasic firing decreases as the animal habituates to the stimuli or novel environment [74, 75]. These lines of evidence suggest that midbrain DA neurons could play a crucial role in the habituation process.
A recent study shows that the medial prefrontal cortex (mPFC) to midbrain DA circuitry is important in controlling habituation to sensory stimuli [76, 77]. Specifically, Lesions of the mPFC DA terminals disrupt habituation on the cellular level, exhibited by prolonged DA release in the nucleus accumbens shell, in response to taste stimuli. An earlier study shows that lesions to the mPFC inhibit normal habituation of locomotor activity [78], which is thought to be mediated by DA tone [79]. It is also observed that young autistic male subjects have increased number of neurons in the prefrontal cortex [80]. Therefore, we can speculate that the abnormal mPFC to midbrain DA circuitry could contribute to impaired habituation to sensory stimuli, when rats are reared in impoverished environments. Future study focusing on characterizing this circuitry is necessary.
4.4. Environmental complexity
There is a large literature showing that the brains of rats reared in simple/impoverished environments are underdeveloped [81], while early intervention, such as postnatal handling and environmental enrichment, can ameliorate the impairment [42, 45, 82, 83]. Because the development of forebrain inputs to midbrain DA neurons is protracted, which can last the entire adolescence and early adulthood [84], these inputs could be readily shaped by environmental factors during postnatal development, considering the high plasticity of the prefrontal cortex [85, 86].
Postnatal handling enhances maternal behaviors [87–89], and consequently provides intensive tactile stimulation during an early sensitive developmental period. Additionally, enriched environment after weaning provides complex physical and social stimuli. Applying postnatal handling and environmental enrichment consecutively has been proven to produce additive beneficial effects [42–44]. Therefore, using the combined approach may facilitate the development of the mPFC to midbrain DA neuronal circuitry, leading to efficient habituation to sensory reinforcers. Indeed, it has been well documented that an increase in environmental complexity induces multiple anatomical (increased neuron size, dendritic branches, and dendritic spine density; enhanced gliogenesis and synaptogenesis; etc.) and neurochemical (e.g., increased expression of growth factors, such as nerve growth factor and brain-derived neurotrophic factor) alterations in cortical areas [90–98]. These events might work in concert to promote the maturation of the essential forebrain to midbrain DA innervation.
Repetitive behaviors caused by developmental disorders do not appear to be treatable pharmacologically. Observations from studies using behavioral therapy show that when one repetitive behavior is ameliorated by eliminating/substituting the corresponding sensory consequences, the results may be just temporary, because patients could start engaging in new repetitive behaviors [1]. Therefore, there is limited efficacy of applying these behavioral therapies. The data from the present study suggest that providing a more complex, enriched environment during early development might be an effective way to decrease reinforcer effectiveness of sensory stimuli, enhance habituation, and thus reduce repetitive behaviors.
Highlights.
Rats reared in less complex environments habituate slower to sensory reinforcers.
Impoverished rearing provides an animal model of repetitive behaviors.
Impaired habituation underlies repetitive behaviors in developmental disorders.
Area under the curve can be used to quantify habituation.
A dishabituation test rules out other explanations for the habituation process.
Acknowledgments
The work was supported by NIH grants AA12435 (RS), AA019482 (RS), DA10588 (JR), and DA026600 (JR). The authors thank Millicent Nwankwo, Marita Paredez, Ying-Ling Shen, and Philip Slepian for their aid in breeding animals, conducting experiments, and collecting data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rapp JT, Vollmer TR. Stereotypy I: A review of behavioral assessment and treatment. Research in Developmental Disabilities. 2005;26(6):527–547. doi: 10.1016/j.ridd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- 3.Lovaas I, Newsom C, Hickman C. Self-stimulatory behavior and perceptual reinforcement. Journal of Applied Behavior Analysis. 1987;20(1):45–68. doi: 10.1901/jaba.1987.20-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkson G, Davenport R. Stereotyped movements of mental defectives: I. Initial Survey. American Journal of Mental Deficiency. 1962 [PubMed] [Google Scholar]
- 5.Lovaas OI, Schaeffer B, Simmons JQ. Building social behavior in autistic children by use of electric shock. Journal of Experimental Research in Personality. 1965 [Google Scholar]
- 6.Patel MR, Carr JE, Kim C, Robles A, Eastridge D. Functional analysis of aberrant behavior maintained by automatic reinforcement: Assessments of specific sensory reinforcers. Research in Developmental Disabilities. 2000;21(5):393–407. doi: 10.1016/s0891-4222(00)00051-2. [DOI] [PubMed] [Google Scholar]
- 7.Saini V, Gregory MK, Uran KJ, Fantetti MA. Parametric analysis of response interruption and redirection as treatment for stereotypy. Journal of Applied Behavior Analysis. 2015;48(1):96–106. doi: 10.1002/jaba.186. [DOI] [PubMed] [Google Scholar]
- 8.Sharp RA, Phillips KJ, Mudford OC. Comparisons of interventions for rumination maintained by automatic reinforcement. Research in Autism Spectrum Disorders. 2012;6(3):1107–1112. [Google Scholar]
- 9.Baker LJ, Milner Y. Sensory reinforcement with autistic children. Behavioural Psychotherapy. 1985;13(4):328–341. [Google Scholar]
- 10.Murphy G. Sensory reinforcement in the mentally handicapped and autistic child: A review. Journal of Autism and Developmental Disorders. 1982;12(3):265–278. doi: 10.1007/BF01531372. [DOI] [PubMed] [Google Scholar]
- 11.Demanche J, Chok JT. The use of wrist weights and vibratory stimulation to treat self-injurious behavior. Journal of Developmental and Physical Disabilities. 2013;25(1):79–90. [Google Scholar]
- 12.Rincover A. Sensory extinction: A procedure for eliminating self-stimulatory behavior in developmentally disabled children. Journal of Abnormal Child Psychology. 1978;6(3):299–310. doi: 10.1007/BF00924733. [DOI] [PubMed] [Google Scholar]
- 13.Shyne A. Meta-Analytic Review of the Effects of Enrichment on Stereotypic Behavior in Zoo Mammals. Zoo Biology. 2006;25(4):317–337. [Google Scholar]
- 14.Beckett C, Bredenkamp D, Castle J, Groothues C, O’Connor TG, Rutter M. Behavior patterns associated with institutional deprivation: A study of children adopted from Romania. Journal of Developmental and Behavioral Pediatrics. 2002;23(5):297–303. doi: 10.1097/00004703-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Mason G, Clubb R, Latham N, Vickery S. Why and how should we use environmental enrichment to tackle stereotypic behaviour? Applied Animal Behaviour Science. 2007;102(3):163–188. [Google Scholar]
- 16.Powell SB, Newman HA, McDonald TA, Bugenhagen P, Lewis MH. Development of spontaneous stereotyped behavior in deer mice: effects of early and late exposure to a more complex environment. Developmental psychobiology. 2000;37(2):100–108. [PubMed] [Google Scholar]
- 17.Bolhuis JE, Schouten WG, de Leeuw JA, Schrama JW, Wiegant VM. Individual coping characteristics, rearing conditions and behavioural flexibility in pigs. Behavioural brain research. 2004;152(2):351–360. doi: 10.1016/j.bbr.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 18.Meehan C, Garner J, Mench J. Environmental enrichment and development of cage stereotypy in Orange-winged Amazon parrots (Amazona amazonica) Developmental Psychobiology. 2004;44(4):209–218. doi: 10.1002/dev.20007. [DOI] [PubMed] [Google Scholar]
- 19.Swaisgood RR, White AM, Zhou X, Zhang G, Lindburg DG. How do giant pandas (Ailuropoda melanoleuca) respond to varying properties of enrichments? A comparison of behavioral profiles among five enrichment items. Journal of Comparative Psychology. 2005;119(3):325. doi: 10.1037/0735-7036.119.3.325. [DOI] [PubMed] [Google Scholar]
- 20.Symons F, Sperry L, Dropik P, Bodfish J. The early development of stereotypy and self-injury: a review of research methods. Journal of Intellectual Disability Research. 2005;49(2):144–158. doi: 10.1111/j.1365-2788.2004.00632.x. [DOI] [PubMed] [Google Scholar]
- 21.Lewis MH, Tanimura Y, Lee LW, Bodfish JW. Animal models of restricted repetitive behavior in autism. Behavioural Brain Research. 2007;176(1):66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bos KJ, Zeanah CH, Smyke AT, Fox NA, Nelson CA. Stereotypies in children with a history of early institutional care. Archives of pediatrics & adolescent medicine. 2010;164(5):406–411. doi: 10.1001/archpediatrics.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd DR, Medina DJ, Hawk LW, Fosco WD, Richards JB. Habituation of reinforcer effectiveness. Frontiers in Integrative Neuroscience. 2014;7 doi: 10.3389/fnint.2013.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiology of learning and memory. 2009;92(2):135–138. doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyd BA, Baranek GT, Sideris J, Poe MD, Watson LR, Patten E, Miller H. Sensory features and repetitive behaviors in children with autism and developmental delays. Autism Research. 2010;3(2):78–87. doi: 10.1002/aur.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritchard WS, Raz N, August GJ. Visual augmenting/reducing and P300 in autistic children. Journal of Autism and Developmental Disorders. 1987;17(2):231–242. doi: 10.1007/BF01495058. [DOI] [PubMed] [Google Scholar]
- 27.Gomot M, Giard M-H, Adrien J-L, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002;39(5):577–584. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- 28.Wiggins LD, Robins DL, Bakeman R, Adamson LB. Breif report: sensory abnormalities as distinguishing symptoms of autism spectrum disorders in young children. Journal of autism and developmental disorders. 2009;39(7):1087–1091. doi: 10.1007/s10803-009-0711-x. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz JM, Hutchinson MR, Bilbo SD. Early-life experience decreases drug-induced reinstatement of morphine CPP in adulthood via microglial-specific epigenetic programming of anti-inflammatory IL-10 expression. The Journal of Neuroscience. 2011;31(49):17835–17847. doi: 10.1523/JNEUROSCI.3297-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self-administration of amphetamine in female and male rats. Psychopharmacology. 2001;155(3):278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- 31.Stairs DJ, Bardo MT. Neurobehavioral effects of environmental enrichment and drug abuse vulnerability. Pharmacology Biochemistry and Behavior. 2009;92(3):377–382. doi: 10.1016/j.pbb.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews K, Robbins TW, Everitt BJ, Caine SB. Repeated neonatal maternal separation alters intravenous cocaine self-administration in adult rats. Psychopharmacology. 1999;141(2):123–134. doi: 10.1007/s002130050816. [DOI] [PubMed] [Google Scholar]
- 33.Moffett M, Vicentic A, Kozel M, Plotsky P, Francis D, Kuhar M. Maternal separation alters drug intake patterns in adulthood in rats. Biochemical pharmacology. 2007;73(3):321–330. doi: 10.1016/j.bcp.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther. 2006;317(3):1210–8. doi: 10.1124/jpet.106.101139. [DOI] [PubMed] [Google Scholar]
- 35.Schneider T, Turczak J, Przewłocki R. Environmental enrichment reverses behavioral alterations in rats prenatally exposed to valproic acid: issues for a therapeutic approach in autism. Neuropsychopharmacology. 2006;31(1):36–46. doi: 10.1038/sj.npp.1300767. [DOI] [PubMed] [Google Scholar]
- 36.Woo CC, Leon M. Environmental enrichment as an effective treatment for autism: a randomized controlled trial. Behavioral neuroscience. 2013;127(4):487. doi: 10.1037/a0033010. [DOI] [PubMed] [Google Scholar]
- 37.Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick C. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. European Journal of Neuroscience. 2004;20(5):1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- 38.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long–Evans rats. Pharmacology Biochemistry and Behavior. 2002;73(1):131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 39.Pamplona FA, Pandolfo P, Savoldi R, Prediger RDS, Takahashi RN. Environmental enrichment improves cognitive deficits in spontaneously hypertensive rats (SHR): relevance for attention deficit/hyperactivity disorder (ADHD) Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(7):1153–1160. doi: 10.1016/j.pnpbp.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Halperin JM, Healey DM. The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: can we alter the developmental trajectory of ADHD? Neuroscience & Biobehavioral Reviews. 2011;35(3):621–634. doi: 10.1016/j.neubiorev.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bredy TW, Lee AW, Meaney MJ, Brown RE. Effect of neonatal handling and paternal care on offspring cognitive development in the monogamous California mouse (Peromyscus californicus) Hormones and Behavior. 2004;46(1):30–38. doi: 10.1016/j.yhbeh.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Fernández-Teruel A, Giménez-Llort L, Escorihuela RM, Gil L, Aguilar R, Steimer T, Tobeña A. Early-life handling stimulation and environmental enrichment: are some of their effects mediated by similar neural mechanisms? Pharmacology Biochemistry and Behavior. 2002;73(1):233–245. doi: 10.1016/s0091-3057(02)00787-6. [DOI] [PubMed] [Google Scholar]
- 43.Escorihuela RM, Tobeña A, Fernández-Teruel A. Environmental enrichment reverses the detrimental action of early inconsistent stimulation and increases the beneficial effects of postnatal handling on shuttlebox learning in adult rats. Behavioural brain research. 1994;61(2):169–173. doi: 10.1016/0166-4328(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 44.Pham TM, Söderström S, Winblad B, Mohammed AH. Effects of environmental enrichment on cognitive function and hippocampal NGF in the non-handled rats. Behavioural brain research. 1999;103(1):63–70. doi: 10.1016/s0166-4328(99)00019-4. [DOI] [PubMed] [Google Scholar]
- 45.Van Praag H, Kempermann G, Gage FH. Neural consequences of enviromental enrichment. Nature Reviews Neuroscience. 2000;1(3):191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 46.Lloyd DR, Kausch MA, Gancarz AM, Beyley LJ, Richards JB. Effects of novelty and methamphetamine on conditioned and sensory reinforcement. Behavioural Brain Research. 2012;234(2):312–322. doi: 10.1016/j.bbr.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myerson J, Green L, Warusawitharana M. Area under the curve as a measure of discounting. Journal of the experimental analysis of behavior. 2001;76(2):235–243. doi: 10.1901/jeab.2001.76-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandervieren E, Hubert M. An adjusted boxplot for skewed distributions, COMPSTAT 2004, proceedings in computational statistics. Springer; Heidelberg: 2004. pp. 1933–1940. [Google Scholar]
- 49.Kling J, Horowitz L, Delhagen J. Light as a positive reinforcer for rat responding. Psychological Reports. 1956;2(2):337–340. [Google Scholar]
- 50.Segal EF. Confirmation of a positive relation between deprivation and number of responses emitted for light reinforcement. Journal of the experimental analysis of behavior. 1959;2(2):165–169. doi: 10.1901/jeab.1959.2-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lloyd DR, Gancarz AM, Ashrafioun L, Kausch MA, Richards JB. Habituation and the reinforcing effectiveness of visual stimuli. Behavioural processes. 2012;91(2):184–191. doi: 10.1016/j.beproc.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neuroscience & Biobehavioral Reviews. 2006;30(7):1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 53.Segovia G, Del Arco A, Mora F. Environmental enrichment, prefrontal cortex, stress, and aging of the brain. Journal of neural transmission. 2009;116(8):1007–1016. doi: 10.1007/s00702-009-0214-0. [DOI] [PubMed] [Google Scholar]
- 54.Moncek F, Duncko R, Johansson B, Jezova D. Effect of environmental enrichment on stress related systems in rats. Journal of neuroendocrinology. 2004;16(5):423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- 55.Wright RL, Conrad CD. Enriched environment prevents chronic stress-induced spatial learning and memory deficits. Behavioural brain research. 2008;187(1):41–47. doi: 10.1016/j.bbr.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green TA, Cain ME, Thompson M, Bardo MT. Environmental enrichment decreases nicotine-induced hyperactivity in rats. Psychopharmacology. 2003;170(3):235–241. doi: 10.1007/s00213-003-1538-3. [DOI] [PubMed] [Google Scholar]
- 57.Brenes JC, Padilla M, Fornaguera J. A detailed analysis of open-field habituation and behavioral and neurochemical antidepressant-like effects in postweaning enriched rats. Behavioural brain research. 2009;197(1):125–137. doi: 10.1016/j.bbr.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 58.Cain ME, Mersmann MG, Gill MJ, Pittenger ST. Dose-dependent effects of differential rearing on amphetamine-induced hyperactivity. Behavioural pharmacology. 2012;23(8):744–753. doi: 10.1097/FBP.0b013e32835a38ec. [DOI] [PubMed] [Google Scholar]
- 59.Würbel H. Behaviour and the standardization fallacy. Nature genetics. 2000;26(3):263–263. doi: 10.1038/81541. [DOI] [PubMed] [Google Scholar]
- 60.Würbel H. Ideal homes? Housing effects on rodent brain and behaviour. Trends in neurosciences. 2001;24(4):207–211. doi: 10.1016/s0166-2236(00)01718-5. [DOI] [PubMed] [Google Scholar]
- 61.Garner JP, Mason GJ. Evidence for a relationship between cage stereotypies and behavioural disinhibition in laboratory rodents. Behavioural brain research. 2002;136(1):83–92. doi: 10.1016/s0166-4328(02)00111-0. [DOI] [PubMed] [Google Scholar]
- 62.Lewis MH. Environmental complexity and central nervous system development and function. Ment Retard Dev Disabil Res Rev. 2004;10(2):91–5. doi: 10.1002/mrdd.20017. [DOI] [PubMed] [Google Scholar]
- 63.Lanovaz MJ. Towards a comprehensive model of stereotypy: Integrating operant and neurobiological interpretations. Research in Developmental Disabilities. 2011;32(2):447–455. doi: 10.1016/j.ridd.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 64.Powell SB, Swerdlow NR, Pitcher LK, Geyer MA. Isolation rearing-induced deficits in prepulse inhibition and locomotor habituation are not potentiated by water deprivation. Physiology & Behavior. 2002;77(1):55–64. doi: 10.1016/s0031-9384(02)00817-x. [DOI] [PubMed] [Google Scholar]
- 65.Zimmermann A, Stauffacher M, Langhans W, Würbel H. Enrichment-dependent differences in novelty exploration in rats can be explained by habituation. Behavioural Brain Research. 2001;121(1–2):11–20. doi: 10.1016/s0166-4328(00)00377-6. [DOI] [PubMed] [Google Scholar]
- 66.Cain ME, Green TA, Bardo MT. Environmental enrichment decreases responding for visual novelty. Behavioural Processes. 2006;73(3):360–366. doi: 10.1016/j.beproc.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gill MJ, Cain ME. Effects of satiety on operant responding in rats raised in enrichment. Behavioural Pharmacology. 2011;22(1):40–48. doi: 10.1097/FBP.0b013e3283425a86. [DOI] [PubMed] [Google Scholar]
- 68.Rose FD, Love S, Dell PA. Differential reinforcement effects in rats reared in enriched and impoverished environments. Physiology & Behavior. 1986;36(6):1139–1145. doi: 10.1016/0031-9384(86)90491-9. [DOI] [PubMed] [Google Scholar]
- 69.Chok JT, Koesler B. Distinguishing obsessive-compulsive behavior from stereotypy: A preliminary investigation. Behavior modification. 2014;38(3):344–373. doi: 10.1177/0145445513509475. [DOI] [PubMed] [Google Scholar]
- 70.Cath DC, Spinhoven P, Hoogduin CA, Landman AD, van Woerkom TC, van de Wetering BJ, Roos RA, Rooijmans HG. Repetitive behaviors in Tourette’s syndrome and OCD with and without tics: what are the differences? Psychiatry research. 2001;101(2):171–185. doi: 10.1016/s0165-1781(01)00219-0. [DOI] [PubMed] [Google Scholar]
- 71.Rapp JT, Vollmer TR. Stereotypy II: A review of neurobiological interpretations and suggestions for an integration with behavioral methods. Research in Developmental Disabilities. 2005;26(6):548–564. doi: 10.1016/j.ridd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 72.Lloyd DR, Hausknecht KA, Richards JB. Nicotine and methamphetamine disrupt habituation of sensory reinforcer effectiveness in male rats. Experimental and clinical psychopharmacology. 2014;22(2):166. doi: 10.1037/a0034741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Research Reviews. 2008;58(2):322–39. doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 74.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 75.Schultz W. Behavioral dopamine signals. Trends in neurosciences. 2007;30(5):203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 76.Bimpisidis Z, Luca MA, Pisanu A, Di Chiara G. Lesion of medial prefrontal dopamine terminals abolishes habituation of accumbens shell dopamine responsiveness to taste stimuli. European Journal of Neuroscience. 2013;37(4):613–622. doi: 10.1111/ejn.12068. [DOI] [PubMed] [Google Scholar]
- 77.De Luca MA. Habituation of the responsiveness of mesolimbic and mesocortical dopamine transmission to taste stimuli. Frontiers in Integrative Neuroscience. 2014;8 doi: 10.3389/fnint.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kolb B. Some tests of response habituation in rats with discrete lesions to the orbital or medial frontal cortex. Canadian Journal of Psychology/Revue canadienne de psychologie. 1974;28(2):260. doi: 10.1037/h0081993. [DOI] [PubMed] [Google Scholar]
- 79.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain Research. 1983;287(2):173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 80.Courchesne E, Mouton PR, Calhoun ME, Semendeferi K, Ahrens-Barbeau C, Hallet MJ, Barnes CC, Pierce K. Neuron number and size in prefrontal cortex of children with autism. Jama. 2011;306(18):2001–2010. doi: 10.1001/jama.2011.1638. [DOI] [PubMed] [Google Scholar]
- 81.Renner MJ, Rosenzweig MR. Enriched and Impoverished Environments: Effects on Brain and Behavior. Springer-Verlag; New York: 1987. [Google Scholar]
- 82.Fernandez-Teruel A, Escorihuela R, Castellano B, Gonzalez B, Tobena A. Neonatal handling and environmental enrichment effects on emotionality, novelty/reward seeking, and age-related cognitive and hippocampal impairments: focus on the Roman rat lines. Behavior genetics. 1997;27(6):513–526. doi: 10.1023/a:1021400830503. [DOI] [PubMed] [Google Scholar]
- 83.Chapillon P, Patin V, Roy V, Vincent A, Caston J. Effects of pre-and postnatal stimulation on developmental, emotional, and cognitive aspects in rodents: A review. Developmental psychobiology. 2002;41(4):373–387. doi: 10.1002/dev.10066. [DOI] [PubMed] [Google Scholar]
- 84.Yetnikoff L, Reichard RA, Schwartz ZM, Parsely KP, Zahm DS. Protracted maturation of forebrain afferent connections of the ventral tegmental area in the rat. Journal of Comparative Neurology. 2014;522(5):1031–1047. doi: 10.1002/cne.23459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kolb B, Gibb R. Plasticity in the prefrontal cortex of adult rats. Front Cell Neurosci. 2015;9:15. doi: 10.3389/fncel.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev. 2007;55(1):78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 87.Fleming AS, Korsmit M, Deller M. Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiology. 1994;22(1):44–53. [Google Scholar]
- 88.Olazábal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, González-Mariscal G, Lévy F, Lucion AB, Morrell JI, Numan M. New theoretical and experimental approaches on maternal motivation in mammals. Neuroscience & Biobehavioral Reviews. 2013;37(8):1860–1874. doi: 10.1016/j.neubiorev.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 89.Pryce CR, Bettschen D, Feldon J. Comparison of the effects of early handling and early deprivation on maternal care in the rat. Developmental psychobiology. 2001;38(4):239–251. doi: 10.1002/dev.1018. [DOI] [PubMed] [Google Scholar]
- 90.Pham T, Ickes B, Albeck D, Söderström S, Granholm A-C, Mohammed A. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94(1):279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- 91.Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22(1):53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 92.Diamond MC, Lindner B, Raymond A. Extensive cortical depth measurements and neuron size increases in the cortex of environmentally enriched rats. Journal of Comparative Neurology. 1967;131(3):357–364. [Google Scholar]
- 93.Diamond MC, Law F, Rhodes H, Lindner B, Rosenzweig MR, Krech D, Bennett EL. Increases in cortical depth and glia numbers in rats subjected to enriched environment. Journal of Comparative Neurology. 1966;128(1):117–125. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- 94.Diamond MC, Ingham CA, Johnson RE, Bennett EL, Rosenzweig MR. Effects of environment on morphology of rat cerebral cortex and hippocampus. Journal of neurobiology. 1976;7(1):75–85. doi: 10.1002/neu.480070108. [DOI] [PubMed] [Google Scholar]
- 95.Altman J, Das GD. Autoradiographic examination of the effects of enriched environment on the rate of glial multiplication in the adult rat brain. Nature. 1964;204(4964):1161–1163. doi: 10.1038/2041161a0. [DOI] [PubMed] [Google Scholar]
- 96.Volkmar FR, Greenough WT. Rearing complexity affects branching of dendrites in the visual cortex of the rat. Science. 1972;176(4042):1445–1447. doi: 10.1126/science.176.4042.1445. [DOI] [PubMed] [Google Scholar]
- 97.Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. Journal of comparative and physiological psychology. 1973;82(2):175. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- 98.Bhide P, Bedi K. The effects of a lengthy period of environmental diversity on well-fed and previously undernourished rats. II. Synapse-to-neuron ratios. Journal of Comparative Neurology. 1984;227(2):305–310. doi: 10.1002/cne.902270213. [DOI] [PubMed] [Google Scholar]