Abstract
The BioSense program was launched in 2003 with the aim of establishing a nationwide integrated public health surveillance system for early detection and assessment of potential bioterrorism-related illness. The program has matured over the years from an initial Centers for Disease Control and Prevention–centric program to one focused on building syndromic surveillance capacity at the state and local level. The uses of syndromic surveillance have also evolved from an early focus on alerts for bioterrorism-related illness to situational awareness and response, to various hazardous events and disease outbreaks. Future development of BioSense (now the National Syndromic Surveillance Program) includes, in the short term, a focus on data quality with an emphasis on stability, consistency, and reliability and, in the long term, increased capacity and innovation, new data sources and system functionality, and exploration of emerging technologies and analytics.
Keywords: Biosense, syndromic surveillance, emergency department data, public health surveillance
As mandated by the Public Health Security and Bioterrorism Preparedness and Response Act of 2002, the Centers for Disease Control and Prevention (CDC) launched the BioSense program in 2003 with the aim of establishing a nationwide integrated public health surveillance system for early detection and assessment of potential bioterrorism-related illness.1,2 The initial BioSense program had 4 goals: (1) improve the nation’s capabilities for conducting near–real-time biosurveillance and health situational awareness; (2) advance analytics for prediagnostic and diagnostic data; (3) increase sharing of approaches and technology among federal, state, and local public health agencies; and (4) promote national system standards and specifications to ensure integration with other public health systems.2,3 BioSense was 1 of 3 initiatives advanced by the 2002 act to improve national preparedness; the other 2 initiatives were BioShield, which focuses on rapid development of vaccines and therapeutics, and BioWatch, a program designed to sample the air in major metropolitan areas for pathogens that terrorists might use.2
A major component of the BioSense system was the infrastructure that CDC developed to receive and securely manage health care–sourced data and to host the BioSense application for analyzing and visualizing data reported to BioSense. The infrastructure included (1) data management processes to receive and process inbound clinical care and related data, (2) analytic processes to bin records into syndrome categories and analyze trends for suspect signals, and (3) a user interface that allowed CDC and state and local staff members to access patient-level data to investigate results, report on notifications, and coordinate responses.3
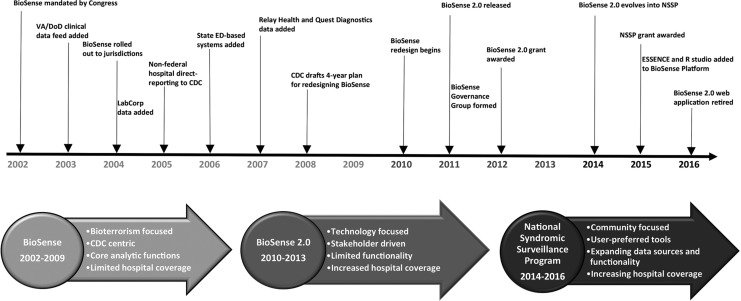
Data from different sources were added to the BioSense system over time, including data from US Department of Veterans Affairs and US Department of Defense hospitals and ambulatory care clinics (2003), test orders from the Laboratory Corporation of America (2004), data from nonfederal hospitals directly reporting to CDC (2005), data from state health departments’ syndromic surveillance systems (2006), anti-infective prescription data from Relay Health outpatient pharmacies (2007), and test orders from Quest Diagnostics (2007). By 2008, the primary data sources for BioSense included 333 Department of Defense and 770 Veterans Affairs hospitals and ambulatory clinics and 532 civilian hospital emergency departments (EDs).3,4
The BioSense application was released to state and local health departments in April 2004. CDC used its secure data network to grant user access, and approximately 300 users from state and local health departments enrolled in the first year.3 CDC also established a BioIntelligence Center to monitor, analyze, and interpret incoming data and identify potential problems and system enhancements. The BioIntelligence Center provided state and local jurisdictions with syndromic surveillance support for major events, such as the 2007 California wildfires.5 The BioIntelligence Center no longer exists, but some of the staff members and functions were merged into CDC’s Division of Health Informatics and Surveillance in 2013. In the fall of 2004, CDC began holding regional meetings with state and local health departments to refine and validate the need for syndromic surveillance systems and standards. Input from these meetings resulted in publication of the Public Health Information Network Preparedness Early Event Detection Guidelines,6 which described the minimum operational requirements necessary to support early event detection.
In 2005, CDC began recruiting nonfederal hospitals to transmit ED and outpatient clinical data to CDC with the goal that, within 3 years, all EDs in the United States would be reporting these data to BioSense. To speed up data acquisition, CDC established servers in large metropolitan hospitals, and data were sent directly to BioSense. By 2007, only about 10% of US EDs were reporting, and BioSense stakeholders became concerned with the slow rate of adding hospitals and increasing the coverage of EDs across the country. State and local public health officials expressed concern about CDC recruiting data sources directly rather than working through the public health departments. Additionally, because syndromic surveillance was a new and untested methodology, state and local health department epidemiologists raised questions about data quality, validity, and utility, and members of the US Congress expressed concerns about the number of false alerts and the cost and effectiveness of BioSense as an early warning system for bioterrorism.7,8 State and local epidemiologists were particularly concerned that the system had been developed and implemented with limited stakeholder input and added little to state surveillance capacity.9–11
As awareness increased about the limits of syndromic surveillance systems for providing early and accurate epidemic alerts, increased emphasis was placed on using the BioSense system to provide timely situational awareness and help public health officials assess the extent and geographic location of illness in their communities. For example, during the 2009-2010 H1N1 influenza pandemic, the BioSense program provided information that helped CDC make decisions about immunization recommendations, school and public building closures, and other response steps.12 After the 2010 Deepwater Horizon oil spill in the Gulf of Mexico, CDC used BioSense and state-based surveillance systems to monitor for health threats that were possibly related to exposures to the oil spill.12,13
BioSense 2.0
From 2008 to 2010, BioSense underwent major changes in response to these concerns of the US Congress and state and local health officials.7–11 In 2008, CDC developed a 4-year plan to redesign BioSense. In 2010, CDC began working collaboratively with stakeholders to develop a more user-friendly, responsive system similar to those being developed by state and local health departments. In November 2011, the redesigned BioSense program, called BioSense 2.0, was implemented in a community-controlled and user-driven environment. CDC funded the Association of State and Territorial Health Officials to host the cloud-based infrastructure and application and develop data use agreements with participating state and local health departments. BioSense 2.0 allowed state and local health departments to access data that supported expansion of their syndromic surveillance systems in accordance with the Meaningful Use program.14 Rather than sending all data directly to CDC (as was the case with the original BioSense), under BioSense 2.0, most data were sent from hospitals to state and local health departments and then to a secure data storage location in the cloud managed by the Association of State and Territorial Health Officials.
A core activity of the redesign process was providing mechanisms for stakeholder involvement, including gathering input on user requirements, user webinars, group meetings at public health conferences, and an Association of State and Territorial Health Officials–facilitated BioSense Governance Group comprising state and local syndromic surveillance stakeholders who represented BioSense 2.0 users. As a consequence of the aim to improve stakeholder involvement, changes to the program initially resulted in CDC staff members losing access to individual-level data and being unable to provide data or system quality assurance or validate the data-processing workflow and the syndromic surveillance binning algorithms. Thus, the BioSense 2.0 application had technical problems such that states and local jurisdictions had little confidence in data quality. Furthermore, BioSense 2.0 users desired a more comprehensive syndromic surveillance system with increased functionalities and additional analytic tools.15
Although some states relied on the partially functional BioSense 2.0 application, others built or bought their own systems. Syndromic surveillance capacity continued to grow across the country, and CDC began supporting this effort through cooperative agreements and other surveillance-related funding. In September 2012, CDC awarded its first BioSense 2.0 program cooperative agreement, allocating financial support to 34 state and local health departments to build syndromic surveillance capacity and support collaboration and innovation among BioSense 2.0 users. The 3-year grant provided state and local health departments with (1) greater capacity for data management and storage, (2) cost savings on information technology and infrastructure, (3) improved and timelier access to regional and national public health surveillance data, and (4) greater access to peers for sharing knowledge and best practices.
As states came together and learned from one another, syndromic surveillance became a core component of public health surveillance, and its use evolved from event detection to documenting and characterizing already known outbreaks or events. For example, New York City learned that ED visits for influenza-like illness correlated well with influenza laboratory diagnoses and were useful for tracking influenza trends.16 King County, Washington, determined that its syndromic surveillance system was useful for detecting clusters of carbon monoxide poisoning due to power outages in certain ZIP codes after a windstorm.17 Using reportable condition queries, syndromic surveillance systems in Florida identified an outbreak of ciguatera fish poisoning from 1 case presenting in the ED, resulting in an official fish recall and investigation.18 In North Carolina, where the syndromic surveillance system NC DETECT (North Carolina Disease Event Tracking and Epidemiologic Collection Tool) includes nearly all hospitals in the state, health department epidemiologists found that opioid-related ED visits correlated temporally and spatially with sales of prescription opioids.19
CDC awarded its second grant20 on September 1, 2015. The new 4-year award provided financial support to 31 state and local health departments and emphasized activities that address lessons learned from the first grant. For example, the grant covered a larger population, more geographic diversity, improved reporting metrics, increased hospital onboarding, improved data quality, and access to the BioSense Platform—a cloud-based syndromic surveillance platform that hosts the BioSense 2.0 web application and other analytic tools and services. The grant built capacity for syndromic surveillance to improve local, state, regional, and national situational awareness and promotes collaboration among participants to further the science and practice of syndromic surveillance. BioSense has evolved from focusing on systems and technology to a community of practice with shared tools, methods, and expertise, as reflected in its new name: the National Syndromic Surveillance Program (NSSP; Figure).
Figure.
Evolution of the Centers for Disease Control and Prevention (CDC) BioSense public health surveillance system, 2002-2016. Abbreviations: ED, emergency department; ESSENCE, Electronic Surveillance System for the Early Notification of Community-Based Epidemics; NSSP, National Syndromic Surveillance Program; VA/DoD, Department of Veterans Affairs/Department of Defense.
National Syndromic Surveillance Program
In early 2014, CDC launched the BioSense Enhancement Initiative as part of CDC’s Surveillance Strategy21 to enhance the NSSP in 2 key areas: (1) establish and support a community of practice to include CDC-funded grantees, nonfunded states, and jurisdictions that contribute data to the BioSense Platform, as well as CDC programs, other federal agencies, partner organizations, hospitals, health care professionals, academic institutions, and public health practitioners who use local syndromic surveillance systems, and (2) modernize the BioSense Platform to improve data processing and provide a suite of analytic and visualization tools for use by all participants.
A pilot project conducted in 2015 with 8 jurisdictions tested the feasibility of using ESSENCE (Electronic Surveillance System for the Early Notification of Community-Based Epidemics)22 and SAS software23 on the BioSense Platform to analyze state and local data. Pilot results and feedback from the user community resulted in a phased implementation to install new tools on the BioSense Platform, rearchitect the data-processing workflow, and transition jurisdictions to the new tools and services. By the end of 2016, ESSENCE, SAS, and R Studio Professional24 were operational on the BioSense Platform; all jurisdictions had transitioned from using the BioSense web application to ESSENCE; and the BioSense web application was discontinued. Today, data from >4000 hospitals are sent to the BioSense Platform, representing about 55% of all ED visits in the country.
Future development of the NSSP and the BioSense Platform will focus on activities that (1) increase data availability and representativeness of ED visits regionally and nationally, (2) improve data quality, and (3) facilitate the use of data for situational awareness and response to hazardous events and disease outbreaks. Changes in Meaningful Use requirements are already resulting in more ambulatory care data being sent to the BioSense Platform. The increasing availability of ambulatory care data offers challenges and opportunities for identifying analytic methods and uses of these data. Several data sources that were available in the original BioSense application but were never integrated and made available through BioSense 2.0 included Department of Veterans Affairs ambulatory care data, Department of Defense military treatment facility ambulatory care data, and laboratory and pharmacy data from vendors. These data will be incorporated into the BioSense Platform and will augment ED data to provide a more comprehensive view of national, regional, and local public health events. Data use agreements and jurisdiction-controlled data access tools determine access to the data and to aggregated analyzed information for all BioSense Platform users.
Improving data quality is an ongoing effort by CDC and the jurisdictions that participate in the NSSP. For example, every jurisdiction is updating and enhancing its master facility table (ie, the inventory of facilities that submit data to the BioSense Platform) to improve the collection and storage of facility-related metadata. CDC is promoting enhanced data quality reporting capacity across jurisdictions by developing national standards for reporting data validity, completeness, and timeliness.25
Establishing and improving the utility of syndromic surveillance data is best realized through a strong community of practice that encourages the sharing of methods, lessons learned, and novel uses of the data. In June 2016, CDC awarded a 3-year cooperative agreement to the International Society for Disease Surveillance to develop, implement, and maintain the NSSP Community of Practice.26
Collaboration is particularly important for the development of event-specific syndrome definitions, algorithms, and test messages. In the past several years, the syndromic surveillance community came together to develop syndrome definitions in response to outbreaks of Middle East respiratory syndrome coronavirus, enterovirus D68, and the chikungunya, Ebola, and Zika viruses.27 The goals of the NSSP Community of Practice are diffusion of syndromic surveillance resources and best practices among public health practitioners and stakeholders; enhanced situational awareness of emergent and chronic public health issues at local, regional, and national levels; and improved public health decision making in response to all hazardous events and outbreaks.
Syndromic surveillance looks very different today than it did when the BioSense program launched 14 years ago. Although BioSense was initially established to identify the health impacts of bioterrorism events, syndromic surveillance is now a core public health practice and is used for situational awareness and for characterizing and monitoring various health conditions that affect population health.
As public health agencies gain increased access to electronic health record data and other data sources and as lessons learned from syndromic surveillance are coupled with advances in technology and analytics, richer and timelier information will be available to monitor and improve public health.
Footnotes
Authors’ Note: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of CDC.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Pub L No. 107-188, 116 Stat 594 (2002).
- 2. Loonsk JW. BioSense: a national initiative for early detection and quantification of public health emergencies. MMWR Morb Mortal Wkly Rep. 2004;53(suppl):53–55. [PubMed] [Google Scholar]
- 3. Bradley CA, Rolka H, Walker D, et al. BioSense: implementation of a national early event detection and situational awareness system. MMWR Morb Mortal Wkly Rep. 2005;54(suppl):11–19. [PubMed] [Google Scholar]
- 4. Tokars JI, English R, McMurray P, et al. Summary of data reported to CDC’s national automated biosurveillance system, 2008. BMC Med Inform DecisMak. 2010;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ginsberg M, Johnson J, Tokars J, et al. Monitoring health effects of wildfires using the BioSense system: San Diego County, California, October 2007. MMWR Morb Mortal Wkly Rep. 2008;57(27):741–744. [PubMed] [Google Scholar]
- 6. Loonks JW, McGarvey SR, Conn LA, et al. The Public Health Information Network (PHIN) preparedness initiative. J Am Med Inform Assoc. 2006;13(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Department of Homeland Security Digital Library. Hearing of the Subcommittee on Bioterrorism and Public Health Preparedness of the Committee on Health, Education, Labor, and Pensions. https://www.hsdl.org/?view&did=481999 . Accessed April 4, 2016.
- 8. Barlas S. CDC’s BioSense network identifying possible bioterrorism, but skeptics abound. Emerg Med News. 2007;29(9):17. [Google Scholar]
- 9. Buehler JW, Whitney EA, Smith D, et al. Situational uses of syndromic surveillance. Biosecur Bioterror. 2009;7(2):165–177. [DOI] [PubMed] [Google Scholar]
- 10. US Government Accountability Office. Health information technology: more detailed plans needed for the Centers for Disease Control and Prevention’s redesigned BioSense program. http://www.gao.gov/new.items/d09100.pdf. Published 2008. Accessed April 16, 2016.
- 11. US Government Accountability Office. Information technology: federal agencies face challenges in implementing initiatives to improve public health infrastructure. http://www.gao.gov/new.items/d05308.pdf. Published 2005. Accessed June 22, 2016.
- 12. Centers for Disease Control and Prevention. National Syndromic Surveillance Program: syndromic surveillance data in action. http://www.cdc.gov/nssp/biosense/action.html. Accessed May 10, 2016.
- 13. Institute of Medicine. Assessing the Effects of the Gulf of Mexico Oil Spill on Human Health: A Summary of the June 2010 Workshop. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 14. Centers for Medicare & Medicaid Services. Electronic health records (EHR) incentive programs. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/index.html. Accessed April 28, 2016.
- 15. Gibson J, Karras BT, Gordon GS. BioSense 2.0 governance: surveying users and stakeholders for continued development. Online J Public Health Inform. 2014;6(1):e19. [Google Scholar]
- 16. Westheimer E, Paladini M, Balter S, et al. Evaluating the New York City emergency department syndromic surveillance for monitoring influenza activity during the 2009-10 influenza season. PLoS Curr. 2012;4:e500563f3ea181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baer A, Elbert Y, Burkom HS, et al. Usefulness of syndromic data sources for investigating morbidity resulting from a severe weather event. Disaster Med Public Health Prep. 2011;5(1):37–45. [DOI] [PubMed] [Google Scholar]
- 18. Klekamp BG, Bodager D, Matthews SD. Use of surveillance systems in detection of a ciguatera fish poisoning outbreak—Orange County, Florida, 2014. MMWR Morb Mortal Wkly Rep. 2015;64(40):1142–1144. [DOI] [PubMed] [Google Scholar]
- 19. Modarai F, Mack K, Hicks P, et al. Relationship of opioid prescription sales and overdoses, North Carolina. Drug Alcohol Depend. 2013;132(1-2):81–86. [DOI] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. The National Syndromic Surveillance Program: enhancing syndromic surveillance capacity and practice. http://www.cdc.gov/nssp/foa.html. Accessed May 27, 2016.
- 21. Richards CL, Iademarco MF, Anderson TC. A new strategy for public health surveillance at CDC: improving national surveillance activities and outcomes. Public Health Rep. 2014;129(6):472–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johns Hopkins University Applied Physics Laboratory. OpenESSENCE user guide. http://www.jhuapl.edu/sages/guides/OE-User-Guide-Feb-2013.pdf. Published 2013. Accessed June 1, 2016.
- 23. SAS Institute, Inc. SAS Version 9.4. Cary, NC: SAS Institute, Inc; 2013. [Google Scholar]
- 24. RStudio, Inc. RStudio Server. Boston, MA: RStudio, Inc; 2016. [Google Scholar]
- 25. International Society for Disease Surveillance. NSSP Data Quality Workgroup. http://www.syndromic.org/cop/nssp/nssp-workgroups/794-bug-data-quality-workgroup. Accessed May 27, 2016.
- 26. Centers for Disease Control and Prevention. National Syndromic Surveillance Program: community of practice. https://www.cdc.gov/nssp/community.html . Accessed May 27, 2016.
- 27. International Society for Disease Surveillance. NSSP Syndrome Definitions Workgroup. http://www.syndromic.org/cop/nssp/nssp-workgroups/797-bug-syndrome-definitions-workgroup. Accessed May 27, 2016.