Abstract
Aim
To evaluate (1) the usefulness of thoracic ultrasound in diagnosis and staging of bronchogenic carcinoma by comparing lesion detectability between thoracic- ultrasound and computed tomography and (2) the outcome of thoracic-ultrasound-guided biopsy in diagnosing bronchogenic carcinoma.
Methods
We conducted a cross-sectional study on 53 patients of confirmed bronchogenic carcinoma. All patients had been investigated by thoracic-ultrasound and chest-computed tomography; data regarding the presence of mass (its size, necrosis), lymph nodes invasion, peritumoural atelectasis, consolidations, pleural effusion, chest wall invasion, and paralysis of the diaphragm were recorded. Thoracic-ultrasound-guided biopsy was done for 41 patients.
Results
Thoracic-ultrasound had significantly higher detection rate of peritumoural atelectasis, paralysis of the diaphragm, and supraclavicular lymph nodes invasion, while it has significantly lower detection rate of pulmonary masses and mediastinal lymph nodes invasion than computed tomography. It has nonsignificant higher detection rate of pleural effusion, consolidations, chest wall invasion and necrosis within mass than computed tomography. Thoracic-ultrasound detects static air-bronchogram and/or fluid bronchogram in 53.3% of bronchogenic carcinoma-associated consolidation. Thoracic-ultrasound-guided biopsy revealed positive yield in 78.0% (32/41) of patients. All patients with negative thoracic-ultrasound biopsy had mass size >5 cm with necrosis within the mass. Self-limited complications occurred in 26.8% after thoracic-ultrasound-guided biopsy (haemoptysis 22.0%, pneumothorax 2.4% and subcutaneous emphysema 2.4%).
Conclusion
Thoracic-ultrasound has a significant complementary role to computed tomography in diagnosis and staging of bronchogenic carcinoma. Thoracic-ultrasound-guided biopsy revealed good positive yield (78%), its yield was negatively affected by mass size and necrosis. It is a simple, practical and accurate procedure without significant patients’ risks.
Keywords: Transthoracic ultrasound, bronchogenic carcinoma, static air-bronchogram, fluid bronchogram
Introduction
Bronchogenic carcinoma is an important and widespread disease that constitutes a major health problem worldwide. It kills over one million people per year.1 Lung cancer is usually suspected in individuals who have abnormal chest radiography (CXR) or have symptoms of either local or systemic metastasis of the tumour. The diagnostic methods of lung cancer depend on the type, size and location of the primary tumour, the presence of metastasis, the overall clinical status of the patient and the anticipated treatment plan.2 Histopathologic examination is regarded as the reference standard for diagnosis of focal lung bronchogenic carcinoma.3
Imaging techniques play a crucial role in the diagnosis, staging and follow-up of patients with lung cancer.4 Computed tomography (CT) scanning of the chest can provide information regarding the location of the tumour, its proximity to local structures, and whether or not lymph nodes (LN) in the mediastinum are enlarged.5 CT is still the imaging method of choice for the diagnosis of thoracic pathologies,6 however, it is of high cost, utilizes ionizing radiation, and there is a risk of contrast agents nephrotoxicity.7 Thoracic ultrasonography (thoracic US) can be considered an important supplementary tool in this setting.6 Although numerous studies have been conducted on the use of thoracic US for the examination of thoracic structures, it is not as widely accepted as abdominal US.6 Ultrasonography is considered a reliable, inexpensive, safe and reproducible diagnostic method for the workup of a variety of thoracic diseases including of the diaphragm, thoracic wall, lung, anterosuperior mediastinum and the pleurae.8 Recently, thoracic US has gained increasing interest in the evaluation of peripheral pulmonary lesions abutting the pleura.9,10 The parenchyma of normally aerated lungs is not visualized by thoracic US; therefore, the central lung tumours are not amenable to thoracic US. However, central lung tumours may cause peritumoural atelectasis and/or post-obstructive pneumonitis which can be detected by thoracic US,11 as fluid bronchograms (FB).12,13 Although chest CT-scan examination can determine the stage of lung cancer, real-time high-resolution thoracic US examination has proven superior to the chest CT-scan in the detection of tumour invasion of pleura and chest wall.14
Imaging-guided percutaneous thoracic US biopsy is a well-established effective and safe minimally invasive technique to obtain sufficient tissue specimens for histopathologic diagnosis of different intrathoracic lesions.15 Percutaneous biopsy yields tissue samples for diagnosis and staging and facilitates differentiation of primary cancer from distant metastasis or infective and inflammatory lesions, which is crucial for correct management of lung lesions.16 Today, thoracic US is widely used to guide transthoracic biopsy of peripheral lung lesions.17 Sonography is considered as effective as CT-guidance in sample accuracy of peripheral lung lesion and mediastinal lesion,3 with the advantages of lower cost, lack of exposure to ionizing radiation, shorter procedure time and lower rates of post-procedural pneumothorax.18,10,19,20,3 Additionally, the use of thoracic US in the detection and sampling of patients with supraclavicular LN invasion in patients with suspected lung cancer is a promising technique; that revealed both a cytological diagnosis and pathological N3 staging.21 Finally, ultrasonography permits immediate bedside identification of post-procedural complications, e.g. pneumothorax.20 Regardless of these advantages, the use of US in malignant lesions of the thorax is still low; in many centres, the CT-guided biopsy is preferred.19 In the current literature, there are no typical patterns of lung neoplasm on US or any other imaging technique. This study was carried out to evaluate (1) the usefulness of thoracic US in diagnosis and staging of bronchogenic carcinoma by comparing lesion detectability between thoracic US and chest CT-scan and (2) the outcome of thoracic US-guided biopsy in diagnosing bronchogenic carcinoma.
Methods
Patient selection
This cross-sectional study was conducted in the period from June 2015 to November 2016. It was carried out at chest diseases department, Al-Zahraa University Hospital, Cairo, Egypt. It included 53 patients with histopathologically confirmed bronchogenic carcinoma out of 79 patients having clinical and/or CXR findings suggestive of bronchogenic carcinoma (Figure 1). Demographic data (e.g., age, sex, smoking history and smoking pack/year (number of packs smoked per day multiplied by the number of years), laboratory data (e.g., platelets count and coagulation profile (international normalization ratio (INR)), main presenting symptoms and chest radiographic findings were tabulated in Table 1. This study was done according to the principles outlined in the Declaration of Helsinki. Every participant gave informed written consent before enrolment into the study and he/she had the right to refuse participation or withdraw from the study at any point without affecting their rights in management. All the data were coded to ensure confidentiality. The majority of the studied cases (83.0%) were male with a mean age (56.7 ± 8.8 years). Around three-quarters (73.6%) were smokers with mean smoking pack/year of 22.7 ± 20.0. More than half of the studied cases (52.8%) had chronic obstructive pulmonary disease (COPD) and 11.3% had diffuse parenchymal lung diseases (DPLD). Among the studied patients, 30.2% had adenocarcinoma, 30.2% had squamous cell carcinoma, 28.3% had large cell carcinoma and 11.3% had small cell lung cancer (SCLC).
Thoracic US examination:
The procedure has been done by the three pulmonologist authors (MRH, ESMS, and SBE), they have four years experience in thoracic US. The thoracic US operator was blinded to the CXR findings, as the author who selects patient to the study did not perform thoracic US examination for that patient. Sonoscape SSI-6000 Medical Systems (Shenzhen, China) with probes of different frequencies were used to examine the chest; a curvilinear transducer (3–5-MHz) was used initially for examination of the lungs. Both thoracic US and chest CT-scan examinations were done on the same day, thoracic US was done first followed by chest CT-scan. The following findings were reported: (1) Mass: its size, presence of necrosis, borders (regular or irregular) and the echogenicity. Necrosis was defined as anechoic area inside clear boundaries lesions, in addition to the absence of blood flow by colour Doppler.19 (2) Presence of consolidations: airbronchogram (AB); static vs. dynamic, FB, and whether a sharp demarcation could be visualized between the mass and distal consolidation. AB was seen as punctiform or linear hyperechoic artifacts within the consolidation. If AB moved ≥1 mm with inspiration it is dynamic, if not it is static. Dynamic AB is likely due to pneumonia, while static AB makes atelectasis more likely. When the bronchial tree is filled with fluid rather than air, a branching pattern of anechoic or hypoechoic tubular structures are seen (FB).22 (3) Peritumoural atelectasis was demonstrated as areas of parenchymal consolidation, with a triangular shape and the apex towards the hilum associated with an obstructive tumoural nodule.19 (4) An associated pleural effusion is identified as echo free space with posterior acoustic enhancement. Swirling sign is seen as numerous, floating, echogenic particles within the effusion that swirl in response to respiratory or cardiac movements.23 (5) Chest wall infiltration: the examination was completed with a 9.5–15 MHz linear transducer, all layers of the chest wall; muscle, fascia, parietal and visceral pleura are identified with TUS.14 Chest wall infiltration is suspected if: (1) the tumour-pleural contact >3 cm, (2) the angle between the tumour and the lung surface is obtuse and (3) the adjacent pleura are thickened. Absent sliding sign (backward and forward movement of parietal and visceral pleura over each other) indicates parietal pleural invasion.24 (6) Assessment of the diaphragm: using M-mode, a low-frequency probe placed in the anterior axillary line at the costal margin and directed upward and dorsally with the directional marker cephalad.4 With this technique, a normal diaphragm will contract caudally, paradoxical movement indicates diaphragmatic paralysis.25 (7) Assessment of lesion vascularity: in malignant tumours, vessels are displaced, being frequently detected at the margin of the tumour, having a spiral or bizarre shape, with variable diameters.26 Contrast enhanced chest CT-scan: The scan was done by using multidetector scanner (160 detectors) (Toshiba, Prime Aquilion Japan). The scans were obtained in the supine position and during full inspiration. Chest CT-scanning was performed from the lower part of the neck to the adrenal gland. Scanning parameters of CT examinations were as follows: slice thickness 5 mm, slice interval 0.5 mm, collimation 2.5 mm, scan time 3.9 seconds, feed/rotation 15 mm. A scout was taken with 120 kV and 100 mA, then helical scanning in caudocranial direction to minimize the respiratory artifacts was done. All scanning was performed after intravenous (IV) administration of contrast agent. In our hospital, all chest CT-scans were reported routinely by an experienced radiologist. Additionally, for this study, the three pulmonologist authors interpreted the chest CT-scan separately and when there is conflict a consensus was held with the radiologist.
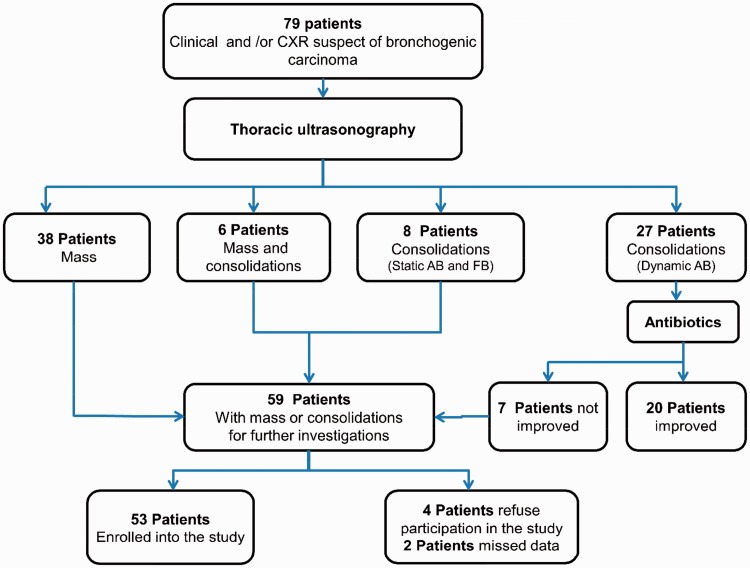
Figure 1.
Flowchart for patient’s selection. This figure demonstrates that 79 patients presented with clinical and or chest radiography findings suggestive of bronchogenic carcinoma were examined by thoracic US. It revealed 38 patients had pulmonary masses, 27 patients had consolidations with dynamic AB, six patients had combined masses and consolidations, and eight patients had consolidations with static AB and FB. Twenty-seven patients who had consolidations with dynamic AB received antibiotics for 10 days; of them 20 patients showed improvement. Patients with masses (n = 38), combined masses and consolidations (n = 6), and those who had static AB and FB consolidations (n = 8) and those who did not improve within clinical and radiological (chest radiography and thoracic US) within three weeks after antibiotics therapy (n = 7), which adds to 59 patients required further investigations. Out of them, six patients were excluded and 53 were enrolled into the study. CXR: chest radiography; AB: air-bronchogram; FB: fluid bronchogram; US: ultrasound.
Table 1.
Personal and clinical characteristics, chest radiography findings, coagulation profile and histopathological types of the studied patients
| Items | |
|---|---|
| Age/year (mean ± SD) | 56.7 ± 8.8 |
| Sex | |
| • Male | 44 (83.0%) |
| • Female | 9 (17.0%) |
| Smoking status | |
| • Smoker | 39 (73.6%) |
| • Non-smoker | 14 (26.4%) |
| Smoking pack/year (mean ± SD) | 22.7 ± 20.0 |
| History of chronic lung diseases | |
| • No | 19 (35.8%) |
| • COPD | 28 (52.8%) |
| • DPLD | 6 (11.3%) |
| Presenting symptomsa | |
| • Cough | 43 (81.1%) |
| • Chest pain | 42 (79.2%) |
| • Dyspnoea | 27 (50.9%) |
| • Haemoptysis | 24 (45.3%) |
| • Fever | 21 (39.6%) |
| • Hoarseness of voice | 9 (15.0%) |
| Chest radiography findingsa | |
| • Hilar LN enlargement | 14 (30.4%) |
| • Mass | 35 (76.1%) |
| • Mass necrosis | 4 (11.4%) |
| • Consolidations | 7 (15.2%) |
| • Collapse | 2 (4.3%) |
| • Pleural effusion | 14 (30.4%) |
| • Elevated dome of the diaphragm | 1 (2.2%) |
| Platelets count | 368.0 ± 9.0 |
| INR | 0.84 ± 0.02 |
| Histopathological types | |
| • Squamous cell carcinoma | 16 (30.2%) |
| • Adenocarcinoma | 16 (30.2%) |
| • Large cell carcinoma | 15 (28.3%) |
| • SCLC | 6 (11.3 %) |
SD: standard deviation, COPD: chronic obstructive pulmonary disease, DPLD: diffuse parenchymal lung diseases.
Percentages add to more than 100% due to multiple presentation of symptoms or CXR findings. INR: international normalization ratio; LN: lymph node; SCLC: small cell lung cancer.
In many cases, multiple types of abnormalities were detected, e.g. mass and peritumoural atelectasis, mass and effusion and intratumoural necrosis and peritumoural atelectasis. The data obtained by thoracic US were compared with those from the chest CT-scan, which was considered the reference method.
The supraclavicular LN are located above the manubrium, lateral to the medial edge of the common carotid artery, and medial to the clavicle. We defined adenopathy as LN with round shape and short axis ≥5 mm on CT or US, with no significant echogenic nodal hilum on sonography.27
-
Biopsy techniques:
Thoracic US-guided biopsy
Forty-one out of 44 patients (93.2%) with lung mass detected by thoracic US examination were subjected to thoracic US-guided biopsy by using automatic disposable soft tissue 16-gauge tru-cut biopsy needle with 20 mm sample notch (Medplus Inc OSMUNDA Medical Device Service GmbH; Keithstr, Berlin, Germany). The puncture site with pleural contact was identified and marked then the direction and the depth of insertion were recognized. A liberal amount of local anesthesia (lignocaine 1%) was infiltrated to provide maximal patient comfort during the procedure. All procedures were subsequently performed with ‘freehand technique’. The needle was inserted over the top of the rib to avoid the neurovascular bundle injury, and it was advanced just to the nearest border of the lesion. The number of specimens was decided on the basis of the quality of the tissue sample evaluated by the thoracic US operator, on average three to five biopsies. Biopsies were directly immersed in formaldehyde (4%) and sent for histopathological examination. Total procedural time was about 30 minutes. The chest was re-examined by thoracic US immediately after the procedure, and a CXR was obtained after 6 hours or immediately after the procedure if the pre- and post-procedure thoracic US findings differed. All patients were observed for 24 hours after the procedure. The presence or absence of haemoptysis, pneumothorax, and subcutaneous emphysema were recorded. US-guided biopsy of supraclavicular LN was done for four patients in addition to thoracic US-guided lung biopsy.
CT-guided or bronchoscopic biopsy
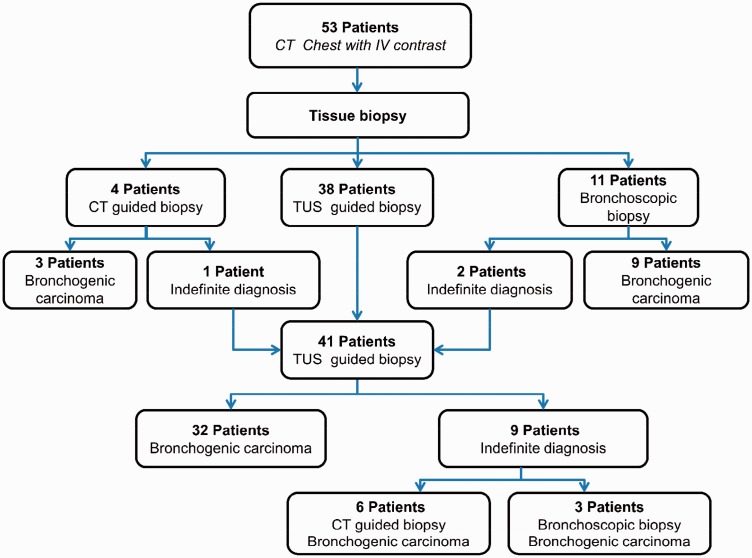
Patients whose lesions were not accessible by thoracic US (n = 15), and those with indefinite thoracic US-guided biopsy yield (n = 9), subjected to either CT-guided biopsy (n = 10) or fibreoptic bronchoscopic (FB) biopsy (n = 14) (Figure 2), which was conducted as soon as the histopathological results of thoracic US-guided biopsy became available (on average 5 days).
Figure 2.
Algorithm for biopsy procedures used to diagnose bronchogenic carcinoma in the study. This figure demonstrates that 53 patients enrolled into this study were subjected to chest CT-scan with IV contrast. Based on CT-scan findings they underwent tissue biopsy; thoracic US-guided biopsy (n = 38), bronchoscopic biopsy (n = 11) and CT-guided biopsy (n = 4). Patients that showed indefinite diagnosis by either bronchoscopy (n = 2) or CT-guided biopsy (n = 1) were added to patients subjected to thoracic US-guided biopsy summed up to 41; out of them 32 confirmed to have bronchogenic carcinoma by thoracic US-guided biopsy, while the remaining were confirmed to have bronchogenic carcinoma either by CT-guided biopsy (n = 6) or bronchoscopic biopsy (n = 3). TUS: thoracic US, CT: computed tomography; US: ultrasound.
Statistical analysis
Data were statistically analyzed by using SPSS version 17 for windows. Data were expressed as mean ± SD for quantitative variables and as frequencies and percentages for qualitative variables. Chi-square-test (X2) and Student's t-test were used for comparison between the two imaging modalities. Statistical significance was considered at p value < 0.05 (with a confidence limit at 95%).
Results
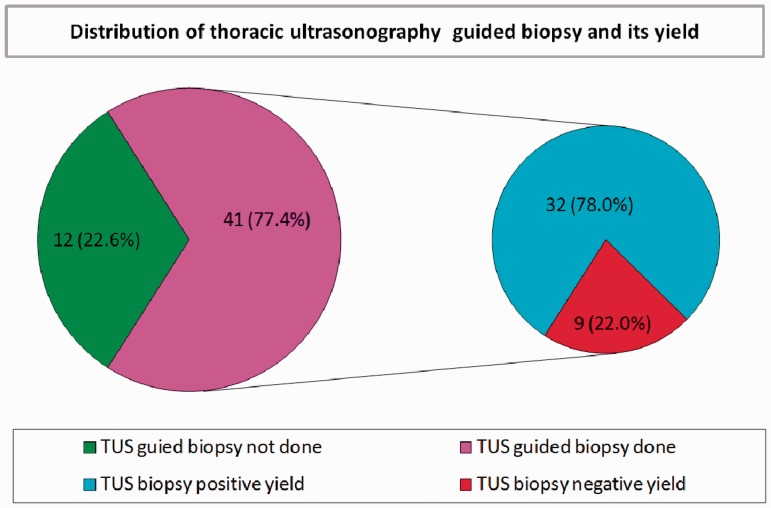
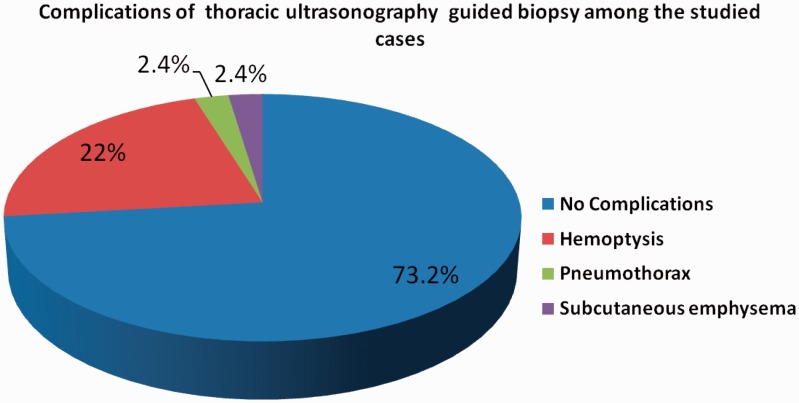
Table 2 demonstrates that thoracic US had a significantly higher detection rate of peritumoural atelectasis, paralysis of the diaphragm and supraclavicular LN invasion than chest CT-scan. It had lower detection rate of pulmonary masses and mediastinal LN invasion than chest CT-scan; also the size of masses detected by thoracic US was nonsignificantly lower than that detected by chest CT-scan. Thoracic US had a high but nonsignificant detection rate of necrosis within the mass, pleural effusion, consolidations and chest wall invasion compared to chest CT-scan. Moreover, thoracic US detected static AB and FB in 53.3% of associated consolidation, and it detects swirling sign in 25% of pleural effusion. Figure 3 shows that thoracic US-guided biopsy was done for 41 (77.4%) patients; positive results were obtained in 32 patients (78.0%), and negative in nine patients (22.0%). Table 3 demonstrates that all bronchogenic carcinoma patients with negative thoracic US-guided biopsy yield had significantly higher mass size ≥5 cm (10.8 ± 2.2 cm vs. 6.8 ± 2.6 cm; p = 0.001) and necrosis within the mass (66.7% of patients vs. 40.6%; p = 0.001). The presence of consolidations, peritumoural atelectasis, chest wall invasion and histopathological types of bronchogenic carcinoma did not affect the yield of thoracic US-guided biopsy. Figure 4 shows that only 22% of patients developed haemoptysis, 2.4% developed pneumothorax, and another 2.4% developed subcutaneous emphysema post-thoracic US-guided tru-cut biopsy. Figures 5, 6 and 7 show CXR, chest CT-scan and thoracic US of three studied patients.
Table 2.
Detection rate of thoracic ultrasound in comparison to chest CT-scan of different types of lesions among the studied patients
| Item | Chest CT-scan (n = 53) |
Thoracic US (n = 53) |
Significance test | p value |
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Mediastinal lymph node | 19 (35.8) | 6 (11.3) | X 2 = 8.8a | 0.003 |
| Mass | 53 (100.0) | 44 (83.0) | X 2 = 9.8a | 0.002 |
| Necrosis within the mass | 17 (32.1) | 19 (35.8%) | X 2 = 0.17 | 0.68 |
| Mass size/cm (mean ± SD) | 6.39 ± 3.79 | 5.96 ± 2.90 | t = 0.65 | 0.51 |
| Consolidations | 10 (18.9) | 15 (28.3) | X 2 = 1.8 | 0.17 |
| • Static AB and fluid bronchogram | – | 8 (53.3) | ||
| • Dynamic AB | – | 7 (46.7) | ||
| Pleural effusion | 23 (43.4) | 32 (60.4) | X 2 = 3.1 | 0.08 |
| • Swirling sign | – | 8 (25.0) | ||
| Peritumoural atelectasis | 16 (30.2) | 30 (56.6) | X 2 = 7.5a | 0.006 |
| Paralysis of diaphragm | 6 (11.3) | 16 (30.2) | X 2 = 5.7a | 0.017 |
| Chest wall invasion | 6 (11.3) | 11 (20.8) | X 2 = 1.7 | 0.18 |
| Supraclavicular LN | 1 (1.2) | 11 (20.8) | X 2 = 9.4a | 0.002 |
CT: computed tomography; thoracic US: thoracic ultrasonography; AB: airbronchogram; LN: lymph node.
Significant p value.
Figure 3.
Distribution of thoracic ultrasonography-guided biopsy and its yields among the studied cases. This Pie-chart showed that thoracic US-guided tru-cut biopsy was done for 41 (77.4%) patients, it yield positive results in 32 (78.0%) patients, and negative in nine patients (22.0%). US: ultrasound; TUS: thoracic ultrasound.
Table 3.
Radiographic characteristics and histopathological types of lesions related to thoracic ultrasound-guided biopsy yield
| Item | Thoracic US-guided biopsy yield |
Significance test | p value | |
|---|---|---|---|---|
| Positive (No. 32) | Negative (No. 9) | |||
| Mass size <5 cm | 9 (28.1) | 0 (0.0) | X2 = 28.0a | 0.001 |
| Mass size >5 cm | 22 (68.8) | 9 (100.0) | ||
| Mass size/cm (mean ± SD) | 6.8 ± 2.6 | 10.8 ± 2.2 | t = 33.7a | 0.001 |
| Mass necrosis | 13 (40.6) | 6 (66.7) | X2 = 10.7a | 0.005 |
| Consolidations | 11 (34.4) | 3 (33.3) | X2 = 1.3 | 0.50 |
| Peritumoural atelectasis | 17 (53.1) | 4 (44.4) | X2 = 2.3 | 0.30 |
| Chest wall invasion | 7 (21.9) | 1 (11.1) | X2 = 0.66 | 0.71 |
| Histopathological types | X2 = 13.37 | 0.34 | ||
| • SCLC | 4 (12.5) | 0 (0.0) | ||
| • Squamous cell carcinoma | 9 (28.1) | 4 (44.4) | ||
| • Adenocarcinoma | 8 (25.0) | 2 (22.2) | ||
| • Large cell carcinoma | 11 (34.4) | 3 (33.3) | ||
AB: airbronchogram, SCLC: small cells lung cancer.
Significant p value.
Figure 4.
Complications of thoracic ultrasonography-guided biopsy complications among the studied cases. This Pie-chart showed that only 22% of patients developed haemoptysis, 2.4% developed pneumothorax and another 2.4% developed subcutaneous emphysema post-thoracic US-guided tru-cut biopsy. US: ultrasound.
Figure 5.
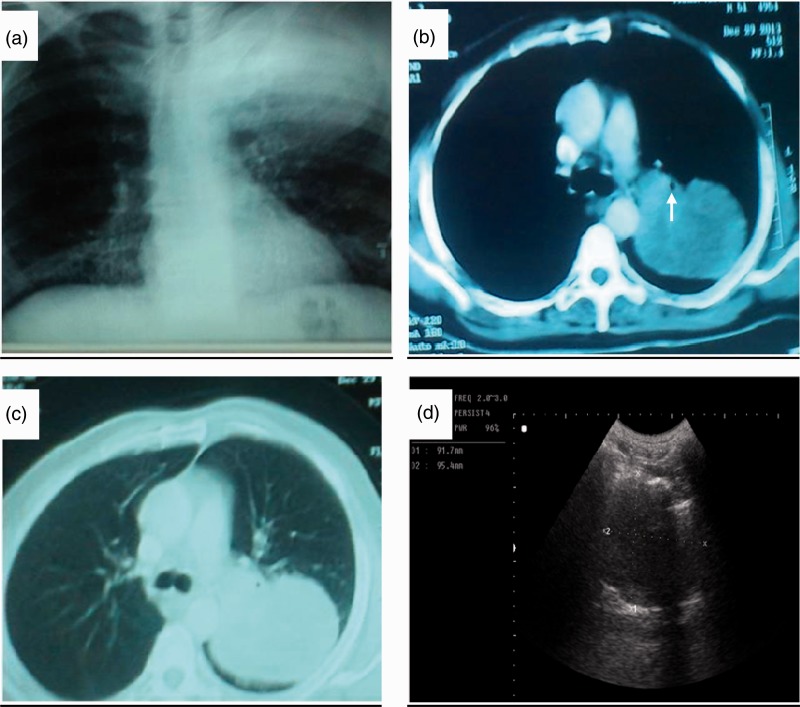
Chest radiography (a), chest CT-scan (b, c) and thoracic ultrasound (d) of patient with confirmed adenocarcinoma of the apicoposterior segments of left lung. (a) Chest radiography shows a homogenous mass measuring 10 x 9.4 cm with smooth outer margin occupying the left upper zone with overcrowded ribs, (b, c) Chest CT-scans with the mediastinal and pulmonary windows show a large semi-homogenous mass measuring 9.4 x 9.1 cm, occupying the apicoposterior segments of the left upper lobe compressing the left upper lobe bronchus (white arrow). (d) Thoracic US shows a large hypoechoic soft mass measuring 9.2 x 9.5 cm, with ill-defined borders and no central necrosis. Thoracic US-guided biopsy revealed as poorly differentiated adenocarcinoma of grade III. CT: computed tomography; US: ultrasound.
Figure 6.
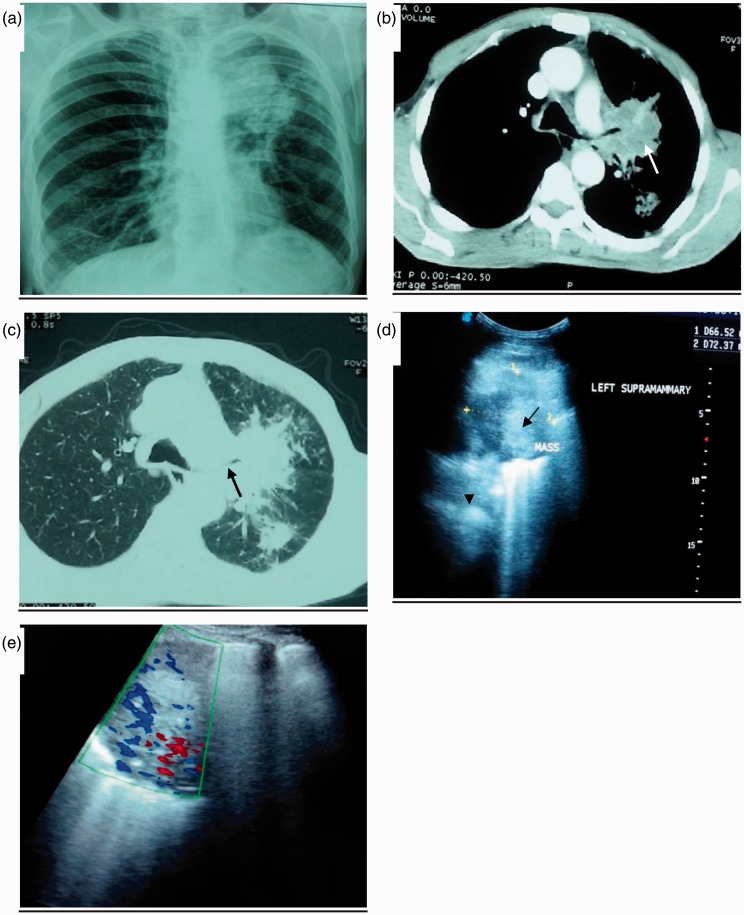
Chest radiography (a), chest CT-scan (b, c) and thoracic ultrasound (d, e) of patient with confirmed adenocarcinoma of the left lung. (a) Chest radiograph shows heterogonous soft tissue mass measuring approximately 7 x 6.9 cm, occupying left paratracheal area with irregular border. (b, c) Chest CT-scan mediastinal and pulmonary windows showing left hilar and parahilar soft tissue mass measuring 7 x 6 cm, with spiculated edge (white arrow) compressing left upper lobe bronchus (black arrow). There is a nodule approximately 2 cm in diameter with irregular border occupying superior segments of left lower lobe. Additionally, there is an associated consolidation with hyperechoic punctiform lesion (airbronchogram) (black arrow head). (d) Thoracic US showing large hypoechoic mass measuring 6.7 x 7.4 cm, with ill-defined borders (black arrow). (e) Echo Doppler showing moderate internal vascularity. Thoracic US-guided biopsy revealed as poorly differentiated adenocarcinoma grade III. CT: computed tomography; US: ultrasound.
Figure 7.
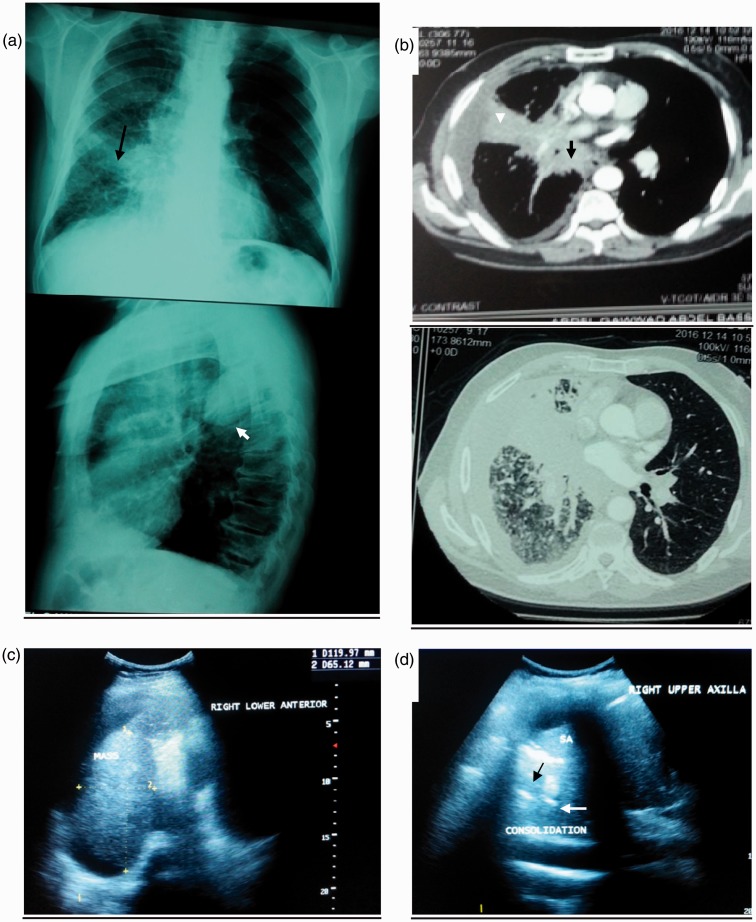
Chest radiography (a), chest CT-scan (b) and thoracic ultrasound (c, d) of patient with confirmed invasive squamous cell carcinoma of the right lung. (a) Chest radiography shows heterogeneous opacity occupying right middle lung zone (black compact arrow) with nodular infiltration of right lower zone and hyper translucency in left lung, lateral view showing effusion encysted at upper part of right oblique fissure (white compact arrow). (b) CT-scan mediastinal and pulmonary windows show right hilar and parahilar soft tissue mass measuring 7 x 6 cm with speculated edge (black dashed arrow) compressing right upper lobe bronchus (dashed arrow head), there is an associated peritumoural atelectasis (white arrow head) and right side minimal pleural effusion. (c) Thoracic US shows large hypoechoic soft mass measuring 6.7 x 7.4 cm, with ill-defined borders. (d) Thoracic US shows punctiform hyperechoic lesion (black arrow), also there is anechoic tubular lesion representing fluid bronchogram (White arrow). Thoracic US-guided biopsy revealed as invasive squamous cell carcinoma. CT: computed tomography; US: ultrasound.
Discussion
Thoracic US has become a valuable diagnostic aid for respiratory physicians and is increasingly being used to guide interventional procedures.28 Tissue sampling for histopathological examination was obtained via thoracic US-guided biopsy in 32 patients, bronchoscopy in 12 patients and CT-guided biopsy in nine patients. Histopathological examination revealed that the most common types of bronchogenic carcinoma among our patients were squamous cell carcinoma and adenocarcinoma (30.2% each) followed by large cell carcinoma (28.3%) and SCLC (11.3%). A similar distribution of pathological classification of bronchogenic carcinoma in Egyptian patients was reported by Abu-Youssef et al.29 Santos-Martínez et al.30 reported that the most common histological types were squamous cell carcinoma (33%) and adenocarcinoma (30%). In another study by Rawat et al.31 reported that squamous cell carcinoma was the most common cell types (44.8%) followed by adenocarcinoma (19.7%) and SCLC (16.75%).
Our study demonstrated that thoracic US has a significantly lower detection rate of pulmonary masses compared to chest CT-scan (83.0% vs. 100.0%, p = 0.002), the remaining patients had no US window to the lesion as they had either central lesions or lesions behind ribs. The mass size was nonsignificantly lower in thoracic US than chest CT-scan (5.96 ± 2.90 vs. 6.39 ± 3.79, p = 0.51), therefore, US might be able to distinguish tumour from its secondary effects, e.g. consolidations and/or peritumoural atelectasis. Islam and Tonn14 postulated that peripheral lung tumours can be detected by US as long as the tumour is attached to the pleura. A similar lower US detection rate for pulmonary masses (62%) had been reported by Abu-Youssef et al.29
The current study demonstrated that thoracic US was significantly superior to chest CT-scan in detection of peritumoural atelectasis (56.6% vs. 30.2%, p = 0.006), with nonsignificantly higher detection rate of consolidations (28.3% vs. 18.9%, p = 0.17). Thoracic US can differentiate between consolidations due to simple pneumonia from that of post-obstructive pneumonia by visualization of static AB and/or FB (53.3%). Similar results reported by Abu-Youssef et al.29 documented that there was a statistically significant difference between thoracic US and chest CT-scan in detecting consolidation and/or peritumoural atelectasis. However, Chira et al.19 reported that the thoracic US is inferior to chest CT-scan in detection of peritumoural atelectasis (33 vs. 38), while it was superior to chest CT-scan in detection of necrosis within mass (87.71% vs. 72.8%).
Regarding the nodal involvement, our study revealed that thoracic US was superior to chest CT-scan in detecting supraclavicular LN invasion (N3) (20.8% vs. 11.3%; p = 0.002) and inferior in the detection of mediastinal LN involvement (11.3% vs. 35.8%; p = 0.003). Ultrasonography-guided biopsy of supraclavicular LN was done for four patients and revealed positive results. These findings denote that thoracic US has an important role in bronchogenic carcinoma staging that precludes unnecessary surgical intervention. The limited ability of thoracic US to detect mediastinal LN invasion is due to the fact that the mediastinal compartment is potentially hidden to thoracic US. The lower detection rate of supraclavicular LN invasion by CT-scan could be attributed to the incoming artifact from the contrast agent in the venous system which severely obscures the visualization of supraclavicular LN.32 Previous studies documented that US has higher sensitivity in detecting supraclavicular LN than both CT-scan33 and clinical examination.29 Another study reported that 12–31% bronchogenic carcinoma patients have sonographically detected supraclavicular LN metastases at the time of presentation.34 Almost 50% of bronchogenic carcinoma patients with mediastinal adenopathy on CT-scan have supraclavicular LN metastases. Therefore, in the presence of mediastinal adenopathy in suspected or known bronchogenic carcinoma, even when supraclavicular LN are not shown on CT-scan, it is recommended to investigate further with US.27
Our study revealed that thoracic US nonsignificantly detected cancerous invasion into the pleura and chest wall for bronchogenic carcinoma staging better than chest CT-scan (20.8% vs. 11.3%, p = 0.18). This is due to the fact that all layers of chest wall have different acoustic impedance and consequently different tissue interfaces that increase the ability of US to differentiate between them. This nonsignificant difference between thoracic US and chest CT-scan in the detection of chest wall invasion is because three of our patients had posterior chest wall invasion located behind the scapula which is difficult to detect with thoracic US. In the same context previous studies documented that the thoracic US had higher sensitivity and specificity for detection of pleural and chest wall invasion than chest CT-scan.14,19,29,35,36
We found that the thoracic US had a higher but nonsignificant detection rate of pleural effusion compared to chest CT-scan (60.4% vs. 43.4%; p = 0.08). Swirling sign was detected by thoracic US in 25% of patients with pleural effusion. Similar results reported by Abu-Youssef et al.29 and Kurian et al.37 found that thoracic US was comparable to chest CT-scan in the detection of pleural effusion. Chian et al.23 reported that the presence of the ‘swirling pattern’ is strongly predictive of a malignant pleural effusion.
The overall thoracic US-guided biopsy diagnostic yield in our study was 78.0% (32/41), which is lower than that reported in previous studies.2,3,18,20,38–42 All patients with negative thoracic US-guided biopsy yield had mass size ≥5 cm with significant necrosis within the mass compared to those with positive yield (66.7% vs. 40.6%, p = 0.005). Therefore, the lower diagnostic yield encountered in our study could be attributed to larger mass size and tissue necrosis. This finding is further supported by histopathological examinations of these patients that revealed non-specific inflammation and necrosis. Other studies concluded that the main cause of false-negative results for malignancy was extensive necrosis3,39 and lesion size.41 However, Scisca et al.43 reported that the tumour size did not affect the diagnostic outcome.
The post thoracic US-guided biopsy complications in our study (haemoptysis 22%, pneumothorax 2.4% and subcutaneous emphysema 2.4%) were trivial and self-limited within 24 hours without intervention. The lower rate of pneumothorax, encountered in this study is due to the wall contact of the tumoural masses as well as large sizes of the lesions. Therefore, thoracic US-guided biopsy is a safe and, well-tolerated procedure for the patients even those with reduced pulmonary reserve as 52.8% of our patients had COPD and 11.3% had DPLD. Previous studies reported similar results documenting infrequent complication rates of thoracic US-guided biopsy. Haemoptysis and pneumothorax are the most frequent complications of transthoracic needle biopsy and are mostly mild and self-limiting.18,38,39,42–44
Conclusions
Thoracic US had a significantly higher detection rate of peritumoural atelectasis, paralysis of the diaphragm, and supraclavicular LN invasion; while it had a lower detection rate of mediastinal LN invasion and pulmonary masses compared to chest CT-scan. Moreover, bedside percutaneous thoracic US-guided biopsy performed by pulmonologists had a good positive yield (78%) beside it is a safe, accurate, practical and effective procedure for obtaining biopsy for diagnosis of bronchogenic carcinoma. Thoracic US has a complementary role to chest CT-scan in diagnosis and staging of bronchogenic carcinoma. Therefore, we recommend the use of thoracic US examination and thoracic US-guided biopsy in the diagnostic workup of suspected bronchogenic carcinoma, especially if peripherally located.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
This study was done according to the principles outlined in the Declaration of Helsinki. Unfortunately we don’t have an ethical committee at our institute.
Guarantor
MRH
Contributors
MRH, ESMS, SBE and OIA researched literature and conceived the study. MRH, ESMS, SBE and OIA designed the audit. MRH, ESMS, SBE and OIA did the data analysis. MRH wrote the first draft of the manuscript. MRH, ESMS, SBE and OIA wrote the final version of the manuscript. All authors reviewed and approved the final version of the manuscript.
Acknowledgements
N/A
References
- 1.Hoque MS, Hashem MA, Hasan S, et al. Role of CT scan in the evaluation of lung tumor with cytopathilogical correlation. Faridpur Med Coll J 2014; 9: 37–41. [Google Scholar]
- 2.Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013; 143: e142S–65S. [DOI] [PubMed] [Google Scholar]
- 3.Cao B, Wu J, Li X, et al. Sonographically guided transthoracic biopsy of peripheral lung and mediastinal lesions. Role of contrast-enhanced sonography. J Ultrasound Med 2011; 30: 1479–1490. [DOI] [PubMed] [Google Scholar]
- 4.Hansell DM, Boiselle PM, Goldin J, et al. Thoracic imaging. Respirology 2010; 15: 393–400. [DOI] [PubMed] [Google Scholar]
- 5.Silvestri GA, Gould MK, Margolis ML, et al. Non-invasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007; 132: 178S–201S. [DOI] [PubMed] [Google Scholar]
- 6.Sperandeo M, Filabozzi P, Varriale A, et al. Role of thoracic ultrasound in the assessment of pleural and pulmonary diseases. J Ultrasound 2008; 11: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira C, Saraiva A. Comparative study between computed tomography and bronchoscopy in the diagnosis of lung cancer. Radiol Bras 2010; 43: 229–235. [Google Scholar]
- 8.Simeone JF, Mueller PR, van Sonnenberg E. The uses of diagnostic ultrasound in the thorax. Clin Chest Med 1984; 5: 281–290. [PubMed] [Google Scholar]
- 9.Sartori S, Tombesi P. Emerging roles for transthoracic ultrasonography in pulmonary diseases. World J Radiol 2010; 2: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sconfienza LM, Mauri G, Grossi F, et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology 2013; 266: 930–935. [DOI] [PubMed] [Google Scholar]
- 11.Nadig SN, Block MI. “Drowned lung” following lobectomy and radiation therapy: a case report. J S C Med Assoc 2003; 99: 26–29. [PubMed] [Google Scholar]
- 12.Koegelenberg CFN, Diacon AH, Bolliger CT. Transthoracic ultrasound of the chest wall, pleura, and the peripheral lung. In: Bollger CT, Herth FJF, Mayo PH, et al. (eds). Progress in Respiratory Research 2009; Vol. 37, Basel: Karger, pp. 22–33.. [Google Scholar]
- 13.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Islam S, Tonn H. Thoracic ultrasound overview. In: Bolliger CT, Herth FJF, Mayo PH, et al. (eds). Clinical chest ultrasound, Sydney: Karger, 2009, pp. 11–20. [Google Scholar]
- 15.Kim GR, Hur J, Lee SM, et al. CT fluoroscopy-guided lung biopsy versus conventional CT-guided lung biopsy: a prospective controlled study to assess radiation doses and diagnostic performance. Eur Radiol 2011; 21: 232–239. [DOI] [PubMed] [Google Scholar]
- 16.Carrafiello G, Laganà D, Nosari AM, et al. Utility of computed tomography (CT) and of fine needle aspiration biopsy (FNAB) in early diagnosis of fungal pulmonary infections: study of infections from filamentous fungi in haematologically immunodeficient patients. Radiol Med 2006; 111: 33–41. [DOI] [PubMed] [Google Scholar]
- 17.Muller NL. Imaging of the pleura. Radiology 1993; 186: 297–309. [DOI] [PubMed] [Google Scholar]
- 18.García-Ortega A, Briones-Gómez A, Fabregat SM, et al. Benefit of chest ultrasonography in the diagnosis of peripheral thoracic lesions in an interventional pulmonology unit. Arch Bronconeumol 2016; 52: 244–249. [DOI] [PubMed] [Google Scholar]
- 19.Chira R, Chira A, Mircea PA. Intrathoracic tumors in contact with the chest wall – ultrasonographic and computed tomography comparative evaluation. Med Ultrason 2012; 14: 115–119. [PubMed] [Google Scholar]
- 20.Blank W. Interventional chest sonography. In: Chest sonography, Berlin, Germany: Springer-Verlag Berlin Heidelberg, 2011, pp. 187–209. [Google Scholar]
- 21.Hoosein MM, Barnes D, Khan AN, et al. The importance of ultrasound in staging and gaining a pathological diagnosis in patients with lung cancer a two year single centre experience. Thorax 2011; 66: 414–417. [DOI] [PubMed] [Google Scholar]
- 22.Lichtenstein D, Mezie're G, Seitz J. The dynamic air bronchogram a lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 2009; 135: 1421–1425. [DOI] [PubMed] [Google Scholar]
- 23.Chian CF, Su WL, Soh LH. Echogenic swirling pattern as a predictor of malignant pleural effusions in patients with malignancies. Chest 2004; 126: 129e34–129e34. [DOI] [PubMed] [Google Scholar]
- 24.Doerschug KC, Schmidt GA. Intensive care ultrasound: III. Lung and pleural ultrasound for the intensivist. Ann Am Thorac Soc 2013; 10: 708–712. [DOI] [PubMed] [Google Scholar]
- 25.Miller A. Practical approach to lung ultrasound. BJA Educ 2016; 16: 39–45. [Google Scholar]
- 26.Rednic N, Orăş an O. Subpleural lung tumours ultrasonography. Med Ultrason 2010; 12: 81–87. [PubMed] [Google Scholar]
- 27.Kumaran M, Benamore RE, Vaidhyanath R, et al. Ultrasound guided cytological aspiration of supraclavicular lymph nodes in patients with suspected lung cancer. Thorax 2005; 60: 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koegelenberg CF, Bolliger CT, Irusen EM, et al. The diagnostic yield and safety of ultrasound-assisted biopsy of mediastinal masses. Respiration 2011; 81: 134–141. [DOI] [PubMed] [Google Scholar]
- 29.Abu-Youssef HA, Kamel KM, Selim S, et al. Study of the added value of transthoracic ultrasound in staging of lung cancer. Egypt J Chest Dis Tuberc 2014; 63: 10–25. [Google Scholar]
- 30.Santos-Martínez MJ, Curull V, Blanco ML, et al. Lung cancer at a university hospital: epidemiological and histological characteristics of a recent and a historical series. Arch Bronconeumol 2005; 41: 307–312. [DOI] [PubMed] [Google Scholar]
- 31.Rawat J, Sindhwani G, Gaur D, et al. Clinico-pathological profile of lung cancer in Uttarakhand. Lung India 2009; 26: 74–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saade C and Sydney NSW/AU. Imaging of the Supraclavicular lymph node in routine oncology screening: an MDCT approach. ECR 2012, Poster No.: C-0563.
- 33.Overhagen VH, Brakel K, Heijenbrok MW, et al. Metastases in supraclavicular lymph nodes in lung cancer: assessment with palpation, US, and CT. Radiology 2004; 232: 75–80. [DOI] [PubMed] [Google Scholar]
- 34.Fultz PJ, Harrow AR, Elvey SP. Sonographically guided biopsy of supraclavicular lymph nodes: a simple alternative to lung biopsy and other more invasive procedures. AJR 2003; 180: 1403–1409. [DOI] [PubMed] [Google Scholar]
- 35.Caroli G, Dell'Amore A, Cassanelli N, et al. Accuracy of transthoracic ultrasound for the prediction of chest wall infiltration by lung cancer and of lung infiltration by chest wall tumours. Heart Lung Circ 2015; 24: 1020–1026. [DOI] [PubMed] [Google Scholar]
- 36.Bandi V, Lunn W, Ernst A, et al. Ultrasound vs. CT in detecting chest wall invasion by tumor. A prospective study. Chest 2008; 133: 881–886. [DOI] [PubMed] [Google Scholar]
- 37.Kurian J, Levin T, Han BK, et al. Comparison of ultrasound and CT in the evaluation of pneumonia complicated by parapneumonic effusion in children. AJR 2009; 193: 1648–1654. [DOI] [PubMed] [Google Scholar]
- 38.El-Shimya WS, El-Emery FA, Abd El-Zaher AH, et al. The diagnostic value of ultrasound-guided percutaneous transthoracic core-needle biopsy versus computed tomography-guided biopsy in peripheral intrathoracic lesions. Egypt J Bronchol 2016; 10: 12–19. [Google Scholar]
- 39.Grasso RF, Luppi G, Giurazza F, et al. Lung core biopsy US-guided: approach to characterise subpleural lesions. Available at: www.myESR.org. Poster No.: C-0197. Congress: ECR 2015. 10.1594/ecr2015/C-0197 (accessed 11 December 2016).
- 40.Montaudon M, Latrabe V, Pariente A, et al. Factors influencing accuracy of CT-guided percutaneous biopsies of pulmonary lesions. Eur Radiol 2004; 14: 1234–1240. [DOI] [PubMed] [Google Scholar]
- 41.Yeow KM, Tsay PK, Cheung YC. Factors affecting diagnostic accuracy of CT-guided coaxial cutting needle lung biopsy: retrospective analysis of 631 procedures. J Vasc Interv Radiol 2003; 14: 581–588. [DOI] [PubMed] [Google Scholar]
- 42.Liao WY, Chen MZ, Chang YL, et al. US-guided transthoracic cutting biopsy for peripheral thoracic lesions less than 3 cm in diameter. Radiology 2000; 217: 685–691. [DOI] [PubMed] [Google Scholar]
- 43.Scisca C, Rizzo M, Maisano R, et al. The role of ultrasound-guided aspiration biopsy of peripheral pulmonary nodules: our experience. Anticancer Res 2002; 22: 2521–2523. [PubMed] [Google Scholar]
- 44.Wallace MJ, Krishnamurthy S, Broemeling LD, et al. CT-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) lesions. Radiology 2002; 225: 823–828. [DOI] [PubMed] [Google Scholar]