SUMMARY
Background
Gluten-free diet (GFD) is the only management available for celiac disease (CeD), a permanent immune intolerance to gluten. Nexvax2® is the first therapeutic vaccine designed to treat CeD. The adjuvant-free formulation of peptides is intended to engage and render gluten-specific CD4+ T cells unresponsive to further antigenic stimulation. We have assessed safety and pharmacodynamics of Nexvax2® in patients with CeD on GFD.
Methods
In two randomized, double-blind, placebo-controlled, phase 1 studies at 12 community sites in Australia, New Zealand and the United States, we screened for HLA-DQ2·5+ CeD patients (aged 18–70 years) on GFD. The screening and post-treatment periods included either a crossover, placebo-controlled, oral gluten challenge (OGC) to mobilize and assess T cells responsive to Nexvax2 or, for the final cohort in each study, endoscopy and duodenal histology without OGC. Participants and study staff were masked to the gluten content of food provided for each interval of the OGCs. One of two sequences of active and placebo challenges was assigned (1:1) by central randomization using a simple block method. The sequence of challenges was active/placebo then active/placebo, or placebo/active then active/placebo for the OGCs in the screening and post-treatment periods, respectively. Participants with a negative interferon (IFN)-γ release assay (IGRA) to Nexvax2 peptides after the screening OGC, or Marsh score >1 were discontinued before dosing. There was temporal allocation of participants to sequential cohorts assessing multiple fixed intradermal doses of Nexvax2 (60µg, 90µg, or 150µg weekly in the 3-dose study; or 150µg, or 300µg two-times weekly in the 16-dose study) in 0.1 mL 0.9% sodium chloride. A maximum tolerated dose (MTD) was administered in the final biopsy cohort in each study. Participants within each cohort were assigned to receive Nexvax2 or placebo by central randomization (2:1, respectively) using simple block method in SAS software Version 9·2. Participants, investigators, and study staff were masked to the treatment assignment, except for the study pharmacist. The primary endpoint was the number and percentage of adverse events in the treatment period. Other safety outcomes included duodenal histology, gastrointestinal symptoms, plasma cytokines, and immune cell frequencies. The main pharmacodynamic endpoint was IGRA to Nexvax2 peptides. All participants who received Nexvax2 or placebo, the safety population, were included in an intention to treat analysis for the primary endpoint. Additional post hoc analyses were also performed. Both trials were completed and closed before data analysis. Trials were registered with Australian New Zealand Clinical Trials Registry, numbers ACTRN12612000355875 and ACTRN12613001331729.
Findings
Participants were screened from November 28, 2012 to August 14, 2014, and August 3, 2012 to September 10, 2013, for the 3-dose and 16-dose studies respectively. Across both studies, 136 (80%) of 169 volunteers met initial eligibility criteria. After OGC, 62 (57%) of 108 participants were randomized, and after endoscopy 20 (71%) of 28 participants were randomized. The number of participants in the 3-dose study randomly allocated to placebo or active treatment arms were 3 and 9 in the first cohort assessing Nexvax2 60 µg, 4 and 9 in the second cohort assessing Nexvax2 90 µg, and 4 and 8 in the third cohort, and 3 and 3 in final (biopsy) cohort, which both assessed Nexvax2 150 µg. The number of participants in the 16-dose study randomly allocated to receive placebo or active treatment were 4 and 8 in the first cohort assessing Nexvax2 150 µg, 3 and 10 in the second cohort assessing Nexvax2 300 µg, and 7 and 7 in the final (biopsy) cohort which assessed Nexvax2 150 µg. The MTD for Nexvax2 was 150 µg due to transient, acute gastrointestinal adverse events with onset at 2 to 5 h after initial doses of Nexvax2, similar to those caused by gluten ingestion. The total number of treatment emergent adverse events and percentage (%) of participants with at least one in the ascending dose cohorts of the 3-dose study were 15 (55%) in the 11 placebo-treated participants, 25 (56%) in 9 who received Nexvax2 60µg, 65 (78%) in 9 who received Nexvax2 90µg, and 16 (63%) in 8 who received Nexvax2 150µg, and 7 (100%) in the 3 placebo-treated participants and 1 (33%) in 3 participants randomized to Nexvax2 150µg in the biopsy cohort; in the 16-dose study, there were 13 (71%) in 7 placebo-treated participants, 21 (75%) 8 who received Nexvax2 150µg, 26 (100%) in 10 who received Nexvax2 300µg, and 24 (86%) in the 7 placebo-treated participants and 18 (71%) in 7 who received Nexvax2 150µg in the biopsy cohort. Vomiting, nausea and headache were the only treatment emergent adverse events that occurred in at least 5% of participants in either study. The total number of treatment emergent adverse events and percentage (%) of participants with at least one occurrence were: vomiting: 2 (22%) in 9 participants receiving Nexvax2 60µg, 5 (56%) in 9 receiving Nexvax2 90µg, 4 (50%) in 8 receiving Nexvax2 150µg in the 3-dose study; and 5 (63%) in 8 receiving Nexvax2 150µg, 4 (40%) in 10 receiving Nexvax2 300µg, 1 (14%) in 7 participants receiving placebo in the biopsy cohort of the 16-dose study; nausea: 1 (11%) with Nexvax2 60µg, 4 (44%) with Nexvax2 90µg, and 2 (25%) with Nexvax2 150µg in the 3-dose study, and none in the 16-dose study; headache: 4 (44%) with Nexvax2 90µg in the 3-dose study; and 3 (43%) for placebo in the 1st and 2nd cohorts, 3 (38%) with Nexvax2 150µg, 5 (50%) with Nexvax2 300µg, and 3 (43%) for placebo and 2 (29%) with Nexvax2 150µg in the biopsy cohort of the 16-dose study. Among Nexvax2-treated participants administered the MTD, the number of gastrointestinal treatment emergent adverse events were 8 in 4 (50%) of 8 participants in the 3rd cohort and none (0%) in 3 participants in the biopsy cohort of the 3-dose study, and 5 in 5 (63%) of 8 participants in the 1st cohort and 3 in 2 (29%) of 7 participants in the biopsy cohort of the 16-dose study. For the biopsy cohort of the 16-dose study, which tested the MTD, Nexvax2 was associated with 5 mild and 2 moderate drug-related adverse events in 4 (57%) of 7 participants compared to 5 mild adverse events in 3 (43%) of 7 placebo-treated participants. Comparing biopsies from screening and after the treatment period, median [interquartile range] villous height to crypt depth ratio in distal duodenal biopsies was not significantly different for Nexvax2 at the MTD with 3 doses over 15 days (2.04 [0.69] versus 2.49 [0·67], n=2), or 16 doses over 53 days (1·74 [0·54] versus 1·56 [0·58], n=7), and for placebo over 15 days (1.75 [0.62] versus 2.09 [0.71], n=3) or 16 doses over 53 days (2·10 [0·25] versus 1·92 [0·35], n=7). In those participants who completed the post-treatment OGC per protocol, IGRA was negative in 2 (22%) of 9 placebo-treated participants in the 3 dose study compared to 2 (33%) of 6 who received Nexvax2 60 µg, 5 (63%) of 8 who received Nexvax2 90 µg, and 6 (100%) of 6 who received Nexvax2 150 µg (p=0.007); and in 0 (0%) of 5 placebo-treated participants in the 16 dose study compared to 6 (75%) of 8 who received Nexvax2 150 µg (p=0.021).
Interpretation
The maximum dose tolerated for Nexvax2 was established as 150µg for two-times weekly intradermal administration over 8 weeks. Administering Nexvax2 at the MTD for 8 weeks modified immune responsiveness to Nexvax2 peptides without deterioration in duodenal histology. The gastrointestinal symptoms that followed the first intradermal administration of Nexvax2 resembled those associated with oral gluten challenge. These findings support continued clinical development of Nexvax2 as a potential therapeutic vaccine for CeD.
Funding
ImmusanT, Inc.
INTRODUCTION
Celiac disease (CeD) is an autoimmune-like disease due to dietary gluten characterized by the presence of highly specific autoantibodies to transglutaminase 2 and damage of epithelial cells in the small intestine.1 The community prevalence of CeD is about 1% in children and adults in many regions including Europe and North America.2 Clinical investigation for CeD is usually prompted by digestive symptoms, associated co-morbidities such as iron deficiency, or screening family members of probands.2 Abnormal CeD-specific serology and duodenal histology showing villous atrophy with crypt hyperplasia and intra-epithelial lymphocytosis support the diagnosis of CeD.2 Currently, the only management for CeD is life-long gluten-free diet (GFD).2 However, GFD is burdensome, and restrictive in social situations, resulting in reduced quality of life and, ultimately, non-adherence.3,4 Consequently, GFD seldom results in complete clinical and histological recovery.
CeD has a remarkably strong association with certain Major Histocompatibility Complex (MHC) haplotypes that accounts for almost half of the total heritable risk of CeD.5 About 90% of patients possess the MHC class II genes HLA-DQA1*05 and HLA-DQB1*02 that together encode the Human Leukocyte Antigen (HLA) heterodimer HLA-DQ2·5.6 HLA-DQ2·5 homozygosity is associated with augmented T-cell activation by gluten peptides.7
MHC class II heterodimers serve a central role in antigen presentation and the induction and maintenance of acquired cellular immune responses.8 The complex formed by short, antigen-derived peptides (epitopes) loaded into the binding groove of MHC class II molecules bind to the T cell antigen receptor (TCR) of CD4+ T cells, which results in highly specific antigen recognition and antigen-specific activation. CD4+ T cells specific for HLA-DQ2.5-restricted gluten peptides that secrete pro-inflammatory cytokines such as interferon(IFN)-γ can be isolated from intestinal tissue.9 One week after commencing a 3-day gluten food challenge, these same CD4+ T cells circulate in blood at increased frequencies.10 The amino acid sequence of immnodominant epitopes recognized by gluten-reactive CD4+ T cells are well established, and are highly consistent amongst HLA-DQ2.5+ CeD patients.9–14
Epitope-specific immunotherapy is a form of antigen-specific immunotherapy that uses peptides instead of whole antigen to target and modify CD4+ T cells.15 In general, higher doses and longer duration of antigen-specific immunotherapy are most clinically effective.16 Evidence supports that clinical benefit is related to disease-specific CD4+ T cells transitioning from being responsive to antigenic stimulation to a state of reversible functional unresponsiveness (anergy), induction of suppressive regulatory T cells, and eventually, over longer periods, to deletion and durable immune tolerance.16
Gluten itself is not suitable for a therapeutic vaccine because it is insoluble, requires deamidation for full immunogenicity, and some gluten peptides and contaminants have direct innate immune activity.17,18 Nexvax2® is an adjuvant-free, particle-free solution of three, highly soluble, synthetic peptides with 15 or 16 amino acids (NPL001, NPL002, and NPL003) (appendix p 10). Nexvax2 has been designed and developed as an epitope-specific immunotherapy for HLA-DQ2.5+ CeD, which is further described in the appendix (pp 6–7). Nexvax2 encompasses at least five immunodominant epitopes that selectively bind to HLA-DQ2.5 and activate gluten-reactive CD4+ T cells isolated from HLA-DQ2·5+ CeD patients (appendix p 10 and p 27).14 These peptides include sequences recognized by anti-gliadin antibodies,19 but are short enough to minimize the likelihood of complement activation by immune complex formation and antibody-mediated hypersensitivity.20
Our aim was to assess safety and pharmacodynamics of repeated intradermal administrations of Nexvax2 in regimens that could potentially modify gluten-specific immunity in HLA-DQ2·5+ CeD patients on GFD.
METHODS
Study design
Two separate randomized, placebo-controlled, double-blind, phase 1 studies were conducted. We tested Nexvax2 administered as 3 fixed doses at weekly intervals over 15 days (“3-dose study”), or 16 fixed doses at 3 or 4-day intervals over 53 days (“16-dose study”) (appendix p 29). The studies were designed to establish a maximum tolerated dose (MTD) by testing incremental dose increases in a series of ascending dose cohorts. To mobilize gluten-specific T cells in blood and allow assessments of their responsiveness to epitopes in Nexvax2, the screening period and post-treatment period for ascending dose cohorts included a crossover, double-blind, placebo-controlled, oral gluten challenge (OGC). After the MTD was established, a “biopsy” cohort was enrolled in each study to test whether duodenal histology deteriorated following fixed dose administration of Nexvax2 at the MTD. Participants in biopsy cohorts did not have OGC before or after the treatment period to avoid the confounding effect of OGC on duodenal histology. Study sites are listed in the appendix (p 3). The studies were conducted concurrently with the 16-dose study recruiting exclusively from community sites in Australia and New Zealand, and the 3-dose study initially recruiting exclusively from community sites in the United States. After completion of the 16-dose study, participants for the second and later cohorts in the 3-dose study were also recruited sites in Australia and New Zealand. Approval was granted by local ethics committees listed in the appendix (pp 3–4). These studies were conducted according to the International Conference on Harmonisation harmonised tripartite guideline E6(R1): Good Clinical Practice. Research Assist (Bridgewater, New Jersey, USA) and CPR Pharma Services (Thebarton, South Australia, Australia) managed the studies.
Participants
The intended study population was patients who were HLA-DQ2·5+ with CeD on a GFD. Participants shown to be immunologically responsive to Nexvax2 assessed by whole IFN-γ release assay (IGRA) six days after commencing active gluten challenge during the screening period OGC were used to determine the MTD. Participants considered to be in histological remission at the screening endoscopy were used to assess the effects of Nexvax2 on duodenal histology. Inclusion criteria required that participants be aged 18 – 70 years, have had a diagnosis of CeD supported by histology and serology,21 followed a GFD for over one year, and were HLA-DQ2·5+. Full eligibility criteria are provided in the appendix (pp 4–5). For randomization to treatment, participants in ascending dose cohorts required a positive IGRA to Nexvax2 peptides on screening day 6 or 13 that had returned to negative one week before dosing, and participants in biopsy cohorts were required to have duodenal histology at the screening gastroscopy that was consistent with modified Marsh type 0 or 1 (no villous atrophy or crypt hyperplasia). All participants provided written informed consent before enrolment.
Randomization and masking
Trial sites assessed consecutive volunteers for eligibility. For each study, ascending dose cohorts were enrolled stepwise, beginning with the lowest dose level, and when they were complete the biopsy cohort was enrolled. Participants in ascending dose cohorts began the screening period with an OGC. The ordering of the gluten-containing (active) and gluten-free (placebo) challenges in the screening period was randomized with half the participants receiving active first, and the other half receiving placebo first. Allocation of participants to the order they received active and placebo gluten challenges in the screening period was by central randomization using simple block method with block size 200. Active was always before placebo gluten challenge in the post-treatment OGC. Cookies used in OGC were matched in physical appearance, consistency, and taste (appendix p 30). Participants were allocated active (Nexvax2) or placebo treatment by central randomization using a simple block method (block size 12) in a 2:1 schema for ascending dose cohorts, and 1:1 in biopsy cohorts. The appearance of syringes, drug product, and volume injected for active and placebo treatment administered in each trial were identical. Enrolment in cohorts and allocation of OGC sequence or treatment were not stratified for any additional factors. Replacements were allowed, and received the same treatment as the participant they replaced. The randomization mechanism for the study was deployed by sites completing a randomization request that was sent to the un-blinded statistician at CRC Pharma Services (Parsippany, NJ, USA) who provided the randomization number to the study site and notified the packager and distributor for OGC cookies (3-dose study: for sites in the USA: Research Assist, and for other sites: Pharmaceutical Packaging Professionals Pty Ltd., Port Melbourne, VIC, Australia; and for the 16-dose study cookies were provided direct to sites by the manufacturer, Shepherd Works, Boxhill North, Victoria, Australia), and for investigational product (Catalent Pharma Solutions; Allendale, NJ, USA). In the 3-dose study investigational product was provided in prefilled syringes with the site pharmacist being masked to treatment allocation. In the 16-dose study, the unmasked site pharmacist was responsible for diluting stock Nexvax2 9 mg/ml from labeled vials using USP 0.9% sodium chloride to the required concentration in 0.1 mL, or to draw USP 0.9% sodium chloride (0.1 mL) into a 1 mL syringes. The un-blinded statistician at CRC Pharma Services was provided the randomization schedule prepared by the central statistician, who was based at CPR Pharma Services, and had no responsibility for monitoring or data management, prepared randomization schedules using SAS software (SAS Institute Inc, Cary, North Carolina, USA) Version 9·2. The unmasked statistician at CRC Pharma, the packager and distributor of cookies and investigational product, and the pharmacist in the 16-dose study were the only other study personnel with copies of the randomization schedules. All study participants, care providers, data managers, sponsor personnel and study site personnel remained blinded to study treatment assignment until the analyses were completed.
Procedures
For ascending dose cohorts, the screening period began on day 1 with participants being provided nine cookies to eat 3 per day on days 1 to 3. When participants returned on day 8 they were provided another set of nine cookies for days 8 to 10. Cookies in each set either contained gluten (3 g per cookie), or were gluten-free (Shepherd Works). Patients with T cells responsive to epitopes in Nexvax2 were identified using fresh blood and overnight IGRA.22 IGRAs were performed with Nexvax2 peptides, and recall viral antigens on days 1, 6, 8, 13, and 29. Plasma cytokines and chemokines, and immune cell frequencies were also measured on day 1 (before cookies were eaten) and 13. If IGRA to Nexvax2 peptides became negative on day 29 the treatment period commenced one week later, but if IGRA remained positive it was repeated weekly until it became negative and the pre-treatment period was extended up to 49 days. For the biopsy cohorts, the screening period was 35 days with a gastroscopy between days 15 and 28; modified Marsh type was determined in duplicate biopsies collected from the bulb, 1st, 2nd, and 3rd parts of the duodenum to assess eligibility.
Three, weekly intradermal injections of placebo or Nexvax2 60µg, 90µg, or 150µg in 0·1 mL were administered in the 3-dose study. In the 16-dose study, 16 two-times weekly doses of placebo or Nexvax2 150µg, or 300µg in 0·1 mL were administered. Injections administered by study staff on site alternated between the supra-deltoid regions on each arm. When at least six participants who received active treatment had completed at least two weeks of treatment, and if the Dose Escalation Committee (DEC) considered that masked clinical and laboratory pathology safety assessments showed an acceptable safety profile according to predefined criteria described in the appendix (p 5), dosage was increased in the next cohort. For additional consideration of safety data and the decision to dose-escalate or discontinue dosing the DEC could consult the independent safety monitor, a designated member of the independent data safety monitoring board (DSMB), or the full DSMB, which could have access to unmasked safety data. MTD was the highest dose reviewed and approved by the DEC.
Safety assessments included the incidence, severity, and dose relation of adverse events. To elicit adverse events, at every study visit, participants were asked, “Have you had any health problems since the previous visit or when you were last asked?” and “Have you had any new symptoms?” The blinded local site investigators were responsible for reporting and coding adverse events using MedDRA v15·0, and grading their severity and causality according to “Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials”.23 Other safety assessments included changes in laboratory haematology and chemistry, and urinalysis variables, self-reported weekly gastrointestinal symptom rating scale (GSRS),24 and self-assessed daily GSRS dimensions of pain (Q1), hunger pains (Q4), nausea (Q5), rumbling (Q6), bloating Q7), and diarrhoea (Q11) (except on days when the GSRS was recorded). Vital signs, physical examination, 12-lead electrocardiograph, cytokine and chemokine measurements, and immune cell frequencies were also safety assessments. Plasma cytokines and chemokines, immune cell frequencies, and IGRA were measured in the treatment period before the first and last doses, on day 8, and in the 16-dose study on day 25 and 39. Plasma cytokines and chemokines were also assessed 6h after the first and last doses. Pharmacokinetics was assessed up to 6h after the first and last dose.
In the 4-week post-treatment period, ascending dose cohorts had a further OGC, and biopsy cohorts had a gastroscopy with duodenal biopsies and quantitative histology within two weeks. IGRA, plasma cytokines and chemokines, and immune cell frequencies were assessed on day 13; IGRA was also performed on the 1st, 6th, and 8th days, and at end of study (EOS).
Serum IgG and IgA specific for the pool of Nexvax2 peptides were measured at screening, treatment period days 1 and 8, and also 25 and 39 in the 16-dose study, and post-treatment on day 1 and at EOS.
Laboratory assays are described in the appendix (pp 6–8).
Post hoc assessments included complement levels in plasma collected for cytokine assessments on treatment days 1, 8 and 53 pre-dose as well as 6h post-dose on day 1 and 53 in both cohorts receiving Nexvax2 150µg of the 16-dose study; and CeD serology in sera collected for anti-Nexvax2 antibody assessments. Post-treatment “responders” to Nexvax2 were defined post hoc as having Nexvax2-specific IGRA positive six or eight days after commencing the gluten segment in OGC, and “non-responders” as Nexvax2-specific IGRA negative six and eight days after commencing gluten only when all nine gluten cookies had been consumed.
Outcomes
The primary endpoint in each study was centrally assessed as the number and percentage of adverse events during the treatment period. Secondary endpoints included other safety and tolerability assessments during the treatment and post-treatment periods, and serum anti-Nexvax2 antibodies. Pharmacokinetics endpoints were maximal concentrations and area under the curve for NPL001, NPL002, and NLP003. Quantitative duodenal histology was an exploratory safety endpoint. Nexvax2-specific IGRA was defined as the main pharmacodynamic endpoint. Post hoc analyses addressed IGRA to recall CMV-EBV-inFluenza (CEF) epitopes; gastrointestinal symptoms and IGRA during the screening OGC.
Statistical analysis
The sample size was pragmatic to assess the safety and tolerability of Nexvax2, while minimizing unnecessary participant exposure. All participants who received Nexvax2 or placebo, the safety population, were included in an intention to treat analysis for the primary endpoint. Additional post hoc analyses were also performed. Both trials were completed and closed before data analysis. No formal per protocol statistical analyses were planned, but post hoc analyses were performed and are described in the appendix (p 8). To address the confounding effects of reduced gluten exposure in the post-treatment OGC, an algorithm was developed post hoc to define the populations for post-treatment pharmacodynamic analysis (appendix p 31). Statistical tests were two-sided with a significance level of p≤0·05. FDR-adjusted p-values, estimated using Benjamini-Hochberg method, were used where indicated to account for multiple hypothesis testing. All analysis was done using MATLAB software. Data were collected by the investigators, managed by CPR, and analysed by Prometrika (Cambridge, Massachusetts, USA) and the academic co-authors. Trials were registered with the Australian New Zealand Clinical Trials Registry, numbers ACTRN12612000355875, and ACTRN12613001331729.
Role of funding source
The funder of the study was involved in the study design, data collection, data analysis, data interpretation, and the writing of this report. GG, LJW, RJX, and RPA had full access to all the data in the study. RPA had final responsibility for the decision to submit for publication.
RESULTS
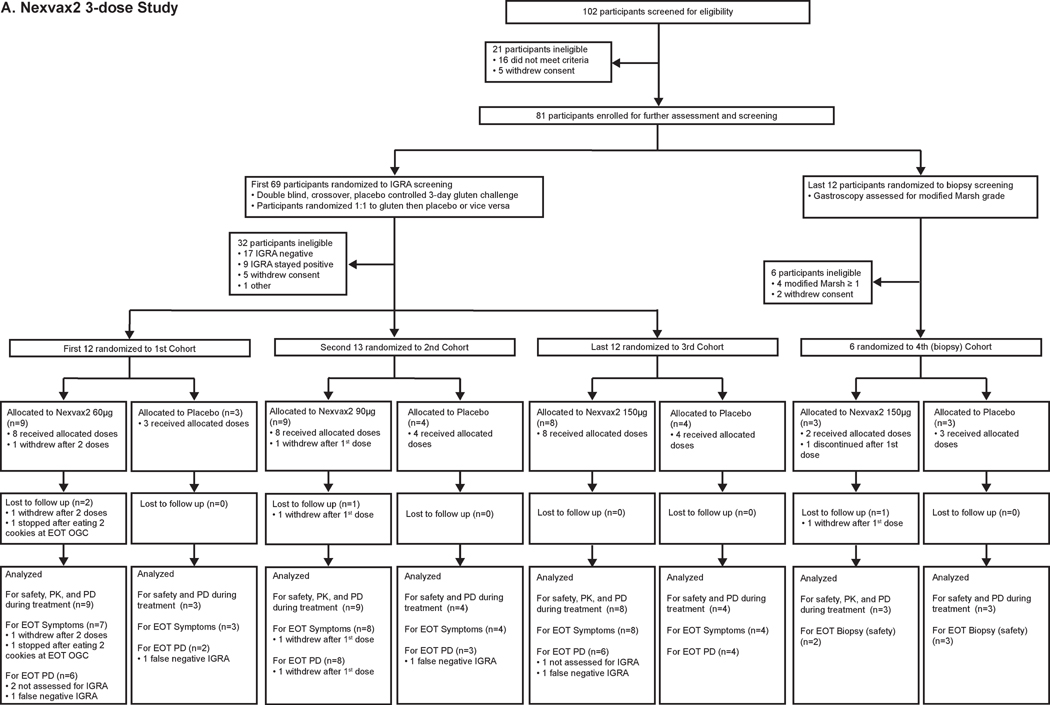
Between 28 November 2012 and 14 August 2014, 102 volunteers were enrolled in the 3-dose study. 21 (21%) of these 102 volunteers did not meet eligibility criteria on screening day 1. Subsequently, 69 (68%) of the original 102 volunteers were screened for ascending dose cohorts and 12 (12%) for the biopsy cohort (Figure 1A). Ultimately, 37 (54%) of 69 participants screened for ascending dose cohorts continued to treatment randomization after OGC. The 32 (46%) of 69 participants screened for ascending dose cohorts who did not continue included 17 (25%) that were IGRA negative, and 9 (13%) with IGRA persisting positive. 6 (50%) of 12 participants screened for the biopsy cohort continued to randomization following gastroscopy after 4 (33%) were excluded with villous atrophy and 2 (17%) withdrew. In the first dose cohort, nine participants were randomly allocated to receive Nexvax2 at 60 µg and three to placebo; in the second dose cohort, nine participants were randomly allocated to receive Nexvax2 at 90 µg and four to placebo; in the third dose cohort, eight participants were randomly allocated to receive Nexvax2 at 150 µg and four to placebo; and in the final (biopsy) cohort three participants were randomly allocated to receive Nexvax2 at 150 µg and three to placebo. Each placebo-treated participant received three doses, but the Nexvax2-treated participants included two who withdrew during dosing from the 60µg and 90µg arms, and another was discontinued due to elevated transaminases pre-dose on the first day of treatment. All participants who completed dosing commenced OGC one day after last dose, or had a second gastroscopy. All 43 participants randomized to treatment were included in the primary endpoint analysis of safety. Table 1 lists the baseline characteristics of the study participants randomized to treatment; 70% were women, mean age was 45 years, and on average CeD had been diagnosed 8 years previously.
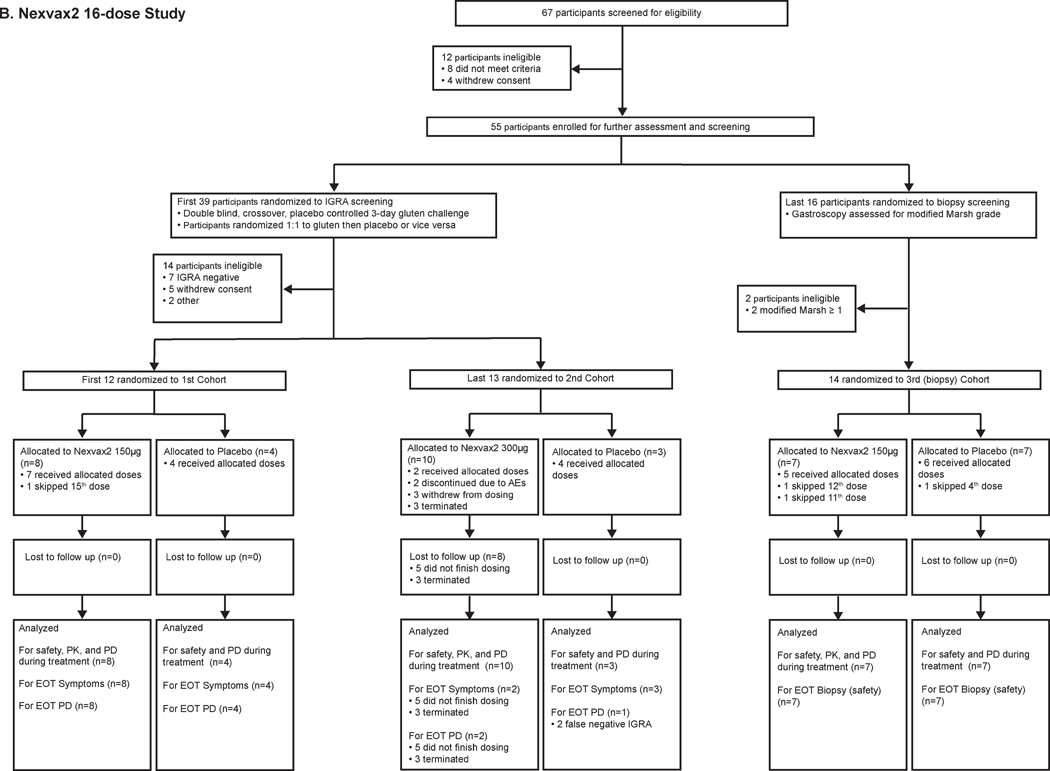
Figure 1. Trial profiles.
Table 1.
Baseline characteristics of participants
| 3-dose study | |||||||
|
| |||||||
| Cohort/s Treatment | 1st, 2nd & 3rd Placebo | 1st Nexvax2 60 µg | 2nd Nexvax2 90 µg | 3rd Nexvax2 150 µg | 4th (biopsy) Placebo | 4th (biopsy) Nexvax2 150 µg | All Participants Dosed |
|
| |||||||
| N | (n=11) | (n=9) | (n=9) | (n=8) | (n=3) | (n=3) | (n=43) |
| Age, years | 44·2 (14·4) | 48·8 (13·5) | 47·6 (12·3) | 47·9 (17·4) | 34·0 (16·8) | 27·3 (11·4) | 44·7 (14·8) |
| Men | 2 (18%) | 3 (33%) | 3 (33%) | 3 (38%) | 0 | 2 (67%) | 13 (30%) |
| Women | 9 (82%) | 6 (67%) | 6 (67%) | 5 (62%) | 3 (100%) | 1 (33%) | 30 (70%) |
| Race, White | 11 (100%) | 9 (100%) | 9 (100%) | 7 (87%) | 3 (100%) | 3 (100%) | 42 (98%) |
| Race, Arab | 0 | 0 | 0 | 1 (13%) | 0 | 0 | 1 (2%) |
| Age at CeD diagnosis, years | 38·5 (13·7) | 42·7 (12·8) | 35·5 (15·5) | 34·0 (19·1) | 28·9 (16·8) | 25·9 (11·4) | 36·4 (15·0) |
| Body mass, kg | 77·5 (14·0) | 94·9 (17·8) | 72·5 (15·5) | 79·6 (18·6) | 67·3 (4·9) | 96·0 (24·3) | 81·1 (18·2) |
| BMI, kg/m2 | 27·1 (3·9) | 32·7 (4·6) | 25·7 (4·8) | 27·2 (3·3) | 24·0 (3·0) | 27·9 (3·8) | 27·8 (4·7) |
| Homozygous HLA-DQ2·5 | 5 (45%) | 4 (44%) | 3 (33%) | 2 (25%) | 0 | 1 (33%) | 15 (35%) |
|
| |||||||
| 16-dose study | |||||||
|
| |||||||
| Cohort Treatment | 1st Placebo | 1st Nexvax2 150 µg | 2nd Placebo | 2nd Nexvax2 300 µg | 3rd (biopsy) Placebo | 3rd (biopsy) Nexvax2 150 µg | All Participants Dosed |
|
| |||||||
| N | (n=4) | (n=8) | (n=3) | (n=10) | (n=7) | (n=7) | (n=39) |
| Age, years | 47·0 (9·8) | 52·0 (11·9) | 39·0 (23·3) | 50·0 (10·1) | 34·6 (15·1) | 42·6 (5·4) | 45·2 (13·0) |
| Men | 1 (25%) | 1 (13% | 1 (33%) | 3 (30%) | 2 (29%) | 2 (29%) | 10 (26%) |
| Women | 3 (75%) | 7 (87%) | 2 (67%) | 7 (70%) | 5 (71%) | 5 (71%) | 29 (79%) |
| Race, white | 4 (100%) | 8 (100%) | 3 (100%) | 10 (100%) | 7 (100%) | 7 (100%) | 39 (100%) |
| Age at CeD diagnosis, years | 42·3 (9·9) | 43·4 (12·7) | 31·1 (17·4) | 42·0 (10·8) | 29·0 (11·8) | 37·6 (5·4) | 38·6 (11·5) |
| Body mass, kg | 63·1 (17·7) | 70·7 (11·2) | 66·8 (12·4) | 85·3 (13·0) | 68·5 (11·8) | 74·4 (11·6) | 73·6 (14·1) |
| BMI, kg/m2 | 22·8 (3·2) | 25·3 (4·3) | 22·7 (2·9) | 29·6 (4·5) | 22·9 (4·7) | 26·1 (2·6) | 25·6 (4·6) |
| Homozygous HLA-DQ2·51 | 0 | 4 (50%) | 0 | 1 (10%) | 0 | 2 (29%) | 7 (18%) |
Data are mean (SD) or n (%). 1 "Homozygous HLA-DQ2·5" indicates no other HLA-DQA or HLA-DQB alleles detected apart from HLA-DQA1*05 & DQB1*02
Between 3 August 2012 and 10 September 2013, 67 volunteers were enrolled in the 16-dose study. 12 (18%) of the original 67 volunteers did not meet eligibility criteria on screening day 1. Subsequently, 39 (58%) of the original 67 volunteers were screened for ascending dose cohorts and 16 (24%) for the biopsy cohort (Figure 1B). Ultimately, 25 (64%) of 39 participants screened for ascending dose cohorts continued to treatment randomization after OGC. 7 (18%) of the participants screened for ascending dose cohorts were discontinued before treatment randomization because IGRA to Nexvax2 peptides was negative. 14 (88%) of 16 participants continued to randomization after gastroscopy in the biopsy cohort after 2 (12%) were excluded with villous atrophy. In the first dose cohort, eight participants were randomly allocated to receive Nexvax2 at 150 µg and four to placebo; in the second dose cohort, ten participants were randomly allocated to receive Nexvax2 at 300 µg and three to placebo; and in the final (biopsy) cohort seven participants were randomly allocated to receive Nexvax2 at 150 µg and seven to placebo. All participants in the first and final cohorts received at least 15 of 16 doses, and then commenced OGC one day after last dose or had a second gastroscopy. In the second cohort, five participants received no more than 3 doses of Nexvax2 300µg, and five participants received between 4 and 16 doses of Nexvax2 and then commenced OGC within 3 days of the last dose. Participants in the second cohort who received placebo had only 5, 10 or 15 doses and then commenced OGC within 3 days of the last dose. All 39 participants randomized to treatment in the 16-dose study were included in the primary endpoint analysis of safety. Baseline characteristics of the study participants randomized to treatment were similar to the 3-dose study (Table 1); 79% were women, mean age was 45 years, and on average CeD had been diagnosed 7 years previously.
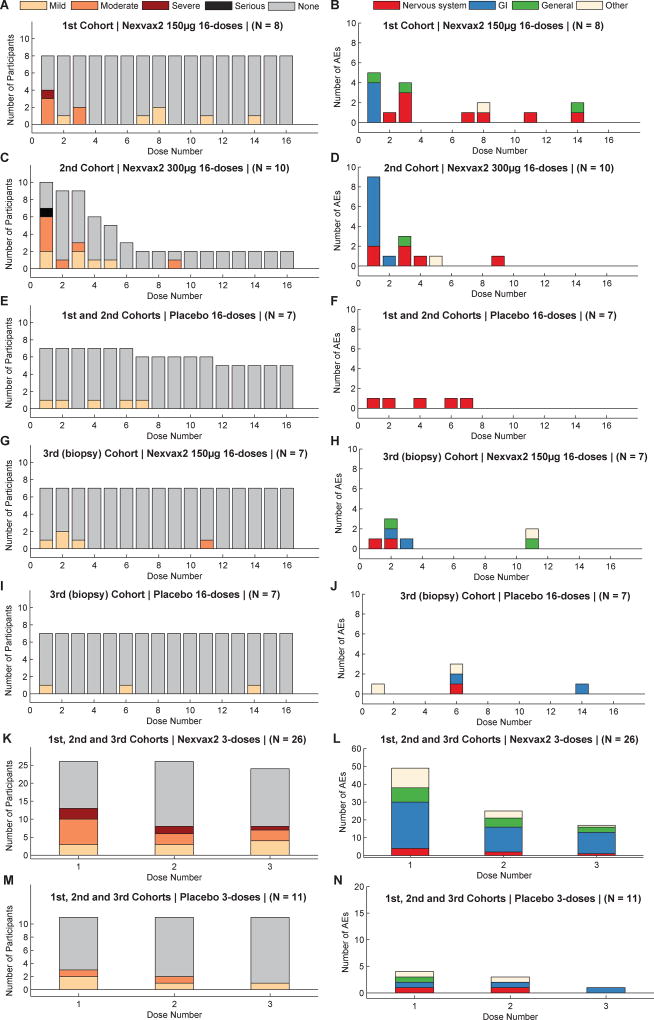
The number, percentage, and severity of adverse events collected during the treatment period for each cohort and in each study are shown in appendix (pp 11–12). The organ systems affected by adverse events are shown in Figure 2 and appendix (pp 13–15). The timing of adverse events in relationship to dosing, and dose number are shown in Figure 2. Adverse events of at least moderate severity are shown in appendix (pp 16–18), and those that affected the gastrointestinal system are summarized in Table 2. Overall, participants in either study who received Nexvax2 were more likely to experience adverse events after the first dose than placebo-treated participants (p=0·0085; Fisher’s Exact test) (Figure 2). In ascending dose cohorts whose screening period always included OGC, the first dose of Nexvax2 was frequently followed by adverse events affecting the gastrointestinal system within 2 to 5 hours (Figure 2); nausea with or without vomiting, abdominal pain, and/or diarrhea were common (Table 2). Adverse events and symptoms with Nexvax2 were reduced with subsequent doses, and were no different from placebo by the third week of dosing (Figure 2A, E). Gastrointestinal and systemic adverse events graded severe included nausea, abdominal pain, vomiting, shivering, clammy skin, and or rigors occurred 2 to 5 hours after the first or second dose of Nexvax2 (appendix pp 16–18). All 7 participants experiencing gastrointestinal and/or systemic adverse events graded severe, including one graded serious – abdominal pain) were in ascending dose cohorts with their screening period including OGC. Five (71%) of the seven participants experiencing these post-dose gastrointestinal adverse events graded severe were homozygous for HLA-DQA1*05 and HLA-DQB1*02, significantly more than among other participants in ascending dose cohorts (9 (26%) of 35 participants, p=0.0313; Fisher’s Exact test) (Table 2 and appendix pp 16–18). All five (100%) of the five participants with an adverse event graded severe or serious after the first dose of Nexvax2 were homozygotes for HLA-DQA1*05 and HLA-DQB1*02. In the 3-dose study, gastrointestinal and/or systemic adverse events graded severe or serious occurred after the first dose in 1 (11%) of 9 participants receiving of Nexvax2 60 µg, in 3 (33%) of 9 participants receiving Nexvax2 90 µg (in 2 after the first dose, and in one after both the second and third doses), and after the second dose in 1 (11%) of 8 participants receiving Nexvax2 150 µg. In the 16-dose study, gastrointestinal adverse events were graded severe in one (13%) of 8 participants after the first dose of Nexvax2 150 µg, and as serious (abdominal pain associated with vomiting) in 1 (10%) of 10 participants after the first dose of Nexvax2 300 µg. Dosing was discontinued in the second cohort of the 16-dose study on the recommendation of the DSMB following unmasked review of safety data summarized in appendix (pp 16–18). According to the dose escalation criteria (appendix pp 5–6), the MTD of 150 µg was established for Nexvax2 administered at intervals of 3 to 4 days by intradermal injection.
Figure 2. Treatment emergent adverse events (TEAE) after each dose of Nexvax2 or placebo.
Number of participants experiencing TEAEs of maximal severity grading mild, moderate, severe, serious or none are shown in A, C, E, G, I, K and M, and total number of TEAEs classified by organ system after each dose are shown in B, D, F, H, J, L, and N (Nervous system disorder was predominantly “Headache”; Gastrointestinal disorder was frequently “Vomiting”, “Nausea”, “Abdominal Pain”, or “Diarrhea”; General disorders included “Injection Site Pain” and “Fatigue”). More participants experienced TEAEs after the first dose of Nexvax2 (A, C, G, and K) (N=54) than participants receiving placebo (E, I, and M) (N=28) (p=0·0085; Fisher’s Exact test), but the only individual dose level that reached significance compared to its matched placebo was Nexvax2 300 µg in 2nd Cohort of 16-dose study (C and E) (p=0·0498; Fisher’s Exact test). Most TEAEs after the first dose of Nexvax2 affected the gastrointestinal system. The number of participants with any TEAE, and the frequency of TEAEs after later doses of Nexvax2 were not significantly different from placebo.
Table 2.
Adverse events graded at least moderate severity in participants experiencing ≥1 gastrointestinal adverse graded at least moderate severity
| Treatment | Participant^ | Gluten challenge in screening |
Last dose number |
Onset after last dose |
Nausea | Abdominal Pain |
Vomiting | Diarrhea | Other |
|---|---|---|---|---|---|---|---|---|---|
| 3-dose study - weekly i.d. doses over 15 days | |||||||||
|
| |||||||||
| Nexvax2 60 µg | S03-01-07 | Yes | 1 | 2.0–2.5h | +++ | +++ | +++ | − | − |
| 2 | 3.5h | ++ | ++ | − | − | − | |||
| 3 | 3.5h | ++ | ++ | − | − | − | |||
|
| |||||||||
| Nexvax2 90 µg | S03-02-13 | Yes | 1 | 3h | +++ | ++ | +++ | − | − |
| 2 | 2h | − | − | + | − | − | |||
|
| |||||||||
| S03-02-08 | Yes | 1 | 0.5–2.75h | ++ | ++ | ++ | − | ||
| 2 | 2.5h | ++ | ++ | ++ | − | − | |||
| 3 | 2.5h | ++ | ++ | ++ | − | − | |||
|
| |||||||||
| S03-02-01 | Yes | 1 | 3.5h | ++ | − | ++ | − | − | |
| 2 | 2.75h | − | +++ | +++ | +++ | − | |||
| 3 | 2.25–3.25h | +++ | +++ | +++ | +++ | − | |||
|
| |||||||||
| S03-02-07 | Yes | 1 | 3–4h | ++ | ++ | ++ | ++ | +++1 | |
|
| |||||||||
| S03-02-10 | Yes | 1 | 3.25h | − | − | ++ | − | − | |
| 2 | 2.4h | − | − | ++ | − | − | |||
|
| |||||||||
| Nexvax2 150 µg | S03-03-03 | Yes | 1 | 4.3h | − | − | ++ | − | − |
| 2 | 3.2h | − | − | +++ | − | − | |||
|
| |||||||||
| S03-03-02 | Yes | 1 | 4h | − | − | ++ | − | − | |
|
| |||||||||
| S03-03-12 | Yes | 1 | 2.75–3.25h | ++ | − | ++ | − | − | |
| 3 | 3h | − | − | ++ | − | − | |||
|
| |||||||||
| S03-03-09 | Yes | 1 | 3h | − | − | ++ | − | − | |
|
| |||||||||
| Placebo | S03-03-05 | Yes | 1 | 0 | ++ | − | − | − | − |
| 2 | 24h | ++ | − | − | − | − | |||
|
| |||||||||
| S03-03-11 | Yes | 1 | 24h | − | − | − | − | +++2 | |
|
| |||||||||
| 16-dose study - twice weekly i.d. doses over 53 days | |||||||||
|
| |||||||||
| Nexvax2 150 µg | S16-01-03 | Yes | 1 | 2.75h | − | − | ++ | − | − |
|
| |||||||||
| S16-01-04 | Yes | 1 | 2.4h | − | − | ++ | − | − | |
|
| |||||||||
| S16-01-12 | Yes | 1 | 2.8–3.25h | +++ | +++3 | ||||
|
| |||||||||
| S16-01-06 | Yes | 1 | 3h | − | − | ++ | − | − | |
|
| |||||||||
| Nexvax2 300 µg | S16-02-07 | Yes | 1 | Same day | − | − | ++ | − | − |
|
| |||||||||
| S16-02-11 | Yes | 1 | 4.1h | − | − | ++ | − | − | |
|
| |||||||||
| S16-02-12 | Yes | 1 | 3.1h | − | − | ++ | − | − | |
|
| |||||||||
| S16-02-13 | Yes | 1 | 2.25h | − | ++++ | + | − | − | |
| 48h | − | +++ | ++ | − | − | ||||
|
| |||||||||
| Placebo | S16-02-04 | Yes | 14 | 24h | ++ | ||||
Participant code (SXX-YY-ZZ) refers to the planned total doses in the study, the cohort number (YY), and order of randomization within the cohort (ZZ)
Adverse event grading +: mild; ++: moderate; +++: severe; ++++: serious (grade 4)
1 Dizziness; Adverse drug reaction, 2 Viral upper respiratory tract infection, 3 rigors
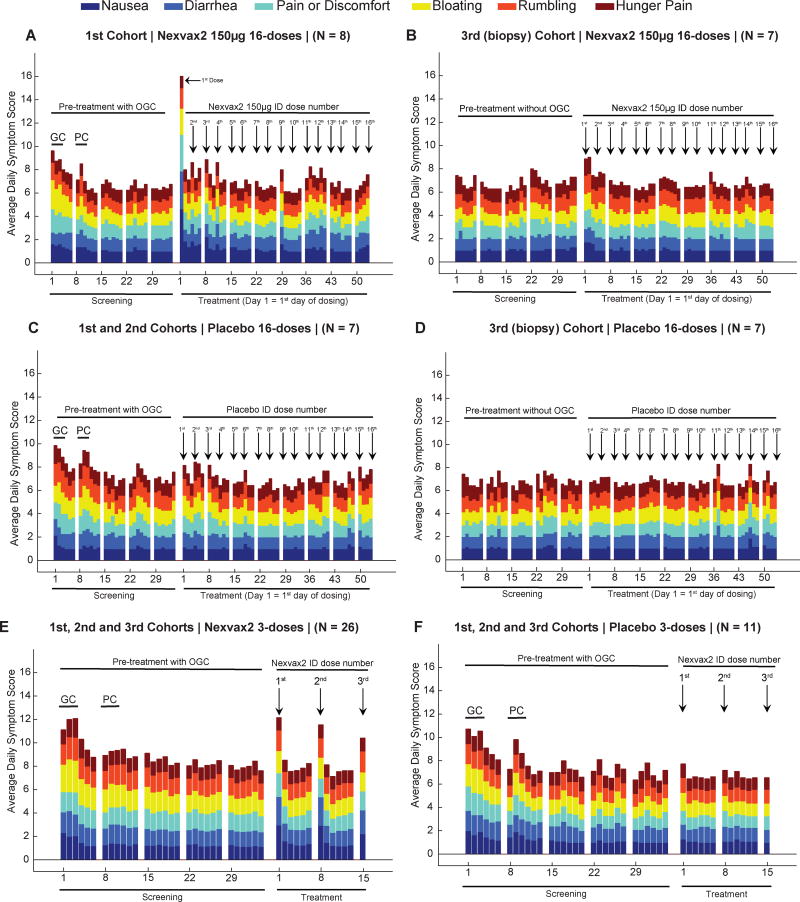
Consistent with adverse events, weekly GSRS scores significantly increased in Nexvax2-treated participants for the first week of twice-weekly dosing (appendix p 19), and there was a trend for gastrointestinal symptoms to increase on each dosing day for Nexvax2-treated participants over the first two weeks of the treatment period in both studies (Figure 3A–F). However, nausea was the only symptom to increase and reach statistical significance on the first day of dosing in any cohort (Figure 3A; p=0·015; Wilcoxon rank sum test). Overall, the clinical effects of administering the first dose of Nexvax2 resembled the symptoms reported by participants during the screening period when they consumed gluten and experienced significantly increased abdominal pain, nausea, rumbling, bloating, and diarrhea (appendix p 20).
Figure 3. Gastrointestinal symptoms.
Participants scored six items in the Gastrointestinal Symptoms Rating Scale (GSRS) from 1 (no discomfort at all) to 6 (very severe discomfort) every day except the last day of each week during the 16-dose (A–D), and 3-dose studies (E and F). In ascending dose cohorts (A, C, E and F), 3-day gluten challenge (GC) corresponds to Screening days 1 to 3, and placebo challenge (PC) corresponds to Screening days 8 to 10; the biopsy cohorts did not have a gluten challenge (B and D). The sum of six symptom scores increased when Nexvax2 was first administered (Treatment day 1) and reached statistical significance in participants receiving Nexvax2 150µg (A) compared to placebo (C) in ascending dose cohorts of the 16-dose study (P=0·015; Wilcoxon rank sum test).
Clinical and laboratory safety assessments including circulating lymphocyte subsets (appendix pp 21–23), and anti-Nexvax2 IgG and IgA (appendix p 32), showed no significant changes over the treatment period. Seroconversion for antibodies specific for transglutaminase 2 and deamidated gliadin peptide was not observed from screening to end of study (appendix p 24). Comparing biopsies from screening and after the treatment period, median [interquartile range] villous height to crypt depth ratio in distal duodenal biopsies was not significantly different for Nexvax2 at the MTD with 3 doses over 15 days (2.04 [0.69] versus 2.49 [0·67], n=2), or 16 doses over 53 days (1·74 [0·54] versus 1·56 [0·58], n=7), and for placebo over 15 days (1.75 [0.62] versus 2.09 [0.71], n=3) or 16 doses over 53 days (2·10 [0·25] versus 1·92 [0·35], n=7). Villous height to crypt depth ratio in proximal duodenal biopsies, density of intra-epithelial lymphocytes, and modified Marsh scores were also not different in biopsies collected before and after dosing with Nexvax2 150µg or placebo weekly over 15 days or two-times weekly over 53 weeks (appendix p 25). Complement levels were stable during the treatment period (appendix p 26). Pre-dose plasma cytokines and chemokines were stable over the study period; post-dose alterations in plasma cytokines and chemokines are reported elsewhere (publication in preparation).
Plasma concentrations of NPL001, NPL002, and NPL003 were above levels of detection (0·05 nM, 0·1 nM, and 0·4 nM, respectively) from ten minutes up to two hours after the first and final administrations of Nexvax2 in both studies, but were at levels below the limits of quantitation (2·6 nM, 5·5 nM, and 5·3 nM, respectively) (appendix p 33).
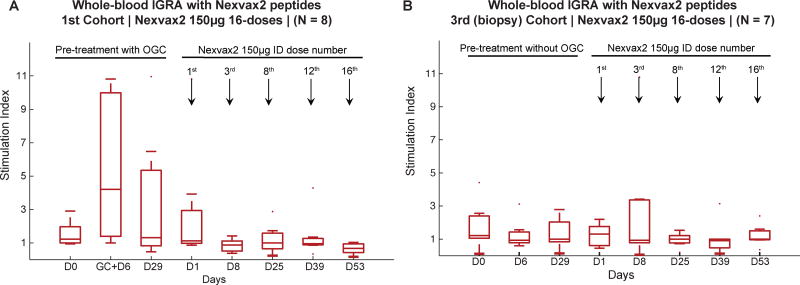
Unlike gluten challenge during the screening period, Nexvax2 administration did not cause the whole blood IGRA for Nexvax2 peptides to become positive (Figure 4). In contrast, IGRA responses to recall epitopes were present and stable throughout the study (appendix p 34).
Figure 4. Activation of T cells.
The presence and functional responsiveness of circulating T cells specific for epitopes in Nexvax2 was tested by ex vivo whole blood interferon(IFN)-γ release assay (IGRA). Fold increase in IFNγ release (stimulation index) stimulated by Nexvax2 peptides compared to medium is shown for participants in the 16-dose study receiving Nexvax2 150µg in the 1st cohort (n=8) (A), and in the 3rd (biopsy) cohort that did not have screening gluten challenge (n=7) (B); median with interquartile range are shown.
Aggregate daily symptoms score during the post-treatment OGC was significantly worse than pre-treatment in placebo-treated participants (p=0·0232, Wilcoxon Signed-Rank Test). Almost half the placebo-treated participants did not consume all gluten-containing cookies during the post-treatment gluten challenge, which was significantly less than Nexvax2-treated participants across both studies (10/18 vs 31/33; p=0·0019, Fisher’s Exact Test, Table 3). Post hoc analysis of Nexvax2-specific IGRA showed that participants treated with Nexvax2 150µg in the 3-dose or 16-dose study were mostly IGRA negative to Nexvax2 peptides when they completed the gluten challenge, but almost all placebo-treated participants who completed gluten challenge were positive (Table 3; 3-dose-150µg: 6/6 versus 2/9, p=0·007; 16-dose-150µg: 6/8 versus 0/5, p=0·021, Fisher’s Exact Test).
Table 3.
Whole blood interferon-γ release assay (IGRA) with Nexvax2 peptides after post-treatment gluten challenge by post hoc analysis
| Study | 3-dose study | 16-dose study | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Cohort/s Treatment |
1st, 2nd & 3rd Placebo |
1st Nexvax 2 60 µg |
2nd Nexvax2 90 µg |
3rd Nexvax2 150 µg |
1st & 2nd Placebo |
1st Nexvax2 150 µg |
2nd Nexvax2 300 µg |
| Participants randomized | (n=11) | (n=9) | (n=9) | (n=8) | (n=7) | (n=8) | (n=10) |
| Participants commenced post-treatment OGC | 11 (100%) | 8 (89%) | 8 (89%) | 8 (100%) | 7 (100%) | 8 (100%) | 2 (20%) |
| Participants completed post-treatment OGC | 7 (78%) | 6 (67%) | 8 (89%) | 7 (88%) | 3 (43%) | 8 (100%) | 2 (20%) |
| Participants eligible for PD analysis* | 9 (82%) | 6 (67%) | 8 (89%) | 6 (75%) | 5 (71%) | 8 (100%) | 2 (20%) |
| Responders of participants eligible for analysis | 7 (78%) | 4 (67%) | 3 (38%) | 0 (0%) | 5 (100%) | 2 (25%) | 0 (0%) |
| P-value# | NA | 1 | 0·1534 | 0·007 | NA | 0·021 | 0·0476 |
Participants who did not finish all study doses, or post-treatment gluten challenge were not included in analysis. IGRA responders were Nexvax2-specific IGRA positive 6 or 8 days after commencing gluten challenge, and “non-responders” were Nexvax2-specific IGRA negative 6 or 8 days after commencing OGC when all 9 gluten cookies had been consumed (Algorithm outlined in Figures S3).
P-value was estimated by Two-tailed Fisher's Exact Test comparing treatment with placebo
DISCUSSION
CeD represents a unique condition amongst the autoimmune diseases since the main components in the etiology and pathogenesis have been recognized: the MHC Class II haplotype contributes almost half of the genetic susceptibility,5 and the hierarchy of epitopes recognized by CD4+ T cells responding to the trigger antigen are well characterized.13 In addition, the causative antigen, gluten, can be reintroduced to patients to assess immune responsiveness and its effects on the target organ.15 For these reasons, CeD is exceptionally well positioned for the development and testing of epitope-specific immunotherapy, complimenting the limited clinical experience of this novel class of antigen-specific immunotherapy in allergy and autoimmunity, and translating insights from preclinical models.25
The phase 1 studies of Nexvax2 are the first to assess the clinical and immunological effects of systemically administered peptides implicated in the adaptive immune response in CeD. The target for Nexvax2 is the HLA-DQ2.5-epitope-TCR complex linking the surfaces of antigen presenting cells and gluten-reactive CD4+ T cells.26 In vitro, the component peptides in Nexvax2 bind selectively to HLA-DQ2.5, but not other CeD-associated HLA-DQ heterodimers. The peptides also activate CD4+ T cell clones from CeD patients that are specific for HLA-DQ2.5-restricted epitopes represented in Nexvax2. In vivo, Nexvax2 engagement with its predicted target was supported by observing that whole blood IGRA for Nexvax2 peptides was converted from positive after pre-treatment OGC to negative after post-treatment OGC in most participants receiving Nexvax2 150µg. Furthermore, to our knowledge, systemic administration of epitopes for CD4+ T cells implicated in an autoimmune or allergic disease has not previously been associated with digestive symptoms, which suggests this may a specific effect of gluten epitopes. Additional indirect evidence of target engagement was supported by observing that the first dose of Nexvax2 is followed by digestive symptoms, which are similar in timing and quality to those associated with gluten exposure.
Despite the first administration of Nexvax2 being followed by symptoms typical of gluten exposure in CeD, repeated dosing over 8 weeks did not affect duodenal histology. Duodenal mucosal histology is slow to recover after institution of gluten-free diet, and would not be expected to show improvement over the duration of the treatment periods in these phase 1 studies. In contrast, daily gluten ingestion can cause symptoms on the first day similar to those after the first dose of Nexvax2 150 µg, and result in pronounced mucosal damage after one week.27 There was a significant reduction in symptoms without changes in pharmacokinetics or anti-Nexvax2 antibodies after the final doses of Nexvax2 in both studies. This was consistent with target T cells becoming functionally unresponsive to antigenic stimulation. This interpretation was supported by whole blood IGRA for Nexvax2 peptides frequently being negative after gluten challenge in the post-treatment period in participants administered Nexvax2. Direct visualization of peripheral blood and intestinal gluten-specific T cells assessed by flow cytometry using MHC-peptide complexes will be required in future studies to address whether CD4+ T cells specific for gluten, and in particular Nexvax2, express surface markers consistent with anergy or regulatory function following treatment with Nexvax2.11
These phase 1 studies were not designed to address the efficacy of Nexvax2 in CeD. Clinically relevant endpoints such as patient reported outcome measurements and quantitative histology after longer periods of treatment will be required to assess efficacy. In this study, we had intended to determine the MTD for Nexvax2 in participants who mobilized CD4+ T cells responsive to Nexvax2. However, a potential limitation to the current studies was the high number of participants ineligible for dosing. The most common reason for exclusion from ascending dose cohorts was negative IGRA to Nexvax2 peptides after gluten challenge in the screening period. Because virtually all HLA-DQ2.5+ CeD patients harbor CD4+ T cells specific for epitopes in Nexvax2,9–14 the most likely explanation for failure to mobilize Nexvax2-specific CD4+ T cells was recent, inadvertent gluten ingestion.28 Exclusion of participants with significant mucosal injury from biopsy cohorts was also most likely due to inadvertent non-adherence to GFD.29
Together these studies support the safety, tolerability, and relevant bioactivity of Nexvax2. Gradual escalation up to the maintenance dose of a peptide immunotherapy may be tolerated better than fixed dose regimens.30 A separate study is addressing whether symptoms associated with initial and maximal doses of Nexvax2 are overcome by gradual dose escalation (ClinicalTrials.gov NCT02528799). These studies provide the basis for future clinical trials to test whether Nexvax2 protects against the damaging effects of gluten in HLA-DQ2·5+ CeD.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched for reviews of emerging treatments for celiac disease (CeD) published before Aug 24, 2016 in PubMed using the terms “Celiac disease” or “coeliac disease”, and “non-dietary therapy”, “immunotherapy”, or “vaccine”. We found 20 reviews of new treatments in development for CeD published since 2009, which confirmed that there are no therapeutics approved specifically for the treatment of CeD. We also searched Clinicaltrials.gov on Aug 24, 2016 for clinical trials using the terms “celiac disease therapy”, “peptide-based immunotherapy”, “peptide immunotherapy”, “antigen-specific immunotherapy”, “epitope-specific immunotherapy”, and “specific immunotherapy”. In total, we found clinical trials assessing 16 different agents for CeD, but Nexvax2 was the only antigen-specific therapeutic and none was at a stage of development more advanced than phase 2. These searches identified only 2 studies that assessed effects of immunogenic peptides for autoimmune diseases, both for type-1 diabetes (NCT01536431 and, not yet commenced, NCT02837094). The authors are also aware of two other recent clinical trials using immunogenic peptides for multiple sclerosis (NCT01097668 and NCT01973491).
Added value of this study
The clinical effects and therapeutic potential of peptides recognized by disease-specific CD4+ T cells has not been systematically evaluated in clinical autoimmune diseases. Unlike other autoimmune diseases, the peptides responsible for the disease-specific CD4+ T-cell response to gluten associated with CeD are well characterized. Nexvax2 is the first antigen-specific immunotherapy under development for CeD and is an adjuvant-free solution of three peptides with immunodominant HLA-DQ2·5-restricted epitopes. The effects of systemically administered gluten peptides have not previously been tested. Intradermal administration of Nexvax2 in HLA-DQ2·5+ CeD participants initially caused gastrointestinal symptoms similar in timing and quality to those triggered by gluten ingestion. Adverse events after later administrations of Nexvax2 were no different from placebo. A maximum tolerated dose of Nexvax2 was established as 150 µg. There was no evidence of deterioration in duodenal histology following two-times weekly intradermal administrations over eight weeks with Nexvax2 150 µg. The recall immune response to gluten was modified in CeD participants receiving Nexvax2 consistent with T cells specific for epitopes in Nexvax2 were rendered unresponsive to further antigenic stimulation.
Implications of all the available evidence
Repeated small, fixed doses of adjuvant-free peptides including immunodominant epitopes for gluten-specific CD4+ T cells are capable of modifying the recall immune response to gluten in CeD patients without causing duodenal injury. The clinical effects of systemically administering immunodominant epitopes for gluten-specific CD4+ T cells are at first similar to the gastrointestinal symptoms following gluten ingestion in CeD patients on GFD, but later doses are well tolerated with effects similar to placebo. These findings are consistent with Nexvax2 peptides engaging and rendering gluten-specific CD4+ T cells unresponsive to further antigenic stimulation. Further assessment and clinical development of antigen-specific immunotherapy for CeD using immunogenic gluten peptides is justified, and may inform the design, immune monitoring, and clinical development of this novel therapeutic class for autoimmune diseases.
Acknowledgments
We thank the celiac disease patients and their family members in support of our research. We thank Dr. Michael Cooreman at ImmusanT, Inc. for critical reading of the manuscript and helpful suggestions. The contribution of flow cytometry data to this publication was made possible with help from the Duke University Center for AIDS Research (CFAR), an NIH funded program (5P30 AI064518).
JMA is a consultant to Abbvie, Abbott, Ferring, Janssen, MSD, Hospira, Pfizer, Takeda, and Shire. JAT-D has received honoraria from ImmusanT, Inc. as a consultant and medical monitor on the clinical trials. JAT-D and RPA are co-inventors of patents, owned or licensed by ImmusanT, Inc. and RPA has also received royalties from those licensed to ImmusanT, Inc., covering the composition of Nexvax2, and utilization of gluten-derived T cell epitopes for use in therapeutics. RPA has additional patents covering the uses of epitopes in diagnostics, food tests and non-toxic cereals, all of which are owned or licensed by ImmusanT, Inc. MM, RJX, LMS and BJ are members of the Scientific Advisory Board of ImmusanT, Inc. MM is a scientific advisor to Celimmune, LLC, and holds a patent on methods and means for detecting gluten-induced diseases that is owned by Tampere University and licensed to Labsystems Diagnostics, Finland. LMS receives honoraria for scientific consulting provided to ImmuanT, Inc., Regeneron, Glenmark, and Celgene, and holds a patent on methods for detection of gluten-specific T-cells. BJ serves as a scientific advisor to Bioniz and Celimmune. LJW, RPA, KEG, SW and JLD are employees of ImmusanT, Inc. PHG is a former employee of ImmusanT, Inc.
Funding Support Dr. Goel and Prof. Xavier are supported by The Paul and Kathy Severino Research Fund. Prof. Mäki and Drs. Popp and Taavela are supported by Competitive Research Funding of the Tampere University Hospital, Pirkanmaa Hospital District, Grant No. 9T040. Prof. Sollid are supported by the Research Council of Norway (grant 179573/V40 through its Centre of Excellence funding scheme and the South-Eastern Norway Regional Health Authority (grant 2011050). Prof. Jabri is supported by grants from the Digestive Diseases Research Core Center (DK42086) at the University of Chicago and from the US National Institutes of Health (RO1DK67180 and R01DK098435 to Prof. Jabri). Dr. Tye-Din is supported by grants from the University of Melbourne, Coeliac Australia, NHMRC Independent Research Institutes Infrastructure Support Scheme and Victorian State Government Operational Infrastructure Support.
Footnotes
Contributors
RPA, LW and PHG designed the studies. TK, AJD, JAM, JK, RK, GB, RF, CFB, RE, TPK, and PBM served as trial site principal investigators. JAT-D was medical monitor and performed immunogenicity testing and immune monitoring along with AG. JT, AP and MM performed histological analysis. JLD, KEG, and SW executed immune monitoring assays. JS performed supplementary MHC Class II peptide binding assays in the lab of AS. Data integration and analysis was performed by GG, RJX, and RPA. Tables and figures were prepared by GG and RPA. BJ and LMS assisted in interpretation of results. GG, BJ and RPA wrote the manuscript. All authors reviewed and approved the manuscript, tables and figures. The authors made the decision to submit the manuscript for publication and vouch for the accuracy of the data and analyses and for the fidelity of this report to the trial protocol. RPA had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests The other authors declared no conflicts of interest.
References
- 1.Sollid LM, Jabri B. Triggers and drivers of autoimmunity: lessons from coeliac disease. Nature Reviews Immunology. 2013;13(4):294–302. doi: 10.1038/nri3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63(8):1210–28. doi: 10.1136/gutjnl-2013-306578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.See JA, Kaukinen K, Makharia GK, Gibson PR, Murray JA. Practical insights into gluten-free diets. Nat Rev Gastroenterol Hepatol. 2015;12(10):580–91. doi: 10.1038/nrgastro.2015.156. [DOI] [PubMed] [Google Scholar]
- 4.Shah S, Akbari M, Vanga R, et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. The American Journal of Gastroenterology. 2014;109(9):1304–11. doi: 10.1038/ajg.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bevan S, Popat S, Braegger CP, et al. Contribution of the MHC region to the familial risk of coeliac disease. J Med Genet. 1999;36(9):687–90. [PMC free article] [PubMed] [Google Scholar]
- 6.Sollid LM, Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993;105(3):910–22. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- 7.Vader W, Stepniak D, Kooy Y, et al. The HLA-DQ2 gene dose effect in celiac disease is directly related to the magnitude and breadth of gluten-specific T cell responses. Proceedings of the National Academy of Sciences, USA. 2003;100(21):12390–5. doi: 10.1073/pnas.2135229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unanue ER, Turk V, Neefjes J. Variations in MHC Class II Antigen Processing and Presentation in Health and Disease. Annu Rev Immunol. 2016;34:265–97. doi: 10.1146/annurev-immunol-041015-055420. [DOI] [PubMed] [Google Scholar]
- 9.Arentz-Hansen H, Korner R, Molberg O, et al. The intestinal T cell response to alpha-gliadin in adult celiac disease is focused on a single deamidated glutamine targeted by tissue transglutaminase. The Journal of Experimental Medicine. 2000;191(4):603–12. doi: 10.1084/jem.191.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson RP, Degano P, Godkin AJ, Jewell DP, Hill AV. In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nature Medicine. 2000;6(3):337–42. doi: 10.1038/73200. [DOI] [PubMed] [Google Scholar]
- 11.Bodd M, Raki M, Bergseng E, Jahnsen J, Lundin KE, Sollid LM. Direct cloning and tetramer staining to measure the frequency of intestinal gluten-reactive T cells in celiac disease. Eur J Immunol. 2013;43(10):2605–12. doi: 10.1002/eji.201343382. [DOI] [PubMed] [Google Scholar]
- 12.Christophersen A, Raki M, Bergseng E, et al. Tetramer-visualized gluten-specific CD4+ T cells in blood as a potential diagnostic marker for coeliac disease without oral gluten challenge. United European Gastroenterol J. 2014;2(4):268–78. doi: 10.1177/2050640614540154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sollid LM, Qiao SW, Anderson RP, Gianfrani C, Koning F. Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics. 2012;64(6):455–60. doi: 10.1007/s00251-012-0599-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tye-Din JA, Stewart JA, Dromey JA, et al. Comprehensive, quantitative mapping of T cell epitopes in gluten in celiac disease. Science Translational Medicine. 2010;2(41):41ra51. doi: 10.1126/scitranslmed.3001012. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RP, Jabri B. Vaccine against autoimmune disease: antigen-specific immunotherapy. Current opinion in immunology. 2013;25(3):410–7. doi: 10.1016/j.coi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wambre E. Effect of allergen-specific immunotherapy on CD4+ T cells. Current opinion in allergy and clinical immunology. 2015;15(6):581–7. doi: 10.1097/ACI.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junker Y, Zeissig S, Kim SJ, et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. The Journal of Experimental Medicine. 2012;209(13):2395–408. doi: 10.1084/jem.20102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanayakkara M, Lania G, Maglio M, et al. An undigested gliadin peptide activates innate immunity and proliferative signaling in enterocytes: the role in celiac disease. The American journal of clinical nutrition. 2013;98(4):1123–35. doi: 10.3945/ajcn.112.054544. [DOI] [PubMed] [Google Scholar]
- 19.Osman AA, Gunnel T, Dietl A, et al. B cell epitopes of gliadin. Clinical and Experimental Immunology. 2000;121(2):248–54. doi: 10.1046/j.1365-2249.2000.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haselden BM, Kay AB, Larche M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. The Journal of Experimental Medicine. 1999;189(12):1885–94. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NIH Consensus Development Conference on Celiac Disease. NIH consensus and state-of-the-science statements. 2004;21(1):1–23. [PubMed] [Google Scholar]
- 22.Ontiveros N, Tye-Din JA, Hardy MY, Anderson RP. Ex-vivo whole blood secretion of interferon (IFN)-gamma and IFN-gamma-inducible protein-10 measured by enzyme-linked immunosorbent assay are as sensitive as IFN-gamma enzyme-linked immunospot for the detection of gluten-reactive T cells in human leucocyte antigen (HLA)-DQ2.5(+) -associated coeliac disease. Clinical and Experimental Immunology. 2014;175(2):305–15. doi: 10.1111/cei.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Administration UDoHaHSFaD. Toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Guidance for Industry. 2007 doi: 10.1016/j.vaccine.2023.07.072. [DOI] [PubMed] [Google Scholar]
- 24.Svedlund J, Sjodin I, Dotevall G. GSRS--a clinical rating scale for gastrointestinal symptoms in patients with irritable bowel syndrome and peptic ulcer disease. Digestive Diseases and Sciences. 1988;33(2):129–34. doi: 10.1007/BF01535722. [DOI] [PubMed] [Google Scholar]
- 25.MacLeod MK, Anderton SM. Antigen-based immunotherapy (AIT) for autoimmune and allergic disease. Curr Opin Pharmacol. 2015;23:11–6. doi: 10.1016/j.coph.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Petersen J, Montserrat V, Mujico JR, et al. T-cell receptor recognition of HLA-DQ2-gliadin complexes associated with celiac disease. Nat Struct Mol Biol. 2014;21(5):480–8. doi: 10.1038/nsmb.2817. [DOI] [PubMed] [Google Scholar]
- 27.Daveson AJ, Jones DM, Gaze S, et al. Effect of hookworm infection on wheat challenge in celiac disease--a randomised double-blinded placebo controlled trial. PloS One. 2011;6(3):e17366. doi: 10.1371/journal.pone.0017366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson RP, van Heel DA, Tye-Din JA, et al. T cells in peripheral blood after gluten challenge in coeliac disease. Gut. 2005;54(9):1217–23. doi: 10.1136/gut.2004.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharkey LM, Corbett G, Currie E, Lee J, Sweeney N, Woodward JM. Optimising delivery of care in coeliac disease - comparison of the benefits of repeat biopsy and serological follow-up. Aliment Pharmacol Ther. 2013;38(10):1278–91. doi: 10.1111/apt.12510. [DOI] [PubMed] [Google Scholar]
- 30.Burton BR, Britton GJ, Fang H, et al. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nature Communications. 2014;5:4741. doi: 10.1038/ncomms5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.