Abstract
Background and aim
Several randomized controlled trials have compared sedation with dexmedetomidine and propofol in gastrointestinal endoscopy, with contradictory results. We conducted a meta-analysis of data from randomized controlled trials that compared dexmedetomidine with propofol.
Methods
We searched PubMed, the Cochrane library, and the Igaku-chuo-zasshi database for randomized trials eligible for inclusion in our meta-analysis. We identified six eligible randomized trials from the database search, and compared the effect of propofol versus dexmedetomidine with respect to: (a) patient’s satisfaction level, (b) body movement or gagging, (c) cardiopulmonary complications, and (d) change in heart rate. Data from eligible studies were combined to calculate pooled risk difference (RD) or weighted mean difference (WMD).
Results
Compared to propofol, dexmedetomidine significantly decreased the patient’s satisfaction level (WMD: –0.678, 95% confidence interval (CI): –1.149 to –0.207, p = 0.0048), and there was no significant heterogeneity among the trial results. The pooled RD for developing body movement or gagging when using dexmedetomidine was 0.107 (95% CI: –0.09 to 0.305, p = 0.288), with no significant differences. Compared with propofol, the pooled RD for hypotension, hypoxia, and bradycardia with dexmedetomidine sedation were –0.029 (95% CI: –0.11 to 0.05), –0.080 (95% CI: –0.178 to 0.018), and 0.022 (95% CI: –0.027 to 0.07), respectively, with no significant differences. Compared to propofol, dexmedetomidine significantly decreased the heart rate (WMD: –10.41, 95% CI: –13.77 to –7.051, p ≤ 0.0001), without significant heterogeneity.
Conclusions
In gastrointestinal endoscopy, patient satisfaction level was higher in propofol administration, when compared to dexmedetomidine. The risk of complications was similar.
Keywords: Dexmedetomidine, propofol, endoscopy, meta-analysis, randomized controlled trial
Introduction
Gastrointestinal endoscopy is an uncomfortable and stressful procedure for most patients. Conscious sedation is a common strategy for improving patient comfort during this procedure. Benzodiazepines (gamma-aminobutyric acid (GABA) agonists) such as midazolam have been used for sedation of patients undergoing gastrointestinal endoscopy. The effective dose ranges of such agents differ considerably among patients, making it difficult to achieve stable sedation.
Propofol, a phenolic derivative, has sedative and hypnotic effects that are mediated by the GABA receptor. It has no analgesic action. It is highly lipophilic, and thus can rapidly cross the blood-brain barrier, resulting in an early onset of action. The most important disadvantage of propofol is the risk of rapidly induced deep sedation, with the possibility of causing respiratory and cardiovascular depression.1 On the other hand, recent meta-analysis shows that the use of propofol as a sedative during gastrointestinal endoscopy provides a shorter recovery time and better sedation than traditional sedative agents without causing an increase in cardiopulmonary complications.2,3
Dexmedetomidine (Precedex; Hospira Japan Co., Osaka, Japan) is an alpha-2 receptor agonist with sedative, analgesic, and anxiolytic properties.4,5 It is a first-line drug for sedation in intensive care units.6 Recent meta-analysis found that dexmedetomidine for gastrointestinal endoscopy provides better sedation than traditional sedative agents without causing an increase in cardiopulmonary complications.7
Several randomized controlled trials (RCTs) have compared sedation with dexmedetomidine versus propofol in gastrointestinal endoscopy, with contradictory results.8–13 We propose that systematic pooling of all data from available studies might provide better insight into differences between dexmedetomidine and propofol as sedative agents. Our objective was to perform a systematic review and meta-analysis of RCTs, with the outcome of comparing the safety and efficacy of dexmedetomidine and propofol for gastrointestinal endoscopy.
Methods
Before performing this meta-analysis, we developed a simplified protocol for search strategies, a specific criterion for selection of studies, methods for extraction of relevant data, and strategies for assessment of study quality, and statistical analysis.
Search strategy
The electronic databases PubMed, the Cochrane library, and the Igaku-chuo-zasshi database of Japan (from 1950 to August 2016) were used for the systematic literature search. A search strategy was constructed using a combination of the following words: (propofol) AND (dexmedetomidine) AND (endoscopy). Articles published in any language were included.
Inclusion and exclusion criteria
Articles were considered eligible if they met the following inclusion criteria: (1) study type: RCT; (2) population: patients undergoing gastrointestinal endoscopy; (3) intervention: active treatment with dexmedetomidine; (4) comparator: propofol; (5) outcome: safety and efficacy of sedation. Duplicate publications, reviews, and abstracts from conferences were excluded.
Data extraction
Standardized data abstraction sheets were prepared. Extracted data included study design, study quality, intervention, outcomes, and adverse effects. The outcome measures examined were patient satisfaction level (1 = least satisfied and 10 = most satisfied), time to full awakening, development of body movement or gagging, cardiopulmonary complications (hypoxia, hypotension, and bradycardia), and heart rate. All articles were examined independently for eligibility by two reviewers (T.N. and H.S.). Disagreements were resolved by consultation with a third reviewer (N.H.).
Assessment of methodology quality
The methodological quality of each study was assessed using the risk-of-bias tool outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0). Two reviewers (T.N. and S.S.) reviewed all studies and assessed six different key aspects that might influence the quality of an RCT, including sequence generation, allocation concealment, blinding of participants, outcome assessors, management of eventual incomplete outcome data, completeness of outcome reporting, and other confounding factors that could potentially undermine the validity of the data.
Statistical analysis
Data were entered into the StatsDirect statistical package (StatsDirect Ltd., Cheshire, UK). Separate analyses were performed for each outcome using a risk difference (RD) or weighted mean difference (WMD). We used a random-effect model to calculate summary odds ratios (ORs) and 95% confidence intervals (CIs).14,15 We always used a random-effect model, regardless of the significance of the heterogeneity. Heterogeneity between studies was assessed by Cochran’s Q and I-squared tests. Because of the low power of the Q test, a cut-off value of less than 0.10 was used to reject homogeneity, which thereby indicated heterogeneity. An I-squared score of ≥ 50% indicates more than moderate heterogeneity. Some trials reported “means” as the measure of the treatment effect, with an accompanying range. For purposes of analysis, standard deviation (SD) was estimated from interquartile range as follows:16 SD = interquartile range × 0.74. However, this addition had no impact on the estimation of the pooled ORs. To evaluate the statistical stability of this meta-analysis, we performed a sensitivity analysis to evaluate the effect of studies with a high risk of bias. Endoscopic submucosal dissection (ESD) and esophageal procedure require special procedures as well as a longer operation time. Therefore, we divided all eligible trials into an endoscopic treatment group and an upper gastrointestinal endoscopy/colonoscopy (endoscopic examination) group and the subgroup analysis was also performed. Finally, we used funnel plot asymmetry to detect any publication bias in the meta-analysis, and Egger’s regression test to measure funnel plot asymmetry.
Results
Search results
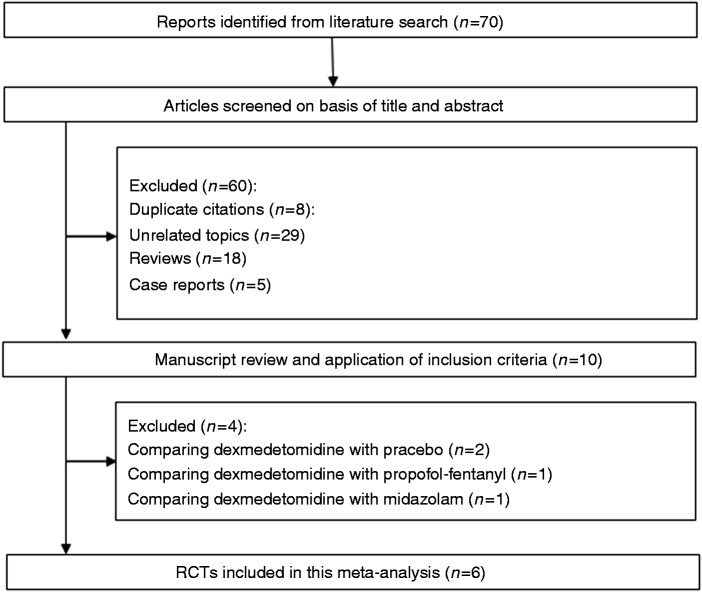
Our database search yielded a total of 70 citations (Figure 1). Of these, 60 studies were removed from consideration after reviewing the abstracts, based on the exclusion criteria (eight duplicate citations, 29 unrelated topics, 18 reviews, and five case reports). The remaining 10 studies were examined in detail. A further four studies were then excluded because of comparison of dexmedetomidine with placebo (n = 2),17,18 comparison of dexmedetomidine with propofol-fentanyl (n = 1),19 and comparison of dexmedetomidine with midazolam (n = 1).20 Finally, six studies were included in the systematic review and meta-analysis. The characteristics of these studies are summarized in Table 1.
Figure 1.
Flow of randomized clinical trials (RCTs) included in the meta-analysis.
Table 1.
Characteristics of studies included in the systematic review and meta-analysis.
| First author, year reference | Country | Procedure | Sedation | Dosage of DEX or PF | Rescue drug | Sample size | Age | Gender (M/F) |
|---|---|---|---|---|---|---|---|---|
| Takimoto, 20118 | Japan | ESD | DEX+Pentazocine | 0.25 µg/kg (5 min) followed by 0.4 µg/kg/h | Midazolam | 30 | 70 (52–80) | 13/17 |
| PF+Pentazocine | 5 mg (bolus) followed by 3 mg/kg/h | Midazolam | 30 | 69 (47–79) | 14/16 | |||
| Hasanin, 20149 | Egypt | EGD/CS | DEX | 2.5 µg/kg (10 min) followed by 2 µg/kg/h | DEX | 40 | 8.4 ± 3.8 | 22/18 |
| PF | 2 mg/kg (bolus) followed by 6 mg/kg/h | PF | 40 | 9.9 ± 4.8 | 24/16 | |||
| Samson, 201410 | India | EGD | DEX | 1 µg/kg (10 min) followed by 0.5 µg/kg/h | Fentanyl | 30 | 36.8 ± 9.6 | 19/11 |
| PF | 1 mg/kg (bolus) followed by 3 mg/kg/h | Fentanyl | 30 | 34.8 ± 10.1 | 17/13 | |||
| Kim, 201511 | Korea | ESD | DEX+Remifentanil | 0.5 µg/kg (5 min) followed by 0.3–0.7 µg/kg/h | PF | 29 | 62.1 ± 10.3 | 19/10 |
| PF+Remifentanil | 0.5 mg/kg (bolus) followed by 1.8 mg/kg/h | PF | 30 | 62.9 ± 12.3 | 22/8 | |||
| Wu, 201512 | China | EGD | DEX+Fentanyl | 1 µg/kg (10 min) followed by 0.5 µg/kg/h | PF | 19 | 52 ± 8.4 | 8/11 |
| PF+Fentanyl | 0.6 mg/kg (bolus), 10–20 mg (additional bolus doses) | PF | 21 | 57 ± 9.5 | 9/12 | |||
| Eberl, 201613 | Netherlands | Esophageal procedure | DEX+Alfentanil | 1 µg/kg (10 min) followed by 0.7 µg/kg/h | PF | 31 | >18 | 28/3 |
| PF+Alfentanil | TCI (targeted plasma concentration 2 µg/ml) | PF | 31 | >18 | 22/9 |
EGD: upper gastrointestinal endoscopy; CS: colonoscopy; ESD: endoscopic submucosal dissection; DEX: dexmedetomidine; PF: propofol; TCI: target controlled infusion system; M: male; F: female.
Quality assessment
The risk of bias in the RCTs is shown in Table 2. In general, the included trials had a low risk of bias, apart from the study by Hasanin and Sira.9 One RCT did not describe the specific methods used for random sequence generation. Allocation concealment and blinding of participants were unclear for one RCT. Blinding of outcomes assessment was not performed in one RCT. All six RCTs were found to adequately assess incomplete outcomes, avoid selective outcome reporting, and were free of other biases.
Table 2.
Bias evaluation of RCTs included in the systematic review.
| First author | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Adequate assessment of incomplete outcome | Selective reporting avoided | No other bias |
|---|---|---|---|---|---|---|---|
| Takimoto8 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hasanin9 | Unclear | Unclear | Unclear | No | Yes | Yes | Yes |
| Samson10 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Kim11 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Wu12 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Eberl13 | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Yes: Low risk of bias. No: High risk of bias. Unclear: Unclear risk of bias. RCTs: randomized controlled trials.
Meta-analysis results
Sedation level
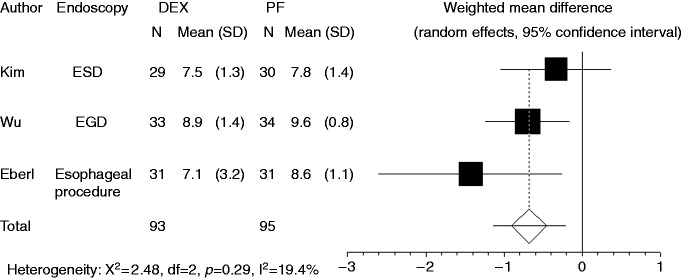
Patients’ satisfaction level was recorded in three studies. Compared to propofol, dexmedetomidine significantly decreased a patient’s satisfaction level (WMD: –0.678, 95% CI: –1.149 to –0.207, p = 0.0048) (Figure 2). There was no significant heterogeneity in the trial results (I-square = 19.4%, p = 0.289). Subgroup analysis indicated that dexmedetomidine reduced patient satisfaction level compared to propofol for the endoscopic examination group (p = 0.01), but no difference was found for the endoscopic treatment group.
Figure 2.
Forest plot displaying the weighted mean difference and 95% confidence interval of each study for patient’s satisfaction level. DEX: dexmedetomidine; PF: propofol; EGD: upper gastrointestinal endoscopy; ESD: endoscopic submucosal dissection.
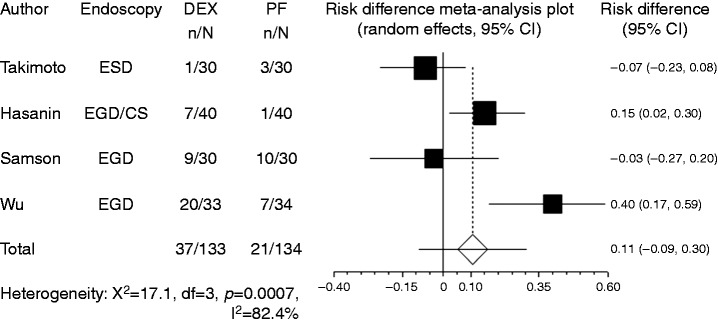
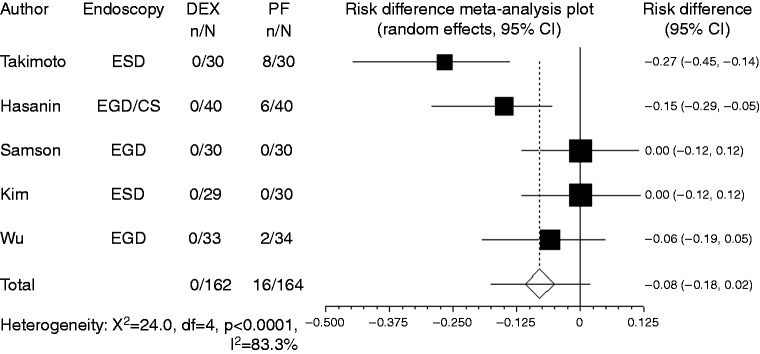
Body movement or gagging was recorded in four studies. Compared with propofol, the pooled RD of developing body movement or gagging when using dexmedetomidine was 0.107 (95% CI: –0.09 to 0.305, p = 0.288), indicating no significant difference between the two groups (Figure 3).
Figure 3.
Forest plot displaying the risk difference and 95% CI of each study for body movement or gagging. DEX: dexmedetomidine; PF: propofol; EGD: upper gastrointestinal endoscopy; ESD: endoscopic submucosal dissection; CI: confidence interval.
Cardiopulmonary complications
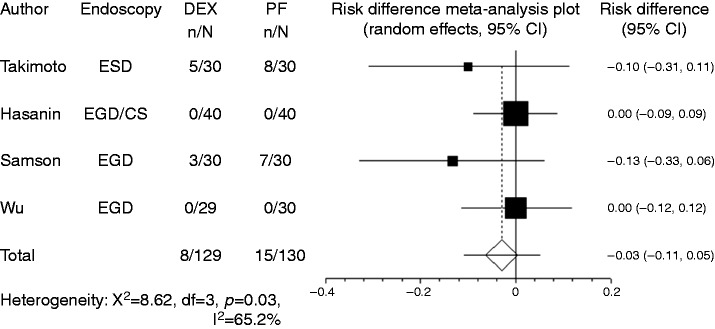
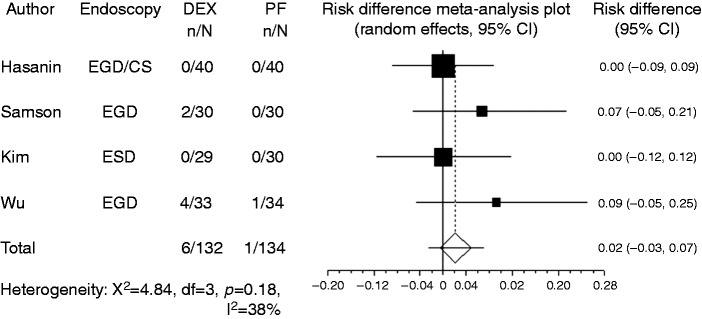
Hypotension was recorded in four studies. In most studies, the criterion for hypotension was blood pressure drop to < 20% of baseline, while Takimoto et al.8 used the criterion of blood pressure drop to < 30% of baseline. Owing to the limited number of reports, studies that used different criteria were combined in the present meta-analysis. Compared with propofol, the pooled RD of developing hypotension when using dexmedetomidine was –0.029 (95% CI: –0.11 to 0.05, p = 0.48), indicating no significant difference between the two groups (Figure 4). For subgroup analysis, both the endoscopic examination group and the endoscopic treatment group had no significant difference in developing hypotension. Hypoxia (percutaneous oxygen saturation level of < 90%) was recorded in five studies. Compared to propofol, the pooled RD of developing hypoxia when using dexmedetomidine was –0.080 (95% CI: –0.178–0.018, p = 0.11) (Figure 5). For subgroup analysis, both the endoscopic examination group and the endoscopic treatment group had no significant difference in developing hypoxia. Bradycardia was recorded in four studies. In most studies, the criterion for bradycardia was a heart rate drop to < 20% of baseline, while Samson et al.10 used the criterion of <60 beats per minute. Again, because of their limited number, studies that used different criteria were included in the present meta-analysis. Compared with propofol, the pooled RD of developing bradycardia when using dexmedetomidine was 0.022 (95% CI: –0.027 to 0.07, p = 0.37), indicating no significant difference between the two groups (Figure 6). For subgroup analysis, both the endoscopic examination group and endoscopic treatment group had no significant difference in developing bradycardia.
Figure 4.
Forest plot displaying the risk difference and 95% confidence interval of each study for hypotension. DEX: dexmedetomidine; PF: propofol; EGD: upper gastrointestinal endoscopy; ESD: endoscopic submucosal dissection; CS: colonoscopy.
Figure 5.
Forest plot displaying the risk difference and 95% CI of each study for hypoxia. DEX: dexmedetomidine; PF: propofol; EGD: upper gastrointestinal endoscopy; ESD: endoscopic submucosal dissection; CS: colonoscopy; CI: confidence interval.
Figure 6.
Forest plot displaying the risk difference and 95% CI of each study for bradycardia. DEX: dexmedetomidine; PF: propofol; EGD: upper gastrointestinal endoscopy; ESD: endoscopic submucosal dissection; CS: colonoscopy; CI: confidence interval.
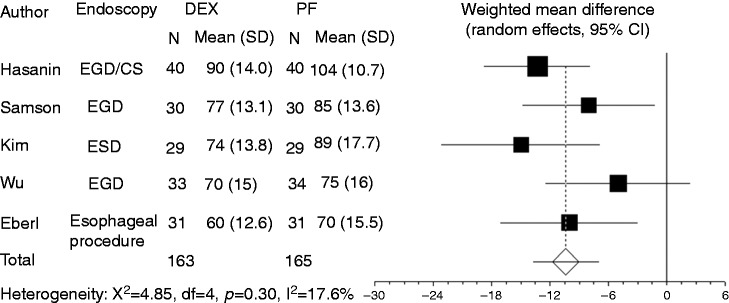
Heart rate was recorded in five studies. Compared to propofol, dexmedetomidine significantly decreased heart rate (WMD: –10.41, 95% CI: –13.77 to –7.051, p ≤ 0.0001, Figure 7). There was no significant heterogeneity among the trial results (I-square = 17.6%, p = 0.303). Results of the Egger test suggested no significant asymmetry of the funnel plot (p = 0.64), indicating no evidence of substantial publication bias (Figure 8). Sensitivity analysis omitting one study with a high risk of bias9 did not alter the findings.
Figure 7.
Forest plot displaying the weighted mean difference and 95% CI of each study for heart rate. DEX: dexmedetomidine; PF: propofol; EGD: upper gastrointestinal endoscopy; ESD: endoscopic submucosal dissection; CS: colonoscopy; CI: confidence interval.
Figure 8.
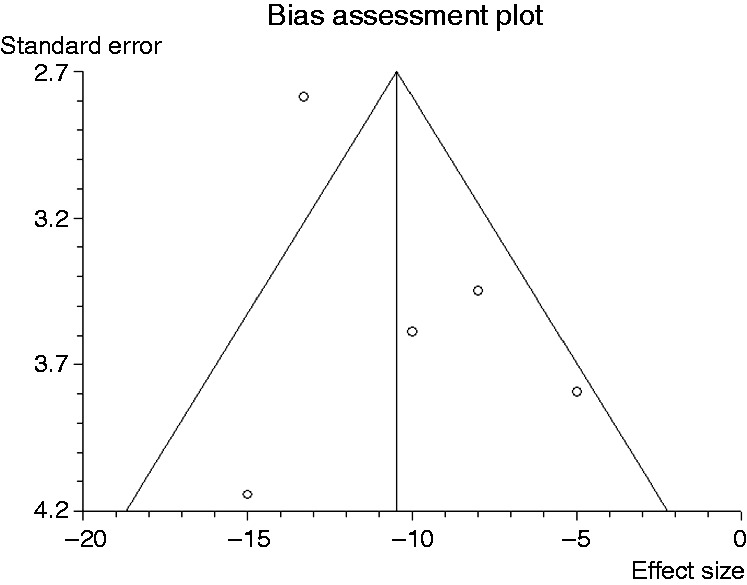
Funnel plot of the included studies for heart rate.
Recovery time
Time to full recovery was recorded in three studies. Pooled results from these studies showed no significant difference between the propofol and dexmedetomidine groups (WMD: 3.539, 95% CI: –8.922 to 15.99, p = 0.578).
Discussion
The current meta-analysis revealed that heart rate and patient’s satisfaction levels were significantly lower with dexmedetomidine than with propofol. In the future, we expect propofol to become an essential sedative agent for endoscopic examination.
Dexmedetomidine is an alpha (α)-2 adrenergic receptor agonist that acts primarily on the locus ceruleus of the pons.21 It exhibits unique sedative activity not found in conventional sedatives and is thus unlikely to cause the respiratory suppression seen with GABA receptor agonists such as midazolam and propofol.22 However, there was no significant difference in hypoxia between dexmedetomidine and propofol in this meta-analysis.
Propofol is a powerful sedative that has recently become the “gold standard” for moderate to deep procedural sedation because of its rapid “onset” and “offset” of action modes.1 Dexmedetomidine is used for light to mild sedation.4,5 Patients might prefer propofol administration for its deeper sedation.
Dexmedetomidine has a short half-life (two to three hours), whereas propofol has a three times shorter half-life (30–60 minutes). Although Hasanin and Sira reported a significantly longer recovery time in the dexmedetomidine group,9 pooled recovery time results showed no significant difference between the propofol and dexmedetomidine groups.
Dexmedetomidine has some disadvantages. First, the method of dexmedetomidine administration is somewhat complicated, as the loading dose (up to 1 µg/kg) should be given over no less than 10 minutes. The slow initial infusion avoids the undesirable hemodynamic changes that occur with faster infusion. Hasanin and Sira reported significantly longer induction times in the dexmedetomidine group (10.51 ± 1.75 minutes versus 3.17 ± 0.72 minutes),9 indicating that dexmedetomidine may be more suitable for relatively longer procedures such as endoscopic submucosal dissection (ESD).
Decreasing of heart rate is a major effect of α2-agonistic agents that is mediated by the activation of α2-adrenoceptors and imidazoline-preferring receptors in the ventrolateral medulla and solitarius nucleus tract.21 In the current meta-analysis, dexmedetomidine significantly decreased heart rate compared to propofol. However, pooled RD of developing bradycardia had no significant difference between the dexmedetomidine and propofol groups. Bradycardia could be managed with atropine during endoscopy in these trials.
Among the included RCTs, no trial estimated the average cost of sedative agents. The cost of dexmedetomidine is 5180 yen (approximately US $51, €47) for one vial (200 µg). The cost of propofol is 1920 yen (approximately US $19, €17) for one vial (500 mg). For a 50 kg patient, one vial (200 µg) of dexmedetomidine could keep for four hours of the sedation. One vial (500 mg) of propofol could keep for three hours. Dexmedetomidine seems to be more expensive than propofol. However, future pharmaco-economic assessment of dexmedetomidine and propofol should be conducted to evaluate their relative effectiveness, safety, and cost.
The present meta-analysis has several limitations. First, we integrated the results of all relevant individual RCTs; however, our conclusions were still based on a relatively small number of trials. This study might therefore be underpowered and may fail to detect unrevealed but statistically important differences between these two drugs. Another limitation is the extensive exclusion criteria in most of the trials, which included cardiovascular disease, renal or hepatic insufficiency, and pregnancy, which limited the applicability of the results to the general critically ill patient population.
In conclusion, patients were more satisfied with propofol administration especially in endoscopic examination, when compared to dexmedetomidine. There were no clear differences in cardiopulmonary complications.
Declaration of conflicting interests
During the last two years, HS received scholarship funds for the research from Otsuka Pharmaceutical Co. Ltd and received service honoraria from Astellas Pharm Inc, Astra-Zeneca K.K., Otsuka Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, Mylan EPD, and Zeria Pharmaceutical Co. Ltd. TK received scholarship funds for the research from Astellas Pharm Inc, Astra-Zeneca K.K., Otsuka Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, Eisai Pharmaceutical Co. Ltd, Zeria Pharmaceutical Co. Ltd, Tanabe Mitsubishi Pharmaceutical Co. Ltd, JIMRO Co. Ltd, Kyorin Pharmaceutical Co. Ltd, and received service honoraria from Astellas Pharm Inc, Eisai Pharmaceutical Co. Ltd, JIMRO Co. Ltd, Tanabe Mitsubishi Pharmaceutical Co. Ltd, Otsuka Pharmaceutical Co. Ltd, Takeda Pharmaceutical Co. Ltd, Miyarisan Pharmaceutical Co. Ltd, and Zeria Pharmaceutical Co. Ltd. N.Y. received scholarship funds for the research from Takeda Pharmaceutical Co. Ltd, Eisai Co, Kaigen Pharm Co. Ltd, Boston Scientific Japan K.K., Nihon Pharmaceutical Co. Ltd, Hoya corporation and Otsuka Pharmaceutical Co. Ltd. OH received scholarship funds for the research from Mochida Seiyaku Co., Ltd. JIMRO Co. Ltd, Takeda Pharmaceutical Co. Limited, Tanabe Mitsubishi Pharmaceutical Co. Ltd, Kyorin Pharmaceutical Co. Limited, Otsuka Pharmaceutical Co. Ltd, Astellas Pharma Inc, Eisai Co. Ltd, Zeria Pharmaceutical Co. Ltd, AstraZeneca K.K., and Boston Scientific Japan K.K. The funding source had no role in the design, practice or analysis of this study.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (B) (16H05291, to HS), MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1411003, to HS) and Keio Gijuku Academic Development Funds (to HS).
References
- 1.Kashiwagi K, Hosoe N, Takahashi K, et al. Prospective, randomized, placebo-controlled trial evaluating the efficacy and safety of propofol sedation by anesthesiologists and gastroenterologist-led teams using computer-assisted personalized sedation during upper and lower gastrointestinal endoscopy. Dig Endosc 2016; 28: 657–664. [DOI] [PubMed] [Google Scholar]
- 2.Wang D, Chen C, Chen J, et al. The use of propofol as a sedative agent in gastrointestinal endoscopy: A meta-analysis. PLoS One 2013; 8: e53311–e53311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishizawa T, Suzuki H, Matsuzaki J, et al. Propofol versus traditional sedative agents for endoscopic submucosal dissection. Dig Endosc 2014; 26: 701–706. [DOI] [PubMed] [Google Scholar]
- 4.Hashiguchi K, Matsunaga H, Hideyuki H, et al. Dexmedetomidine for sedation during upper gastrointestinal endoscopy. Dig Endosc 2012; 20: 178–183. [Google Scholar]
- 5.Coursin DB, Maccioli GA. Dexmedetomidine. Curr Opin Crit Care 2001; 7: 221–226. [DOI] [PubMed] [Google Scholar]
- 6.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013; 41: 263–306. [DOI] [PubMed] [Google Scholar]
- 7.Nishizawa T, Suzuki H, Sagara S, et al. Dexmedetomidine versus midazolam for gastrointestinal endoscopy: A meta-analysis. Dig Endosc 2015; 27: 8–15. [DOI] [PubMed] [Google Scholar]
- 8.Takimoto K, Ueda T, Shimamoto F, et al. Sedation with dexmedetomidine hydrochloride during endoscopic submucosal dissection of gastric cancer. Dig Endosc 2011; 23: 176–181. [DOI] [PubMed] [Google Scholar]
- 9.Hasanin A, Sira A. Dexmedetomidine versus propofol for sedation during gastrointestinal endoscopy in pediatric patients. Egypt J Anaesth 2014; 30: 21–26. [Google Scholar]
- 10.Samson S, George S, Vinoth B, et al. Comparison of dexmedtomidine, midazolam, and propofol as an optimal sedative for upper gastrointestinal endoscopy: A randomized controlled trial. J Dig Endosc 2014; 5: 51–57. [Google Scholar]
- 11.Kim N, Yoo YC, Lee SK, et al. Comparison of the efficacy and safety of sedation between dexmedetomidine-remifentanil and propofol-remifentanil during endoscopic submucosal dissection. World J Gastroenterol 2015; 21: 3671–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Zhang Y, Hu X, et al. A comparison of propofol vs. dexmedetomidine for sedation, haemodynamic control and satisfaction, during esophagogastroduodenoscopy under conscious sedation. J Clin Pharm Ther 2015; 40: 419–425. [DOI] [PubMed] [Google Scholar]
- 13.Eberl S, Preckel B, Bergman JJ, et al. Safety and effectiveness using dexmedetomidine versus propofol TCI sedation during oesophagus interventions: A randomized trial. BMC Gastroenterol 2013; 13: 176–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishizawa T, Suzuki H, Akimoto T, et al. Effects of preoperative proton pump inhibitor administration on bleeding after gastric endoscopic submucosal dissection: A systematic review and meta-analysis. United European Gastroenterol J 2016; 4: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishizawa T, Suzuki H, Fujimoto A, et al. Effects of carbon dioxide insufflation in balloon-assisted enteroscopy: A systematic review and meta-analysis. United European Gastroenterol J 2016; 4: 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraser GL, Devlin JW, Worby CP, et al. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: A systematic review and meta-analysis of randomized trials. Crit Care Med 2013; 41(9 Suppl 1): S30–S38. [DOI] [PubMed] [Google Scholar]
- 17.Mazanikov M, Udd M, Kylänpää L, et al. Dexmedetomidine impairs success of patient-controlled sedation in alcoholics during ERCP: A randomized, double-blind, placebo-controlled study. Surg Endosc 2013; 27: 2163–2168. [DOI] [PubMed] [Google Scholar]
- 18.Hammer GB, Sam WJ, Chen MI, et al. Determination of the pharmacodynamic interaction of propofol and dexmedetomidine during esophagogastroduodenoscopy in children. Paediatr Anaesth 2009; 19: 138–144. [DOI] [PubMed] [Google Scholar]
- 19.Muller S, Borowics SM, Fortis EA, et al. Clinical efficacy of dexmedetomidine alone is less than propofol for conscious sedation during ERCP. Gastrointest Endosc 2008; 67: 651–659. [DOI] [PubMed] [Google Scholar]
- 20.Akarsu Ayazoğlu T, Polat E, Bolat C, et al. Comparison of propofol-based sedation regimens administered during colonoscopy. Rev Med Chil 2013; 141: 477–485. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi Y, Guo TZ, Maze M. Desensitization to the behavioral effects of alpha 2-adrenergic agonists in rats. Anesthesiology 1995; 82: 954–962. [DOI] [PubMed] [Google Scholar]
- 22.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: A randomized trial. JAMA 2009; 301: 489–499. [DOI] [PubMed] [Google Scholar]