Abstract
Background
Recent data imply young patients (age ≤50 years) undergoing small-bowel (SB) capsule endoscopy (CE) for iron deficiency anaemia (IDA) show higher diagnostic yield (DY) for sinister pathology. We aimed to investigate DY of CE in a large cohort of young IDA patients, and evaluate factors predicting significant SB pathology.
Materials and methods
This was a retrospective, multicentre study (2010–2015) in consecutive, young patients (≤50 years) from 18 centres/12 countries, with negative bidirectional gastrointestinal (GI) endoscopy undergoing SBCE for IDA. Exclusion criteria: previous/ongoing obscure-overt GI bleeding; age <19 or >50 years; comorbidities associated with IDA. Data retrieved: SBCE indications; prior investigations; medications; SBCE findings; final diagnosis. Clinical and laboratory data were analysed by multivariate logistic regression.
Results
Data on 389 young IDA patients were retrieved. In total, 169 (43.4%) were excluded due to incomplete clinical data; data from 220 (122F/98M; mean age 40.5 ± 8.6 years) patients were analysed. Some 71 patients had at least one clinically significant SBCE finding (DY: 32.3%). They were divided into two groups: neoplastic pathology (10/220; 4.5%), and non-neoplastic but clinically significant pathology (61/220; 27.7%). The most common significant but non-neoplastic pathologies were angioectasias (22/61) and Crohn’s disease (15/61). On multivariate analysis, weight loss and lower mean corpuscular volume(MCV) were associated with significant SB pathology (OR: 3.87; 95%CI: 1.3–11.3; p = 0.01; and OR: 0.96; 95%CI: 0.92–0.99; p = 0.03; respectively). Our model also demonstrates association between use of antiplatelets and significant SB pathology, although due to the small number of patients, definitive conclusions cannot be drawn.
Conclusion
In IDA patients ≤50 years with negative bidirectional GI endoscopy, overall DY of SBCE for clinically significant findings was 32.3%. Some 5% of our cohort was diagnosed with SB neoplasia; lower MCV or weight loss were associated with higher DY for SB pathology.
Keywords: Capsule endoscopy, iron deficiency anaemia, young, small bowel, neoplasia
Background
In the developed world, iron deficiency anaemia (IDA) is estimated to affect about 5–10% of premenopausal women and 2–5% of men and postmenopausal women.1,2 Therefore, IDA accounts for up to 13% of gastrointestinal (GI) referrals, representing a significant burden of GI disease.3,4 Despite an increased uptake of bidirectional GI endoscopy in the diagnostic evaluation of IDA,3 30–50% of patients remain undiagnosed.4 In these patients, recent guidelines based on moderate to weak evidence3 suggest initial treatment with iron supplementation. Although current guidelines do not recommend further direct visualisation of the small bowel (SB), unless there are symptoms suggestive of SB disease or refractory IDA, anecdotally it is now routine to perform SB capsule endoscopy (SBCE) in patients following negative bidirectional endoscopy.
Small-bowel tumours (or malignancy) have a prevalence of 3–9% in patients undergoing SB evaluation5,6 and although uncommon, they are of particular importance due to their poor prognosis. Furthermore, an increasing incidence of SB malignancy has been documented over the past few decades.7,8 Previous SBCE studies have shown that the aetiology of GI blood loss differs with patient demographics. Young patients are more likely to bleed from SB malignancies, Dieulafoy lesions, Meckel’s diverticula, polyps or Crohn’s disease (CD). Conversely, those older than 40 are more likely to have angioectasias or non-steroidal anti-inflammatory drug (NSAIDs)-induced ulceration.9–11 Therefore, due to current guidelines, younger patients may be at risk of delayed diagnosis, which could adversely impact outcomes.12
Young patients represent a small proportion of patients undergoing SBCE. Moreover, to the best of our knowledge, only a couple of studies focusing on young IDA patients undergoing SB evaluation are available to date.10,11 With this retrospective study, we aimed to estimate the diagnostic yield (DY) of SBCE for SB pathology – in particular, the prevalence of SB neoplasia – in a large cohort of young patients (age ≤ 50 years) with IDA and negative bidirectional GI endoscopy. We also aimed to assess possible predictive factors associated with the occurrence of significant SB pathologies.
Materials and methods
This was a retrospective study. High-volume SBCE providers (>100 CE cases/ year) were invited to contribute data on consecutive patients undergoing SBCE between 2010 and 2015. Inclusion criteria were age 19–50 (inclusive), presenting with IDA based on the World Health Organization criteria (Hb <13 g/dl in men and <12 g/dl in women, with evidence of iron deficiency: mean corpuscular volume (MCV) <80 or ferritin <12–15 µg/l), and negative upper and lower GI endoscopy evaluation. Exclusion criteria were previous (or ongoing) obscure-overt GI bleeding (to homogenise the included patients), patients referred for SBCE for indications other than IDA, or presence of any comorbidity that could also cause IDA (e.g. known inflammatory bowel disease, coeliac disease, end-stage renal failure, prosthetic heart valve). We only included women with recent complete gynaecological evaluation to exclude any cause of excessive gynaecological blood loss.
Structured data collection questionnaires were sent to all participating centres. We collected data on patient demographics (age, gender), medical history including weight loss and comorbidities, indications for SBCE, investigations performed before SBCE (haemoglobin(Hb) at time of SBCE and lowest recorded value if available, MCV, GI endoscopies/cross-sectional imaging, duodenal biopsies/coeliac serology), medications (NSAIDs, antiplatelet agents, warfarin/heparin), findings, final diagnosis and outcomes (if known or if followed-up within the study period). SBCE videos were analysed by local SBCE readers as part of standard clinical care; no further central CE reading was performed. Local investigators were asked to categorise findings according to their clinical relevance using the Saurin score;13 SBCE were deemed positive when containing at least one P2 SB finding, that is, a finding which could explain symptoms and/or guide further workup. For the purpose of further analysis, P2 CE findings were eventually categorised as neoplastic or non-neoplastic but clinically significant. In order to allow for variations in practice between participating centres and to accommodate missing data, a minimum data set was defined for inclusion: patients had to have had Hb at time of SBCE, MCV, negative bidirectional GI endoscopies and CE results.
Continuous variables are presented as means (SD) or medians (IQR), as appropriate. Categorical variables are presented as numbers (%). Due to the number of variables, CE findings were analysed by multivariate logistic regression using five multiple imputed datasets to adjust for missing values of ferritin and lowest recorded Hb in some patients. This allowed maximal use of our data while minimising bias from missing values.14,15 Further variable selection was done using backwards elimination. For model comparison, the log likelihood test was used, with a p-value of 0.157 deemed to be of statistical significance.16,17
All patient identifiable data were anonymised during collection. No specific ethical approval needed to be obtained as all data were collected during routine patient care.
Results
Cases were collected from 18 centres in 12 countries. Data on 389 patients (262 F/127 M; mean age 39.4 ± 9.3 years) were scrutinised. In total, 220 patients (122 F/98 M; mean age 40.5 ± 8.6 years) had sufficient data for inclusion in the final analysis, as defined by the minimum data set (Figure 1). The patients’ clinical characteristics are summarised in Table 1. At presentation, the mean Hb for our patient group was 9.27 ± 2.36 g/dl, mean MCV was 71.54 ± 9.59 fl and mean ferritin was 13.16 ± 29.65 µg/l.
Figure 1.
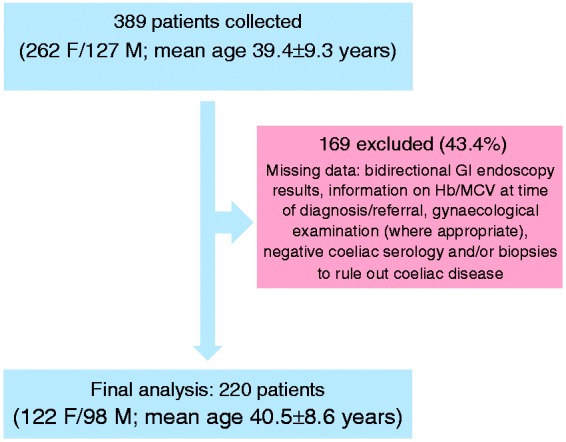
Patient selection in our study.
Table 1.
Characteristics of our patient group (n = 220).
| Demographic details | |
| Gender | 122 F/98 M |
| Age (mean ± SD), years | 40.5 ± 8.6 |
| Past medical history | |
| Gastrointestinal disease n (%) | 38 (17.3%) |
| Cardiovascular disease n (%) | 25 (11.4%) |
| Previous malignancy n (%) | 5 (2.3%) |
| Renal disease n (%) | 2 (0.9%) |
| Other past medical history n (%) e.g. diabetes, rheumatological conditions | 65 (29.5%) |
| Family history of GI malignancy n (%) | 23 (10.5%) |
| Characteristics at presentation | |
| Patients presenting with weight loss n (%) | 17 (7.7%) |
| Hb at presentation (mean ± SD) | 9.27 ± 2.36 g/dL |
| Lowest Hb recorded (n = 193) (mean ± SD) | 8.53 ± 2.2 g/dL |
| MCV at presentation (mean ± SD) | 71.54 ± 9.59 fL |
| Ferritin at presentation (n = 181) (mean ± SD) | 13.16 ± 29.65 µg/L |
| Relevant medications n (%) | None: 201 (91.4%) Yes: 19 (8.6%) Antiplatelet medications (aspirin/clopidogrel): 7 NSAIDs: 7 Anticoagulants: 5 More than 1 medication: 2 |
| Prior imaging investigations | |
| Patients previously investigated with CT abdomen n (median; range) | 60 (1; 1–3) |
| Patients previously investigated with MRE n (median; range) | 15 (1; 1–3) |
The number of patients is specified where data was not available for all patients.
CT: computed tomography; GI: gastrointestinal; Hb: haemoglobin; MCV: mean corpuscular volume; MRE: magnetic resonance enterography; NSAIDs: non-steroidal anti-inflammatory drugs; SD: standard deviation
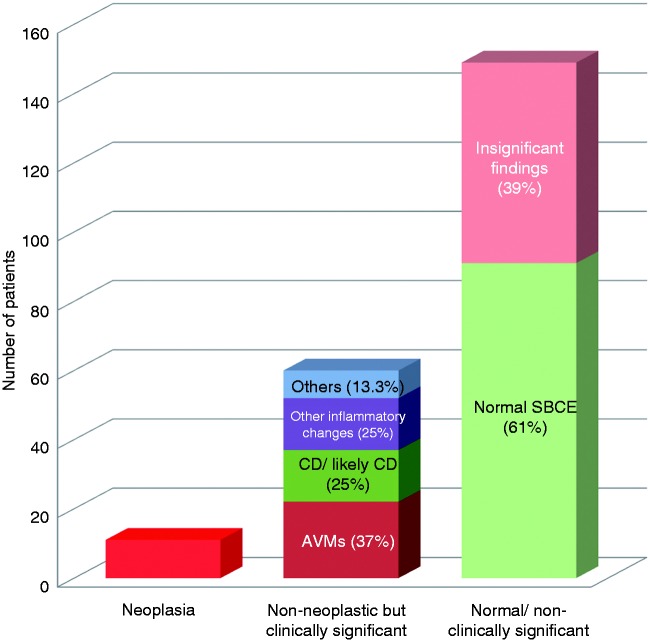
Among the 220 patients, 71 had a positive CE (DY 71/220; 32.3%). Subsequently, patients with positive CE were divided according to final diagnosis into two groups (Figure 2): patients with neoplastic SB pathology (10/220; 4.5%), and non-neoplastic albeit clinically significant CE findings (61/220; 27.7%). The most common non-neoplastic but significant findings were SB angioectasias (22/61) and SB Crohn’s disease (15/61) (Table 2). In total, 17 patients reported weight loss at presentation. Two of this group eventually had neoplastic pathology and nine had non-neoplastic but significant findings, that is, 2/10 (20%) of patients with neoplasia presented with weight loss, compared with 9/61 (14.8%) of patients with non-neoplastic findings and 6/149 (4%) of patients with normal or insignificant findings. In the patients with neoplasia, 6/10 had undergone computed tomography (CT) or magnetic resonance (MR) imaging prior to CE with no pathology yield (hence the investigation with CE). Some 22/61 of patients with significant non-neoplastic pathology, and 40/149 of patients with normal CE, had had previous CT or MR imaging.
Figure 2.
Breakdown of capsule endoscopy findings in our group of patients.
AVMs: arteriovenous malformations/angioectasias; CD: Crohn’s Disease; SBCE: small bowel capsule endoscopy.
Table 2.
CE small bowel findings in our group of patients.
| Type of findings | Number of patients (%) Details below |
|---|---|
| Neoplastic | 10 (4.5%) Malignant neoplasia: 4 adenocarcinoma, 3 GIST, 1 lymphoma Benign neoplasia: 1 Vanek tumour, 1 hamartoma |
| Non-neoplastic (clinically significant) | 61 (27.7%) 22 angioectasias, 15 Crohn’s Disease, 5 nonspecific inflammation, 5 ulcers, 5 NSAID enteropathy, 9 others (2 Meckel’s, 1 inflammation due to rheumatoid arthritis, 1 coeliac, 1 strictures, 1 Dieulafoy lesion, 1 hereditary haemorrhagic telangiectasia, 1 pinworms, 1 mucosal bulge) |
| Normal/minimal and not clinically significant | 149 (67.7%) 91 normal, 58 minor/insignificant findings (e.g. lymphangiectasias, red spots) |
All possible predictive factors included were subjected to variable selection to identify the best predictors of significant SB pathology (both neoplastic and non-neoplastic). These were: ferritin, MCV, presence of weight loss and use of antiplatelet pharmacologic agents (see supplementary material). On multivariate analysis (Table 3), lower MCV was associated with clinically significant SB pathology (OR 0.96; 95%CI 0.92–0.99; p = 0.03), that is, the odds of diagnosing significant SB pathology in CE were increased 4% for every unit of decrease in MCV. Furthermore, the presence of weight loss at clinical presentation increased the odds of significant SB pathology 3.85 times (OR 3.85; 95%CI 1.31–11.13; p = 0.01). Lastly, our model suggests a possible association between the use of antiplatelet medications and the presence of significant findings (OR 3.74; 95%CI 0.765–18.313; p = 0.10); however, due to the small number of patients receiving this specific pharmacological treatment, no valid conclusion can be drawn.
Table 3.
Final model with predictive factors.
| Variables in initial model | OR | SE(logOR) | Pr(>|t|) | 95% CI |
|---|---|---|---|---|
| (Intercept) | 2.226 | 1.26 | 0.530 | 0.188–26.301 |
| Weight loss (Y/N) | 3.857 | 0.55 | 0.010 | 1.313–11.336 |
| Initial MCV | 0.961 | 0.02 | 0.030 | 0.924–0.999 |
| Antiplatelet use | 3.743 | 0.81 | 0.100 | 0.765–18.313 |
| NSAID use | 2.586 | 0.94 | 0.310 | 0.410–16.320 |
| Lowest Hb | 1.150 | 0.09 | 0.120 | 0.964–1.372 |
CI: confidence interval; Df: degrees of freedom; Hb: haemoglobin; MCV: mean corpuscular volume; NSAID: non-steroidal anti-inflammatory drug; OR: odds ratio; SE: standard error
In our cohort, 136/220 patients had resolution of IDA on follow-up (which was variable between centres). At the time of writing, 18/220 were lost to follow-up. Table 4 details outcomes for our patient group following CE. Seven of the 10 patients diagnosed with neoplasia had resolution of IDA following surgical management. Of the three in whom IDA did not resolve, two were diagnosed with adenocarcinoma and one had been diagnosed with a hamartoma.
Table 4.
Outcomes in our patient group following CE.
| Group (n = patients where information on follow-up was available) | Resolution of IDA |
No Resolution |
||
|---|---|---|---|---|
| Active treatment | Conservative management only | Active treatment | Conservative management only | |
| Neoplastic pathology (n = 10) | 7: all surgical management | – | 2: further enteroscopy (1 for retrieval of retained capsule, 1 for biopsy) 1: surgical resection | – |
| Non-neoplastic but significant pathology (n = 57) | 14: treatment for CD 10: further enteroscopy 4: repeat ileocolonoscopy 2: repeat CE 1: repeat UGIE 5: surgical management | 13 | 9: further SB evaluation with deep enteroscopy | 5 |
| No significant pathology (n = 135) | 2: repeat ileocolonoscopy 1: Meckel’s scan | 82 | 2: further SB evaluation 1: repeat CE 1: repeat UGIE and ileocolonoscopy | 45 |
CD: Crohn’s disease; CE: capsule endoscopy; SB: small bowel; UGIE: upper gastrointestinal endoscopy
Supplementary material. Variable selection from all predictive factors analysed.
In the group of patients with non-neoplastic but clinically significant pathology, 44/61 (72.1%) had IDA resolution on follow-up. Eighteen of these 44 patients required further GI endoscopy (upper gastrointestinal endoscopy, ileocolonoscopy, repeat CE and/or deep enteroscopy including push enteroscopy and double-balloon enteroscopy). Five patients required surgical management: two underwent resection of Meckel’s diverticula, one required surgery for removal of the retained capsule, one had haemorrhoids banded and one underwent SB resection for CD. Thirteen out of 44 patients were managed conservatively; 10 had angioectasias, two had nonspecific SB inflammation and one had pinworms. Thirteen out of 61 patients with non-neoplastic but clinically significant pathology (21.3%) did not have resolution of IDA on follow-up. Seven of these 13 patients had angiodysplasias. In this group, 9/13 had undergone further SB evaluation by deep enteroscopy.
Of the patients with no significant pathology on CE, 85/149 (57.0%) had resolution of IDA on follow-up; 82 of these patients were managed conservatively; two underwent further ileocolonoscopy and one had a negative Meckel’s scan.
Discussion
A significant proportion of patients with IDA (approximately 30%) remain undiagnosed following bidirectional GI endoscopy, prompting SB evaluation.3 Our data are in agreement with existing studies on the epidemiology of SB blood loss and show that younger patients, presenting with IDA, are at higher risk of SB neoplasia compared with older patients. Zhang et al. found that SB angioectasias, while the most common cause of occult GI bleeding in patients >65 accounting for 54% of cases, were present in only 9% of patients 40 years old or less.18 Likewise, only 10% (22/220) of patients in our cohort were found to have SB angioectasias. In contrast, about 5% of our patients had SB neoplasia, similar to the estimated population prevalence of 3–9%.5,6 Previously, we reported that sinister or significant pathology appears in 25% of patients below 40 years old but only 7.5% of patients over 40 years.10
A study by Sidhu et al. demonstrated angioectasias in 10% of patients younger than 50 years old who underwent CE for IDA, and SB tumours in 3% of the same patient cohort.11 Interestingly, SB angioectasias are known to occur more frequently alongside other comorbidities including cardiovascular disease, chronic kidney disease and/or chronic respiratory conditions; consequently, SB angioectasias may be less common in younger, fitter patients such as our group.19 Therefore, this large multicentre study underscores the importance of having a high index of suspicion in young patients presenting with IDA.
Small-bowel neoplasia was the diagnosis we considered the most significant in our patient group. Of the 10 patients from our cohort diagnosed with neoplasia, eight had malignant histopathology. According to US and UK data, carcinoid tumours and adenocarcinomas are the most common SB neoplasias.7,8 The UK data also show an increasing incidence of SB tumours since the 1980s.8 The prognosis of SB malignancy remains poor; for example, SB adenocarcinoma still has a 5-year survival of less than 30%.7 This could be due to factors such as location of the malignancy – significant proportions of these SB tumours were located in the ileum, thus out of reach of conventional endoscopy8 – and the resulting diagnostic delay.20 Modlin et al. found patients with SB carcinoid tumours were more likely to have disseminated disease at diagnosis compared with gastric carcinoids. The same study showed minimal change in survival rates for carcinoid tumours over the past 50 years, implying failure to identify these lesions in a timely manner, or a lack of information to guide effective treatment.21
Notably, only a small proportion of patients in our group had weight loss as a symptom at the time of presentation, and only two out of 10 patients with neoplastic pathology experienced weight loss. This emphasises the minimal or nonspecific symptoms which SB malignancies initially present with.5 On the other hand, a larger proportion (20%) of the group with neoplastic diagnoses reported weight loss compared with patients with significant non-neoplastic pathology (14.8%) and those with normal CE results (4%). These differences suggest that young patients presenting with weight loss should be investigated more extensively and earlier.
To the best of our knowledge, there are few studies attempting to quantitatively correlate risk of significant SB findings with red cell indices as markers of IDA. As MCV decreased for our group of patients, there appeared to be a proportionate increase in the likelihood of SB tumours. In anaemic patients the probability of IDA increases with decreasing MCV.22 This could be related to the duration of IDA, or because the anaemia had failed to resolve over a period of time thus indicating ongoing or progressive pathology. For such patients with more severe IDA, the current UK guidelines suggesting 1–3 months of empiric oral iron replacement therapy following negative bidirectional endoscopy may cause further diagnostic delay.
There is a lack of data on the outcomes for patients with unexplained IDA, and existing studies imply that the current management of IDA alone is often incomplete or inadequate.23 Our study has attempted to address some of these gaps so as to improve patient care.
Limitations of this study include its retrospective study design, meaning that clinical data were incomplete for several patients (almost half in our cohort). This could have led to some overestimation of our results. We have attempted to minimise this possible effect using multivariate analysis as detailed. Secondly, many of our centres were high-volume or tertiary referral centres, which would therefore have taken a disproportionate number of complex patients or those suspected of having sinister pathology. Finally, our study used MCV as a marker of iron deficiency in anaemic patients, although drawbacks exist to the use of MCV to quantify iron deficiency. Other red cell indices such as mean cell haemoglobin (MCH) (i.e. markers of hypochromia rather than microcytosis) may correlate better with severity of IDA than MCV.24 Current guidelines state that MCV alone is not enough to make a diagnosis of IDA and other parameters, namely ferritin, should be used to assess iron status,22 as ferritin correlates well with total body iron stores and is a better marker of iron deficiency; low MCV occurs only in the later stages of iron deficiency.25 Data on ferritin were not available for all the patients in our group, and MCV was used in this study due to its widespread use and availability. Both markers are less reliable in elderly and/or hospitalised patient populations, and in several other comorbidities, for example inflammation and anaemia of chronic disease,26 but may be more reliable in the younger group that overall has a lower rate of comorbidities.
Conclusion
In patients ≤50 years old presenting with IDA, the overall diagnostic yield of SBCE for significant SB findings was 32.3%. Around 5% in our group were diagnosed with SB neoplasia. In this cohort, lower MCV and weight loss were associated with higher risk of a diagnosis of significant SB findings. We propose that in young patients with certain clinical features such as low MCV and weight loss, CE should be prioritised.
Supplementary Material
Acknowledgements
This study was designed and led by ER and AK. Overall data collection and collation was carried out by DEY; data analysis was done by AG, TA and DEY. DEY, AK and ER wrote up the manuscript; JNP, ET and RE provided their expert input and guidance throughout the process. All other authors contributed cases and data from their centres and provided input on the manuscript. The members of the research group contributed and collected data for the cases from their centre. AK is the guarantor of this manuscript.
Declaration of conflicting interests
X Dray: Lecture fees from Given Imaging/Coviden/Medtronic, R Eliakim: Consultant/ lecture fees from Given Imaging/Medtronic, L Elli: financial support from Coviden/Medtronic for organization of congresses and study protocols, GW Johansson: Lecture fees from Medtronic, E Toth: Lecture fees from Medtronic. A Koulaouzidis: honoraria from and member of advisory board for Dr Falk Pharma UK; material support for research from Synmed UK. All other authors: nothing to declare.
Funding
DE Yung received a Dr Falk/ Core F1/F2 Award 2015 for this study.
References
- 1.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014; 123: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Iron deficiency anaemia: Assessment, prevention, and control. A guide for programme managers. World Health Organization, 2001: 114.
- 3.Goddard AF, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut 2011; 46: iv1–iv5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zuckerman G, Prakash C, Askin M, et al. AGA technical review on the evaluation and management of occult and obscure gastrointestinal bleeding. Gastroenterology 2000; 118: 201–221. [DOI] [PubMed] [Google Scholar]
- 5.Rondonotti E, Pennazio M, Toth E, et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: A multicenter European study. Endoscopy 2008; 40: 488–495. [DOI] [PubMed] [Google Scholar]
- 6.Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer 2006; 107: 22–27. [DOI] [PubMed] [Google Scholar]
- 7.Partridge BJ, Tokar JL, Haluszka O, et al. Small bowel cancers diagnosed by device-assisted enteroscopy at a U.S. referral center: A five-year experience. Dig Dis Sci 2011; 56: 2701–2705. [DOI] [PubMed] [Google Scholar]
- 8.Shack LG, Wood HE, Kang JY, et al. Small intestinal cancer in England & Wales and Scotland: Time trends in incidence, mortality and survival. Aliment Pharmacol Ther 2006; 23: 1297–1306. [DOI] [PubMed] [Google Scholar]
- 9.Scaglione G, Russo F, Franco MR, et al. Age and video capsule endoscopy in obscure gastrointestinal bleeding: A prospective study on hospitalized patients. Dig Dis Sci 2011; 56: 1188–1193. [DOI] [PubMed] [Google Scholar]
- 10.Koulaouzidis A, Yung DE, Lam JHP, et al. The use of small-bowel capsule endoscopy in iron-deficiency anemia alone; be aware of the young anemic patient. Scand J Gastroenterol 2012; 47: 1094–1100. [DOI] [PubMed] [Google Scholar]
- 11.Sidhu PS, McAlindon ME, Drew K, et al. The utility of capsule endoscopy in patients under 50 years of age with recurrent iron deficiency anaemia: Is the juice worth the squeeze? Gastroenterol Res Pract 2015; 948574. [DOI] [PMC free article] [PubMed]
- 12.North JH, Pack MS. Malignant tumors of the small intestine: A review of 144 cases. Am Surg 2000; 66: 46–51. [PubMed] [Google Scholar]
- 13.Saurin JC, Delvaux M, Gaudin JL, et al. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: Blinded comparison with video push-enteroscopy. Endoscopy 2003; 35: 576–584. [DOI] [PubMed] [Google Scholar]
- 14.Van Buuren S, Groothuis-Oudshoorn K. Multivariate imputation by chained equations. J Stat Softw 2011; 45: 1–67. [Google Scholar]
- 15.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009; 338: b2393–b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royston P and Sauerbrei W. Multivariable model-building: A pragmatic approach to regression analysis based on fractional polynomials for modelling continuous variables. John Wiley & Sons, 2008.
- 17.Lindsey JK, Jones B. Choosing among generalized linear models applied to medical data. Stat Med 1998; 17: 59–68. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Chen C, Li Y. Capsule endoscopy examination identifies different leading causes of obscure gastrointestinal bleeding in patients of different ages. Turk J Gastroenterol 2012; 23: 220–225. [DOI] [PubMed] [Google Scholar]
- 19.Holleran G, Hall B, Hussey M, et al. Small bowel angiodysplasia and novel disease associations: A cohort study. Scand J Gastroenterol 2013; 48: 433–438. [DOI] [PubMed] [Google Scholar]
- 20.Dabaja BS, Suki D, Pro B, et al. Adenocarcinoma of the small bowel. Cancer 2004; 101: 518–526. [DOI] [PubMed] [Google Scholar]
- 21.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003; 97: 934–959. [DOI] [PubMed] [Google Scholar]
- 22.Galloway MJ, Smellie WSA. Investigating iron status in microcytic anaemia. BMJ 2006; 333: 791–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yates JM, Logan ECM, Stewart RM. Iron deficiency anaemia in general practice: Clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad Med J 2004; 80: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jolobe OMP. Prevalence of hypochromia (without microcytosis) vs microcytosis (without hypochromia) in iron deficiency. Clin Lab Haematol 2000; 22: 79–80. [DOI] [PubMed] [Google Scholar]
- 25.Hastka J, Lasserre JJ, Schwarzbeck A, et al. Laboratory tests of iron status: Correlation or common sense? Clin Chem 1996; 42: 718–724. [PubMed] [Google Scholar]
- 26.Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem 2002; 48: 1066–1076. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.