Abstract
The principal molecular mechanisms underlying the cardiovascular and renal adverse effects of nonsteroidal anti-inflammatory drugs (NSAIDs), such as myocardial infarction and hypertension, are understood in more detail than most side effects of drugs. Less is known, however, about differences in the cardiovascular safety profile between chemically distinct NSAIDs and their relative predisposition to complications. Here, we discuss how heterogeneity in the pharmacokinetics and pharmacodynamics of distinct NSAIDs may be expected to affect their cardiovascular risk profile. We consider evidence afforded by studies in model systems, mechanistic clinical trials, a meta-analysis of randomized controlled trials, and two recent large clinical trials, SCOT and PRECISION, designed specifically to compare the cardiovascular safety of the cyclooxygenase (COX)-2 selective NSAID, celecoxib, with traditional NSAIDs. We conclude that SCOT and PRECISION have apparently not compared equipotent doses and have other limitations that bias them towards underestimation of the relative risk of celecoxib.
NSAIDs and Adverse Events
Chronic inflammatory pain is a major global health problem, which affects tens of millions in North America and Europe alone [1, 2] and has contributed to the opioid epidemic in the United States [3]. Inhibitors of prostanoid biosynthesis, nonsteroidal anti-inflammatory drugs (NSAIDs), are the commonest option available for non-addictive treatment of mild to moderate inflammatory pain. They are amongst the most frequently consumed drugs, and compounds such as ibuprofen, naproxen and diclofenac or their metabolites are detectable in bodies of water worldwide. It has been estimated that more than one in ten adults in the United States uses NSAIDs at least three times a week for at least three months per year [4]. Short term exposure to NSAIDs is particularly prevalent in individuals at risk for acute and chronic musculoskeletal injuries. For example, >80% of all active duty United States army personal was prescribed an NSAID at least once in 2014 [5] and perhaps half of the participants in endurance sports, such as triathlons or marathons, consume NSAIDs for analgesia during the event [6, 7]. With such a large population exposure, even small safety signals may have considerable public health impact and while several NSAIDs are considered safe enough to sell over-the-counter, they have the potential to cause serious complications. Thus, NSAIDs may damage the gastrointestinal mucosa, raise blood pressure, cause heart attack, stroke and heart failure and perhaps arrhythmias and sudden cardiac death.
Serious gastrointestinal complications of NSAIDs – bleeding and perforated ulcers, and obstruction – were estimated to contribute to tens of thousands of hospitalizations and as many as 6,000 – 7,000 deaths per year in the United States in the late 1980s, although the individual risk for patients was low [8]. This problem was the driving force behind the rapid development of cyclooxygenase (COX)-2 selective NSAIDs once the second isoform of the drug target was discovered in the 1990s. However, at that time, the biology of COX-2 was insufficiently understood – COX-2 was assumed to be the exclusive source of prostanoids in inflammation and perhaps cancer. Thus, selective inhibition of COX-2 was expected to provide analgesia, while avoiding completely gastrointestinal complications, and the new drugs were aggressively advertised to consumers and physicians as “safer NSAIDs”. However, physiological roles for COX-2 in the vasculature and kidney had been discovered prior to the market launch of the first inhibitors, raising the possibility of cardiovascular adverse events, including heart attacks [9, 10]. Subsequently, this was confirmed in a series of randomized, placebo-controlled trials, designed to explore the utility of COX-2 inhibition in the prevention of colon cancer and post-operative analgesia [11, 12]. Eventually most selective COX-2 inhibitors were withdrawn from the market or their use restricted and the development of novel compounds halted [13, 14]. Conservative estimates suggest that approximately 70,000 additional heart attacks and 26,000 deaths were caused in the United States alone in the first five years following their introduction by prescribing COX-2 selective NSAIDs to millions of patients [15]. To put this into a public health perspective, this is more than the deaths averted by the flu vaccine within a five year period.
The development of COX-2 selective NSAIDs and detection of their cardiovascular hazard has prompted extensive research into the underlying molecular mechanism, the biochemistry of COX inhibition, drug-drug and drug-gene interactions, the clinical cardiovascular safety profile of COX-2 selective and non-isoform selective NSAIDs, and novel approaches to anti-inflammatory pain therapy. These investigations have generated an unprecedented body of information in model organisms and hundreds of thousands of patients – more than exists for any other adverse drug effect. Yet, there is still uncertainty as to which of the many chemically distinct NSAIDs still on the market are the safest to use in arthritic patients with comorbidities such as heart disease or hypertension – a population that increases with older age. In its most recent evaluation of NSAID safety in 2014, the USA Food and Drug Administration (FDA) concluded that there was insufficient comparative clinical trial information to answer this question and strengthened labelling for all NSAIDs to warn of potential cardiovascular complications [16]. The results of two large randomized controlled clinical studies designed to compare the cardiovascular safety of NSAIDs were since reported [17–20]. Here, we discuss molecular mechanisms underlying the cardiovascular hazard, how they may cause differences in the safety profile between chemically distinct NSAIDs and in how far the recent clinical trial data can inform decision making.
Cardiovascular Mechanisms of Cyclooxygenase Inhibition
NSAIDs are inhibitors of the COX enzymes, COX-1 and COX-2, which both metabolize arachidonic acid to an unstable precursor, prostaglandin (PG) H2. A variety of prostaglandin isomerases use PGH2 as the substrate to form prostaglandins, prostacyclin (PGI2) and thromboxane (Tx) A2. As with all drugs, adverse reactions of NSAIDs may either derive from inhibition of their target enzymes, the COXs, expressed at sites where they have physiological roles and are not involved in the inflammatory process, or from inhibition of unrelated targets – so called off-target effects. Off-target effects have indeed been identified for most NSAIDs; however, most were detected at supratherapeutic concentrations in cell culture experiments, and those few that occur at reasonably low concentrations have not been shown to cause thrombosis, blood pressure or heart failure in model organisms or humans. Instead, inhibition of physiological COX functions in the cardiovascular system can explain sufficiently a cardiovascular risk.
A cardiovascular risk was first predicted from studies in healthy volunteers that revealed suppression of PGI2 by COX-2 selective NSAIDs, such as celecoxib and rofecoxib, before the first such drug came on the market [9, 10]. PGI2 was known to be a potent platelet inhibitor in vitro and its biosynthesis, estimated by measurement of its stable metabolites in urine, was elevated in patients in syndromes of accelerated platelet vessel wall interactions, such as severe peripheral vascular disease, unstable angina and myocardial infarction [21, 22]. Thus, it had been hypothesized that PGI2 was synthesized in the vasculature as part of a complex negative feedback mechanism, in concert with thrombomodulin, nitric oxide, the ectonucleotidase CD39 and other mediators, to restrict the growth of a thrombus at the site of vascular injury. These interdependent regulatory molecules act potently to terminate the explosive feed-forward reactions that otherwise accelerate the clotting process to assure hemostatic integrity. For example, the potency of the CD39 mechanism, which reduces local concentrations of the platelet activator adenosine diphosphate (ADP), is illustrated by the cardioprotective effects of thienopyridine platelet inhibitors, such as clopidogrel and cangrelor, which block the platelet ADP receptor, P2Y12.
Several lines of evidence in support of the hypothesis that PGI2 is part of this regulatory system have been identified (reviewed in detail here [13] and here [14]): Mice deficient in the PGI2 receptor (the IP) show gene-dose dependent accelerated thrombotic responses following vascular injury [23] and humans with genetic variants in the IP or the enzymes that synthesize PGI2 have an elevated risk of atherothrombotic events [24]. PGI2 interacts with multiple components of the vascular system that terminate coagulation. It is the most potent endogenous platelet inhibitor, upregulates thrombomodulin [25] and depresses tissue factor expression [26]. Accelerated thrombotic responses have also been observed in mice, hamsters, and dogs treated with COX-2 selective NSAIDs and in COX-2 deficient mice [14, 26]. In humans, a cardiovascular hazard with 3 structurally distinct COX-2 selective NSAIDs was detected in 10 placebo controlled trials [14], in meta-analyses of over 700 randomized trials including individual data from ~300,000 patients [27], and in numerous epidemiological studies [28]. The vascular origin of COX-2 derived PGI2 was established in mice using site-specific deletion of COX-2 in just endothelial (tie2-Cre) or smooth muscle cells (sm22-Cre) or both [29]. These experiments found that biosynthesis of PGI2 (measured as its stable urinary metabolite, 2,3 dinor-6-keto PGF1α), PGI-M, which is formed through enzymatic β-oxidation (largely in liver peroxisomes) was reduced when COX-2 was removed from the vasculature and showed that vascular COX-2 deletion was sufficient to accelerate thrombosis and elevate blood pressure in vivo [29].
The role of vascular COX-2 as a component of the endogenous antithrombotic system was challenged in reports that detected only low quantities of COX-2 in the vascular wall while COX-1 was abundantly expressed [30]. Although in the absence of standard curves, these were not valid comparative measurements of protein expression [31], it was suggested that the majority of detectable urinary PGI2 metabolites stem from other tissue sources and that the vascular contribution of COX-2 derived PGI2 is small in humans. We know that this is not true in mice, where, as mentioned above, tissue specific knockout experiments proved that the vasculature is a significant contributor to COX-2 derived urinary PGI2 metabolite excretion [29]. Attributing a tissue of origin to a systemic marker of PG formation in humans is a greater challenge. With another COX derived urinary biomarker, a stable metabolite of TxA2, 11-dehydro-TxB2 (Tx-M), the task is made simpler in the case of the anucleate platelet, where recovery after platelet inhibition by aspirin requires de novo platelet synthesis over 7–10 days. Here, evaluation of the dynamics of this marker in situations of cardiovascular health and disease identify platelets as a significant source of urinary Tx-M [21, 22]. Low doses of aspirin inhibit platelet TxA2 formation ex vivo and depress urinary excretion of Tx-M in healthy volunteers, indicating that even in apparently healthy individuals a small degree of platelet activation occurs constantly and results in the production of small, but detectable background levels of platelet derived TxA2 [32]. In patients with established atherosclerotic lesions and presumably enhanced platelet vessel wall interactions, such as those with severe peripheral artery disease, urinary Tx-M excretion is increased further and can be depressed with low dose aspirin [21]. Finally, in patients with acute myocardial infarction – a platelet thrombus forming in a coronary artery – urinary Tx-M excretion is markedly increased, but again can be attributed to platelets by studies with aspirin [22]. Together, these observations suggest strongly that platelet derived TxA2 is usually a significant source of urinary TxM. The fractional contribution of platelets to total TxA2 depends on the context, such as the degree of vascular disease and consequent platelet activation. However, if another cellular source of TxA2 is augmented, for example macrophage activation in smokers, there is considerable capacity for the macrophage contribution to urinary Tx-M, reflecting overall systemic Tx biosynthesis, to increase [33]. Very similar dynamics have been observed with PGI2. Parallel with augmented platelet derived urinary Tx-M, urinary excretion of the PGI-M increases, consistent with the hypothesis that it acts as a restraint on accelerated platelet-vessel wall interactions in humans [21, 22] (Figure 1).
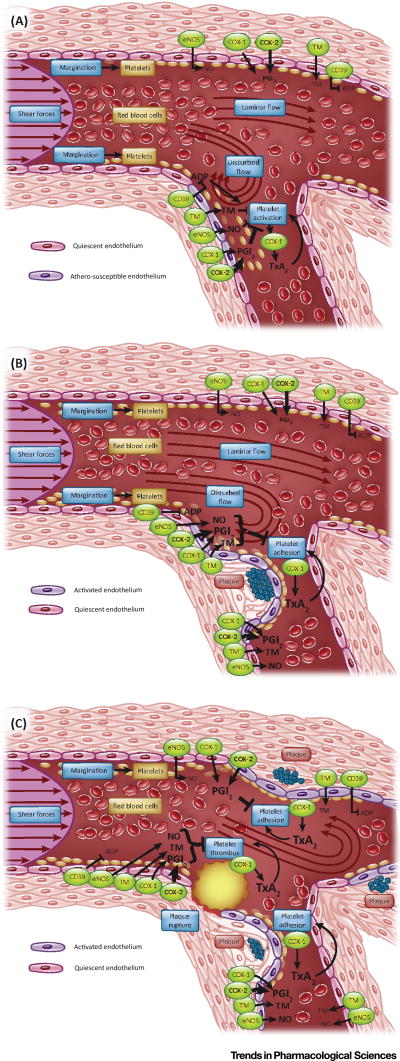
Figure 1. Local formation of pro- and anti-thrombotic mediators in healthy and atherosclerotic arteries.
A) Disturbed flow at branching points in healthy arteries induces an pro-inflammatory state of the endothelium and results in platelet-vessel wall interactions which contribute to low levels of pro-and anti-inflammatory mediators, including thromboxane (Tx) and prostacyclin formation (PGI2) being formed in healthy individuals. B) The formation of atherosclerotic plaque in susceptible locations such as branching points results in more intense platelet-vessel wall interactions, reflected by augmented release of pro and antithrombotic mediators. C) Plaque rupture induces thrombus formation through the explosive release of prothrombotic mediators in a feed forward loop. The coincident substantial release of antithrombotic mediators in the direct vicinity is an important regulatory mechanism to restrict thrombus formation to the site of rupture.
CD39: Ectonucleoside triphosphate diphosphohydrolase-1 (hydrolyzes purinergic receptor ligands); TM: thrombomodulin (converts thrombin to an anticoagulant enzyme); eNOS: endothelial nitric oxide synthase; COX: cyclooxygenase; ADP: adenosine diphosphate
It has long been known that less COX-2 than COX-1 is expressed in the vasculature; sometimes COX-2 is difficult to detect at all post mortem, perhaps because it is induced by flow [34]. It is indeed remarkable that despite such lower expression levels, selective inhibition of COX-2 in both humans and mice, and global or vascular COX-2 deletion in mice all reduce time integrated markers of systemic PGI2 formation (urinary excretion of stable PGI2 metabolites, such as 2,3 dinor-6-keto PGF1α) by 20–70 % in the absence of inflammation. A likely explanation for the divergence between low vascular expression levels and urinary biomarker excretion is that prostanoid formation by COX-2 is much more efficient than formation by COX-1. While the enzyme kinetics of COX-1 and COX-2 are nearly identical, activation of COX-2 requires about 10-fold lower peroxide concentrations than COX-1 [35]. Structure function studies revealed that the COX enzymes operate as homodimers consisting of allosteric and catalytic subunits. Cellular fatty acids that are not COX substrates differentially regulate COX-1 and COX-2 activity through interactions with their allosteric subunits [36, 37]. Thus, COX-1, but not COX-2, is kept in a tonically inhibited state, which can only be overcome by high substrate concentrations, e.g. high arachidonic acid concentrations [38]. COX-2 on the other hand, is stimulated by these same fatty acids, lowering effectively the concentration of arachidonic acid needed for activation [36, 37]. This leads to the preferential production of COX-2 derived prostanoids at low substrate concentrations. It also explains why primarily COX-1 derived prostanoids can be detected in blood vessels subjected in vitro or ex vivo to excessive stimulation that results in unphysiologically high concentrations of arachidonic acid [30].
In addition to its roles in thrombosis, PGI2 inhibition may contribute to the development of atherosclerotic lesions through at least two mechanisms: direct effects on plaque development [39, 40], and its regulatory function in blood pressure control. Elevated blood pressure contributes to atherogenesis, myocardial infarction and stroke. The role of prostanoids in the regulation of blood pressure has long been known and the largest randomized controlled NSAID trial reported the development of clinically manifest hypertension in 5–8% of osteoarthritis and rheumatoid arthritis patients [41]. The role of the COX enzymes in the kidney is complex – with opposing actions depending on when and where the enzymes are expressed. For example, COX-2 dependent vasodilator prostanoids (PGI2 and PGE2) formed in renal medullary interstitial cells play an important role in the adaptive regulation of arterial pressure through pressure diuresis driven by medullary perfusion [42]. Older individuals, in particular, depress medullary blood flow and increase blood pressure on NSAIDs. Younger, healthy individuals, in most of whom both nonselective COX inhibition and selective COX-2 inhibition have no effects on arterial pressure, still show transient changes in the glomerular filtration rate (GFR) and sodium handling on initiation of dosing. This is due to COX-2 inhibition in the renal cortex where dilator prostanoids (PGI2 and PGE2) help maintain the patency of afferent arterioles. However, inhibition of COX-2 in the macula densa system can also have a blood pressure lowering effect – depending on context and timing – as it is a component of the tubuloglomerular feedback mechanism controlling renin release [43]. Renal injury from NSAIDs may result from both inhibition of medullary and glomerular perfusion.
Recently a link between renal COX-2 and the vascular system to terminate thrombus formation has been proposed [44]. A study reported a dysregulation of genes involved in the formation and degradation of forms of methylated arginine, asymmetrical dimethylarginine (ADMA) and N-monomethyl-L-arginine (L-NMMA) [45], in the kidneys of COX-2 deficient mice. These investigators detected elevated plasma levels of ADMA and L-NMMA in mice in which COX-2 had been either conventionally deleted or inhibited by a small molecule and an elevation of ADMA in volunteers treated with a COX-2 inhibitor [45]. Methylarginines are released into the extracellular space and circulation following the post-translational methylation of arginine residues within proteins and their subsequent proteolysis. ADMA and L-NMMA act as endogenous inhibitors of nitric oxide biosynthesis and would be expected to inhibit formation of nitric oxide in the vessel wall (Figure 1), potentially synergizing with the inhibition of COX-2 derived vascular PGI2 to accelerate thrombogenesis and elevate blood pressure. Elevated ADMA concentrations have been associated with major cardiovascular risk factors, including hypertension, hypercholesterolaemia, homocysteine, inflammation, obesity and diabetes mellitus [46] and an independent association between plasma levels of ADMA and major cardiovascular events has been established in population studies [47]. While a role of methylarginines in the cardiovascular toxicity of NSAIDs is an intriguing concept, it seems unlikely as a primary mechanism. The conventional prenatal COX-2 knockout mice used in these studies are born with renal defects, including markedly reduced numbers of glomeruli, leading to kidney failure within weeks and death. Methylated arginines are elevated in chronic renal failure in humans and, indeed, this is the condition in which they were first described in [45]. Here the elevated ADMA and L-NMMA levels were detected in knockouts already in renal failure. The NSAID used, parecoxib, was administered at concentrations ~100 times the maximal permitted human daily dose. Neither its selectivity for inhibition of COX-2 nor its impact on renal function was reported [44]. In a small study in healthy volunteers, NSAIDs increased the concentration of ADMA in plasma [44]. However, here again, renal function was apparently compromised (an average ~1.5 fold increase in creatinine) when compared to the small changes that have been previously reported in healthy individuals [9]. Thus, it remains an open question whether these changes can be validated in larger populations, the degree to which they reflect renal compromise, and if so, how robust an association might exist between NSAID induced renal functional impairment and the cardiovascular complications that result from administration of these drugs. Remarkably little information is extant in in vivo systems on the interaction between nitric oxide and prostaglandin formation. However, in mice, suppression of COX-2 derived PGI2 in the vasculature reduces expression of endothelial nitric oxide synthase (eNOS) and generation of nitric oxide [29], suggesting that its reduced formation might amplify the predominant mechanism by which NSAIDs cause cardiovascular complications, irrespective of a role for endogenous inhibitors, such as ADMA.
The Comparative Cardiovascular Safety of NSAIDs
Mechanistic considerations
All NSAIDs have in common the inhibition of COX-2-derived prostanoid synthesis that primarily alleviates inflammation, pain and fever. However, NSAIDs are a chemically heterogeneous group of compounds distinguished by multiple characteristics with potential impact on their risk / benefit profile, such as the potency of COX-2 inhibition, the degree of coincident COX-1 inhibition (the degree of selectivity for COX-2), and their duration of action. Importantly, these variables are continuous – all ~40 chemically distinct NSAIDs marketed worldwide can be ranked by each variable – and the combination of the pharmacodynamic and -kinetic variables produce an NSAID’s unique therapeutic characteristics. This is the mechanistic context in which differences in their clinical impact has to be evaluated.
Compound specific characteristics of frequently consumed drugs include the following:
-
(i)
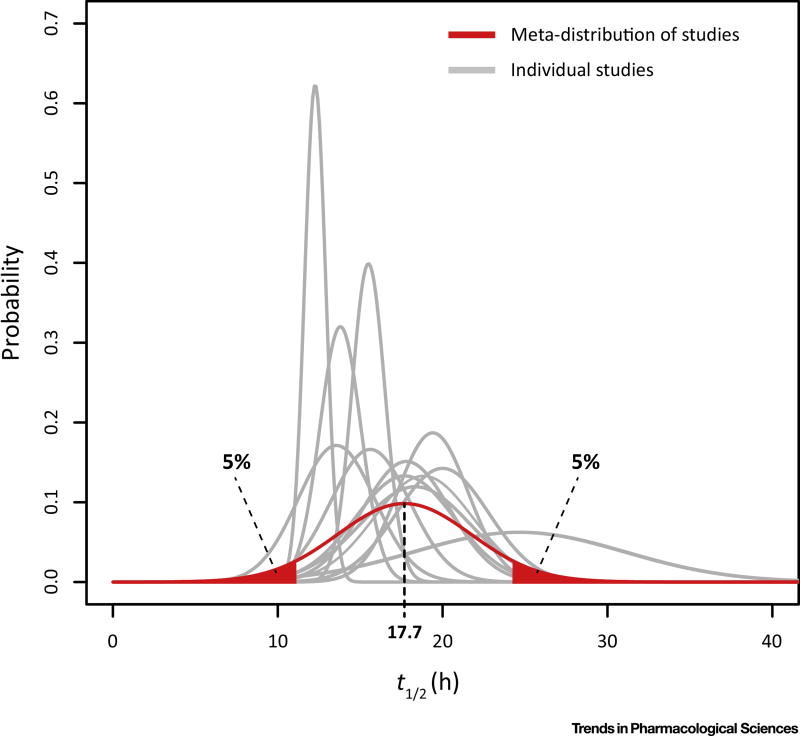
Naproxen is non-selective, inhibiting both COX-1 and COX-2. Naproxen is usually taken every 12 hours and can be expected to provide equal COX-1 and COX-2 inhibition throughout. It is not a very potent COX inhibitor, probably because it acts on the allosteric rather than the catalytic monomers of the COX enzymes [48]. However, while its half-life is highly variable between individuals ranging from 9 to 26 hrs (Figure 2), it is, on average, comparatively long amongst NSAIDs [49–61]. Thus, naproxen given in relatively high doses twice daily results in predictable plasma drug levels and provides stable COX inhibition and analgesia. However, sustained platelet COX-1 inhibition occurs in those who receive high doses of naproxen and/or have particularly long half-lives (see below) [62].
-
(ii)
Ibuprofen, like naproxen, is an inhibitor of both COX isoforms; however, its half-life is shorter (t½ 2–4 hrs). Thus, it has to be given frequently (3–4 times per day) to provide sufficient analgesia and because it is not a very potent COX inhibitor, high doses are given, resulting in wide fluctuations of plasma concentrations even under steady state conditions.
-
(iii)
Diclofenac is a much more potent inhibitor then naproxen and ibuprofen. However, although it was introduced long before COX-2 selective inhibitors were developed, it happens to be as selective for COX-2 as celecoxib, the only purposefully developed COX-2 inhibitor on the US market [63]. It has, however, a very short half-life (t½ 1–2 hrs). Thus, analgesic plasma levels can only be maintained until the end of the dosing interval when diclofenac is administered at doses that greatly exceed those necessary for inhibition of COX-2. Its plasma concentration undergoes wide fluctuations with the consequence that diclofenac inhibits both COX isoforms equally at the beginning of the dosing interval, but when plasma levels drop its selectivity for COX-2 becomes apparent during the majority of the dosing interval.
-
(iv)
Celecoxib is as COX-2 selective as diclofenac, but longer acting (t½ 6–12 hrs) and its potency for COX-2 inhibition lies in between that of diclofenac and naproxen. Thus, it does not need to be dosed as highly as diclofenac to attain COX-2 inhibition throughout the dosing interval and the initial inhibition of COX-1 after dosing, while detectable, is small.
Figure 2. Interindividual and inter-study heterogeneity of the half-life of Naproxen.
The distributions of the half-life, determined in 15 pharmacokinetic analyses [49–61], was plotted and the underlying distribution estimated by sampling from the study populations.
The heterogeneous impact these pharmacokinetic and dynamic features of NSAIDs may have on the dynamics of COX inhibition and organ function is illustrated in Figure 3 [64–67].
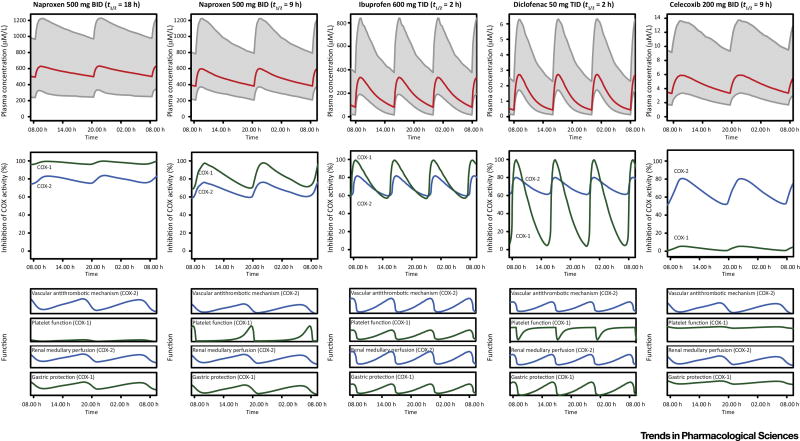
Figure 3. Illustration of the relationship of the pharmacokinetic and pharmacodynamic properties of naproxen, ibuprofen, diclofenac and celecoxib.
Top panels: Plasma concentration vs time curves under steady state conditions after 10 days of dosing. Grey area: 90% confidence interval. Middle panels. Expected dynamics of cyclooxygenase (COX)-1 and −2 function, given the degree of COX-2 selectivity of the drug and its pharmacokinetic properties. Bottom panels: Expected dynamic changes in organ function. Platelets have a markedly steeper COX inhibition-response relationship than other tissues. Thus, changes in platelet function are less gradual then changes in the function of other tissues. The dynamics for 2 distinct half-lives (9 and 18hrs) of naproxen are shown. (The plasma concentration vs time curves are based on simulations using two-compartment model parameters [64]. The illustration of the expected impacted on COX-1 and COX-2 inhibition is informed by published data [65–67]. The drawings of the expected changes in organ function are based on our understanding of COX biology.)
We hypothesized previously that the dynamics of the inhibition of COX-2 and COX-1 during the dosing interval might be relevant to the safety profiles of distinct NSAIDs because both isoforms play roles in the mechanisms underlying complications [13]. The most obvious such mechanism is that inhibition of COX-1 is largely (but not exclusively) responsible for severe gastrointestinal complications of NSAIDs. Thus, minimizing inhibition of COX-1 reduces the risk of ulcer complications [27] and this was the rationale driving the development of COX-2 selective inhibitors [63]. The issue is more complex with cardiovascular complications. COX-1 has an established role in platelet aggregation, where its product TxA2 acts as one of the amplification signals (ADP is another one) that enhance platelet activation and their recruitment to the thrombus (Figure 1). Inhibition of platelet COX-1 is the mechanism sufficient to explain cardioprotection by low dose aspirin. However, inhibition of platelet COX-1 results in inhibition of platelet function and, consequently, cardioprotection, only if COX-1 derived TxA2 formation is suppressed almost completely [68]. The capacity of platelets to produce TxA2 greatly exceeds what is needed to activate platelets – a feature of the feed-forward loop in platelet function that results in a rapid clotting response [68]. At steady state, low dose (<100 mg/day) aspirin suppresses TxA2 almost completely during the dosing interval because it inhibits COX-1 irreversibly and anucleate platelets have a very limited capacity to synthesize new protein [69]. By contrast, all other NSAIDs are reversible COX inhibitors. They inhibit platelet COX-1 only partially (if they are relatively COX-2 selective) and/or for a limited period of time during the dosing interval (if they are short acting) and thus, do not produce the stable platelet inhibition that is the basis of cardioprotection. Thus, COX-1 inhibition by non-isoform selective NSAIDs, such as ibuprofen, does not offset the predisposition to thrombosis consequent to suppression of COX-2 derived PGI2. An exception, however, may be long acting NSAIDs with a pronounced COX-1 inhibition sustained throughout the dosing interval, such as naproxen, which may result in long acting platelet inhibition. Naproxen inhibits platelet COX-1 and platelet function throughout the dosing interval in some individuals – especially those with longer half-lives, at the tail end of the pharmacokinetic distribution [64, 70].
Clinical evidence
While most NSAIDs which inhibit platelet COX-1 cannot be expected to mitigate the prothrombotic effect of COX-2 inhibition through this mechanism, they have the potential to interfere with the cardioprotective effects of low dose aspirin [64, 71]. Indeed, a range of mixed COX inhibitors, including ibuprofen and naproxen, can prevent acetylation of platelet COX-1 by aspirin [64, 70–77]. Acetylation of serine-529 close in the hydrophobic substrate binding channel of COX-1 causes irreversible inhibition of the enzyme by blocking access of the substrate arachidonic acid. Reversible COX inhibitors block the substrate binding channel (e.g. ibuprofen) or change its steric configuration through interaction with the allosteric monomer of the COX homodimer (e.g. naproxen), which prevents not only the entry of arachidonic acid, but also that of aspirin. When the drug-drug interaction occurs, the transient inhibition of platelet COX-1 by the reversible NSAID predominates over irreversible inhibition caused by aspirin as its half-life in the circulation is the shortest of all COX inhibitors (t½ 20–30 min); it is quickly hydrolyzed spontaneously or by intestinal and plasma esterases. Initial proof-of-concept studies designed to detect the drug-drug interaction [71] were misinterpreted by some, which led to the erroneous recommendation that ingestion of aspirin two hours before the NSAIDs would avoid the drug-drug interaction. Trough plasma levels of naproxen and ibuprofen under steady-state conditions are sufficiently high to prevent aspirin acetylation of COX-1 irrespective of the order of drug administration [64, 71].
COX-1 is also expressed in the kidney and its inhibition can have opposing effects to inhibition of COX-2. While baseline blood pressure is not affected by COX-1 inhibition and deletion in mice, coincidental limitation of sodium intake results in reduced blood pressure [42, 78]. However, the dose response curve relating inhibition of renal COX-1 and the consequent impact on function would be more linear than in the platelet. Thus, partial COX-1 inhibition by isoform unselective COX inhibitors or haploinsufficiency in mice may well have modest functional effects on blood pressure regulation. This suggests that the hypertensive adverse effects of COX-2 selective and non-isoform selective NSAIDs may differ in magnitude and mechanism, reflecting the degree of inhibition of COX-2 and the selectivity with which it is attained [79]. While it has been known for decades that all NSAIDs have the potential to raise blood pressure and cause heart failure, the development of COX-2 selective NSAIDs and their assessment in randomized controlled studies led to the detection of the thrombotic risk – primarily heart attacks (reviewed here [13] and here [14]). Whether or not some NSAIDs have a more favorable side effect profile than others has been the subject of numerous pharmacoepidemiological studies [28], metaanalyses [27] and more recently prospective controlled randomized trials [18, 20]. As with other drug classes, all of these approaches have strengths and weaknesses which need to be considered when evaluating the evidence. Pharmacoepidemiological studies perhaps represent best what is frequently termed a “real world experience” and they can compare multiple NSAIDs, if they have been prescribed frequently enough. However, epidemiological studies with NSAIDs usually fail to account for coincidental consumption of over-the-counter NSAIDs including aspirin and selection bias is difficult to control (e.g. differences in disease burden between NSAID non-users and users, differences in comorbidities between users of low vs. high doses, etc.). Meta-analyses of randomized controlled trials conducted during the development of the COX-2 selective NSAIDs have the advantage that they can provide sufficient power to detect differences in relatively rare events such as myocardial infarction, but comparisons of multiple drugs are usually indirect and the baseline cardiovascular risk of those enrolled in studies during the development process of the COX-2 inhibitors was, on average, low. Prospective randomized controlled trials designed to compare the cardiovascular safety of NSAIDs should certainly be the gold standard, but they are large, costly and lengthy and, thus, can compare only a limited number of NSAIDs and a limited number of “real world” cofactors – if any – that might affect the primary outcome. They are also subject to the limitations of many aspects of trial design (see below).
The largest meta-analysis to address differences in cardiovascular risk between NSAIDs, the Coxib and traditional NSAID Trialists’ (CNT) Collaboration, combined patient level data (and aggregate data where these were not available) from 754 randomized controlled NSAID studies contributing >230,000 patient-years [27]. Trials with COX-2 selective NSAIDs conducted during the development of these drugs contributed most of the data; they were compared to placebo or traditional NSAIDs. Thus, comparisons between traditional NSAIDs and placebo were indirect. The combined COX-2 selective NSAID analysis included celecoxib, rofecoxib, etoricoxib, lumiracoxib and valdecoxib. The risk of major vascular events (non-fatal myocardial infarction, non-fatal stroke, or vascular death) was increased in those allocated to a COX-2 selective NSAID vs. placebo (rate ratio [RR] 1.37, 95% CI 1.14–1.66, p=0.0009) and diclofenac vs. placebo (1.41, 1.12–1.78, p=0.0036), consistent with behavior of the latter as a COX-2 selective inhibitor during part of the dosing interval. Major coronary events (non-fatal myocardial infarction or coronary death) contributed most to the risk, but very few strokes occurred, and the risk was already detectable within the first months of treatment. Naproxen was not associated with an increased risk of major vascular events (0.93, 0.69–1.27), consistent with a protective effect through inhibition of platelet function in a sufficiently large number of patients. Ibuprofen, for which by far the lowest number of patient-years of exposure was available for analysis (~ a third of those for naproxen and a ninth of those for diclofenac), was not associated with a detectable excess of vascular events (1.44, 0.89–2.33, p=0.14), but increased major coronary events (2.22, 1.10–4.48, p=0.0253). This was consistent with insufficient platelet inhibition during the dosing interval and thus, a lack of an aspirin-like protective effect. The cardiovascular safety profile of ibuprofen may also have been confounded by the drug-drug interaction discussed above. Most of the original studies with ibuprofen and a COX-2 selective NSAID, which contributed the majority of patient-years, allowed concurrent consumption of aspirin for cardioprotection. Aspirin consumption was approximately 30% in the largest ibuprofen studies [80]. Thus, a portion of the vascular events detected with ibuprofen may reflect a disruption of aspirin’s cardioprotective effect rather than a direct effect of ibuprofen.
All four treatments (COX-2 selective NSAIDs, diclofenac, naproxen and ibuprofen) increase the heart failure rate significantly by approximately two-fold. Heart failure is at least in part linked to the renal COX-2 mediated effects on blood pressure and sodium retention (and possibly direct effects on the myocardium [81]), and thus the similar increase between the treatments indicates that COX-2 inhibition was, on average, equipotent. The risk of gastrointestinal complications was elevated by all treatments vs. placebo, but most pronounced by naproxen (RR 4.22, 2.71–6.56) followed by ibuprofen (RR 3.97, 2.22- 7.10), reflective of gastrointestinal COX-1 inhibition, which in contrast to platelet COX-1 inhibition, would be expected to result in a gradual and linear dose dependent impairment of function.
Interestingly, while the doses of the traditional NSAIDs were quite homogenous across trials in the CNT meta-analysis and at the upper end of the permitted dosing range (naproxen 1000 mg/d, ibuprofen 2400 mg/d, diclofenac 150mg/d), individual doses of the COX-2 selective drugs varied substantially across trials reflective of dose finding strategies during the development of these drugs. Thus, while this meta-analysis provides the best available evidence in support of a class effect of COX-2 inhibition induced atherothrombotic risk, it does not necessarily provide an estimate of risk at doses that are actually most commonly prescribed for inflammatory pain. There was a larger proportional increase of the risk of major vascular events with higher celecoxib doses in placebo controlled trials (p for trend=0.0117). However, the analysis was not powered for the evaluation of individual doses. Thus, while the unapproved dose of 800 mg celecoxib per day was associated with an excess risk of 2.96 (CI 1.21 – 7.25), a dose of 400 mg per day, licensed for rheumatoid arthritis, showed a numerical increase of 1.29 (CI 0.81 – 2.04). This was similar to the most frequently studied dose of rofecoxib, 25 mg/d, which alone also included an insufficient number of events to detect the risk (RR 1.33, 0.94 – 1.89). The number of events available for analysis at the lower celecoxib doses – as are approved for treatment of osteoarthritis (100–200 mg/d) was even lower.
Two randomized controlled trials were conducted to assess the cardiovascular safety of lower doses of celecoxib prospectively. The Standard Care vs. Celecoxib Outcome Trial (SCOT) in osteoarthritis and rheumatoid arthritis patients free of established cardiovascular disease [17, 18] and the Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen or Naproxen (PRECISION) trial, which intended to enroll osteoarthritis and rheumatoid arthritis patients with concomitant cardiovascular disease or elevated risk for cardiovascular disease [19, 20]. Both were sponsored by Pfizer, the maker of celecoxib.
The SCOT was conducted in Scotland, England, Denmark and the Netherlands and randomized patients 65 years or older, who had never had a major cardiovascular event and who were currently requiring chronic treatment with traditional NSAIDs to either continuation of the current therapy or switching to celecoxib [17, 18]. Patients randomized to continue their ongoing NSAID were stratified into ibuprofen or diclofenac treatment arms and all other NSAIDs pooled into a single stratum. The traditional NSAIDs and celecoxib were given in standard doses and adjusted as necessary up to the maximum approved dose (celecoxib up to 400 mg/d) to provide adequate pain relief. The primary endpoint was the same as in the CNT meta-analysis, major vascular events (non-fatal myocardial infarction, non-fatal stroke, or vascular death). The study was initially powered at 90% for a non-inferiority limit of a hazard ratio (HR) = 1.3 to exclude a poorer outcome on celecoxib compared with traditional NSAIDs [17]. However, the power of the study was revised to 80% and the non-inferiority limit to HR=1.4 when fewer than anticipated major vascular events occurred in the study population [18]. Some 7297 patients were randomized of which around 1933 were assigned to celecoxib. The HR of the primary cardiovascular (CV) outcome for celecoxib vs all traditional NSAIDs combined was 1.12, with a 95% confidence interval of 0.81–1.55 in the on-treatment analysis, which failed to show non-inferiority. The on-treatment population usually provides higher sensitivity for the detection of adverse drug events and, accordingly, was the main prespecified statistical analysis in the SCOT. Thus, the SCOT is consistent with an increased risk of major cardiovascular events on celecoxib vs. traditional NSAIDs.
Interestingly, the major conclusion of the study is based on the secondary analyses, which showed non-inferiority of celecoxib vs. traditional NSAIDs in the intention-to-treat population: “In subjects 60 years and over, free from CV disease and taking prescribed chronic non-selective (ns)NSAIDs, CV events were infrequent and similar on celecoxib and nsNSAIDs.” [18] However, there are reasons to have concern regarding the validity of this conclusion based on the intention-to-treat analysis. First, the drop-out rates were significantly higher in the celecoxib group (51% withdrew) than in the traditional NSAID group (30% withdrew), which was twice as frequently attributed to lack of efficacy in the celecoxib group than in the traditional NSAID group (23% vs. 10%). Indeed, the original design included a run in phase with dose titration of celecoxib in order to exclude from randomization patients who attained less symptomatic relief from celecoxib than from the NSAID they had previously been prescribed. However, celecoxib failed to achieve equivalent pain control in about a third of patients and the run-in phase was removed from the study protocol after over 1400 patients were excluded due to insufficient pain relief on celecoxib. This probably shifted withdrawals related to lack of efficiency to a later stage of the trial after the study protocol was changed. Second, SCOT was an open label trial and knowing which drug the patients were on may have affected perception of efficacy. Furthermore the asymmetric dropout in the celecoxib arm raises the possibility that COX-2 was less potently inhibited in a subset of patients on celecoxib. Both issues would have biased the trial towards the detection of non-inferiority.
Another question is whether the selected non-inferiority limit was appropriate in the first place. At the completion of the trial 22,600 person-years had been accrued for analysis (7297 participants, median 3-year follow-up), which is similar to the number of person-years available in the CNT meta-analysis (20,068 person –years) for the comparison of celecoxib vs. placebo where a risk of RR=1.36 (95% CI 1.00 – 1.84, p=0.05, see above) was just about detectable for the same primary endpoint [27]. The frequency of major vascular events was similar in the CTN analysis (1.13% p.a. in the celecoxib group) and SCOT (0.95 % p.a. in the celecoxib group), reflective of the low cardiovascular baseline risk in both cohorts. Thus, had SCOT been a comparison between celecoxib and placebo it may or may not have just about detected celecoxib’s risk with a non-inferiority limit of 1.4. However, the comparison was against all traditional NSAIDs and ~40% of these patients received diclofenac. As discussed above, the CNT study showed an elevated cardiovascular risk of diclofenac likely due to its selectivity for COX-2. This would be expected to “dilute” the signal from celecoxib, biasing the study towards confirmation of non-inferiority. Thus, the detected point estimate HR=1.12 may not be an unreasonable estimate of the excess risk of celecoxib vs all traditional NSAIDs combined in a population with low cardiovascular baseline risk.
It was the intention of the PRECISION trial to compare the cardiovascular safety of celecoxib with traditional NSAIDs in patients with established cardiovascular risk [19, 20]. The PRECISION trial compared celecoxib with ibuprofen and naproxen in patients with osteoarthritis or rheumatoid arthritis. Originally, the primary analysis was designed to test non-inferiority of any of the regimens using a 97.5% upper CI of the HR ≤1.33 and point estimate ≤1.12 for both on treatment and intent-to-treat populations [19]. Just like in the SCOT, this was later relaxed to an upper CI limit of 1.4 for the on treatment population, due to the accrual of fewer than anticipated events [20]. Some 24,081 patients were studied. The primary outcome, major vascular events, defined as in CNT and the SCOT, occurred in 1.7% – 1.9% of patients in the three treatment arms - an event rate corresponding to an intermediate Framingham risk score. In the on-treatment analysis, the HR for celecoxib vs. naproxen was 0.90; 95% CI, 0.71 to 1.15, the HR for celecoxib vs. ibuprofen, 0.81; 95% CI, 0.65 – 1.02. Non-inferiority was formally confirmed. However, there are also substantial concerns about the conclusions that can be drawn from the PRECISION study.
First, as in SCOT, an assumption underlying the PRECISION trial was that equipotent doses of the NSAIDs would be compared. As in SCOT, NSAID doses were titrated to provide sufficient pain relief. Given that PG dependent pain is largely mediated by COX-2, this approach should also result in equal inhibition of vascular and renal COX-2 on average across treatment groups. Dose adjustments by practitioners resulted in mean (±SD) daily doses of 209±37 mg /d (permitted dose 200 or 400 mg/d) for celecoxib, 852±103 mg /d (750 or 1000 mg/d) for naproxen and 2045±246 mg/d (1800 or 2400 mg/d) for ibuprofen. The mean administered doses of naproxen and ibuprofen fall within the middle of the permitted dosing range [19, 20], while the mean celecoxib dose is close the lower dosing limit. Indeed, comparison of the distributions of doses indicate that 2–3 times more patients in the naproxen and ibuprofen arms received the higher daily dose than in the celecoxib arm [82]. This is likely because the maximal celecoxib dose was capped at 200 mg/d for treatment of osteoarthritis patients by regulatory agencies in most countries where the trial was performed and ~90 % of the study population were osteoarthritis patients. Several lines of evidence suggest that celecoxib was, on average, less potent in the relief of pain than the comparator drugs [82]. (i) The rate of discontinuation due to lack off efficacy was numerically higher in the celecoxib arm (9.4% celecoxib, 8.4% ibuprofen, 8.2% naproxen), although a p-value was not reported [20]. (ii) The anti-arthritic efficacy assessed on a visual analog scale was significantly lower (p<0.001) on celecoxib than on naproxen [20]. (iii) The incidence of both arthralgia and osteoarthritis flares were higher (p<0.003) on celecoxib compared to ibuprofen [20]. (iv) Increases in blood pressure (p=0.001) and creatinine (p<0.001), which are dose-dependent NSAID responses, were more frequent on ibuprofen than celecoxib [20]. More than two-thirds of patients dropped out of PRECISION, further augmenting these signals. A comparatively lower dose – resulting in less pronounced inhibition of COX-2 at sites of inflammation, in the kidney and in the vasculature – in the celecoxib group can mask its relative cardiovascular risk, because of the dose dependency of the CV side effects [27].
Second, approximately 50% of all patients in PRECISION were on low dose aspirin for cardioprotection [20]. We have previously shown that ibuprofen and naproxen, but not celecoxib prevent acetylation by aspirin of the target amino acid in the substrate binding channel of platelet COX-1. PRECISION investigators attempted to avoid such a drug-drug interaction by recommending, while the trial was ongoing, the consumption of aspirin 2 hours before the NSAIDs. However, as mentioned above, this does not prevent the interaction during chronic dosing, because NSAID trough levels are sufficiently high to compete with aspirin acetylation of COX-1 [64]. Thus, the interaction would be expected to undermine the cardioprotective effect of aspirin in the ibuprofen and naproxen groups, but not in the celecoxib group, rendering assessment of the comparative direct effects of the NSAIDs uninterpretable. There is insufficient power for a subgroup analysis by aspirin consumption in PRECISION and, indeed, determining who consumed aspirin is not trivial without a functional measurement of drug action. The only reported information on verbally recorded aspirin consumption was at the time of study initiation. Thereafter, patients may have ceased drug consumption as the study proceeded or started taking it, given its ready availability over-the-counter. As was suggested before PRECISION commenced [83], such a situation was avoidable, either by performing a study with sufficient power to assess the impact of the aspirin interaction or by using an alternative antiplatelet drug, such as clopidogrel, where the interaction is likely irrelevant. These and other limitations (e.g. high drop-out rate (~70%), duration of the study >10 years, no enrollment of patients in the European Union, where that study did not receive regulatory approval because of ethical concerns regarding the exposure of patients with heart disease to a COX-2 selective NSAIDs) render PRECISION unhelpful in guiding clinical practice [84].
Concluding Remarks
Mechanistic evidence suggests that differences in the cardiovascular risk between chemically distinct NSAIDs may exist. Placebo-controlled clinical trials in patients with pain are not ethical, thus, prospective trials have compared celecoxib head-to-head with traditional NSAIDs. Both prospective trials, however, have apparently not compared equipotent analgesic doses of the NSAIDs and have other limitations that bias them towards underestimation of the risk of celecoxib. Both SCOT and PRECISION were funded by the maker of celecoxib. They provide no conclusive answer as to which of the compared NSAIDs have the most favorable safety profile for patients who require daily pain relief for arthritis, particularly when they have also cardiovascular risk factors or established cardiovascular disease (see Outstanding Questions Box). The SCOT and PRECISION studies do not exonerate celecoxib from contributing to cardiovascular events at doses that relief arthritic pain as effectively as standard doses of traditional NSAIDs, a note of caution particularly relevant to the proposal to make celecoxib available without prescription [85]. Future studies should focus on comparisons of equipotent doses, for example using measures of drug exposure and biochemical markers of drug activity, carefully monitored blood pressure and renal function as functional markers of COX-2 inhibition and measurements of symptom relief. Given the complexity of such approaches and the shifting economic incentives – celecoxib is now off patent – megatrials are unlikely to be performed. Thus, smaller populations carefully stratified based on mechanistic insights (e.g. those with long naproxen half-lives, those with genotypes linked to slow celecoxib metabolism, etc.) might be studied using deep-phenotyping methods, including remote sensing approaches and linkage to their electronic health records.
QUESTION BOX.
Is there a difference amongst different NSAIDs, at similarly efficacious doses, in terms of their propensity to cause cardiovascular risk?
Do people differ in their analgesic or anti-inflammatory responsiveness to different NSAIDs?
Do people differ in terms of their cardiovascular risk from individual NSAIDs?
Can we develop and validate predictive algorithms that improve our ability to select an individual NSAID, a dose and perhaps a time of dosing that is likely to work effectively and safely in an individual patient?
It is time for studies of NSAID efficacy and toxicity to shift from large poorly designed trials seeking to detect, on average, “non-inferiority” to translational approaches designed to predict increasingly, the efficacy and toxicity of these drugs at the individual level, thereby moving the clinical pharmacology of NSAIDs into the era of precision medicine.
“..we must take the current when it serves, or lose our ventures”. Julius Caesar Act 4, Scene 3.
Trends Box.
The dynamics of the inhibition of cyclooxygenase (COX)-2 and COX-1 during the dosing interval are relevant to the cardiovascular safety profiles of distinct NSAIDs.
The largest meta-analysis to address differences in cardiovascular risk between NSAIDs, the CNT Collaboration, supports a class effect of COX-2 inhibition induced atherothrombotic risk. It does not provide an estimate of risk at doses of COX-2 selective NSAIDs that are most commonly prescribed for inflammatory pain.
Two randomized controlled trials were conducted to compare the cardiovascular safety of lower doses of celecoxib with traditional NSAIDs, the SCOT and the PRECISION trial.
SCOT and PRECISION have apparently not compared equipotent analgesic doses of the NSAIDs and have other limitations that bias them towards underestimation of the risk of celecoxib.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement.
T.G., E.R. and G.A.F declare no conflicts of interest.
References
- 1.Reid KJ, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Current medical research and opinion. 2011;27:449–462. doi: 10.1185/03007995.2010.545813. [DOI] [PubMed] [Google Scholar]
- 2.Insitute of Medicine. Relieving pain in America. A Blueprint for transforming prevention, care, education and research. The National Academies Press. 2011 [PubMed] [Google Scholar]
- 3.Murthy VH. Ending the Opioid Epidemic - A Call to Action. The New England journal of medicine. 2016;375:2413–2415. doi: 10.1056/NEJMp1612578. [DOI] [PubMed] [Google Scholar]
- 4.Zhou Y, et al. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf. 2014;23:43–50. doi: 10.1002/pds.3463. [DOI] [PubMed] [Google Scholar]
- 5.Walker LA, et al. Widespread Use of Prescription Nonsteroidal Anti-Inflammatory Drugs Among U.S. Army Active Duty Soldiers. Military medicine. 2017;182:e1709–e1712. doi: 10.7205/MILMED-D-16-00183. [DOI] [PubMed] [Google Scholar]
- 6.Gorski T, et al. Use of NSAIDs in triathletes: prevalence, level of awareness and reasons for use. British journal of sports medicine. 2011;45:85–90. doi: 10.1136/bjsm.2009.062166. [DOI] [PubMed] [Google Scholar]
- 7.Kuster M, et al. Consumption of analgesics before a marathon and the incidence of cardiovascular, gastrointestinal and renal problems: a cohort study. BMJ open. 2013:3. doi: 10.1136/bmjopen-2012-002090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjorkman DJ. Current status of nonsteroidal anti-inflammatory drug (NSAID) use in the United States: risk factors and frequency of complications. The American journal of medicine. 1999;107:3S–8S. doi: 10.1016/s0002-9343(99)00362-9. discussion 8S-10S. [DOI] [PubMed] [Google Scholar]
- 9.Catella-Lawson F, et al. Effects of specific inhibition of cyclooxygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. The Journal of pharmacology and experimental therapeutics. 1999;289:735–741. [PubMed] [Google Scholar]
- 10.McAdam BF, et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the human pharmacology of a selective inhibitor of COX-2. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:272–277. doi: 10.1073/pnas.96.1.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bresalier RS, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. The New England journal of medicine. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114:1028–1035. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 13.Grosser T, et al. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. The Journal of clinical investigation. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grosser T, et al. Emotion recollected in tranquility: lessons learned from the COX-2 saga. Annual review of medicine. 2010;61:17–33. doi: 10.1146/annurev-med-011209-153129. [DOI] [PubMed] [Google Scholar]
- 15.Brownstein JS, et al. The tell-tale heart: population-based surveillance reveals an association of rofecoxib and celecoxib with myocardial infarction. PloS one. 2007;2:e840. doi: 10.1371/journal.pone.0000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Food and Drug Administration Center for Drug Evaluation and Research. Summary Minutes of the Joint Arthritis Advisory Committee and Drug Safety and Risk Management Advisory Committee Meeting February 10–11. [Accessed 05/23/2017];Food and Drug Administration. 2014 http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM395527.pdf.
- 17.Macdonald TM, et al. Methodology of a large prospective, randomised, open, blinded endpoint streamlined safety study of celecoxib versus traditional non-steroidal anti-inflammatory drugs in patients with osteoarthritis or rheumatoid arthritis: protocol of the standard care versus celecoxib outcome trial (SCOT) BMJ open. 2013:3. doi: 10.1136/bmjopen-2012-002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald TM, et al. Randomized trial of switching from prescribed non-selective non-steroidal anti-inflammatory drugs to prescribed celecoxib: the Standard care vs. Celecoxib Outcome Trial (SCOT) European heart journal. 2016 doi: 10.1093/eurheartj/ehw387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker MC, et al. Rationale, design, and governance of Prospective Randomized Evaluation of Celecoxib Integrated Safety versus Ibuprofen Or Naproxen (PRECISION), a cardiovascular end point trial of nonsteroidal antiinflammatory agents in patients with arthritis. Am Heart J. 2009;157:606–612. doi: 10.1016/j.ahj.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Nissen S, et al. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. The New England journal of medicine. 2016;375(26):2519–29. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 21.FitzGerald GA, et al. Increased prostacyclin biosynthesis in patients with severe atherosclerosis and platelet activation. The New England journal of medicine. 1984;310:1065–1068. doi: 10.1056/NEJM198404263101701. [DOI] [PubMed] [Google Scholar]
- 22.Fitzgerald DJ, et al. Platelet activation in unstable coronary disease. The New England journal of medicine. 1986;315:983–989. doi: 10.1056/NEJM198610163151602. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, et al. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science. 2002;296:539–541. doi: 10.1126/science.1068711. [DOI] [PubMed] [Google Scholar]
- 24.Arehart E, et al. Acceleration of cardiovascular disease by a dysfunctional prostacyclin receptor mutation: potential implications for cyclooxygenase-2 inhibition. Circulation research. 2008;102:986–993. doi: 10.1161/CIRCRESAHA.107.165936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabausch K, et al. Regulation of thrombomodulin expression in human vascular smooth muscle cells by COX-2-derived prostaglandins. Circulation research. 2005;96:e1–6. doi: 10.1161/01.RES.0000153150.27690.f2. [DOI] [PubMed] [Google Scholar]
- 26.Barbieri SS, et al. Cyclooxygenase-2-derived prostacyclin regulates arterial thrombus formation by suppressing tissue factor in a sirtuin-1-dependent-manner. Circulation. 2012;126:1373–1384. doi: 10.1161/CIRCULATIONAHA.112.097295. [DOI] [PubMed] [Google Scholar]
- 27.Coxib and traditional NSAID Trialists’ Collaboration et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382:769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS medicine. 2011;8:e1001098. doi: 10.1371/journal.pmed.1001098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Y, et al. Vascular COX-2 modulates blood pressure and thrombosis in mice. Science translational medicine. 2012;4:132ra154. doi: 10.1126/scitranslmed.3003787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirkby NS, et al. Cyclooxygenase-1, not cyclooxygenase-2, is responsible for physiological production of prostacyclin in the cardiovascular system. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17597–17602. doi: 10.1073/pnas.1209192109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricciotti E, et al. COX-2, the dominant source of prostacyclin. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E183. doi: 10.1073/pnas.1219073110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grosser T, et al. Drug resistance and pseudoresistance: an unintended consequence of enteric coating aspirin. Circulation. 2013;127:377–385. doi: 10.1161/CIRCULATIONAHA.112.117283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reilly M, et al. Modulation of oxidant stress in vivo in chronic cigarette smokers. Circulation. 1996;94:19–25. doi: 10.1161/01.cir.94.1.19. [DOI] [PubMed] [Google Scholar]
- 34.Topper JN, et al. Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, et al. Hydroperoxide dependence and cooperative cyclooxygenase kinetics in prostaglandin H synthase-1 and −2. The Journal of biological chemistry. 1999;274:20301–20306. doi: 10.1074/jbc.274.29.20301. [DOI] [PubMed] [Google Scholar]
- 36.Dong L, et al. Different Fatty Acids Compete with Arachidonic Acid for Binding to the Allosteric or Catalytic Subunits of Cyclooxygenases to Regulate Prostanoid Synthesis. The Journal of biological chemistry. 2016;291:4069–4078. doi: 10.1074/jbc.M115.698001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong L, et al. Fatty Acid Binding to the Allosteric Subunit of Cyclooxygenase-2 Relieves a Tonic Inhibition of the Catalytic Subunit. The Journal of biological chemistry. 2016;291:25641–25655. doi: 10.1074/jbc.M116.757310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.So OY, et al. The dynamics of prostaglandin H synthases. Studies with prostaglandin h synthase 2 Y355F unmask mechanisms of time-dependent inhibition and allosteric activation. The Journal of biological chemistry. 1998;273:5801–5807. doi: 10.1074/jbc.273.10.5801. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi T, et al. Roles of thromboxane A2 and prostacyclin in the development of atherosclerosis in apoE-deficient mice. The Journal of clinical investigation. 2004;114:784–794. doi: 10.1172/JCI21446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Egan KM, et al. COX-2-derived prostacyclin confers atheroprotection on female mice. Science. 2004;306:1954–1957. doi: 10.1126/science.1103333. [DOI] [PubMed] [Google Scholar]
- 41.Cannon CP, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet. 2006;368:1771–1781. doi: 10.1016/S0140-6736(06)69666-9. [DOI] [PubMed] [Google Scholar]
- 42.Qi Z, et al. Opposite effects of cyclooxygenase-1 and −2 activity on the pressor response to angiotensin II. The Journal of clinical investigation. 2002;110:61–69. doi: 10.1172/JCI14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang T, et al. Renin expression in COX-2-knockout mice on normal or low-salt diets. American journal of physiology. Renal physiology. 2000;279:F819–825. doi: 10.1152/ajprenal.2000.279.5.F819. [DOI] [PubMed] [Google Scholar]
- 44.Ahmetaj-Shala B, et al. Evidence that links loss of cyclooxygenase-2 with increased asymmetric dimethylarginine: novel explanation of cardiovascular side effects associated with anti-inflammatory drugs. Circulation. 2015;131:633–642. doi: 10.1161/CIRCULATIONAHA.114.011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vallance P, et al. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- 46.Caplin B, Leiper J. Endogenous nitric oxide synthase inhibitors in the biology of disease: markers, mediators, and regulators? Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1343–1353. doi: 10.1161/ATVBAHA.112.247726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leiper J, Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nature reviews. Drug discovery. 2011;10:277–291. doi: 10.1038/nrd3358. [DOI] [PubMed] [Google Scholar]
- 48.Dong L, et al. Human cyclooxygenase-2 is a sequence homodimer that functions as a conformational heterodimer. The Journal of biological chemistry. 2011;286:19035–19046. doi: 10.1074/jbc.M111.231969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palazzini E, et al. Multiple-dose pharmacokinetics of naproxen from a controlled-release tablet in healthy volunteers. Int J Clin Pharmacol Res. 1990;10:277–284. [PubMed] [Google Scholar]
- 50.Vree TB, et al. Pharmacokinetics of naproxen, its metabolite O-desmethylnaproxen, and their acyl glucuronides in humans. Biopharmaceutics & drug disposition. 1993;14:491–502. doi: 10.1002/bdd.2510140605. [DOI] [PubMed] [Google Scholar]
- 51.Choi HG, et al. Pharmacokinetic comparison study of a combination containing 500 mg of Naproxen and 20 mg of Esomeprazole: a randomized, single-dose, 2-way crossover, open-label study in healthy Korean men. Clinical therapeutics. 2015;37:83–93. doi: 10.1016/j.clinthera.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Frost C, et al. Evaluation of the effect of naproxen on the pharmacokinetics and pharmacodynamics of apixaban. British journal of clinical pharmacology. 2014;78:877–885. doi: 10.1111/bcp.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yilmaz B, et al. HPLC method for naproxen determination in human plasma and its application to a pharmacokinetic study in Turkey. Journal of chromatographic science. 2014;52:584–589. doi: 10.1093/chromsci/bmt080. [DOI] [PubMed] [Google Scholar]
- 54.Berges A, et al. Pharmacokinetics, Safety, and Tolerability Following the Administration of a Single Dose of a Combination Tablet Containing Sumatriptan and Naproxen Sodium in Adolescent Patients With Migraine and in Healthy Adult Volunteers. Clinical pharmacology in drug development. 2012;1:85–92. doi: 10.1177/2160763X12447302. [DOI] [PubMed] [Google Scholar]
- 55.Wang-Smith L, et al. Pharmacokinetics and relative bioavailability of a fixed-dose combination of enteric-coated naproxen and non-enteric-coated esomeprazole magnesium. Journal of clinical pharmacology. 2012;52:670–680. doi: 10.1177/0091270011405500. [DOI] [PubMed] [Google Scholar]
- 56.Anttila M, et al. Pharmacokinetics of naproxen in subjects with normal and impaired renal function. European journal of clinical pharmacology. 1980;18:263–268. doi: 10.1007/BF00563009. [DOI] [PubMed] [Google Scholar]
- 57.Caille G, et al. Single dose pharmacokinetics of ketoprofen, indomethacin, and naproxen taken alone or with sucralfate. Biopharmaceutics & drug disposition. 1987;8:173–183. doi: 10.1002/bdd.2510080208. [DOI] [PubMed] [Google Scholar]
- 58.Niazi SK, et al. Dose dependent pharmacokinetics of naproxen in man. Biopharmaceutics & drug disposition. 1996;17:355–361. doi: 10.1002/(SICI)1099-081X(199605)17:4<355::AID-BDD960>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 59.Strocchi E, et al. Pharmacokinetics of a controlled release preparation of naproxen. International journal of clinical pharmacology, therapy, and toxicology. 1991;29:253–256. [PubMed] [Google Scholar]
- 60.Ling TL, et al. A multiple-dose pharmacokinetic comparison of naproxen as a once-daily controlled-release tablet and a twice-daily conventional tablet. Journal of clinical pharmacology. 1987;27:325–329. doi: 10.1002/j.1552-4604.1987.tb03024.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhou D, et al. Single- and multiple-dose pharmacokinetic comparison of a sustained-release tablet and conventional tablets of naproxen in healthy volunteers. Journal of clinical pharmacology. 1998;38:625–629. doi: 10.1002/j.1552-4604.1998.tb04469.x. [DOI] [PubMed] [Google Scholar]
- 62.Capone ML, et al. Clinical pharmacology of platelet, monocyte, and vascular cyclooxygenase inhibition by naproxen and low-dose aspirin in healthy subjects. Circulation. 2004;109:1468–1471. doi: 10.1161/01.CIR.0000124715.27937.78. [DOI] [PubMed] [Google Scholar]
- 63.FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. The New England journal of medicine. 2001;345:433–442. doi: 10.1056/NEJM200108093450607. [DOI] [PubMed] [Google Scholar]
- 64.Li X, et al. Differential impairment of aspirin-dependent platelet cyclooxygenase acetylation by nonsteroidal antiinflammatory drugs. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16830–16835. doi: 10.1073/pnas.1406997111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capone ML, et al. Pharmacodynamic of cyclooxygenase inhibitors in humans. Prostaglandins & other lipid mediators. 2007;82:85–94. doi: 10.1016/j.prostaglandins.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 66.Schwartz JI, et al. Comparative inhibitory activity of etoricoxib, celecoxib, and diclofenac on COX-2 versus COX-1 in healthy subjects. Journal of clinical pharmacology. 2008;48:745–754. doi: 10.1177/0091270008317590. [DOI] [PubMed] [Google Scholar]
- 67.Fries S, et al. Marked interindividual variability in the response to selective inhibitors of cyclooxygenase-2. Gastroenterology. 2006;130:55–64. doi: 10.1053/j.gastro.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Reilly IA, FitzGerald GA. Inhibition of thromboxane formation in vivo and ex vivo: implications for therapy with platelet inhibitory drugs. Blood. 1987;69:180–186. [PubMed] [Google Scholar]
- 69.Pedersen AK, FitzGerald GA. Dose-related kinetics of aspirin. Presystemic acetylation of platelet cyclooxygenase. The New England journal of medicine. 1984;311:1206–1211. doi: 10.1056/NEJM198411083111902. [DOI] [PubMed] [Google Scholar]
- 70.Capone ML, et al. Pharmacodynamic interaction of naproxen with low-dose aspirin in healthy subjects. Journal of the American College of Cardiology. 2005;45:1295–1301. doi: 10.1016/j.jacc.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 71.Catella-Lawson F, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. The New England journal of medicine. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 72.Rosenstock M, et al. Prostaglandin H synthase-2 inhibitors interfere with prostaglandin H synthase-1 inhibition by nonsteroidal anti-inflammatory drugs. European journal of pharmacology. 2001;412:101–108. doi: 10.1016/s0014-2999(00)00931-6. [DOI] [PubMed] [Google Scholar]
- 73.Gladding PA, et al. The antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. Am J Cardiol. 2008;101:1060–1063. doi: 10.1016/j.amjcard.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 74.Hohlfeld T, et al. Pyrazolinone analgesics prevent the antiplatelet effect of aspirin and preserve human platelet thromboxane synthesis. Journal of thrombosis and haemostasis : JTH. 2008;6:166–173. doi: 10.1111/j.1538-7836.2007.02800.x. [DOI] [PubMed] [Google Scholar]
- 75.Anzellotti P, et al. Low-dose naproxen interferes with the antiplatelet effects of aspirin in healthy subjects: recommendations to minimize the functional consequences. Arthritis and rheumatism. 2011;63:850–859. doi: 10.1002/art.30175. [DOI] [PubMed] [Google Scholar]
- 76.Galliard-Grigioni KS, Reinhart WH. A randomized, controlled study on the influence of acetaminophen, diclofenac, or naproxen on aspirin-induced inhibition of platelet aggregation. European journal of pharmacology. 2009;609:96–99. doi: 10.1016/j.ejphar.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 77.Angiolillo DJ, et al. Impact of a fixed-dose combination of naproxen and esomeprazole magnesium on serum thromboxane B2 inhibition by low-dose aspirin over 5 days in healthy adults: a phase I, randomized, double-blind, placebo-controlled, noninferiority trial. Clinical therapeutics. 2011;33:1883–1893. doi: 10.1016/j.clinthera.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 78.Francois H, Coffman TM. Prostanoids and blood pressure: which way is up? The Journal of clinical investigation. 2004;114:757–759. doi: 10.1172/JCI22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.FitzGerald GA. The choreography of cyclooxygenases in the kidney. The Journal of clinical investigation. 2002;110:33–34. doi: 10.1172/JCI16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schnitzer TJ, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet. 2004;364:665–674. doi: 10.1016/S0140-6736(04)16893-1. [DOI] [PubMed] [Google Scholar]
- 81.Wang D, et al. Cardiomyocyte cyclooxygenase-2 influences cardiac rhythm and function. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7548–7552. doi: 10.1073/pnas.0805806106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grosser T. Cardiovascular Safety of Celecoxib, Naproxen, or Ibuprofen for Arthritis. The New England journal of medicine. 2017;376:1389. doi: 10.1056/NEJMc1702534. [DOI] [PubMed] [Google Scholar]
- 83.Couzin J. Massive Trial of Celebrex Seeks to Settle Safety Concerns. Science. 2005;310:1890–1891. doi: 10.1126/science.310.5756.1890a. [DOI] [PubMed] [Google Scholar]
- 84.FitzGerald GA. Imprecision: Limitations to Interpretation of a Large Randomized Clinical Trial. Circulation. 2017;135:113–115. doi: 10.1161/CIRCULATIONAHA.116.026324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.LaMattina J. PRECISION Results Open The Door For OTC Celebrex. Forbes. 2016 Nov 15; [Google Scholar]