Abstract
Patents are designed to protect and encourage creativity and innovation. Patenting a biomedical discovery can be a requirement before a pharma or biotech entity will invest in the lengthy and costly clinical testing necessary to achieve patient benefit. Although scientists and clinicians are well versed in research publication requirements, patent descriptions and claims are formatted in a manner quite different from a research paper. Patents require a) a series of logical statements clearly delineating the boundaries of the novel aspects of the invention and b) sufficient disclosure of the invention so that it can be reproduced by others. Patents are granted only for inventions that meet three conditions: novelty, non-obviousness and usefulness. This chapter provides basic guidelines and definitions for inventions, inventorship, and patent filing which are summarized using a question and answer format.
Keywords: disclosure, discovery, invention, non-disclosure agreement, patent, technology transfer
1. Introduction
Patenting biomedical discoveries is an important requirement for commercialization. A pharmaceutical company, a diagnostic company, or a biotechnology firm will not spend the considerable resources to develop a new product that can benefit patients suffering from disease, if the product is not covered by an issued or pending patent (Jones 2005 reference). Even experienced academic scientists may not understand that inventorship, an invention, and a patent are quite different from a scientific publication. In many ways, obtaining a granted patent can be much harder and longer than publishing a research study in a peer reviewed journal. This is because of the requirement for absolute novelty and utility, as judged by the lengthy examining process by the US Patent and Trademark Office (USPTO, www.uspto.gov) (Jones 2005; Gholz 2007 reference). Patent claims must be written in a form of an independent clause or multiple dependent clauses that logically delineate the novel features claimed under the invention. Licensing and assignment of intellectual property rights (inventions) provide employers/inventors with a means to effectively sell or “rent” the invention under specific conditions. (Merges 1999 reference).
Molecular profiling and individualized therapy are providing new insights into disease treatment while at the same time providing new technologies and therapies. Although the concept of genomic and proteomic analysis is not new, the wealth of patentable information gleaned from these molecular insights is constantly challenging current health and patent laws (Kelton; Jones, Shi reference). It is our intent in this chapter to explain basic guidelines applicable to biomedical inventions and patents, including a tutorial for writing a patent application (based on the Patent Cooperative Treaty and United States Patent and Trademark Office requirements).
2. Types of Patents
Question: What constitutes an invention that can be patented?
Answer: Inventions can be patented if they are novel, non-obvious, and useful (Jones 2005; Shi 2005 reference).
Meaning of Novel, Non-obvious, and Useful in Patent Terms
New and Novel: For a United States patent the invention must never have been disclosed in public in any way, anywhere in the world, more than one year before the date on which the patent application is filed. In other countries, the inventor does not have a one year grace period.
Original and Non-obvious: An invention involves a creative, inventive step. When compared with what is already known, it would not be obvious to someone experienced in the subject matter, or would be unexpected or contrary to established theories or findings. Useful: This means that the invention must take the practical form of a machine, apparatus, device, diagnostic kit, pharmaceutical compound, it has to accomplish something of practical value to society.
A utility invention can fulfill any of the following definitions: a Kit for accomplishing a useful goal, a Method or Process of synthesis or production, a Machine, an Article of Manufacture, a Composition of Matter (such as a chemical compound), or an improvement of any of the above categories.
Design patents are for the new ornamental design of an article of manufacture.
Plant patents provide patent protection for any asexually reproduced distinct and new variety of plant.
2.1. Issued Patents
Question: What is an issued patent and what protection does it afford to the inventor?
Answer: The term “patent” is derived from “letters of patents patent”; an open letter by which a sovereign entity conferred a special privilege or right on subject. The first recorded patent was granted to Filippo Brunelleschi in 1421 in Florence, Italy for an industrial invention. Since then countries have set their own rules to grant patents, including the duration, types of patents and filing rules.
An invention is a property right (an owned article of property that comes into existence the instant it is invented) for an invention granted by a government to the inventor. A United States patent gives inventors the right “to exclude others from making, using, offering for sale, or selling their invention throughout the United States or importing their invention into the United States” for a limited time.
Utility and plant patents are granted for a term that begins with the date of the grant and usually ends 20 years from the date the applications were filed. You must make the timely payment of the appropriate maintenance fees.
Design patents last 14 years from the date you are granted the patent. No maintenance fees are required for design patents.
The patent is a personal property: so it can be sold, assigned or transferred as determined by the owner. As such there can be disputes, in which case the authority or jurisdiction concerned has to mediate and investigate infringement. If infringement is found then a determination must be made to grant penalties to the violator and award damages to the rightful owner.
In the 1990’s the establishment of World Trade Organization set forth a common minimum set of rights that should be granted to all patent owners by governments, as well as a period of 20 years (from the date the application filed) as the term of the patent.
Table 1 lists links to patent offices/organizations/procedural guideline sources for selected countries, world bodies and interest groups.
Table 1.
Resources for patent offices/organizations/procedural guidelines for selected countries, world bodies, and interest groups.
| Country/Entity | Web site resources |
|---|---|
| US | How to get a Patent in US http://www.tutorial-reports.com/innovation/patent/howtogetus.php |
| India | How to get a Patent in India http://www.tutorial-reports.com/innovation/patent/howtogetindia.php |
| Europe | european-patent-office.org |
| Japan | http://www.jpo.go.jp/ |
| Korea | www.kipo.go.kr |
| Italy | http://www.info-brevetti.org/ |
| Canada | http://strategis.ic.gc.ca/sc_mrksv/cipo/ |
| Australia | http://www.ipaustralia.gov.au/ |
| African Region | http://www.aripo.wipo.net/ |
| New Zealand | http://www.iponz.govt.nz/ |
| Singapore | http://www.ipos.gov.sg/ |
| UK | www.ukpats.org.uk |
| WIPO (World Intellectual Property Organization) | http://www.wipo.int/patentscope/en/ |
| Patent Cooperation Treaty (PCT) World Trade Organization (WTO) |
http://www.wto.org/english/tratop_e/trips_e/trips_e.htm http://www.pctlearningcenter.org/ |
| The Public Patent Foundation PUBPAT | http://www.pubpat.org/index.html |
| Intellectual Property Owners Association | http://www.ipo.org/ |
2.1.2. Non-patentable articles
Question: What cannot be patented?
Answer: Unmodified pre-existing articles of nature cannot be patented. You cannot patent an unmodified natural chemical, gene, protein, or animal, or plant species (Jones 2005; Shi 2005 reference). However, you can patent a modified form of an article of nature if the modification serves a useful purpose. You can patent the use of existing articles of nature in devices, compounds, or diagnostic kits that are useful.
A 2010 court case ruling highlights the controversy concerning the patenting of genes and proteins (1, 2). A US district court ruled in March 2010 that the claims were not valid in seven patents covering genetic testing using breast cancer susceptibility genes. The ruling followed a lawsuit against the company Myriad Genetics and the University of Utah Research Foundation, which hold the patents on the BRCA1 and BRCA2 breast cancer susceptibility genes (1). A woman who tests positive has on average an 82% risk of developing breast cancer in her lifetime and a 44% risk of developing ovarian cancer, according to Myriad. The plaintiffs who brought the lawsuit were the Association for Molecular Pathology and the American College of Medical Genetics and they were represented by the American Civil Liberties Union (ACLU) and the New York–based Public Patent Foundation. Judge Robert Sweet of the US District Court for the Southern District of New York ruled that both Myriads’ composition and method claims are invalid under the law, because a product of nature, in this case a gene, even if isolated, can not be patented as an invention (1). While this case is being appealed, many experts worry that the ruling will have a chilling impact on the biotechnology industry. Nevertheless, if the ruling is upheld it does not prevent the patenting of diagnostic tests using genes or proteins to predict disease or guide therapy. The take home message is to craft patent claims around the non-obvious practical use of a gene or protein, or its modified form, instead of trying to patent the gene or protein itself as a composition of matter.
You cannot patent: laws of nature, physical phenomena, abstract ideas, literary, dramatic, musical, and artistic works. These can be copy write protected. You cannot patent inventions that are considered not useful or physically impossible by the USPTO (for example perpetual motion machines) or considered offensive to public morality.
2.2. Persons qualifying as inventors or co-inventors
Question: If two people or groups make the same invention around the same time, who gets the patent?
Answer: First to Invent Rule: The United States grants a patent to the first inventor who conceives and reduces the invention to practice, e.g. a working prototype or well-written description. Other countries use the first to file rule granting a patent and all rights to the first person who files a patent application for an invention (3, 4).
Clause 101 of US Code 35 states:
“Whoever invents or discovers any new and useful process, machine, manufacture, or composition of matter, or any new and useful improvement thereof, may obtain a patent therefore, . . .”
This has further been defined by the following case law:
“He who first perfected a thing is the inventor although others might have experimented with the idea.” Agawam Co. v Jordan (1869) 74 US583, 19 L Ed 177, and “Crude and imperfect experiments are not sufficient to confer right to patent; until invention is perfected and adapted to use it is not patentable and he who first perfects it and adapts it to use is first inventor in sense of patent law and entitled to patent.” Seymour v Osborne (1871) 78 US516, 20 L Ed 33.
With effect from 1st January, 1996, clause 104 of US Code 35 was changed with the effect that residents of World Trade Organization member countries can now rely on the law of “first to invent” in establishing invention priority in the USA (5).
“In proceedings in the Patent and Trademark Office, in the courts, and before any other competent authority, an applicant for a patent, or a patentee, may not establish a date of invention by reference to knowledge or use thereof, or other activity with respect thereto, in a foreign country other than a NAFTA country or a WTO member country . . .” (http://www.uspto.gov/web/offices/pac/mpep/consolidated_laws.pdf reference)(6).
2.2.1. Co-inventors
Question: Who qualifies to be a co-inventor?
Answer: Each co-inventor must have made an independent creative contribution to at least one of the claims specified in the patent. This definition is quite different from co-authorship on a scientific publication. Thus, those who creatively and directly generated the invention, qualifying to be co-inventors, may be only a small subset of co-authors listed on a research publication relevant to the subject invention (7).
2.3. Provisional Patent Application
Question: When should I file a Provisional application?
Answer: You may be in a hurry to get a patent because you see immediate commercial application or you want to beat a competitor. In the US, you can take the option of filing a Provisional Application for Patent. The Provisional application discloses detailed information about the invention, but not to the depth required in the regular application. You must file a regular patent application on the invention of your Provisional application within one year.
The Provisional application establishes a registration date for your invention that is much earlier in time than the ultimate date of patent issue after filing the regular application. The Provisional in no way resembles a regular utility patent, as it expires in a year’s time, cannot be searched, and “it does not start a 20 year patent term running”.
Provisional applications are usually filed for reasons of urgency to establish priority. However, sometimes there are many good reasons not to file a Provisional application, such as higher overall costs and extra time delay before the patent is granted (prosecution will begin only on the utility application). US patent laws have been amended to allow upgrading of the Provisional application into a Utility patent application.
An alterative means of proving that you were the first to think of the idea is a Disclosure Document. A Disclosure Document is an “evidence of conception” of an idea or an invention. In no way does it substitute for a Provisional application or a regular utility patent application. For a fee of $10, it enables the applicant a to have a recorded proof of date of conception provided it is followed up by the regular patent application within two years of receipt of the disclosure document at the USPTO. Unlike a Provisional application, the date of the Disclosure Document cannot be considered an effective filing date. Because the Provisional application serves the purpose of providing an earlier filing date, most intellectual property offices recommend filing the Provisional application and not bothering with the Disclosure Document.
2.4. Non-disclosure agreements
Question: What are the proper components of a Non-disclosure Agreement?
Answer: Nondisclosure Agreement
A Nondisclosure Agreement is an agreement under which a party (the “Recipient”) agrees not to disclose proprietary and confidential information (“Confidential Information”) that it receives from another party (the “Owner”).
No Warranty. There is a possibility that the Confidential Information could contain mistakes or errors, or be based on assumptions that later prove to be incorrect. Therefore, it is common for Owners to include a “no warranty” provision that specifies that the Owner will not be responsible for any damages that the Recipient might incur from using the Confidential Information.
Risk of Accepting Disclosure. The Owner may also want to provide that any disclosure made by the Recipient of any information is at the Recipient’s risk. Because the Owner has already stated that it will not warrant the accuracy of the information, the Owner can further provide that the Recipient will bear the risk of using the information in violation of the agreement. For example, if the Recipient acts on some of the information and the information was inaccurate, the Recipient cannot hold the Owner responsible for the harm caused by the inaccurate information.
Limited License. Generally, the Owner and the Recipient intend that the Confidential Information will only be used by the Recipient for the limited purpose of reviewing the information to determine whether the parties might have interest in future transactions. A “limited license” provision specifies that the Recipient is not acquiring the right to use the Confidential Information for any other than the specified purpose.
General Provisions. A Nondisclosure Agreement (see Note 1–3) should include provisions that (i) provide a detailed description of the Confidential Information and the purpose of disclosing, (ii) require all Confidential Information be identified as such and all oral disclosures reduced to writing within a specific time frame, (iii) include a termination date (ie. 2–5 years) and return policy, (iv) acknowledge an obligation under Federal and State Freedom of Information Act (FOIA), (v) require amendments (changes) to the agreement to be in writing and signed by both parties, (vi) specify the state whose laws will govern and interpret disputes between the parties regarding the matters covered by the agreement, and (vii) prohibit the parties from assigning their obligations under the agreement to third parties. Generally, the state whose laws should govern the agreement should be the state of the Owner
3. Preparing a patent application
3.1. Records of the invention
Question: What is the proper format for an inventor’s notebook record?
Answer: The inventor’s logbook ideally should be a separate book or witnessed highlighted pages or entries in an ongoing laboratory notebook. Detailed records of the concepts, test results, and other information related to making an invention should be kept in a logbook (3, 8).. Look for sequentially pre-printed numbered pages, fade-away backgrounds, spaces for you and a witness to sign & date. Never use a loose leaf notebook or a 3-ring binders as a log book. Never use a legal pad or any glued together notebook. Use a notebook with bound or sewn pages. The pages must be bound so that you can prove in a legal patent dispute that you did not simply add the notebook record later and back date it (3, 4).
3.2. Patent Specifications
Question: What are the essential features of the Patent specification and the sections of the application?
Answer: The specification is a written detailed description of the invention and how to make and use the invention. The specification must be written such that a person that is skilled in the technology could make and use your invention. The components of the application are listed below (5, 6, 9).
TITLE OF INVENTION
CROSS-REFERENCE TO RELATED APPLICATIONS
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR DEVELOPMENT
REFERENCE TO A SEQUENCE LISTING, A TABLE, OR A COMPUTER PROGRAM, LISTING COMPACT DISC APPENDIX
BACKGROUND OF THE INVENTION
BRIEF SUMMARY OF THE INVENTION
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWING
DETAILED DESCRIPTION OF THE INVENTION
CLAIM OR CLAIMS
ABSTRACT OF THE DISCLOSURE
DRAWINGS (When Necessary)
OATH OR DECLARATION
SEQUENCE LISTING (When Necessary)
TITLE OF INVENTION
The title of the invention may have up to 500 characters, and should be as short and specific as possible.
CROSS-REFERENCE TO RELATED APPLICATIONS
Any non-provisional utility patent application claiming the benefit of one or more prior filed co-pending non-provisional applications (or international applications) under laws 120, 121 or 365(c) must contain in the first sentence of the specification following the title, a reference to each prior application, identifying it by the application number or international application number and international filing date, and indicating the relationship of the applications, or include the reference to the earlier application. Cross-references to other related patent applications may be made when appropriate.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR DEVELOPMENT
The application should contain a statement as to rights to inventions made under federally sponsored research grants or intramural programs if applicable.
BACKGROUND OF THE INVENTION
This section should include a statement of the field of endeavor to which the invention pertains. This section may also include a paraphrasing of the applicable U.S. patent Classification Definitions or the subject matter of the claimed invention. In the past, this part of this section may have been titled “FIELD OF INVENTION” or “TECHNICAL FIELD.” This section should also contain a description of information known to you, including references to specific documents, which are related to your invention. It should contain, if applicable, references to specific problems involved in prior art or missing gaps or needs in existing technology.
BRIEF SUMMARY OF THE INVENTION (different from the Abstract)
This section should present the substance, objective, or general idea of the claimed invention in summarized form. The summary may point out the advantages of the invention and how it solves previously existing problems identified in the BACKGROUND OF THE INVENTION.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWING
Where there are drawings, you must include a listing of all figures by number (e.g., Figure 1A) and with corresponding statements explaining what each figure depicts.
Figure 1.
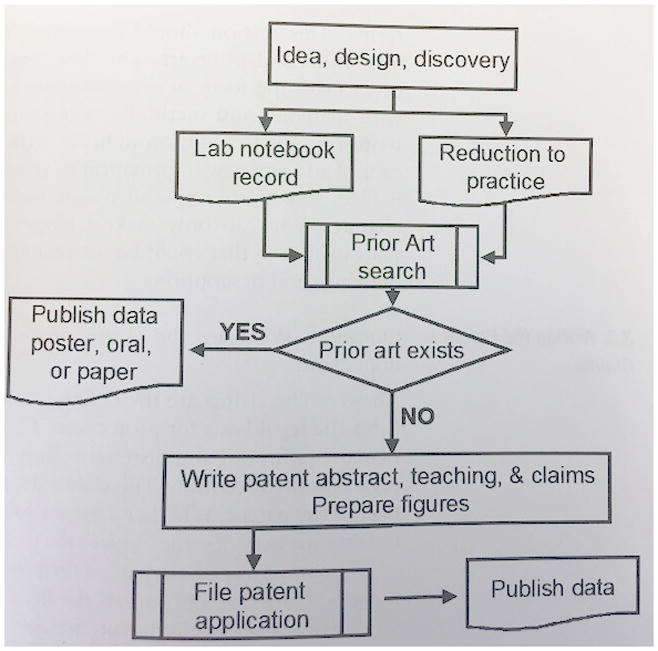
DETAILED DESCRIPTION OF THE INVENTION
The specification is the description along with the claims. In this section, the invention must be explained along with the process of making and using the invention in full, clear, concise, and exact terms. This section should distinguish the invention from other inventions and prior art. The description for biomedical patents often takes the form of experimental examples that are presented with materials and methods, results and conclusions. You must write a complete and thorough, yet broad, description because you cannot add any new information to your patent application once it is filed. If you are required by the patent examiner to make any changes, you can only make changes to the subject matter of your invention that could be reasonably inferred from the original drawings and description.
3.3. Writing the patent claims
Question: What are the claims of the patent and why are they important?
Answer: The claims are the defining features of the invention and form the legal basis for protection. The claim or claims must particularly point out and distinctly claim the subject matter that you regard as the invention. The claims define the scope of the protection of the patent. Whether a patent will be granted is determined, in large measure, by the choice of wording of the claims.
The most important part of the patent is the claims. The claims must be crafted to encompass the invention as broadly as possible while not overstepping the requirements of novelty and utility. The material covered in the patent application is often referred to as “teaching” because an important requirement of the patent is that it “teaches” others how to carry out the invention.
The terms in the claims must refer back to words used in the teaching (body) of the patent. Importantly, a claim in the patent cannot extend very far beyond what is reduced to practice in the data and examples given in the patent. The breadth of a claim becomes a balance between what can be argued is novel beyond the prior art, and what is disclosed or logically inferred from the teaching of the patent. This is best explained as an example of an invention and a discovery in the field of molecular diagnostics.
Imagine that you have discovered that the phosphorylation of a specific site on the molecule Beclin-1 shows a strong correlation with disease free survival in patients with multiple myeloma who have been treated with dexamethasone. You could file a patent with the following claim:
“We claim a method of selecting therapy for a patient with multiple myeloma comprising the steps a) measuring the phosphorylation level of Beclin-1 in a cell sample derived from the patient, and b) determining if the level of phosphorylation is above a defined threshold, and c) administering dexamethasone to the patient.” This claim would not cover the use of phosphorylated Beclin-1 to choose the therapy for any other type of disease, or any other type of therapy besides dexamethasone. A broader claim therefore, might be as follows, if there is data or rationale provided in the patent teaching:
“We claim a method of selecting therapy for a patient with a hematologic malignancy, comprising the steps of a) measuring the phosphorylation level of Beclin-1 in a cell sample derived from the patient, b) determining if the level of phosphorylation is above a defined threshold, and c) administering a steroid alone or in combination with another treatment modality.” This broader claim can be argued because a) dexamethasone is classified as a steroid, and b) multiple myeloma is one of many types of hematologic malignancies including myelodysplasias and leukemias.
3.3.1
The claims section must begin with the statement, “What I claim as my invention is...” or “I (We) claim...” followed by the statement of what you regard as your invention. One or more claims may be presented in dependent form, referring back to and further limiting another claim or claims in the same application. All dependent claims should be grouped together with the claim or claims to which they refer to the extent practicable. Any dependent claim that refers to more than one other claim shall refer to such other claims in the alternative only.
3.3.2. In “Claims” every word is important
Claims are the parts of a patent that define the boundaries of patent protection. Patent claims are the legal basis for your patent protection. They form a protective boundary line around your patent that lets others know when they are infringing on your rights. The limits of this line are defined by the words and phrasing of your claims (see Note 4).
a. Scope
Each claim should have only one meaning which can be either broad or narrow, but not both at the same time. In general, a narrow claim specifies more details than a broader claim. Having many claims, where each one is a different scope, allows you to have legal title to several aspects of your invention.
b. Important Characteristics
Three criteria to take note of when drafting your claims are that they should clear, complete, and supported in the application. Any terms you use in the claims must be either found in the description or clearly inferred from the description.
c. Structure of a claim
A claim is a single sentence composed of three parts: the introductory phrase, the body of the claim, and the link that joins the two.
The introductory phrase identifies the category of the invention and sometimes the purpose for example, “a diagnostic test kit”, or “a composition for treating cancer”. The body of the claim is the specific legal description of the exact invention that is being protected.
The linking consists of words and phrases such as: “which comprises”, “which consists of”, “including”, “consisting of” or “consisting essentially of”. The linking word or phrase describes how the body of the claim relates to the introductory phrase. The linking words are also important in assessing the scope of the claim as they can be restrictive or permissive in nature.
d. Merit of the claim
Each claim will be evaluated by the Patent Examiner on its own merit. It is important to make claims on all aspects of your invention to ensure that you receive the most protection possible. One way of ensuring that specific inventive features are included in several or all claims is to write an initial claim and refer to it in claims of narrower scope. Thus all the elements in the first claim are also included in the subsequent claims. As more features are added the claims become narrower in scope.
3.4. Patent Abstract
Question: What are the essential features of the Patent Abstract?
Answer: Abstracts are limited to 150 words and are used primarily for searching patents. They should be written in a way to make the invention easily understood by those with a background in the field. The abstract should summarize your invention and how it is useful, but does not discuss the scope of your claims.
3.5. Patent Drawings and Sequence Tables
Question: What are the essential features of the Patent Drawings and Sequence tables?
Answer: A patent application is required to contain drawings if drawings are necessary for the understanding of the subject matter to be patented. The drawings must show every feature of the invention as specified in the claims. Omission of drawings may cause an application to be considered incomplete. All patent drawings must show every feature of the invention specified in the claims, and is required to be in a particular form. The reason for specifying the standards in detail is that the patent drawings are printed and published in a uniform style when the patent issues, and patents must provide enough information so that someone with proper expertise can reproduce the invention (6).
3.6. Amino acid and nucleotide sequence listing
If they apply to your invention, amino acid and nucleotide sequences must be included as they are considered part of the description. You must prepare this section, for the disclosure of a nucleotide and/or amino acid sequence, with a listing of the sequence that complies with the following patent rules: 1.821, 1.822, 1.823, 1.824, and 1.825 (37 CFR 1.821 Nucleotide and/or amino acid sequence disclosures in patent applications and WIPO Standard ST.25 (1998)) (6, 9).
3.7. International agreement for patent application filing
Question: How do I obtain patent protection in a foreign country? What is a PCT Application?
Answer: To be protected in any country you must attain an issued patent in that country. For this reason most new patents are filed under an international agreement for sharing patent application among countries. (http://www.pctlearningcenter.org/) The Patent Cooperation Treaty or PCT is an international agreement for filing patent applications having effect in up to 117 countries. Although the PCT system does not provide for the grant of an international patent, the system is designed to a) simplify the process of filing patent applications across multiple countries, b) delay the expenses associated with applying for patent protection in other countries, and d) to allow the inventor more time to evaluate the commercial viability of his/her invention. Under the PCT, an inventor can file one international patent application in one language with one patent office in order to simultaneously seek protection for an invention in up to 117 countries.
Filing a PCT application does not mean that a separate application is automatically filed in all the countries covered in the agreement. The invention must still be individually filed in each country and the inventor must follow the rules specific to that country. The specifications of the PCT patent application are similar to the US patent application, but each country has its unique requirements. Filing fees for the PCT application are considerable and the inventor still has to pay additional fees for each foreign country filing. In the end the invention may be granted in some countries but not others and the patent claims allowed for the same invention may be different in each country.
3.8. Publication of the patent application
Question: When is the application published?
Answer: Publication (making copies available to the public) of patent applications is required for most plant and utility patent applications. Publication of patent applications is one of the functions of the WIPO (World Intellectual Property Organization) and the USPTO. On filing of a US plant or utility application, an applicant may request that the application not be published, but only if the invention has not been and will not be the subject of an application filed in a foreign country that requires publication 18 months after filing (or earlier claimed priority date) or under the Patent Cooperation Treaty (PCT).
Publication occurs after expiration of an 18-month period, both by the USPTO and WIPO/PCT patent applications, following the earliest effective filing date or priority date claimed by an application. Following publication, the application for patent is no longer held in confidence by the US PTO or WIPO Office and any member of the public may request access to the entire file history of the application.
3.9. Patent examination of the patent office
Question: What happens after the patent application is filed? Once the patent is filed your attorney will receive a notice of the filing and the identifying registration number. If the resolution of the figures, the size of the tables, or the format of the application has any errors, the patent office may request corrections to made, will provide a deadline, and will charge a fee. When you make the corrections you can not change any of the text or the data. Once the application is in order it will then be reviewed by an Examiner who has expertise in the field of the invention. The Examiner will evaluate every claim for novelty and utility. Your attorney will eventually receive a report from the Examiner rejecting or accepting one or more Claims. For any Claim that is rejected, the Examiner will cite the reason for rejection and will provide a citation of literature that the Examiner believes anticipate the specific Claim or Claims. Your attorney can appeal the decision in a written rebuttal to each of the Examiner’s rejections. In response to the rebuttal, the Examiner may, or may not, agree with your arguments. Your attorney may request a meeting with the Examiner to discuss your rebuttal in person. At some point in the exchange the Examiner will declare their decision as “final”. Any Claims that are allowed at this stage will become the Claims of the published patent.
3.10. Benefits of publication for the inventor
As a result of publication, an applicant may assert provisional rights. These rights provide a patentee with the opportunity to obtain a reasonable royalty from a third party that infringes a published application claim. Thus, damages for pre-patent grant infringement by someone violating one or more of the claims of the invention are now available.
3.10.1. Patent assignment
Question: What is a patent assignment?
Answer: Assigning your patent is like selling your house. You do not own it any longer. Licensing your patent is like renting your house, you can evict the renter’s if they violate the terms of the lease.
Patent law provides for the transfer or sale of a patent by a written agreement called an “assignment” that can transfer the entire interest in the patent. The assignee, when the patent is assigned to him or her, becomes the owner of the patent and has the same rights that the original patentee had. Patent law also provides for the assignment of a part interest, that is, a half interest, a fourth interest, etc., in a patent. An assignment can be granted for a particularly specified part of the invention or a specific field of use.
The US Patent Office records assignments, grants, and similar instruments sent to it for recording, and the recording serves as notice. If an assignment, grant, or conveyance of a patent or an interest in a patent (or an application for patent) is not recorded in the US Patent Office within three months from its date, there can be no subsequent purchaser(s).
3.10.2. Patent licensing and joint ownership
Patents may be owned jointly by two or more persons as in the case of a patent granted to joint inventors, or in the case of the assignment of a part interest in a patent. Any joint owner of a patent, no matter how small the part interest, may make, use, offer for sale and sell and import the invention for his or her own profit provided they do not infringe another’s patent rights, without regard to the other owners, and may sell the interest or any part of it, or grant patent licensing to others, without regard to the other joint owner.
A patent licensing agreement is in a promise by the licensor not to sue the licensee for patent infringement. No particular form of license is required; a license is a written contract and may include whatever provisions the parties agree upon, including the payment of royalties, etc.
3.11. Patent infringement
Question: What do I do if someone is infringing my patent?
Answer: Patent infringement consists of the “unauthorized making, using, offering for sale or selling any patented invention within the United States or United States Territories, or importing into the United States of any patented invention during the term of the patent.” (35 United States Code 271; http://www.uspto.gov/web/offices/pac/mpep/consolidated_laws.pdf) (5).
When patent infringement happens, the patentee may sue for relief in the appropriate Federal court. The patentee may ask the court for an injunction to prevent the continuation of the patent infringement and may also ask the court for an award of damages because of the patent infringement (Adamo 2007 reference).
The defendant usually challenges the validity of the patent, which is then decided by the court. An invalid patent, for example, could be wrongly granted because the Examiner did not know about a prior art reference, produced by the defendant that anticipated the invention. The defendant may also try to say that what is being done does not constitute infringement. Infringement is determined primarily by the specific language of the claims. The defendant will argue that the specific terms of the claims are not violated.
Suits for infringement of patents follow the rules of procedure of the Federal courts. From the decision of the district court, there is an appeal to the Court of Appeals for the Federal Circuit. The Supreme Court may thereafter take a case by writ of certiorari. If the United States Government infringes a patent, the patentee has a remedy for damages in the United States Court of Federal Claims. The Government may use any patented invention without permission of the patentee, but the patentee is entitled to obtain compensation for the use by or for the Government.
3.12. Product patent documentation
Question: If I buy a product or kit and there is no patent number listed on the product, can it still be covered by a patent?
Answer: No. Anyone who sells patented articles, is required to mark the articles with the word “Patent” and the number of the patent. The penalty for failure to mark is that the patentee may not recover damages from an infringer unless the infringer was duly notified of the infringement and continued to infringe after the notice. The marking of an article as patented when it is not in fact patented is against the law and subjects the offender to a penalty.
3.12.1 Patent Pending
Articles can be sold with the terms “Patent Applied For” or “Patent Pending.” These phrases have no legal effect, but only give information that an application for patent has been filed in the US Patent and Trademark Office or WIPO. The protection afforded by a patent does not start until the actual grant of the patent. False use of these phrases or their equivalent is prohibited.
Acknowledgments
The authors thank Richard Peet for invaluable discussion regarding patent law and intellectual property.
Footnotes
Question: In a typical confidential disclosure agreement, what is proprietary information, and how long must the information be kept secret? To be considered “Proprietary Information” all such information must clearly and conspicuously be identified as “confidential” or “proprietary” to the Disclosing Party, and all oral proprietary information must be reduced to writing or other tangible form and delivered identified as confidential or proprietary within 20 days of disclosure. A university may have an obligation under Federal and state or province statutes to disclose certain information in the possession of University to the public. Unless terminated by either party, the obligations set forth herein shall remain in full force and effect for a period of two (2) years from the date hereof. Termination of Agreement and Return of Proprietary Information: Either party may terminate this agreement without cause by giving written notice of such termination by certified mail, express or overnight mail, or by telephone facsimile. Such termination shall be effective immediately upon receipt of the notification by the other party. Upon termination the parties shall immediately: (i) return to the Disclosing Party all items of Proprietary Information (including all copies thereof) of the Disclosing Party, upon written request or (ii), at the option of the disclosing party, destroy any notes or personal memoranda which include or make reference to such Proprietary Information.
Question: What are the requirements for a patent figure or table.
The figure must be on A4 paper, with one inch margins, 32mm text (14 point font) and must be in black and white. The resolution must be adequate for reproduction (300 dpi). A listing of a nucleic acid sequence must comply with the following patent rules: 1.821, 1.822, 1.823, 1.824, and 1.825, and may be in paper or electronic form (See: 35 United States Code 271; http://www.uspto.gov/web/offices/pac/mpep/consolidated_laws.pdf.) (5)
Question: How much does it cost to file a patent?
Filing a patent is very expensive, and this cost must be weighed in the decision to file a patent and where to file a patent. If the inventor files a patent only in her/his home country the costs incurred will include the a) filing fee, b) additional fees depending on the number of claims, c) maintenance fees, and d) additional fees required during the prosecution of the case. In addition to the cost of a PCT application, if the invention is filed in a foreign country, each country will have its own set of fees. The inventor will have to pay fees to translate the patent into the appropriate language for each country. On top of all the filing fees for the application, the inventor or the sponsor must factor in the legal fees paid to the patent attorney. Filing a patent therefore can range from $2,000.00 to $10,000.00, and the total cost for an issued patent may be much greater than $20,000.
Each individual claim is evaluated by the patent examiner. Each claim is either allowed or not allowed based on its individual merit. For this reason the language of the claim is often redundant in stating the novelty of the invention.
Common pitfalls encountered by inventors are listed below in Notes 6–9. Failure to record the invention process. You should keep detailed records of the concepts, data, and other information related to making an invention in a logbook. Start a logbook entry series from the very first moment you think of an idea. Proper record keeping can be used as proof of the conception date of an invention. The best way to prove that an idea is yours is by maintaining an inventor’s journal or logbook, recording the experiments and discussions including with whom you discussed the invention, and having a witness who can testify that you made the invention (3, 8).
Failure to Actually Make Your Invention. You can’t sell ideas - you can only sell inventions. You must create a working prototype, provide example data demonstrating utility, or at least describe you invention in enough detail so that it would be expected to work by someone knowledgeable in the field (10).
Pursuing an invention that has no commercial market. Before using your children’s college funds to heavily invest in your invention, assess the true value in the market compared to the existing state of the art. Does your invention offer true advantages in performance or economy? Know when it’s time to move on to your next great idea (11).
Revealing your invention prematurely. In the US, a one-year countdown begins the instant you reveal your invention to the public or anybody that has not signed a confidentiality agreement with you (see definition of novelty above). You only have one year to patent your invention in the US. For other countries, if you reveal the invention, this is no one-year grace period and you lose the rights.
It has already been invented. At any point in time, scientists all over the world are exposed to similar information and perceive similar needs for new products or treatments. There is a good chance that someone is thinking of, or has already patented, your new idea. Conduct a search for prior art to see if anybody else has already patented an invention similar or identical to yours. This can be done in the scientific literature as well as patent databases.
References
- 1.Ledford H. US government wants limits on gene patents. 2011. Nature News. Nature. 2010 doi: 10.1038/news.2010.576. [DOI] [Google Scholar]
- 2.Wadman M. Breast cancer gene patents judged invalid. 2011. Nature News. Nature. 2010 doi: 10.1038/news.2010.160. [DOI] [Google Scholar]
- 3.Dolak LA. Patents Without Paper: Proving a Date of Invention With Electronic Evidence. Houston Law Review. 1999;36:472–530. [Google Scholar]
- 4.Gholz CL. First-toFile or First-to-Invent? J Pat & Trademark Office Society. 2000;82:891–895. [Google Scholar]
- 5.(2007) United States Code Title 35-Patents. 35, in Patent Laws, L1-L88.
- 6.Manual of Patent Examining Procedure (MPEP) United States Government Printing Office; Washington, D.C: 2010. 2011. [Google Scholar]
- 7.Matt J. Searching for an Efficacious Joint Inventorship Standard. Boston College Law Review. 2002;44:245–287. [Google Scholar]
- 8.Merges RP. The Law and Economics of Employee Inventions. Harvard Journal of Law and Technology. 1999;13:1–53. [Google Scholar]
- 9.(2011) Title 37 Patents, Trademarks, and Copyrights. in United States Code Title 37 Parts 1–199,
- 10.Gholz CL. A Critique of Recent Opinions in Patent Interferences. J Pat & Trademark Office Society. 2007;89:1–43. [Google Scholar]
- 11.Kelton T. Pharmacogenomics:The rediscovery of the concept of tailored drug therapy and personalized medicine. The Health Lawyer. 2007;19:1–10. [Google Scholar]


