Introduction
Chronic itch is the most troublesome symptom of psoriasis, a chronic autoimmune skin disease of scaling and inflammation. Epidermal hyperinnervation by itch-signaling fibers is considered to be one cause of chronic itch [32], and increased epidermal nerve density has been frequently reported in humans with chronic itch conditions, including psoriasis [12; 18; 29–32], with some exceptions [19; 24]. Nociceptive fibers are expressed throughout the epidermis and often categorized as peptidergic or nonpeptidergic fibers [7]. The former contain the neuropeptides calcitonin gene–related peptide (CGRP) and/or substance P, whereas the latter express the purinergic receptor P2X3 and isolectin B4. Ablation of the peptidergic or non-peptidergic subset of fibers produces modality-specific deficits in response to heat and mechanical stimuli, respectively [8; 40]. Enhanced intraepidermal nonpeptidergic nerve density has been reported previously in animal models of dry skin itch [25; 34].
The majority of nonpeptidergic sensory neurons express glial cell line-derived neurotrophic factor (GDNF) family receptor (GFR)α-1 and/or GFRα-2, which show preferential binding for GDNF and neurturin (NRTN), respectively [36]. These neurotrophic growth factors are important for the development and maintenance of nonpeptidergic sensory neurons [5; 9; 13; 15; 35; 41]. As such, overexpression of these growth factors could lead to increased nonpeptidergic nerve density, possibly leading to increased itch.
We have reported that the imiquimod-induced psoriasis mouse model is useful for the investigation of chronic itch in psoriasis [23]. Utilizing this model, we presently investigated whether the epidermal density of nonpeptidergic nerves and the expression of their neurotrophic growth factors (GDNF and NRTN) are increased in psoriasis-like skin. Because this model exhibits spontaneous scratching that is histamine-dependent in the early phase (Day 2) and histamine-independent in the late phase (Day 7), we measured nerve fiber density at these time points. In addition, we tested whether inhibition of NRTN could affect the density of intraepidermal fibers and spontaneous scratching behavior.
Materials and Methods
Imiquimod application and drug treatment
Experiments were performed using adult male C57BL/6J mice (19–29 g) under a protocol approved by the Temple University Animal Care and Use Committee. Fur on the rostral back was trimmed with electric clippers and then removed with an electric shaver. Each mouse received a daily topical application of 62.5 mg Aldara cream (5% imiquimod, Meda Pharmaceuticals) on the shaved back skin (2.5 cm x 2 cm) for seven consecutive days.
In NRTN-neutralizing antibody experiments, each animal received an intradermal injection of either goat IgG control antibody (0.4 μg/50 μL; R&D Systems, Minneapolis, MN) or a neutralizing antibody to mouse NRTN (low dose: 0.04; high dose: 0.4 μg/50 μL; AF477; R&D Systems) [11] into the imiquimod treatment area after behavior recording and before each topical application of Aldara cream for Days 1–7.
Behavioral tests
Mice were habituated twice to a Plexiglas recording arena for 60 min before testing. At baseline (Day 0) and 20 to 22 hours after each topical application, mice were videotaped from above for 60 min. The number of videotaped scratch bouts was counted by a trained observer blinded to the treatment condition. A scratch bout was defined as one or more rapid back-and-forth hind paw motions directed toward and contacting the treated area and ending with licking or biting of the toes or placement of the hind paw on the floor. Hind paw movements directed away from the treated area (e.g., ear-scratching) and grooming movements were not counted [2–4]. Scratch bouts were counted for mice treated with imiquimod alone, imiquimod plus goat IgG control, imiquimod plus low-dose NRTN-neutralizing antibody, and imiquimod plus high-dose NRTN-neutralizing antibody at Days 0, 2, and 7.
Real-time qRT-PCR
On Days 0, 1, and 6 of imiquimod treatment, animals were euthanized under sodium pentobarbital anesthesia, and skin samples were immediately collected, submerged in RNAlater (Qiagen, Valencia, CA), and stored at −80°C. Total RNA was extracted using Direct-zol RNA Mini Prep (Zymo Research, Irvine, CA). Reverse transcription of 0.5 μg total RNA was performed using ProtoScript® II First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA). Amplification of GAPDH cDNA was used for normalization. Real-time RT-PCR was performed using Fast Plus EvaGreen qPCR Master Mix (Biotium, Hayward, CA) on a 7500 Real Time PCR System (Applied Biosystems, Grand Island, NY). Forty cycles of amplification were performed involving sequential denaturation at 95°C for 5 s, annealing at 55–60°C for 5 s, and extension at 72°C for 33 s. Assays were validated using serial dilutions and confirmation of equal amplification efficiencies of the cDNA of interest and the GAPDH cDNA. Fold differences in expression were calculated using the comparative ΔCt method by standardizing against GAPDH expression. The following primers were used for analyses of gene expression: mGDNF-F, 5′CCAGTGACTCCAATATGCCTG3′; mGDNF-R, 5′CTCTGCGACCTTTCCCTCTG3′; mNRTN-F, 5′GGGCTACACGTCGGATGAG3′; mNRTN-R, 5′CTTCTCCTCCGAGGCATAGC3′; mGAPDH-F, 5′TCCACTGGCGTCTTCAC3′; mGAPDH-R, 5′GGCAGAGATGATGACCCTTTT3′.
Immunohistochemistry
On Days 0, 2, and 7 of imiquimod treatment (with or without drug treatment), animals were euthanized under sodium pentobarbital anesthesia, and the skin was immediately dissected. Skin was fixed in Zamboni Fixative solution (Newcomer Supply, Middleton, WI) followed by 30% sucrose, frozen in optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura Finetek, Torrance, CA), and cut in 16- and 40-μm sections on a cryostat. The 16-μm sections were incubated with 5% donkey serum and 0.2% Triton X-100 in PBS, then immunostained with a goat NRTN antibody (10 μg/ml; R&D Systems Inc., Minneapolis, MN) at 4°C overnight, followed by incubation with the corresponding secondary antibody conjugated with Alexa Fluor 488 (1:300; Life Technologies Inc., Grand Island, NY) for 2 hr.
The 40-μm sections were incubated with 5% donkey serum and 0.2% Triton X-100 in PBS, and then immunostained with either rabbit CGRP (1:300; Peninsula Laboratories International Inc., San Carlos, CA) or P2X3 (1:200; NeuromicsInc., Edina, MN) antibody at 4°C overnight, followed by incubation with the corresponding secondary antibody conjugated with AlexaFluor 594 (1:300; Life Technologies Inc., Grand Island, NY) for 2 hr. Subsequently, the sections were incubated with 5% goat serum and 0.2% Triton X-100 in PBS for 2 hr, and then incubated with donkey anti-mouse IgG (1:10; Jackson ImmunoResearch Laboratories Inc., West Grove, PA) in PBS for 1 hr. The sections were immunostained with mouse β-tubulin antibody (1:500; BioLegendInc., San Diego, CA) at 4°C overnight, followed by incubation with goat anti-mouse IgG2a, Fcγ specific, conjugated with Alexa Fluor 488 (1:300; Jackson ImmunoResearch Laboratories Inc.) for 2 hr. For GFRα-1 and GFRα-2 experiments, the sections were incubated with CGRP or P2X3 antibody followed by rabbit secondary antibody conjugated with AlexaFluor 594 as described above. Then, the sections were immunostained with either goat GFRα-1 (1:1000; R&D Sytems Inc.) or GFRα-2 antibody (1:1600; R&D Systems Inc.) at 4°C overnight, followed by incubation with the corresponding secondary antibody conjugated with Alexa Fluor 488 for 2 hr. All sections were counterstained with 4′,6-diamino-2-phenylindole (DAPI) in the mounting medium (Vector Laboratories, Burlingame, CA). Images were captured from 10–12 skin sections from each animal at 20X magnification (4–6 mice per group) and evaluated by a trained observer blinded to the treatment condition. The number of labeled nerve fibers crossing the dermal-epidermal junction was counted. To accurately count CGRP+ or P2X3+ nerve fibers, only those coexpressing β-tubulin were counted. The length of the dermal-epidermal junction (in mm) was measured using ImageJ. Epidermal nerve fiber density (ENFD) was calculated as the number of epidermal nerve fibers divided by the length of the dermal-epidermal junction.
Data analysis
Between-group comparisons were made by one-way ANOVA followed by the Bonferroni post-test or by unpaired t-test. In all cases p<0.05 was considered to be significant.
Results
Increase in epidermal nonpeptidergic fiber density in psoriatic skin
To investigate whether total epidermal nerve fiber density (ENFD) is altered in imiquimod-induced psoriasis skin, we quantified epidermal nerve fibers on Day 0 (naive control), Day 2 (early phase of model), and Day 7 (late phase of model) of imiquimod treatment [23]. An antibody against β-tubulin was used in order to visualize all nerve fibers present in the epidermis. Total ENFD was not different among the naive control, early phase, and late phase groups (Day 0: 33.9±6.4 nerve fiber counts/mm epidermis; Day 2: 30.5±2.9 counts/mm; Day 7: 29.9±2.9 counts/mm) (Fig. 1A–C). Next, ENFD was quantified with a focus on peptidergic and nonpeptidergic fibers. Antibodies against CGRP and P2X3 were used to visualize peptidergic and nonpeptidergic fibers in the epidermis, respectively. The density of CGRP-positive peptidergic fibers was significantly decreased on Days 2 and 7 compared to the control group (Day 0: 22.5±1.4 counts/mm; Day 2: 6.7±1.0 counts/mm; Day 7: 3.3±0.3 counts/mm) (Fig. 1D–F). In contrast, the density of P2X3-positive nonpeptidergic fibers was significantly increased on Days 2 and 7 compared to the control group (Day 0: 11.6±0.4 counts/mm; Day 2: 21.5±2.7 counts/mm; Day 7: 19.4±1.9 counts/mm) (Fig. 1G–I).
Fig. 1. Increase in nonpeptidergic epidermal nerve fiber density in psoriasis model.
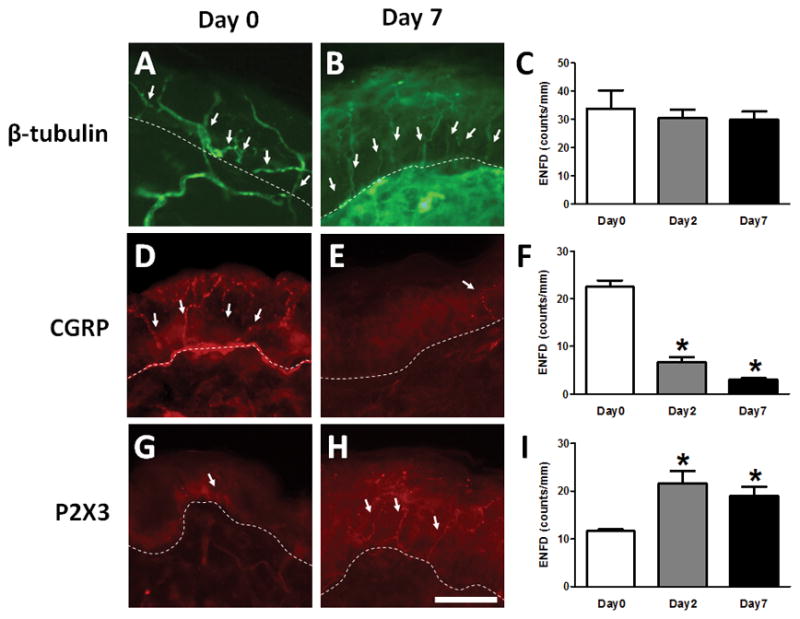
(A–I) Skin was dissected from mice left untreated (Day 0, A,C,D,F,G,I) or after 2 (C,F,I) or 7 (B,C,E,F,H,I) days of imiquimod treatment. (A,B) Skin sections were immunostained with an antibody for β-tubulin to visualize all nerve fibers. (C) Total ENFD (epidermal nerve fiber density) was quantified at Day 0 (open bars), Day 2 (grey bars), and Day 7 (black bars). Error bars are S.E.M. (D,E). Skin sections were immunostained with antibodies for CGRP, a marker for peptidergic nerves. (F) As in C for peptidergic fibers immunostained with a CGRP antibody. * p< 0.05, significant difference from Day 0 (one-way ANOVA followed by Bonferroni post-test, F(2,9)=105.766, n = 4/group). (G,H) Skin sections were immunostained with antibodies for P2X3, a marker for nonpeptidergic nerves. The scale bar indicates 50 μm. (I) As in C for nonpeptidergic fibers immunostained with a P2X3 antibody. * p< 0.05, significant difference from Day 0 (one-way ANOVA followed by Bonferroni post-test, F(2,9)=7.303, n = 4/group).
GFRα-1 and GFRα-2 expression in epidermal nonpeptidergic fibers
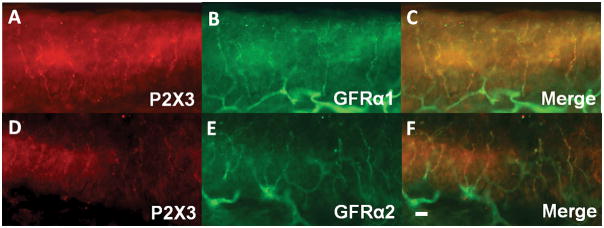
To confirm that the majority of nonpeptidergic epidermal nerve fibers express GFRα-1 and/or GFRα-2 in the imiquimod-treated psoriasis model, as under normal conditions, we double-immunostained Day 0, Day 2 and Day7 skin with antibodies against P2X3 and either GFRα-1 or GFRα-2. Most P2X3-positive epidermal fibers were also either GFRα-1-positive or GFRα-2-positive (GFRα-1: 88.6±4.3% on Day 0, 78.5±2.9% on Day 2 and 77.4%±2.9% on Day 7; GFRα-2: 90.0±1.6% on Day 0, 79.4±1.6% on Day 2 and 80.4±0.5% on Day 7; n=4/group) (Fig. 2). There was a significant decrease in the proportion of GFRα-2-positive P2X3 nerves from Day 0 to Day 2 (10.6% decrease) and from Day 0 to Day 7 (9.6% decrease) (p<0.05, one-way ANOVA followed by Bonferroni post-test, F(2,9)=19.68). In contrast, only about 25% of CGRP-positive epidermal nerves co-expressed either GFRα-1 or GFRα-2, with no change between Day 0 and Day 2 (GFRα-1: 24.9±1.8% on Day 0 and 24.6±5.3% on Day 2; GFRα-2: 21.9±1.8% on Day 0 and 26.4±7.4% on Day 2; n=4/group).
Fig. 2. Coexpression of P2X3 with GFRa-1 and GFRa-2 in psoriasis model.
Skin sections from imiquimod-treated mice were immunostained with antibodies for P2X3 (red; A,D) and either GFRa-1 (green; B) or GFRa-2 (green; E) antibody. Merged images (C,F). The scale bar indicates 30 μm.
Increased NRTN expression in psoriatic skin
To investigate the role of growth factors in increased nonpeptidergic ENFD in psoriatic skin, mRNA expression of GDNF and NRTN was measured on Days 0, 1, and 6. These time points were chosen to allow time for translation of mRNA, signaling, and nerve growth before the observed changes in ENFD on Days 2 and 7. GDNF mRNA decreased gradually over time and showed a significant reduction on Day 6 (p<0.05, F(2,15)=7.726, Fig. 3A). NRTN mRNA increased gradually over time and showed a significant increase on Day 6 (p<0.05, F(2,11)=6.52, Fig. 3B). These results imply that NRTN, and not GDNF, is responsible for the increase in epidermal nonpeptidergic fiber density. Additionally, NRTN immunoreactivity was strongly detectable in keratinocytes on Day 2 (Fig. 3D) and moderately detectable on Day 7 (Fig. 3E) compared to Day 0 (Fig. 3C). NRTN immunoreactivity was completely abolished by preabsorption of antibodies with NRTN (Fig. 3F).
Fig. 3. Increase of NRTN in imiquimod treated skin.
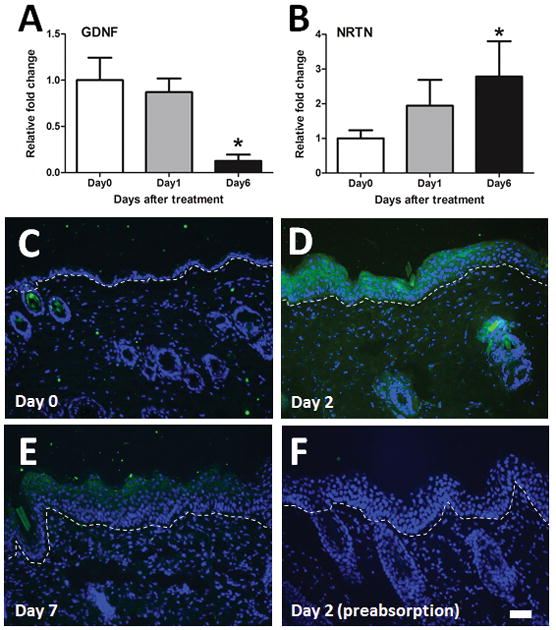
(A) GDNF mRNA was measured in the skin of imiquimod-treated mice at Day 0 (white bars), Day 1 (grey bars), and Day 6 (black bars). Error bars are S.E.M. * p< 0.05, significant difference from Day 0 group (one-way ANOVA followed by Bonferroni post-test, F(2,11)=6.52, n = 4–5/group). (B) As in A, for NRTN. * p< 0.05, significant difference from Day 0 group (one-way ANOVA followed by Bonferroni post-test, F(2,15)=7.726, n = 6/group). (C–F) Typical examples of NRTN (green) and DAPI (blue) staining in the skin treated with imiquimod on Day 0 (C), Day 2 (D), and Day 7 (E). NRTN detection on Day 2 was blocked by preabsorption of the primary antibody with NRTN (F). The scale bar indicates 50 μm.
Reduction in epidermal nonpeptidergic fiber density and spontaneous scratching by blockage of NRTN/GFRα-2 signaling
Finally, we investigated whether blocking NRTN/GFRα-2 signaling with an NRTN-neutralizing antibody could reduce spontaneous scratching in the imiquimod-induced psoriasis model by inhibiting the increase of nonpeptidergic ENFD. Either a low dose or high dose of the neutralizing antibody was injected intradermally into the imiquimod treatment area once daily for Days 1–7 of the model. These mice were compared to those injected with a goat IgG control antibody. The neutralizing antibody for NRTN significantly reduced P2X3-positive ENFD on Days 2 and 7 (Day 2 control: 26.5±2.8 counts/mm; Day 2 NRTN (high dose): 14.9±0.9 counts/mm; Day 7 control: 22.9±3.1 counts/mm; Day 7 NRTN (low dose): 20.4±1.5 counts/mm; Day 7 NRTN (high dose): 11.8±3.1 counts/mm; p<0.05 unpaired-t-test or one-way ANOVA followed by Bonferroni test, 4–6 mice/group) (Fig. 4A). In contrast, NRTN-neutralizing antibody treatment did not affect the CGRP-positive ENFD on either Day 2 or 7 (Day 2 control: 7.4±0.9 counts/mm; Day 2 NRTN (low dose): 6.0±0.2 counts/mm; Day 7 control: 7.5±0.5 counts/mm; Day 7 NRTN (low dose): 7.0±0.9 counts/mm; Day 7 NRTN (high dose): 7.8±0.5 counts/mm; p<0.05 unpaired-t-test or one-way ANOVA followed by Bonferroni test, 4–6 mice/group) (Fig. 4B). Intriguingly, the neutralizing antibody for NRTN dose-dependently reduced spontaneous scratching behavior in the imiquimod-induced psoriasis model on Day 7, but not Day 2 (Fig. 4C). Of note, we did not observe a recovery in external skin appearance, such as scaliness and erythema, after treatment with the neutralizing antibody (data not shown).
Fig. 4. Reduction of spontaneous scratching and nonpeptidergic nerve fiber density by NRTN neutralizing antibody.
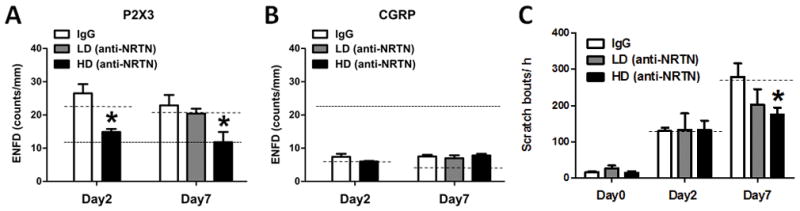
Goat IgG control antibody (white bars), low-dose NRTN neutralizing antibody (LD, 0.04 μg/50 μL, gray bars) or high-dose NRTN neutralizing antibody (HD, 0.4 μg/50 μL, black bars) was intradermally injected into the imiquimod-treated area before each topical application of Aldara cream. (A) On Day 2 (left) or Day 7 (right), the skin was dissected and immunostained with a P2X3 antibody. ENFD (epidermal nerve fiber density) was counted. For comparison, dotted and dashed lines indicate data for Day 0 mice and mice treated with only Aldara cream, respectively (data from Fig. 1). Error bars are S.E.M. * p< 0.05, significant difference from IgG control-treated group (unpaired t-test or one-way ANOVA followed by Bonferroni post-test, F(2,11)=15.436, n = 4–6/group). (B) As in A for CGRP antibody. (C) Spontaneous scratching behavior was observed on Days 0, 2, and 7. Dashed lines indicate data for mice treated only with Aldara cream. Error bars are S.E.M. * p< 0.05, significant difference from IgG control-treated group (one-way ANOVA followed by Bonferroni post-test, F(2,15)=3.99, n = 5–7/group).
Discussion
Psoriatic itch is often intractable, and its mechanisms are still unknown. Here we show that: 1) the density of nonpeptidergic epidermal nerve fibers was significantly increased in the imiquimod-induced psoriasis model, while the density of peptidergic fibers significantly decreased. 2) NRTN expression increased in the skin of imiquimod-treated mice, while GDNF expression decreased. 3) Injection of an NRTN-neutralizing antibody into the imiquimod treatment area significantly inhibited the increase in nonpeptidergic nerve density as well as spontaneous scratching, a behavior associated with chronic itch. These findings indicate that NRTN, by promoting an increase in nonpeptidergic epidermal nerve fibers, contributes to chronic itch in imiquimod-treated mice. Therefore, inhibition of NRTN could be a potential treatment for chronic itch in psoriasis.
In the present study, NRTN expression was increased in the epidermis of imiquimod-treated mice. Previous studies have shown that NRTN KO mice exhibited a reduction in GFRα-2-positive neurons [9], and GFRα-2 KO mice showed a reduced density of nonpeptidergic nerve innervation [13; 15]. Conversely, genetically induced NRTN overexpression in keratinocytes increased the total epidermal nerve density without affecting epidermal peptidergic fiber density [35]. These findings support the idea that NRTN is a crucial mediator of nonpeptidergic ENFD. In the present study, upregulation of NRTN in psoriatic skin plausibly contributes to the increase in epidermal nonpeptidergic fiber density via GFRα-2. The percent of GFRα-2-positive P2X3 fibers significantly decreased from Day 0 to Day 2, perhaps due to overexpression of NRTN leading to downregulation of GFRα-2 [20]. Additionally, though NRTN preferentially binds to GFRα-2, it can also act via GFRα-1 [1; 6; 27]. Thus, NRTN may increase nonpeptidergic ENFD partially through GFRα-1. By blocking these pathways, the NRTN-neutralizing antibody treatments prevented the increase in epidermal nonpeptidergic fiber density in psoriatic skin.
The majority of epidermal P2X3-immunoreactive fibers expressed GFRα-2. Recent studies using single-cell RNA-sequencing analyses of dorsal root ganglia neurons revealed that the cluster containing P2X3- and GFRα-2-positive neurons had high expression of Mas-related G-coupled protein receptor (Mrgpr) D, MrgprB5, transient receptor potential (TRP) ankyrin 1, TRP canonical 3, voltage-gated sodium channel 1.8/9, lysophosphatidic acid receptors 3 and 5, and serotonin 2A receptors [14; 33]. These receptors have established roles in itch transmission [10; 16; 37; 39]. This suggests that nonpeptidergic nerve fiber density may be an important factor in many different itch conditions. Further studies are needed to determine which endogenous mediators are most important for psoriatic itch.
We previously reported that the imiquimod-induced psoriasis model exhibited histamine-dependent itch in the early phase (Day 2) and histamine-independent itch in the late phase (Day 7) [23]. In the present study, although the NRTN-neutralizing antibody inhibited the increase in nonpeptidergic ENFD in both the early and late phases of the psoriasis model, it only inhibited spontaneous itch in the late phase. Thus, it is plausible that nonpeptidergic fibers are not involved in spontaneous itch in the early phase, even though their epidermal density increased. We speculate that the increase in nonpetidergic nerve density may precede the availability of ligands that activate these fibers to evoke itch. A previous study found an increase in ENFD in mice after only one acetone treatment, though three daily acetone/ether applications are typically necessary to evoke scratching behavior in the dry skin itch mouse model [31; 34].
We also report a decrease in peptidergic ENFD in this model. There is conflicting evidence as to whether peptidergic epidermal nerve fibers are increased or decreased in psoriasis patients [12; 21; 22; 29]. These differences could be due to duration of the lesions and/or variations in the progression of the disease [17; 19]. Recently, Wong et al. reported that total ENFD was increased in imiquimod-treated psoriasis mice through the action of vascular endothelial growth factor-A (VEGF-A) [38]. However, they measured ENFD by total area of PGP9.5-positive nerve staining in the epidermis, which may indirectly reflect factors such as branching or fiber thickness. In our study, we counted the number of epidermal nerve fibers crossing epidermal-dermal junction and did not find an increase in total ENFD in imiquimod-induced psoriasis mice, which is consistent with another study using a similar counting method [28]. VEGF-A receptors are expressed by a majority of sensory nerves, including both peptidergic and non-peptidergic nerves [26], leaving open the possibility that VEGF-A may also be involved in psoriatic itch mediated by nonpeptidergic nerve fibers.
In summary, this is the first study to examine the role of nonpeptidergic nerve fibers in a model of psoriasis. We demonstrate that NRTN is involved in enhanced nonpeptidergic ENFD in the imiquimod-induced mouse model of psoriasis, and these nonpeptidergic fibers may be linked to late-phase, histamine-independent itch. Further studies are necessary to fully explore the factors that drive increased NRTN expression and the endogenous pruritogens that act on these epidermal nonpeptidergic fibers. Finally, it will be of significant importance to clarify the role of NRTN in pruritic dermatological diseases in humans.
Acknowledgments
This work was supported by grants from the National Institutes of Health (AR063228 to T. A.) and Pfizer (ASPIRE to T.A. and L.J.).
Footnotes
Conflict of Interest
Y.G. has served as a consultant for Pfizer.
References
- 1.Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 2.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151(2):378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama T, Merrill AW, Zanotto K, Carstens MI, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J Pharmacol Exp Ther. 2009;329(3):945–951. doi: 10.1124/jpet.109.152256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama T, Nguyen T, Curtis E, Nishida K, Devireddy J, Delahanty J, Carstens MI, Carstens E. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain. 2015;156(7):1240–1246. doi: 10.1097/j.pain.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albers KM, Woodbury CJ, Ritter AM, Davis BM, Koerber HR. Glial cell-line-derived neurotrophic factor expression in skin alters the mechanical sensitivity of cutaneous nociceptors. J Neurosci. 2006;26(11):2981–2990. doi: 10.1523/JNEUROSCI.4863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baloh RH, Tansey MG, Johnson EM, Jr, Milbrandt J. Functional mapping of receptor specificity domains of glial cell line-derived neurotrophic factor (GDNF) family ligands and production of GFRalpha1 RET-specific agonists. J Biol Chem. 2000;275(5):3412–3420. doi: 10.1074/jbc.275.5.3412. [DOI] [PubMed] [Google Scholar]
- 7.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106(22):9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM, Jr, Milbrandt J. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron. 1999;22(2):253–263. doi: 10.1016/s0896-6273(00)81087-9. [DOI] [PubMed] [Google Scholar]
- 10.Jones DE. Pathogenesis of cholestatic itch: old questions, new answers, and future opportunities. Hepatology. 2012;56(4):1194–1196. doi: 10.1002/hep.25847. [DOI] [PubMed] [Google Scholar]
- 11.Knox SM, Lombaert IM, Haddox CL, Abrams SR, Cotrim A, Wilson AJ, Hoffman MP. Parasympathetic stimulation improves epithelial organ regeneration. Nature communications. 2013;4:1494. doi: 10.1038/ncomms2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kou K, Nakamura F, Aihara M, Chen H, Seto K, Komori-Yamaguchi J, Kambara T, Nagashima Y, Goshima Y, Ikezawa Z. Decreased expression of semaphorin-3A, a neurite-collapsing factor, is associated with itch in psoriatic skin. Acta Derm Venereol. 2012;92(5):521–528. doi: 10.2340/00015555-1350. [DOI] [PubMed] [Google Scholar]
- 13.Kupari J, Airaksinen MS. Different requirements for GFRalpha2-signaling in three populations of cutaneous sensory neurons. PLoS One. 2014;9(8):e104764. doi: 10.1371/journal.pone.0104764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li CL, Li KC, Wu D, Chen Y, Luo H, Zhao JR, Wang SS, Sun MM, Lu YJ, Zhong YQ, Hu XY, Hou R, Zhou BB, Bao L, Xiao HS, Zhang X. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 2016;26(1):83–102. doi: 10.1038/cr.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindfors PH, Voikar V, Rossi J, Airaksinen MS. Deficient nonpeptidergic epidermis innervation and reduced inflammatory pain in glial cell line-derived neurotrophic factor family receptor alpha2 knock-out mice. J Neurosci. 2006;26(7):1953–1960. doi: 10.1523/JNEUROSCI.4065-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by beta-alanine. J Neurosci. 2012;32(42):14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naukkarinen A, Harvima I, Paukkonen K, Aalto ML, Horsmanheimo M. Immunohistochemical analysis of sensory nerves and neuropeptides, and their contacts with mast cells in developing and mature psoriatic lesions. Arch Dermatol Res. 1993;285(6):341–346. doi: 10.1007/BF00371834. [DOI] [PubMed] [Google Scholar]
- 18.Ostlere LS, Cowen T, Rustin MH. Neuropeptides in the skin of patients with atopic dermatitis. Clin Exp Dermatol. 1995;20(6):462–467. doi: 10.1111/j.1365-2230.1995.tb01378.x. [DOI] [PubMed] [Google Scholar]
- 19.Pergolizzi S, Vaccaro M, Magaudda L, Mondello MR, Arco A, Bramanti P, Cannavo SP, Guarneri B. Immunohistochemical study of epidermal nerve fibres in involved and uninvolved psoriatic skin using confocal laser scanning microscopy. Arch Dermatol Res. 1998;290(9):483–489. doi: 10.1007/s004030050340. [DOI] [PubMed] [Google Scholar]
- 20.Pierchala BA, Milbrandt J, Johnson EM., Jr Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation. J Neurosci. 2006;26(10):2777–2787. doi: 10.1523/JNEUROSCI.3420-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pincelli C, Fantini F, Romualdi P, Sevignani C, Lesa G, Benassi L, Giannetti A. Substance P is diminished and vasoactive intestinal peptide is augmented in psoriatic lesions and these peptides exert disparate effects on the proliferation of cultured human keratinocytes. J Invest Dermatol. 1992;98(4):421–427. doi: 10.1111/1523-1747.ep12499846. [DOI] [PubMed] [Google Scholar]
- 22.Remrod C, Lonne-Rahm S, Nordlind K. Study of substance P and its receptor neurokinin-1 in psoriasis and their relation to chronic stress and pruritus. Arch Dermatol Res. 2007;299(2):85–91. doi: 10.1007/s00403-007-0745-x. [DOI] [PubMed] [Google Scholar]
- 23.Sakai K, Sanders KM, Youssef MR, Yanushefski KM, Jensen L, Yosipovitch G, Akiyama T. Mouse model of imiquimod-induced psoriatic itch. Pain. 2016;157(11):2536–2543. doi: 10.1097/j.pain.0000000000000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuhknecht B, Marziniak M, Wissel A, Phan NQ, Pappai D, Dangelmaier J, Metze D, Stander S. Reduced intraepidermal nerve fibre density in lesional and nonlesional prurigo nodularis skin as a potential sign of subclinical cutaneous neuropathy. Br J Dermatol. 2011;165(1):85–91. doi: 10.1111/j.1365-2133.2011.10306.x. [DOI] [PubMed] [Google Scholar]
- 25.Schuttenhelm BN, Duraku LS, Dijkstra JF, Walbeehm ET, Holstege JC. Differential Changes in the Peptidergic and the Non-Peptidergic Skin Innervation in Rat Models for Inflammation, Dry Skin Itch, and Dermatitis. J Invest Dermatol. 2015;135(8):2049–2057. doi: 10.1038/jid.2015.137. [DOI] [PubMed] [Google Scholar]
- 26.Selvaraj D, Gangadharan V, Michalski CW, Kurejova M, Stosser S, Srivastava K, Schweizerhof M, Waltenberger J, Ferrara N, Heppenstall P, Shibuya M, Augustin HG, Kuner R. A Functional Role for VEGFR1 Expressed in Peripheral Sensory Neurons in Cancer Pain. Cancer Cell. 2015;27(6):780–796. doi: 10.1016/j.ccell.2015.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev. 2001;12(4):361–373. doi: 10.1016/s1359-6101(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi N, Tominaga M, Kosaka R, Kamata Y, Umehara Y, Matsuda H, Sakaguchi A, Ogawa H, Takamori K. Involvement of micro-opioid Receptors and kappa-opioid Receptors in Itch-related Scratching Behaviour of Imiquimod-induced Psoriasis-like Dermatitis in Mice. Acta Derm Venereol. 2017 doi: 10.2340/00015555-2704. [DOI] [PubMed] [Google Scholar]
- 29.Taneda K, Tominaga M, Negi O, Tengara S, Kamo A, Ogawa H, Takamori K. Evaluation of epidermal nerve density and opioid receptor levels in psoriatic itch. Br J Dermatol. 2011;165(2):277–284. doi: 10.1111/j.1365-2133.2011.10347.x. [DOI] [PubMed] [Google Scholar]
- 30.Tobin D, Nabarro G, Baart de la Faille H, van Vloten WA, van der Putte SC, Schuurman HJ. Increased number of immunoreactive nerve fibers in atopic dermatitis. J Allergy Clin Immunol. 1992;90(4 Pt 1):613–622. doi: 10.1016/0091-6749(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 31.Tominaga M, Ozawa S, Tengara S, Ogawa H, Takamori K. Intraepidermal nerve fibers increase in dry skin of acetone-treated mice. J Dermatol Sci. 2007;48(2):103–111. doi: 10.1016/j.jdermsci.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Tominaga M, Takamori K. Sensitization of Itch Signaling: Itch Sensitization-Nerve Growth Factor, Semaphorins. In: Akiyama T, CE, editors. Itch Mechanisms and Treatment. Boca Raton, FL: CRC Press; 2014. p. 293. [PubMed] [Google Scholar]
- 33.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18(1):145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- 34.Valtcheva MV, Samineni VK, Golden JP, Gereau RWt, Davidson S. Enhanced nonpeptidergic intraepidermal fiber density and an expanded subset of chloroquine-responsive trigeminal neurons in a mouse model of dry skin itch. J Pain. 2015;16(4):346–356. doi: 10.1016/j.jpain.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang T, Jing X, DeBerry JJ, Schwartz ES, Molliver DC, Albers KM, Davis BM. Neurturin overexpression in skin enhances expression of TRPM8 in cutaneous sensory neurons and leads to behavioral sensitivity to cool and menthol. J Neurosci. 2013;33(5):2060–2070. doi: 10.1523/JNEUROSCI.4012-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang T, Molliver DC, Jing X, Schwartz ES, Yang FC, Samad OA, Ma Q, Davis BM. Phenotypic switching of nonpeptidergic cutaneous sensory neurons following peripheral nerve injury. PLoS One. 2011;6(12):e28908. doi: 10.1371/journal.pone.0028908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisshaar E, Ziethen B, Gollnick H. Can a serotonin type 3 (5-HT3) receptor antagonist reduce experimentally-induced itch? Inflamm Res. 1997;46(10):412–416. doi: 10.1007/s000110050213. [DOI] [PubMed] [Google Scholar]
- 38.Wong LS, Otsuka A, Yamamoto Y, Nonomura Y, Nakashima C, Honda T, Dainichi T, Kitoh A, Nakajima S, Hirakawa S, Miyachi Y, Kabashima K. Vascular endothelial growth factor partially induces pruritus via epidermal hyperinnervation in imiquimod-induced psoriasiform dermatitis in mice. J Dermatol Sci. 2016;83(2):148–151. doi: 10.1016/j.jdermsci.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35(2):77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Cavanaugh DJ, Nemenov MI, Basbaum AI. The modality-specific contribution of peptidergic and non-peptidergic nociceptors is manifest at the level of dorsal horn nociresponsive neurons. J Physiol. 2013;591(4):1097–1110. doi: 10.1113/jphysiol.2012.242115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, Albers KM. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. 2002;22(10):4057–4065. doi: 10.1523/JNEUROSCI.22-10-04057.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]