Abstract
Down syndrome is the most common cause of intellectual disability among live born children and is amenable to prenatal detection. Screening for Down syndrome on a population basis requires a thorough understanding of the principles involved in the screening tests. We discuss the rationale behind the commonly available screening tests and the Indian scenario in this setting.
Keywords: Down syndrome, Prenatal screening, Combined screening test, Quadruple screening test, Indian scenario
“…tests must be validated before they are applied to case-finding; harm may result to public health agencies’ relationships with the public (not to mention the direct harm to the public), and with the medical profession, from large numbers of fruitless referrals for diagnosis…”
Wilson and Jungner, 1968 [1]
Introduction
Down syndrome (DS) may be said to have its independent existence in medical literature since 1866, when Langdon John Down, a British physician who practiced at London and Earlswood, described the unique physical and mental characteristics of a subgroup of intellectually challenged individuals under his care [2]. Down asserted, on the basis of his observations, that the different races of humans represented variations in the same species and not different species as was believed in that era. Subsequently, the group of individuals described by him were referred to as “mongoloids,” and the disease, as “mongolism” based on the racial classification existent then. In 1965, World Health Organisation (WHO) dropped the term mongolism; in 1975, the National Institute of Health, USA, recommended the term Down syndrome to replace all the other names describing such a phenotype.
Although the medical recognition of this syndrome as a separate entity is quite recent, anthropologists have put forth arguments favouring its existence since antiquity. In his review, Starbuck has suggested the syndrome exists in non-human primates as trisomy 22, the primate equivalent of trisomy 21. In addition, on the basis of evidence from material culture and skeletal remains, he concluded that it was possible that trisomy 21 existed since ancient past [3].
Origins of Prenatal Screening
About a century after the phenotypic description of DS, Le Jeune et al. in 1959 reported the occurrence of an extra copy of chromosome 21 in the cultured fibroblast cells from patients with DS. Later in 1968, Valenti et al. [4] and Nadler et al. [5] independently reported the first prenatally diagnosed cases of trisomy 21 from cultured amniotic fluid cells. Amniocentesis and access to fetal cells opened up the possibility of prenatal diagnosis of many genetic and metabolic conditions, with a spree of publications in the 1970s [6]. Also, around this time in England, medical abortion became legalized. The combination of the ability to identify prenatally fetuses with trisomy 21 and the possibility of legal termination of such pregnancies imposed such sociopolitical pressure that the government reportedly decided to offer a fixed number (about 30,000) of amniocenteses annually [7]. This number corresponded to roughly 5% of the total pregnant women in England at that time. This figure of 5% has since become a very important figure in the prenatal screening programs world over; however, its origin was completely arbitrary. Choosing which 5% of pregnant women would undergo amniocentesis was based on the previous work on the risk factors for DS.
Penrose [8] in 1933 had demonstrated a linear relation between increasing maternal age at delivery and the risk of DS in the offspring in his analysis of 150 families that included 154 offsprings with DS among a total of 727 children. Several other investigators had also reported this observation before Penrose.
The mothers with 35 years of age and above constituted the oldest 5% of the pregnant population in England and consequently formed the 5% with the highest risk of DS. During this early era, imprecise estimation of the maternal age-specific rates of DS births put the risk of a 35-year-old at 1 in 250; at the same time, some quarters conveniently quoted the procedure-related pregnancy loss following amniocentesis to be 1 in 250 [9]. Neither of these assumptions were correct: population-based studies from Belgium and Sweden with almost complete ascertainment indicated the risk of a 35-year-old as 1 in 350–370 [10]; the risk of miscarriage from amniocentesis estimated from the Danish trial was about 1% [11] and the most recent meta-analyses estimate this risk as about 0.1% (1 in 1000) [12] or even less [13]. In addition, the Swedish study indicated that the maternal age-specific risk inflects at around the 32 years’ mark rather than at 35 years [10].
A few unquestioned axioms became ingrained in prenatal screening for DS due to these initial empirical practices: firstly, 35 years being taken as the cutoff maternal age to be considered as high risk; secondly, the screen-positive rate (i.e., the total number of women in a screening program who would be marked as high risk requiring diagnostic testing) should be around 5%; thirdly, a risk cutoff of 1 in 250 to be considered as the equivalent of the maternal age of 35 years and hence to be considered as the screen-positive cutoff value. Over the decades, these empirical rules shaped the evolution of various screening strategies for the prenatal detection of DS.
Justification and Principles of Down Syndrome Screening
The most common argument to screen for DS rather than any other chromosomal/genetic disease is that it is the commonest cause of mental retardation among children accounting for 15–20% of all children with mental retardation in the UK [14]. The most common chromosomal aberration among all conceptions is trisomy 16. However, such fetuses almost always die in utero. Similarly, the other common chromosomal abnormalities such as trisomy 18 (Edward syndrome) and trisomy 13 (Patau syndrome) have as high as 80% in utero mortality between the first trimester and term, while this figure is about 30% for trisomy 21 [15]. Also, the vast majority of fetuses with Edward and Patau syndrome exhibit ultrasound detectable structural defects, whereas only about 50% of DS fetuses have any structural defects identifiable on prenatal ultrasound. Postnatally, infants with Patau syndrome rarely survive beyond the first week, and Edward syndrome beyond the first year, whereas a significant proportion of those with Down syndrome reach adulthood.
Elaboration of the ethical challenges involved in the prenatal screening for DS is beyond the scope of this paper; the social and financial burden of caring for these patients is proportionate to their postnatal survival. Therefore, among the genetic etiologies of intellectually differently-abled, DS represents the group that is “asymptomatic” and hence less likely to be detected during a prenatal ultrasound examination. Also, it poses a high cost of care in State-funded healthcare systems. Loosely, these aspects seem to fulfill the Wilson’s criteria required for a health condition to be considered for population-wide screening [1]. Not surprisingly therefore, research efforts focused into developing screening tests for this condition in Europe, UK and the USA. These screening tests utilized the observations that DS fetuses exhibited certain ultrasound manifestations (larger nuchal translucency, absent nasal bone, abnormal flow across tricuspid valve and ductus venosus) and produced certain biochemical analytes (alpha fetoprotein [AFP], human chorionic gonadotropin [hCG] and its beta subunit, unconjugated estriol [UE3], inhibin A, pregnancy-associated plasma protein-A [PAPP-A])in a different range compared to normal fetuses, and that these observations were independent of the effect of maternal age and independent of each other. The screening tests use the likelihood ratio of each of these manifestations to derive the posttest probability of DS [16]. The fundamental component of this mathematical derivation of the test result for an individual patient is the background risk of that particular condition in the population, known as the a priori risk. In the case of DS, the background risk has been shown to be dependent on the maternal age [8] as discussed before and subsequently large population-based studies have established the maternal age-specific risks of Down syndrome in England [15], Europe [10, 17–19], Taiwan [20] and North America among many other regions.
Down Syndrome Prevalence and Ethnicity
Most authors who studied the Caucasian population or the mixed population have generally agreed that there is no reason to suspect different prevalence based on ethnicity. However, the argument is based on logical reasoning in the absence of data rather than true evidence. Compiling population-based data on the birth prevalence of DS is a resource-intense task for most developing countries, and hence, such data are expectedly scarce. There are data from other ethnicities, for example, from Jews that indicate a higher prevalence of Down syndrome compared to White population. The Jerusalem Perinatal Study [21] documented a higher overall prevalence of Down syndrome in the native Jewish population compared to white population. Although such data do not form concrete evidence of ethnicity-based difference in prevalence, they certainly do not lend strength to the traditional view of “equal prevalence across ethnicities.”
Screening Tests for Down Syndrome
Several reviews have evaluated the screening tests for Down syndrome, including one in our journal [22]. It has been customary to evaluate the performance of prenatal screening tests for DS using the arbitrarily fixed false-positive (or screen-positive) rate of 5%. It is important to note that the performances of the various tests have been validated in the western population [23].
Several factors affect the performance of any screening test, and this is true for Down syndrome screening tests too. First, the yield of the test (defined as the number of “cases” picked up by the screening program) will depend on the prevalence of the disease in the population. The validity of the test depends on the accuracy and this in turn on the correct and reproducible measurement of the various test components such as the nuchal translucency, nasal bone and the serum analytes mentioned before. In addition, as these measurements vary with gestation, they have to be standardized by converting to the multiples of median. Therefore, it is vital that the distribution of the test component measurements in the screening population is accurately known before the MoMs are applied. Another important point about test validity is the requirement for ongoing quality assurance programs that periodically validate the measurements against acceptable standards. The Fetal Medicine Foundation and the United Kingdom National External Quality Assessment Service (UK NEQAS), for example, provide the service of continual audit for various components of the screening programs.
Indian Scenario
Awareness About Screening Versus Diagnostic Tests
The lay public who are the end-users of the screening program may hardly be aware of the differences between a screening test and a diagnostic test. A screening test, such as the combined test for Down syndrome, only categorizes women into high and low risk and can never confirm a fetus as having Down syndrome. The purpose of a screening test is only to identify a subset from the screened population to whom the diagnostic test (e.g., chorionic villus sampling/amniocentesis) needs to be offered. A positive screening test should never be equated to an affected fetus. The odds of the fetus being affected when the test is screen positive are generally between 1 in 30 and 1 in 20. These are very important concepts that need to be informed in an understandable way to the patient before performing the test (pretest counseling). The onus of making the end-user understand the test before performing rests with the agency that runs the screening program. In India, Down syndrome screening is a not a priority for the State’s health program and therefore screening is offered mostly in the private sector only. Few hospitals and practitioners make an effort to provide meaningful pretest counseling. It is certainly not uncommon to encounter women who have undergone a “brain function test” for their fetus and then undergone termination of pregnancy since the test was abnormal. If the western world is ruing the loss of normal fetuses due to unnecessary invasive tests after screen positivity, we are faced with the shameful situation of losing normal fetuses due to misinformation and lack of information. It is worthy of mention that prenatal screening for Down syndrome is still an “informed choice” and the couple have the right to decide whether they would like to go through a screening test or not.
A Priori Risk or Prevalence Data
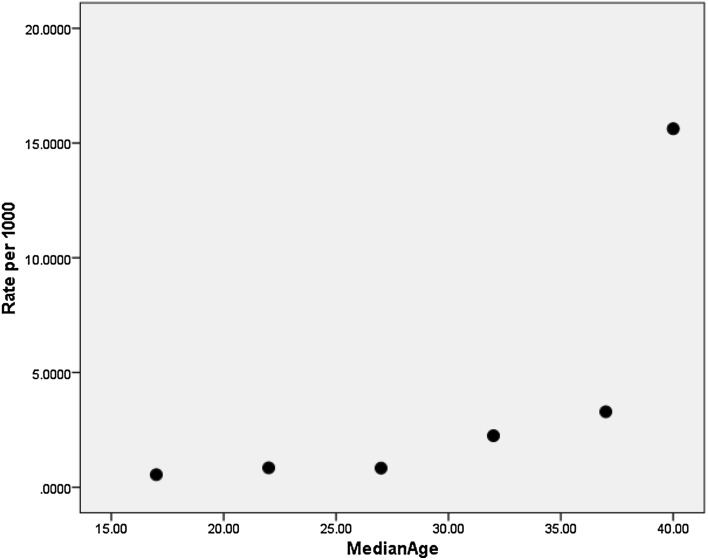
Unfortunately, population-based data on the birth prevalence of Down syndrome are critically lacking in the Indian medical literature. Our population is quite diverse with more than 4500 anthropologically defined groups [24]. The single largest data set on Down syndrome prevalence is from the “Study on Malformations and Down Syndrome in India” [SOMDI] project that presented data from three regions—viz Baroda [25], Bombay [26] and Delhi [27]. Three papers presented data from each of these regions, and all were derived from births in tertiary-level hospitals. Technically, they do not represent the community-wide prevalence. The data from Baroda are much more useful for gauging the prevalence than the other two. The papers from Bombay and Delhi could not provide maternal age-specific prevalence for Down syndrome owing to lack of adequate number of Down syndrome cases. The Baroda paper reported the prevalence for 5-year intervals of maternal age (Table 1). It is clear from Table 1 that the overall prevalence of DS differs between the three regions studied. Scatter plot of the data from the Baroda paper shows an inflection in the prevalence rate from about 30 years onward (Fig. 1). Another population-based study on the prevalence of DS among a tribal population in Madhya Pradesh was limited by the numbers [28]. In the 2767-strong population, the authors identified 4 cases of DS, setting a prevalence of about 1 in 692. Since this is not birth prevalence, it is likely to underestimate the true birth prevalence. Even so, this number is higher than the prevalence noted from the previous hospital-based studies.
Table 1.
Selected Indian data on Down syndrome
| Down syndrome prevalence | |||||
|---|---|---|---|---|---|
| Region | Maternal age | Total births | Total DS births | Prevalence | References |
| Baroda | [25] | ||||
| 15–19 | 1825 | 1 | 1/1825 | ||
| 20–24 | 16,572 | 14 | 1/1183 | ||
| 25–29 | 9588 | 8 | 1/1198* | ||
| 30–34 | 3118 | 7 | 1/445 | ||
| 35–39 | 608 | 2 | 1/304 | ||
| ≥40 | 64 | 1 | 1/64 | ||
| All | 31,775 | 33 | 1/962 | ||
| Delhi | All | 23,367 | 19 | 1/1230 | [27] |
| Bombay | All | 42,304 | 28 | 1/1511 | [26] |
| Chandigarh | All | 7400 | 8 | 1/925 | [33] |
| Madhya Pradesh, Tribal | All | 2767 | 4 | 1/692 | [28] |
| Cytogenetic abnormalities | ||||||
|---|---|---|---|---|---|---|
| Author | n | Translocation (%) | Mosaicism (%) | Pure trisomy (%) | Other cytogenetic findings (%) | References |
| Jyothy et al. 2000 | 1001 | 4.4 | 7.7 | 87.9 | [29] | |
| Thomas et al. 1992 | 316 | 7.6 | 5.8 | 86.6 | [30] | |
| Isaac et al. 1985 | # | 3.5 | 96.5 | [31] | ||
| Mandava et al. 2010 | 1572 | 7.1 | 1.8 | 89.1 | 0.3 | [32] |
| Sheth et al. 2007 | 382 | 8.9 | 3.9 | 84.8 | 2.4 | [33] |
Fig. 1.
Scatter dot plot of the data derived from reference [25]
Several others have reported the frequency of cytogenetic abnormalities [29–33] among children referred to genetic clinics and/or laboratories with a clinical diagnosis of DS. These clearly do not contribute to the database that would help estimate the a priori risk of Indian women. Some authors have suggested a younger age distribution of the mothers with DS children [34, 35] in some Indian populations. Advanced grand maternal age has been implicated to explain such a finding [36].
Following Empirical Practice
Few papers attempt to clinically validate the performance of screening test in our population. Kaur et al. [37] presented the clinical performance of the triple screening test (TST) from a government hospital setup in Chandigarh. DS prevalence in their series was limited to 8 cases from a population of 7400 pregnancies that were screened. The screen-positive rate was 4.5% for DS, and the detection rate was about 88%. This rate is higher than that reported for TST from other international papers probably due to small sample studied.
Most Indian laboratories that report DS screening tests have fixed a screen-positive cutoff at 1 in 250 without validating the assumption that underpins this cutoff: that the population undergoing screening would distribute itself such that 5% of the population would have a final adjusted risk at or greater than 1 in 250. The entire performance of any screening test would depend on the cutoff that is considered as screen positive: large-scale data on the distribution of the risks and the risk determinants in our population are distinctly lacking. We have analyzed our data from 27,647 singleton pregnancies [38] that further confirms the urgent need for a nationwide data on the risk distribution to scientifically choose the appropriate screen-positive cutoff point.
Quality Control Programs and Regulatory Requirements
Certainly, the excuse of a “busy clinic” cannot be accepted for not providing pretest counseling while offering Down syndrome screening since the implication of a misunderstood test result is of a grave nature to the unborn patient. Professional bodies, women representative agencies and Government should constitute committees that would ensure that physicians who perform NT measurements and laboratories that perform biochemical screening maintain a certain standard on an ongoing basis. Such committees would also have the responsibility of ensuring the end-user understanding of screening program through mass awareness programs, media, patient information leaflets, hospital and practitioner involvement and other methods.
Noninvasive Prenatal Test
The technological advancement of the recent years has introduced another screening test for Down syndrome, the noninvasive prenatal test. By amplifying, counting and comparing the cell free DNA fragments in the maternal blood, it is now possible to screen for trisomy 21 to a very high degree of accuracy. The high cost of the test at present precludes it from being adopted as a first-line screening test. As in other screening tests, NIPT also needs to be validated within our population before widespread adoption can be prescribed.
Conclusions
The prenatal screening and subsequent diagnosis of Down syndrome seem practical in the Indian scenario. However, given the different demographics of this population, the basic elements of the screening program such as population awareness, risk distribution and performance of the screening test need to be established at least at a regional level on an urgent basis. This calls for a nationwide integrated large-scale data sharing and cooperation between the relevant stakeholders.
Dr K. Manikandan
graduated from Madras Medical College in 2003. He set his focus on Fetal Medicine following an inspirational oration by Dr S. Suresh in 2002. He completed his MD (Ob Gyn) at JIPMER, Pondicherry, in 2006. After completing 3-year Senior Residency at JIPMER and a brief stint at Harris Birthright Research Centre for Fetal Medicine under Professor Nicolaides, he was appointed as Faculty of Obstetrics and Gynaecology at JIPMER. He resigned as Associate Professor to join his mentor Dr S Suresh at Mediscan Systems, Chennai, where he is currently pursuing the Fellowship in Fetal Medicine. Dr Manikandan was awarded the “Phani Seshadri Memorial Award” for the best postgraduate dissertation in 2007. He is a member of the Royal College of Obstetricians and Gynaecologists, London (MRCOG) since 2012. Currently, along with Dr S Suresh and Dr Sudarshan Suresh, he is pursuing a DBT-funded project on developing Fetal Growth Charts for the south Indian population. His research focus is on preeclampsia prevention.
Compliance with Ethical Standards
Conflict of interest
SS is Honorary Clinical Director of PerkinElmer Laboratory, Chennai. KM declares that he has no conflict of interest.
Human and Animals Rights
The authors declare that no human research participants or animals were involved in composing this invited review article.
Informed Consent
The authors declare that informed consent was not required for this invited review article as no human research participants were involved.
Footnotes
K Manikandan is a Senior Research Consultant at Fetal Care Research Foundation and a Fellow in Fetal Medicine, Mediscan Systems, Chennai, India; Suresh Seshadri is the Director and Senior Consultant at Fetal Medicine Department, Mediscan systems, Chennai, India.
References
- 1.Wilson JMG, Gunnar J. Principles and practice of screening for disease [Internet]. World Health Organisation. 1968 [cited 2017 May 31]. http://apps.who.int/iris/bitstream/10665/37650/17/WHO_PHP_34.pdf.
- 2.Langdon J, Down H. Observations on an ethnic classification of idiots1. Heredity. 1966;21(4):695–697. doi: 10.1038/hdy.1966.69. [DOI] [Google Scholar]
- 3.On the antiquity of trisomy 21: moving towards a quantitative diagnosis of down syndrome in historic material culture [Internet]. [cited 2017 Feb 21]. http://connection.ebscohost.com/c/articles/70399984/antiquity-trisomy-21-moving-towards-quantitative-diagnosis-down-syndrome-historic-material-culture.
- 4.Valenti C, Schutta EJ, Kehaty T. Prenatal diagnosis of Down’s syndrome. Lancet Lond Engl. 1968;2(7561):220. doi: 10.1016/S0140-6736(68)92656-1. [DOI] [PubMed] [Google Scholar]
- 5.Nadler HL. Antenatal detection of hereditary disorders. Pediatrics. 1968;42(6):912–918. [PubMed] [Google Scholar]
- 6.Gertner M, Hsu LY, Martin J, et al. The use of amniocentesis for prenatal genetic counseling. Bull N Y Acad Med. 1970;46(11):916–921. [PMC free article] [PubMed] [Google Scholar]
- 7.Nicolaides K. Fetal medicine [Internet]. Abu Dhabi first international conference in fetal medicine, pediatrics, pediatric gastroenterology, hepatology and nutrition. 2015 [cited 2017 Mar 22]; Abu Dhabi. https://www.youtube.com/watch?time_continue=2&v=t8CKcModb58.
- 8.Penrose LS. The relative effects of paternal and maternal age in mongolism. 1933. J Genet. 2009;88(1):9–14. doi: 10.1007/s12041-009-0002-5. [DOI] [PubMed] [Google Scholar]
- 9.Nicolaides KH. Screening for fetal chromosomal abnormalities: need to change the rules. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 1994;4(5):353–354. doi: 10.1046/j.1469-0705.1994.04050353.x. [DOI] [PubMed] [Google Scholar]
- 10.Hook EB, Lindsjö A. Down syndrome in live births by single year maternal age interval in a Swedish study: comparison with results from a New York State study. Am J Hum Genet. 1978;30(1):19–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Tabor A, Madsen M, Obel E, et al. Randomised controlled trial of genetic amniocentesis in 4606 low-risk women. Lancet. 1986;327(8493):1287–1293. doi: 10.1016/S0140-6736(86)91218-3. [DOI] [PubMed] [Google Scholar]
- 12.Akolekar R, Beta J, Picciarelli G, et al. Procedure-related risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2015;45(1):16–26. doi: 10.1002/uog.14636. [DOI] [PubMed] [Google Scholar]
- 13.Wulff CB, Gerds TA, Rode L, et al. Risk of fetal loss associated with invasive testing following combined first-trimester screening for Down syndrome: a national cohort of 147 987 singleton pregnancies. Ultrasound Obstet Gynecol. 2016;47(1):38–44. doi: 10.1002/uog.15820. [DOI] [PubMed] [Google Scholar]
- 14.Rutter S. Down’s syndrome | intellectual disability and health [Internet]. [cited 2017 May 28]. http://www.intellectualdisability.info/diagnosis/articles/downs-syndrome.
- 15.Snijders RJ, Sundberg K, Holzgreve W, et al. Maternal age- and gestation-specific risk for trisomy 21. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 1999;13(3):167–170. doi: 10.1046/j.1469-0705.1999.13030167.x. [DOI] [PubMed] [Google Scholar]
- 16.Manikandan K, Suresh S, Seshadri S. Basic biostatistical concepts for the fetal physician—I: the 2 × 2 table and Its derivatives. J Fetal Med. 2016;3(4):151–157. doi: 10.1007/s40556-016-0100-4. [DOI] [Google Scholar]
- 17.Reimand T, Ounap K, Zordania R, et al. Descriptive epidemiology of Down’s syndrome in Estonia. Paediatr Perinat Epidemiol. 2006;20(6):512–519. doi: 10.1111/j.1365-3016.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 18.Brajenović-Milić B, Prpić I, Petrović O, et al. The prevalence of live birth Down syndrome in the region of Primorsko-goranska County in Croatia, 1996–2005: the impact of screening and amniocentesis. Matern Child Health J. 2008;12(5):620–623. doi: 10.1007/s10995-007-0272-6. [DOI] [PubMed] [Google Scholar]
- 19.Wortelboer MJ, De Wolf BT, Verschuuren-Bemelmans CC, et al. Trends in live birth prevalence of down syndrome in the Northern Netherlands 1987-96: the impact of screening and prenatal diagnosis. Prenat Diagn. 2000;20(9):709–713. doi: 10.1002/1097-0223(200009)20:9<709::AID-PD910>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 20.Jou H-J, Kuo Y-S, Hsu J-J, et al. The evolving national birth prevalence of Down syndrome in Taiwan. A study on the impact of second-trimester maternal serum screening. Prenat Diagn. 2005;25(8):665–670. doi: 10.1002/pd.1220. [DOI] [PubMed] [Google Scholar]
- 21.Harlap S. Down’s syndrome in West Jerusalem. Am J Epidemiol. 1973;97(4):225–232. doi: 10.1093/oxfordjournals.aje.a121503. [DOI] [PubMed] [Google Scholar]
- 22.Khalil A, Pandya P. Screening for Down syndrome. J Obstet Gynaecol India. 2006;56(3):205–211. [Google Scholar]
- 23.Wald NJ, Rodeck C, Hackshaw AK, et al. SURUSS in perspective. Semin Perinatol. 2005;29(4):225–235. doi: 10.1053/j.semperi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Mastana SS. Unity in diversity: an overview of the genomic anthropology of India. Ann Hum Biol. 2014;41(4):287–299. doi: 10.3109/03014460.2014.922615. [DOI] [PubMed] [Google Scholar]
- 25.Modi UJ, Nayak U, Aiyer S, Bharani S, Master DC, Shah T, et al. Study of malformations and Down syndrome in India (SOMDI): Baroda region. Httpwwwijhgcomarticleaspissn0971-6866year1998volume4issue1spage93epage98aulastModitype2 [Internet]. 1998 [cited 2017 Jun 5]. http://imsear.hellis.org/handle/123456789/159843.
- 26.Bharucha BA. Study of malformations and Down syndrome in India (SOMDI): Bombay region. Httpwwwijhgcomarticleaspissn0971-6866year1998volume4issue1spage88epage92aulastBharuchatype2 [Internet]. 1998 [cited 2017 Jun 6]. http://imsear.hellis.org/handle/123456789/159842.
- 27.Verma IC, Anand NK, Kabra M, et al. Study of malformations and Down syndrome in India (SOMDI): Delhi region. Indian J Hum Genet. 1998;4(1):84–87. [Google Scholar]
- 28.Lakhan R, Kishore MT. Down syndrome in tribal population in India: a field observation. J Neurosci Rural Pract. 2016;7(1):40–43. doi: 10.4103/0976-3147.172167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jyothy A, Kumar KS, Rao GN, et al. Cytogenetic studies of 1001 Down syndrome cases from Andhra Pradesh, India. Indian J Med Res. 2000;111:133–137. [PubMed] [Google Scholar]
- 30.Thomas IM, Rajangam S, Hegde S. Cytogenetic investigations in Down syndrome patients & their parents. Indian J Med Res. 1992;96:366–371. [PubMed] [Google Scholar]
- 31.Isaac GS, Krishnamurty PS, Reddy YR, et al. Down’s syndrome in Hyderabad, India. Acta Anthropog. 1985;9(4):256–260. [PubMed] [Google Scholar]
- 32.Mandava S, Koppaka N, Bhatia V, et al. Cytogenetic analysis of 1572 cases of Down syndrome: a report of double aneuploidy and novel findings 47, XY, t (14; 21) (q13; q22.3) mat, + 21 and 45, XX, t (14;21) in an Indian population. Genet Test Mol Biomark. 2010;14(4):499–504. doi: 10.1089/gtmb.2009.0167. [DOI] [PubMed] [Google Scholar]
- 33.Sheth F, Rao S, Desai M, et al. Cytogenetic analysis of Down syndrome in Gujarat. Indian Pediatr. 2007;44(10):774–777. [PubMed] [Google Scholar]
- 34.Rao VB. Mean maternal age of Down’s syndrome in Hyderabad, India. J Indian Med Assoc. 1999;97:25. [PubMed] [Google Scholar]
- 35.Vundinti BR, Ghosh K. Incidence of down syndrome: Hypotheses and reality. Indian J Hum Genet. 2011;17(3):117–119. doi: 10.4103/0971-6866.92080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malini SS, Ramachandra NB. Influence of advanced age of maternal grandmothers on Down syndrome. BMC Med Genet. 2006;7(1):4. doi: 10.1186/1471-2350-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaur G, Srivastav J, Kaur A, et al. Maternal serum second trimester screening for chromosomal disorders and neural tube defects in a government hospital of North India. Prenat Diagn. 2012;32(12):1192–1196. doi: 10.1002/pd.3984. [DOI] [PubMed] [Google Scholar]
- 38.Manikandan K, Rangaraj A, Ganesan P, et al. The first trimester combined screening test in the Indian population: insights from a cohort of 27,647 pregnancies. J Fetal Med [Internet] 2017 [Google Scholar]