Abstract
Background
Microsurgical tenets and peritoneal conditioning during laparoscopic surgery (LS) decrease postoperative adhesions and pain. For a trial in human, the strong beneficial effects of N2O needed to be confirmed in open surgery (OS).
Results
In a mouse model for OS, the effect of the gas environment upon adhesions was evaluated. Experiment I evaluated desiccation and the duration of exposure to CO2, N2O or CO2 + 4%O2. Experiment II evaluated the dose-response curve of adding N2O to CO2. Experiment III compared humidified CO2 + 10% N2O during LS and OS.
In OS, 30- and 60-min exposure to non-humidified CO2 caused mortality of 33 and 100%, respectively. Mortality was prevented by humidification, by dry N2O or dry CO2 + 4%O2. Adhesions increased with the duration of exposure to CO2 (p < 0.0001) and decreased slightly by humidification or by the addition of 4% O2. N2O strongly decreased adhesions at concentrations of 5% or greater. With humidified CO2 + 10% N2O, adhesion formation was similar in OS and LS.
Conclusions
The drug-like and strong beneficial effect of low concentrations of N2O is confirmed in OS.
Keywords: Postoperative adhesions, N2O, Conditioning, Humidification, Microsurgery, Microsurgical principle
Background
The peritoneal cavity with its peritoneal fluid is a specific environment different from that of plasma. The mesothelial cell lining of the peritoneal cavity and its organs facilitates the gliding of the bowels and actively regulates homeostasis and transport of fluids, molecules and cells. In males, the volume of peritoneal fluid is small. In women of reproductive age, follicular exudation increases the volume and adds high concentrations of steroid hormones. The peritoneal cavity is not vascularised and constitutes a sterile cavity that does not belong to the body homeostasis. Any trauma in the peritoneal cavity causes an inflammatory reaction and a mesothelial cell retraction, exposing the basal membrane. This abolishes the blood-peritoneal fluid barrier and permits the entry of immunocompetent cells and facilitates diffusion of larger molecules as immunoglobins, which is an efficient defence mechanism to intruders [1, 2].
The large and flat mesothelial cells react within seconds to any trauma by retraction and bulging [1, 2] causing an acute inflammation [3] which increases with the duration and severity of the trauma. Identified traumas are surgical manipulation, mesothelial cell hypoxia by CO2 pneumoperitoneum, deeper ischaemia at an intraperitoneal pressure of more than 8 mmHg and ischaemia-reperfusion at desufflation [4], oxidative stress [5] or reactive oxygen species (ROS) induced by exposure to air with 20% of oxygen, desiccation and saline as irrigation liquid. The severity and the duration of this acute inflammation of the entire peritoneal cavity create an inversely proportional reduction of fibrinolysis. This, in turn, increases the potential of adhesion formation through a reduction in tissue plasminogen activator (tPA) and an increase in plasminogen activator inhibitor (PAI) [6, 7]. During laparoscopic surgery, the retraction and bulging of mesothelial cells cause a progressive increase in CO2 resorption. The acute peritoneal inflammation increases postoperative C-reactive protein concentrations (CRP) and causes postoperative pain [2].
During laparoscopic surgery, prevention of the mesothelial cell retraction and the subsequent acute inflammation effectively prevents or decreases the associated consequences including postoperative adhesion formation and postoperative pain. In addition, it accelerates recovery and in animal experiments decreases tumour metastasis. The most effective preventive factors are the addition of more than 5% of nitrous oxygen to the CO2 pneumoperitoneum, cooling of the peritoneal cavity below 31 °C, minimalising mechanical trauma and ROS production, using Ringer’s lactate instead of saline and administering one or two doses dexamethasone postoperatively [2]. If used together with a barrier [8], this approach results in virtually adhesion free surgery [9].
The similarity between our current knowledge derived largely from animal experiments, and the microsurgical tenets developed in the early 1970s empirically, but controlled by systematic second-look laparoscopy, 8–12 weeks after the initial operation, is striking. These principles were developed for open surgery and soon after applied in laparoscopic surgery [10]. These microsurgical principles indeed are a combination of gentle tissue handling, judicious use of electrical and/or laser energy, use of inert sutures, continuous irrigation with Ringer’s lactate at room temperature during the procedure to avoid desiccation, shielding the bowels from the ambient air, thorough lavage of the peritoneal cavity at the end of the procedure, instillation of Ringer’s lactate solution containing a minimum of 500 mg of hydrocortisone succinate into the peritoneal cavity before closure and administration of one or two doses of dexamethasone after surgery.
Microsurgical tenets were proven to decrease adhesion formation and to increase pregnancy rates in open and laparoscopic surgery [10, 11]. The relative importance of each of these factors that decrease acute inflammation and adhesion formation was investigated only recently in a laparoscopic mouse model with proof of concept trials in human [9]. However, the addition of low doses of N2O which is the single most effective factor was investigated during laparoscopic surgery with an insufflation pressure only. Since there is no insufflation pressure in open surgery, we, therefore, decided to evaluate the effect of N2O in a mouse model for open surgery before undertaking a trial in human.
Methods
Animals and the experimental set-up (anaesthesia, ventilation, laparoscopic surgery, adhesion induction and scoring) were as previously described [3, 12, 13].
Animals
Inbred 9 to 10-week-old female BALB/c OlaHsd mice of 18 to 20 g (Harlan Laboratories B.V., Venray, The Netherlands) mice were used to decrease experimental variability. They were kept under standard laboratory conditions and diet at the animal facilities of the Katholieke Universiteit Leuven (KUL). The study was approved by the Institutional Review Animal Care Committee (KUL: P040/2010).
The laparoscopic mouse model
Following anaesthesia and pneumoperitoneum induction (Thermoflator, Karl Storz, Tüttlingen, Germany) with humidified gas (Humidifier, 204,320 33, Karl Storz) and standardised 10 × 1.6 mm bipolar lesions (20 W, standard coagulation mode, Autocon 350, Karl Storz, Tüttlingen, Germany) were made on both right and left uterine horns and on abdominal walls using a 2 mm endoscope (Karl Storz, Tüttlingen Germany) and two 14-gauge catheters (Insyte-W, Vialon, Becton Dickinson, Madrid, Spain) as secondary ports. The insufflation pressure was 15 mmHg. Since adhesion formation increases with body temperature, the latter was strictly controlled [13]. Therefore, mice and equipment were placed in a closed chamber at 37 °C (heated air, WarmTouch, Patient Warming System, model 5700, Mallinckrodt Medical, Hazelwood, MO). Anaesthesia and ventilation [14] and the timing between anaesthesia (T 0), intubation (at 10 min, T 10) and the onset of the experiment (at 20 min, T 20) were standardised.
The only variable in this model of adhesion formation thus was the duration of the pneumoperitoneum and the type and humidification of the gas used.
A mouse model for open surgery
All factors validated for the laparoscopic model were kept identical, i.e. animals, anaesthesia, intubation, ventilation, temperature control, timing, type of lesions, the equipment used for gas insufflation and humidification and the scoring of adhesions. The only difference was that instead of a laparoscopy with a pneumoperitoneum, a laparotomy was performed and the mice were kept with the open abdomen in a box exposed to the specific gas environment.
Following some pilot experiments, the model was standardised as follows. Following anaesthesia (T 0), shaving and disinfection of the abdomen, the mouse was placed on a warm pillow in a transparent plexiglas box measuring 22 ×10 ×30 cm, closed with a sliding transparent cover that could be removed to perform at 20 min, T 2 surgery and lesions (Fig. 1). The box had one hole that accommodates the ventilation tube without gas leaks and two holes of 1 cm diameter each. The upper hole permitted escape of gas without pressure. The lower hole permitted insufflation of gas standardised in these experiments at 2 L/min. Since densities of CO2 and N2O are higher than air (δCO2 = 1.842, δN2O = 1.872, δ air = 1.205 at room temperature and atmospheric pressure), the box fills progressively until the gas escapes by overflow. In order to perform the surgical procedure, the box had to be opened; this causes the insufflated gas to partially mix with the ambient air. A midline xyphopubic incision was performed, and the abdomen kept open with two pins. Standard 10× 2 mm bipolar lesions were created similar to the laparoscopic model. Following surgery, the cover was placed over the box and the mouse kept with the abdomen open exposed to the insufflated gas. This cover was necessary, since otherwise the insufflated gas would mix partially with the ambient air varying with the height of the box, the diameter of the opening and the flow rate of the gas insufflated. At the end of the experiment, the abdomen was closed with nylon 3-0 sutures.
Fig. 1.
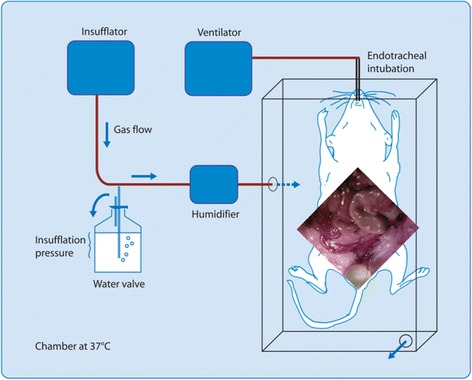
The mouse model for open surgery. Image modified from Binda et al. [13], Corona R et al, Gynecol Surg, 2017 + ref
Scoring of adhesions
Postoperative adhesions were scored blindly after 7 days as previously described during a second laparotomy using a stereomicroscope. The terminology of Pouly et al. [15] was used to describe de novo adhesion formation as adhesions formed at non-surgical sites.
Study design
Randomisation and factorial design
All experiments were block randomised by day as done in all previous experiments. Thus, one animal of each experimental group was operated at random on the same day in order to avoid eventual differences by day.
A factorial design [16] was used since a two by two factorial design results for each of the two variables in an almost similar statistical power as if two experiments had been performed with the same total number of animals in each experiment.
Mixture of N2O and CO2
In these experiments, we used either premixed gas with 90% CO2 and 10% N2O (Ijsfabriek, Strombeek, Belgium) or two Thermoflators one delivering CO2 and the other N2O, or the premixed gas. The gases from both insufflators were subsequently mixed in a mixing chamber, and the excess gas was permitted to escape from a water valve, the flow of both gases entering the box was limited to 2 L/min with a stopcock.
Pilot experiments
The first pilot experiment for open surgery consisted of mice (n = 3) with the abdomen open exposed to the ambient air at 37 °C (chamber at 37 °C) for 60 min. After 60 min, bowels were macroscopically dry and all mice died within 2 days. Mortality was thought to be caused by desiccation and maybe the damaging effect of 20% of O2 in air. Therefore, the box was designed as described in order to control the gas environment, and CO2 was used as a carrier gas in order to be comparable with laparoscopy and because CO2 is heavier than air and thus will fill the box progressively from the bottom.
A second pilot experiment was performed to evaluate in open surgery gas conditions known from the laparoscopy model. Humidified CO2 for 60 min confirmed the absence of mortality with humidification; humidified 50% CO2 + 50% N2O and humidified 100% N2O confirmed the strong adhesion preventing the effect of N2O in concentrations over 5% (six mice, two mice per group).
Experiment I
The first experiment was designed to evaluate in open surgery the effect of humidification and of the duration of exposure to either 100% CO2 or 100% N2O or 96% CO2 + 4%O2 upon adhesion formation. A factorial design was used with non-humidified or humidified gas (two factors), during 30 or 60 min (two factors), and the three gas compositions (three factors). With three mice/cell for two humidification factors and two duration factors and three gas factors (total mice = 3×2×2×3 = 36), an almost similar statistical power for each variable was obtained as if in three consecutive experiments with 36 mice each would have been done.
Experiment II
A dose response of the addition of various concentrations of N2O to the CO2 was evaluated in open surgery. Mice were exposed for 30 min to humidified CO2 with concentrations of N2O varying from 0 to 0.3, 1, 3, 10 and 100%. For 100% CO2, 100% N2O and 10% N2O + 90% of CO2, a Thermoflator was used with CO2, N2O or a premixed gas (10% N2O + 90% CO2), respectively. For the other concentrations two Thermoflators were used, one with CO2 and the other with premixed gas (10% N2O + 90% CO2). The final concentrations of 3, 1, and 0.3% N2O were obtained by combining various flow rates of 4 and 2 L/min, 9 and 1 L/min and 14.5 and 0.5 L/min of 100% CO2 and premixed gas with 10% of N2O (six mice/group, total mice = 36).
Experiment III
The third experiment was designed in order to compare adhesion formation following laparoscopic and open surgery and to evaluate whether the addition of 4% of O2 had an additive effect when 10% of N2O had been added to the CO2. Using a factorial design, mice were exposed for 60 min to humidified 90% CO2 + 10% N2O or to 86% CO2 + 10% N2O + 4% O2 either during laparoscopy or during open surgery. Since adhesions were known to be very low with 10% of N2O, 10 mice per cell were used in order to have a power of almost 40 mice for each factor (total mice = 40).
Statistics
Differences were calculated with the SAS System (SAS Institute, Cary, NC) [17] using Wilcoxon/Kruskal Wallis unpaired test for comparison of individual data and a two-way analysis of variance (Proc GLM) for experiments with a factorial design. Results are expressed as a mean and standard deviations unless indicated otherwise.
Results
Experiment I
As observed in the pilot experiment for open surgery with non-humidified air for 60 minutes, open surgery with non-humidified CO2 for 60 min resulted in 100% mortality (3/3). Even exposure to 30 min non-humidified CO2 resulted in 33.3% mortality (1/3) (Fig. 2). As expected, there was no mortality when humidified gas was used. To our surprise, there was also no mortality when 10% of N2O or 4% of O2 were added to the non-humidified CO2.
Fig. 2.
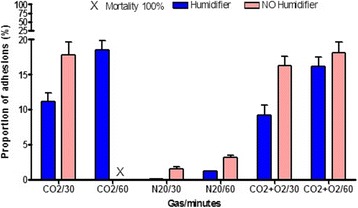
Effect of duration, humidification and gas type upon mortality and adhesion formation during open surgery: 30 or 60 min of non-humidified or humidified CO2, N2O or 96% CO2 + 4%O2 Corona R et al, Gynecol Surg, 2017 + ref were used. Proportions of adhesions are given (mean and SD)
Adhesions at the surgical lesion site increased when the duration of exposure was longer (p < 0.0001) and when non-humidified gas was used (p < 0.0001) When 100% N2O was used, adhesions were very scant in comparison with 100% CO2 and 96% CO2 + 4%O2 with and without humidification (all comparisons p < 0.0001). When 96% CO2 + 4% O2 was used, adhesions were slightly less than with 100% CO2 (non-humidified gas for 30 min p < 0.0001; humidified gas for 30 min p = 0.0011 and for 60 min p = 0.003).
Whereas in the laparoscopic mouse model, de novo adhesions in the upper abdomen have never been observed; in this experiment, de novo adhesions were seen in the upper abdomen between bowels, and between bowels and sidewalls when non-humidified CO2 or non-humidified 96% CO2 + 4%O2 were used (Fig. 2).
Experiment II
The addition of increasing concentrations of N2O to CO2 decreases exponentially adhesion formation, with a half maximum effect around 2.5% and a maximal effect from 5% onwards (Fig. 3). The difference between 10 and 100% N2O was not significant (p = 0.1551). Differences between 0 and 3%, 0 and 10% and between 3 and 10% N2O were p = 0.0061, p = 0.0006 and p = 0.03, respectively.
Fig. 3.
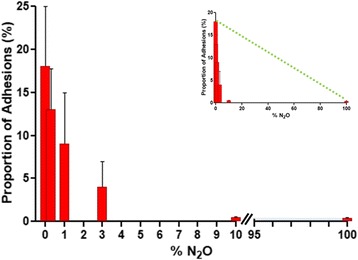
Dose-response curve of the addition of 0.3 to 100% N2O to humidified CO2 upon adhesion formation during open surgery demonstrating the drug-like effect. In inset, the dotted yellow line indicates adhesion formation if the effect of N2O would have been by replacing CO2 irritation. Proportions of adhesions are given (mean and SD) Corona R et al, Gynecol Surg, 2017 + ref
Experiment III
When humidified CO2 with 10% of N2O was used, adhesion formation was as expected very low in all groups (Fig. 4). The extent of adhesions was similar after both laparoscopic and open surgery with (NS) or without (NS) 4% of oxygen. The addition of 4% of oxygen to CO2 with 10% of N2O had a very small additive effect which however turned out to be significant (p < 0.01) caused by the high power of 20 mice in each group (factorial design, 10 mice/cell) and the low variability of inbred strains,
Fig. 4.
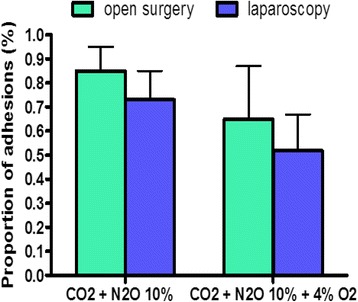
The additional effect of 4% of O2 on adhesion formation when 10% of N2O is used was investigated during laparoscopic and open surgery using humidified CO2. Adhesion formation was comparable between laparoscopic and open surgery. The additive effect of 4% of O2 was marginal (p < 0.01). Proportions of adhesions are given (mean and SD) Corona R et al, Gynecol Surg, 2017 + ref
Discussion
These experiments confirm that the prevention of adhesion formation by conditioning is similar in both open and laparoscopic surgery. The main damaging effect of CO2, thus, is caused by mesothelial cell hypoxia and retraction, and less by tissue and ischaemia-reperfusion. Adhesions indeed increase with the duration of exposure to CO2 and with desiccation. N2O in concentrations of more than 5% appears to be the single most effective factor with a marginal beneficial effect when 4% of oxygen is added to this gas mixture. Although not all observations made during laparoscopic surgery were repeated in open surgery, we conclude that the mechanisms involved are similar in both open and laparoscopic surgery. The key factors are mesothelial cell damage and acute inflammation in the entire peritoneal cavity, as a reaction to trauma, hypoxia, ROS, oxidative stress and desiccation.
The exact mechanisms involved in the peritoneal cavity that enhance or prevent adhesion formation are not fully understood. The half maximal effect around 2.5% of N2O indicates that N2O has a drug-like effect, the mechanism of which is unknown. It is also not understood why mortality is 100% after exposure for 60 min to non-humidified CO2 and no mortality when non-humidified N2O is used, although the desiccated aspect of the bowels is the same. We only can speculate that mortality is not only caused by the desiccation but mainly by the severity of the inflammatory process since N2O strongly and O2 slightly decrease the inflammatory reaction [18].
The observed effects of 5 to 10% of N2O in open surgery at atmospheric pressure shed new light on the pathophysiology of adhesion formation. CO2 pneumoperitoneum at an insufflation pressure of 15 mmHg decreases peritoneal oxygenation, triggers hypoxemia inducible factor (HIF) and decreases tissue plasminogen activator and upregulates PAI for several days. These effects of CO2 pneumoperitoneum are less and/or of shorter duration at lower insufflation pressures and disappear at insufflation pressures below 8 mmHg in human and 2 mmHg in mice [3, 6–8]. Taking into account the differences in size between man and mice and Pascal’s law, these pressures are considered the pressures at which vascular compression of the peritoneum, hypoxia and oxidative stress start. Since at atmospheric pressure N2O still decreases adhesion formation caused by the CO2 environment, we must conclude that key mechanism driving the subsequent events is mesothelial hypoxia and retraction. In addition, the observations that with laparoscopic surgery in the presence of a 10% N2O environment at 15 mmHg pressure, the extent of adhesion formation is similar to open surgery at atmospheric pressure strongly suggests that 10% of N2O prevents mesothelial cell oxidative stress hypoxia and its consequences including mesothelial cell retraction and decreased fibrinolysis enhanced adhesion formation and postoperative pain. However, whether N2O also has a protective effect on oxidative stress caused by partial oxygen pressures higher than 75 mmHg (or more than 10% O2 at atmospheric pressure) as in air remains to be investigated.
Despite the differences that exist between oxidative stress caused by 20% CO2 in ambient air in open surgery and the detrimental effect of the CO2 pneumoperitoneum and insufflation pressure, the prevention of adhesion formation and postoperative pain are similar in both open and laparoscopic surgery. Beside the use of a proper atraumatic surgical technique and precise haemostasis, the important adhesion preventive factors in open surgery are to avoid ROS formation, caused by the 20% oxygen concentration in ambient air; the use of N2O in concentrations of 5% or more; cooling the peritoneal cavity; avoiding desiccation; the use of Ringer’s lactate solution instead of saline; being toxic for mesothelial cells [19–25] for intraoperative irrigation and terminal thorough lavage and administration of one or two doses of dexamethasone after surgery. Although, it has not been investigated as yet whether N2O can prevent the damaging effects of exposure to 20% of oxygen concentration, the flooding of the surgical site in open surgery with 5 to 10% of N2O will require a carrier gas for which both CO2 and nitrogen (N2) seem suitable. The importance of cooling in open surgery has indirectly been confirmed in rats using cold saline infusions [26, 27]. Adhesions were also less when the abdominal cavity was exposed to the atmosphere of the operating theatre (21% O2, 21 °C, 40–47% relative humidity) than to CO2 + 4% of oxygen and 95–100% relative humidity at 37 °C [28]. Prevention of desiccation is much more important in open surgery than in laparoscopic surgery considering the 100% mortality of mice when exposed to dry CO2 for 60 min. The toxicity of saline for the peritoneum was known since the early 1970s [19, 20] and has recently been confirmed [21–25]. The use of dexamethasone and another tenet of microsurgery was only proven to be effective after conditioning in laparoscopic surgery in an animal model. In any case, adhesion formation following open and laparoscopic surgery appears to be remarkably similar in an atmosphere of 10% N2O in CO2 without desiccation.
The implementation of these principles to open surgery should be carried out judiciously. That saline should be abandoned, and a richer solution should be used for irrigation is obvious. Prevention of desiccation can be achieved by continuous irrigation as done in microsurgery, by covering bowels with moistened inert towels and/or by flooding the operative field with humidified CO2 [29, 30]. The latter indeed decreased adhesion formation in open cardiac surgery. The instillation of humidified CO2 deep into the surgical field also decreased oxidative stress since the organs were no longer exposed to the 20% of O2 in ambient air. A similar effect was achieved by shielding the organs in microsurgery. The exposure of the surgical field to the temperature of the operating theatre has never been an issue in open surgery. We can be happy today that cooling unexpectedly has a beneficial effect. The administration of dexamethasone after surgery, eventually at the end of surgery, may be beneficial to reduce inflammation and adhesion formation and accelerate recovery, while its use might aggravate an eventual infection. The proven very strong beneficial effect of 5 to 10% of N2O with no explosion risk demands a trial in open surgery. As described for humidified CO2 in cardiac surgery, [29, 30] the deep instillation of gases heavier than air will fill and flood progressively the operation field. CO2 seems obvious as a carrier gas since it is heavier than air with minimal irritative effect at atmospheric pressure. N2O, which fortunately also is heavier than air, should be used in concentrations of 5 to 10% of N2O. For this reason, we used the same combination for these experiments, the efficacy of which had furthermore already been proven in animal models. It will obviously be necessary to prevent or reduce contamination of the operating theatre with N2O. The suggested upper threshold for N2O is 25 ppm [31]. We speculate that this can be achieved with aquarium-like drapings extending above the operating field with aspiration at the borders to prevent overflow; the opening of the draping would be a compromise between being sufficiently large to permit surgery but small enough to prevent mixture with the ambient air. Indeed even without aspiration contamination with 2 L/min with 10% of N2O would result in only 15 ppm N2O in a normal sized (e.g. 40 m3) and ventilated (e.g. refresh rate of 20 cycles/h) operating room.
Conclusions
In conclusion, the mechanisms of mesothelial cell damage and their prevention are the same with open and with laparoscopic surgery. The application of microsurgical tenets, which enabled to decrease inflammation in the peritoneal cavity, reduce adhesion formation and improve fertility outcomes, can benefit further from flooding the operative field with 5 to 10% of N2O, which has proven to be a most effective factor. Prevention of mesothelial cell damage and the subsequent acute inflammation may even be more important in open surgery than in laparoscopic surgery, especially when bowels are exteriorised and subjected to desiccation and exposed to ambient air that creates oxidative stress. Since both CO2 and N2O are heavier than air, it is also possible to instil humidified CO2 with 5 to 10% of N2O deep into the abdominal cavity during the procedure. Given the absence of side effects, as demonstrated in laparoscopic surgery, a study in open surgery may well demonstrate a virtually adhesion free surgery, a reduction of postoperative pain and a shortened recovery period, as has been observed in laparoscopic surgery.
Acknowledgements
We thank Anastasia Ussia (Rome, Italy), Karina Mailova (Moscow, Russia), Jasper Verguts (Hasselt, Belgium) and Michel Camus and Herman Tournaye (Brussels, Belgium) for the review and discussions. eSaturnus NV (Sony), Fisher and Paykel and Storz AG are acknowledged for supplying the equipment for these experiments. This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors, except royalties from patents hold at the university.
Condensation
Peritoneal conditioning is equally important for open surgery as for laparoscopic surgery to prevent postoperative adhesions and pain.
Authors’ contributions
RC and MMB carried out the experiments (study design, data collections) coordinated by PK. RC, MMB and PK have performed the data analysis and writing. LA made the basic observations on N2O, and all were closely involved with the design and finalisation of the study. VG actively contributed to the understanding of these observations as an update of microsurgical tenets. All authors read and approved the final manuscript.
Competing interests
Roberta Corona, Maria Mercedes Binda, Leila Adamyan and Victor Gomel have nothing to declare. Philippe R Koninckx is a stockholder of EndoSAT NV.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Roberta Corona, Email: coronaroberta@gmail.com, Email: rcorona@barbadosivf.com.
Maria Mercedes Binda, Email: Mercedes.binda@gmail.com.
Leila Adamyan, Email: adamyanleila@gmail.com.
Victor Gomel, Email: victorgomel1@gmail.com.
Philippe R. Koninckx, Email: pkoninckx@gmail.com
References
- 1.Mutsaers SE, Prele CM, Pengelly S, Herrick SE. Mesothelial cells and peritoneal homeostasis. Fertil Steril. 2016;106:1018–1024. doi: 10.1016/j.fertnstert.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Koninckx PR, Gomel V, Ussia A, Adamyan L. Role of the peritoneal cavity in the prevention of postoperative adhesions, pain, and fatigue. Fertil Steril. 2016;106:998–1010. doi: 10.1016/j.fertnstert.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Corona R, Verguts J, Schonman R, Binda MM, Mailova K, Koninckx PR. Postoperative inflammation in the abdominal cavity increases adhesion formation in a laparoscopic mouse model. Fertil Steril. 2011;95:1224–1228. doi: 10.1016/j.fertnstert.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Matsuzaki S, Jardon K, Maleysson E, D'Arpiany F, Canis M, Bazin JE, Mage G (2010) Carbon dioxide pneumoperitoneum, intraperitoneal pressure, and peritoneal tissue hypoxia: a mouse study with controlled respiratory support. Surg Endosc. 2010;24:2871–80 [DOI] [PubMed]
- 5.Donnez J, Binda MM, Donnez O, Dolmans MM. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil Steril. 2016;106:1011–1017. doi: 10.1016/j.fertnstert.2016.07.1075. [DOI] [PubMed] [Google Scholar]
- 6.Shimomura M, Hinoi T, Ikeda S, Adachi T, Kawaguchi Y, Tokunaga M, Sasada T, Egi H, Tanabe K, Okajima M, Ohdan H. Preservation of peritoneal fibrinolysis owing to decreased transcription of plasminogen activator inhibitor-1 in peritoneal mesothelial cells suppresses postoperative adhesion formation in laparoscopic surgery. Surgery. 2013;153:344–356. doi: 10.1016/j.surg.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki S, Botchorishvili R, Jardon K, Maleysson E, Canis M, Mage G. Impact of intraperitoneal pressure and duration of surgery on levels of tissue plasminogen activator and plasminogen activator inhibitor-1 mRNA in peritoneal tissues during laparoscopic surgery. Hum Reprod. 2011;26:1073–1081. doi: 10.1093/humrep/der055. [DOI] [PubMed] [Google Scholar]
- 8.Diamond MP. Reduction of postoperative adhesion development. Fertil Steril. 2016;106:994–997. doi: 10.1016/j.fertnstert.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 9.Koninckx PR, Corona R, Timmerman D, Verguts J, Adamyan L. Peritoneal full-conditioning reduces postoperative adhesions and pain: a randomised controlled trial in deep endometriosis surgery. J Ovarian Res. 2013;6:90. doi: 10.1186/1757-2215-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomel V, Koninckx PR. Microsurgical principles and postoperative adhesions: lessons from the past. Fertil Steril. 2016;106:1025–1031. doi: 10.1016/j.fertnstert.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 11.Gomel V. Reconstructive tubal microsurgery and assisted reproductive technology. Fertil Steril. 2016;105:887–890. doi: 10.1016/j.fertnstert.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 12.Corona R, Verguts J, Koninckx R, Mailova K, Binda MM, Koninckx PR. Intraperitoneal temperature and desiccation during endoscopic surgery. Intraoperative humidification and cooling of the peritoneal cavity can reduce adhesions. Am J Obstet Gynecol. 2011;205:392–397. doi: 10.1016/j.ajog.2011.06.091. [DOI] [PubMed] [Google Scholar]
- 13.Binda MM, Molinas CR, Hansen P, Koninckx PR. Effect of desiccation and temperature during laparoscopy on adhesion formation in mice. Fertil Steril. 2006;86:166–175. doi: 10.1016/j.fertnstert.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 14.Molinas CR, Tjwa M, Vanacker B, Binda MM, Elkelani O, Koninckx PR. Role of CO2 pneumoperitoneum-induced acidosis in CO2 pneumoperitoneum-enhanced adhesion formation in mice. Fertil Steril. 2004;81:708–711. doi: 10.1016/j.fertnstert.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Pouly JL, Seak-San S. Adhesions: laparoscopy versus laparotomy. Peritoneal surgery. New York: Springer-Verlag; 2000. [Google Scholar]
- 16.Armitage P, Berry G (1987) Factorial designs. Statistical methods in medical research, 2nd edn, Oxford: Blackwell Scientific Publications; 227–39
- 17.Inc SI (1988). In: Inc SI (ed) Sas/stat users guide. Cary NC : SAS Institute Inc,
- 18.Corona R, Binda MM, Mailova K, Verguts J, Koninckx PR. Addition of nitrous oxide to the carbon dioxide pneumoperitoneum strongly decreases adhesion formation and the dose-dependent adhesiogenic effect of blood in a laparoscopic mouse model. Fertil Steril. 2013;100:1777–1783. doi: 10.1016/j.fertnstert.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 19.Ryan GB, Grobety J, Majno G. Postoperative peritoneal adhesions. A study of the mechanisms. Am J Pathol. 1971;65:117–148. [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan GB, Grobety J, Majno G. Mesothelial injury and recovery. Am J Pathol. 1973;71:93–112. [PMC free article] [PubMed] [Google Scholar]
- 21.Cwalinski J, Breborowicz A, Polubinska A. The impact of 0.9% NaCl on mesothelial cells after intraperitoneal lavage during surgical procedures. Adv Clin Exp Med. 2016;25:1193–1198. doi: 10.17219/acem/44381. [DOI] [PubMed] [Google Scholar]
- 22.Cwalinski J, Staniszewski R, Baum E, Jasinski T, Mackowiak B, Breborowicz A. Normal saline may promote formation of peritoneal adhesions. Int J Clin Exp Med. 2015;8:8828–8834. [PMC free article] [PubMed] [Google Scholar]
- 23.Polubinska A, Breborowicz A, Staniszewski R, Oreopoulos DG. Normal saline induces oxidative stress in peritoneal mesothelial cells. J Pediatr Surg. 2008;43:1821–1826. doi: 10.1016/j.jpedsurg.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Breborowicz A, Polubinska A, Breborowicz M, Simon M, Wanic-Kossowska M, Oreopoulos DG. Peritoneal effects of intravenous iron sucrose administration in rats. Transl Res. 2007;149:304–309. doi: 10.1016/j.trsl.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Breborowicz A, Oreopoulos DG. Is normal saline harmful to the peritoneum? Perit Dial Int. 2005;25(Suppl 4):S67–S70. [PubMed] [Google Scholar]
- 26.Lin HF, Wu CY, Wu MC, Chou TH, Lin GS, Yen ZS, Chen SC (2014) Hypothermia decreases postoperative intra-abdominal adhesion formation. Am J Surg. 2014;208:419–24 [DOI] [PubMed]
- 27.Fang CC, Chou TH, Lin GS, Yen ZS, Lee CC, Chen SC. Peritoneal infusion with cold saline decreased postoperative intra-abdominal adhesion formation. World J Surg. 2010;34:721–727. doi: 10.1007/s00268-009-0378-7. [DOI] [PubMed] [Google Scholar]
- 28.de Vries A, Marvik R, Kuhry E. To perform operative procedures in an optimized local atmosphere: can it reduce post-operative adhesion formation? Int J Surg. 2013;11:1118–1122. doi: 10.1016/j.ijsu.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Frey JM, Janson M, Svanfeldt M, Svenarud PK, van der Linden JA. Local insufflation of warm humidified CO2 increases open wound and core temperature during open colon surgery: a randomized clinical trial. Anesth Analg. 2012;115:1204–1211. doi: 10.1213/ANE.0b013e31826ac49f. [DOI] [PubMed] [Google Scholar]
- 30.van der Linden J, Persson M. CO2 field flooding may also reduce oxidative stress in open surgery. Anesth Analg. 2009;109:683–684. doi: 10.1213/ane.0b013e3181a90846. [DOI] [PubMed] [Google Scholar]
- 31.Anesthetic Gases: Guidelines for Workplace Exposures. (2000) United States Department of Labour: Occupational Safety and Health Administration. https://www.osha.gov/dts/osta/anestheticgases/. Accessed 2017 2000


