Significance
Future production of renewable transportation fuels such as ethanol must rely on abundant nonfood plant sources also known as lignocellulosic biomass. However, a major historical barrier to low-cost production of ethanol from biomass is the low ethanol yields and titers that result from fermentation of biomass solids at high solids when compared with simple sugar fermentations. Here, we show that combining a cosolvent-enhanced lignocellulosic fractionation (CELF) pretreatment process with subsequent simultaneous saccharification and fermentation (SSF) can achieve similar high ethanol yields and titers that match that of separate pure glucose fermentations. We demonstrate a strategy whereby direct fermentation of biomass to ethanol is now limited by the microbe rather than by the process.
Keywords: biomass, pretreatment, fermentation, ethanol, yield
Abstract
Simultaneous saccharification and fermentation (SSF) of solid biomass can reduce the complexity and improve the economics of lignocellulosic ethanol production by consolidating process steps and reducing end-product inhibition of enzymes compared with separate hydrolysis and fermentation (SHF). However, a long-standing limitation of SSF has been too low ethanol yields at the high-solids loading of biomass needed during fermentation to realize sufficiently high ethanol titers favorable for more economical ethanol recovery. Here, we illustrate how competing factors that limit ethanol yields during high-solids fermentations are overcome by integrating newly developed cosolvent-enhanced lignocellulosic fractionation (CELF) pretreatment with SSF. First, fed-batch glucose fermentations by Saccharomyces cerevisiae D5A revealed that this strain, which has been favored for SSF, can produce ethanol at titers of up to 86 g⋅L−1. Then, optimizing SSF of CELF-pretreated corn stover achieved unprecedented ethanol titers of 79.2, 81.3, and 85.6 g⋅L−1 in batch shake flask, corresponding to ethanol yields of 90.5%, 86.1%, and 80.8% at solids loadings of 20.0 wt %, 21.5 wt %, and 23.0 wt %, respectively. Ethanol yields remained at over 90% despite reducing enzyme loading to only 10 mg protein⋅g glucan−1 [∼6.5 filter paper units (FPU)], revealing that the enduring factors limiting further ethanol production were reduced cell viability and glucose uptake by D5A and not loss of enzyme activity or mixing issues, thereby demonstrating an SSF-based process that was limited by a strain’s metabolic capabilities and tolerance to ethanol.
Abundant lignocellulosic biomass in the form of agricultural and woody residues or energy crops presents a near-term solution to supporting energy demands that can alleviate global climate change caused by the consumption of fossil resources (1–3). The biological conversion of lignocellulosic biomass to fuel ethanol typically centers around three major interdependent steps (4): (i) pretreatment by physical (5, 6) or thermochemical (7) methods to enhance the accessibility and reactivity of biomass polysaccharides to hydrolytic breakdown (8), (ii) enzymatic hydrolysis of (hemi) cellulose-rich solids produced from pretreatment to fermentable sugars (9, 10), and (iii) microbial fermentation of the sugars to produce ethanol (11). The latter two steps can be consolidated into a single operation known as simultaneous saccharification and fermentation (SSF) to potentially reduce capital and operating costs through integrating processes, reducing end-product inhibition of enzymes, and reducing enzyme demand (12, 13). Separate hydrolysis and fermentation (SHF) is a competing strategy that decouples enzymatic hydrolysis and fermentation steps as separate processes that relies on newer enzyme formulations to produce high-gravity sugar solutions (14). However, high enzyme costs and low yields still challenge overall competitiveness of ethanol production from lignocellulosic biomass, factors that an improved SSF strategy could overcome (13).
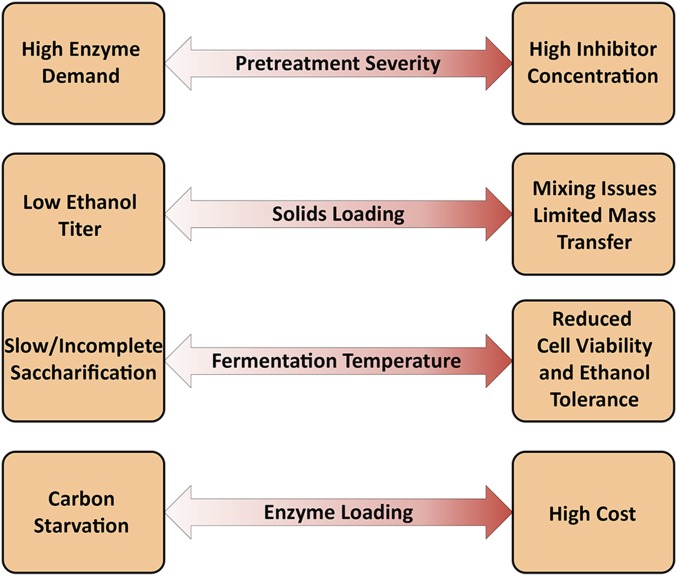
As outlined in Fig. 1, there are a number of limiting factors that prevent high-yield ethanol production from biomass using SSF that depend on processing choices such as pretreatment severity, solids loading, culture conditions, and enzyme loading. The reaction conditions affecting pretreatment severity must be carefully optimized: too low severity reduces pretreatment effectiveness necessitating higher enzyme demand, whereas too high severity results in sugar loss and degradation to unwanted microbial inhibitors such as furfural. For SSF, culture temperatures must be compromised between high temperatures at about 50 °C that are optimal for fungal enzymes and lower temperatures of about 30 °C that are favored by many fermentative microorganisms. Furthermore, SSF must be capable of operating at high solids to achieve sufficiently high ethanol titers, but the high lignin content of biomass reduces the effective glucan loadings that can be mixed effectively and exacerbates mass transfer limitations. Finally, maintaining high enzymatic activity is crucial for preventing carbon starvation, but too high enzyme loadings lead to prohibitively high processing costs (15). To address these limiting factors, development of effective pretreatment methods is needed (8). Even then, saccharification and fermentation of substrates prepared by traditional hydrothermal or dilute acid pretreatments tend to be slow, particularly at reduced enzyme loadings that are more economical. However, if ethanol yield and titer from SSF can be improved to be comparable to those from starch/sugarcane-derived or pure sugar fermentations while minimizing enzyme demands, then significant cost savings can be achieved over current SHF strategies to advance cellulosic ethanol production (16).
Fig. 1.
Competing factors to different process considerations that limit economic ethanol production from pretreated biomass using SSF. Double arrows represent the different process parameters that can be reduced (Left) or increased (Right), leading to different limitations.
When applying SSF to solids prepared by current conventional pretreatment technologies such as dilute acid or steam explosion, ethanol yields suffered as solid loadings were increased past 15 wt %, and ethanol titers were unable to significantly exceed 50 g⋅L−1, a concentration that was determined as the lower threshold to supporting economic recovery of ethanol from water (17–19). For example, Mohagheghi et al. (19) showed that SSF of dilute acid-pretreated wheat straw at 24.2 wt %, 28.2 wt %, and 32.3 wt % solids loadings resulted in ethanol titers of 57, 40, and 34 g⋅L−1 and corresponding yields of only 54.5%, 33.2%, and 23.9%, respectively. The reduced ethanol yields from SSF suffered as enzyme loadings were reduced or solids loadings were increased due to cellulolytic enzymes losing activity over extended hydrolysis times, high-solids loadings limiting mass transfer and mixing, and/or inhibition of the enzymes by sugars, oligomers, and/or ethanol (18). Thus, a strategy to sustain high ethanol yields during high-solids SSF is needed.
Recently, we developed a highly effective pretreatment method called cosolvent-enhanced lignocellulosic fractionation (CELF) that augments dilute acid pretreatment with tetrahydrofuran (THF) as a water cosolvent. THF promotes delignification of biomass and hydrolysis of recalcitrant cellulose in water to produce a highly digestible glucan-rich solid. We applied CELF pretreatment on corn stover to recover over 95% of total C5 and C6 sugars after enzymatic hydrolysis at enzyme loadings as low as 2 mg protein⋅g glucan−1 (20). Surprisingly, enzyme activity was sustained over long culture times, highlighting the enhanced stability of cellulolytic enzymes when applied to CELF-pretreated material that enables comparable sugar release at 90% lower enzyme doses than from dilute acid or hydrothermal pretreatments. THF is also a low boiling solvent that can be easily removed after pretreatment resulting in two additional benefits: (i) concentration of hemicellulose sugars in the liquid stream, and (ii) precipitation of extracted lignin as a solid powder (20). It was then shown that SSF of CELF-pretreated corn stover could achieve ethanol titers exceeding 50 g⋅L−1, if solids loading could exceed 15.5 wt % while maintaining low enzyme loadings (21).
In our prior studies, maintaining moisture in the solids after corn stover pretreatment was beneficial to improved enzymatic digestibility; however, too high moisture limited effective solids loadings during SSF and thereby constrained ethanol titers (21). To overcome that limitation, in this study, we pretreated corn stover with CELF and then hydraulically pressed the wet pretreated solids to reduce its moisture content without air/oven drying that would negatively impact its digestibility (22–25). As a result, a denser, more glucan-rich material was produced from corn stover that is suitable for high-solids SSF. In this study, we (i) evaluated the impact of fermentation stresses on ethanol production from Saccharomyces cerevisiae D5A in glucose fermentations; (ii) elucidated process limitations by mapping cell viability, glucose concentration, and ethanol yields at different solids loadings; (iii) determined the minimum enzyme loadings needed to maximize ethanol yield; and (iv) explored different culture strategies to improve performance at low enzyme loadings.
Results and Discussion
Factors Limiting Ethanol Titers in Glucose Fermentations.
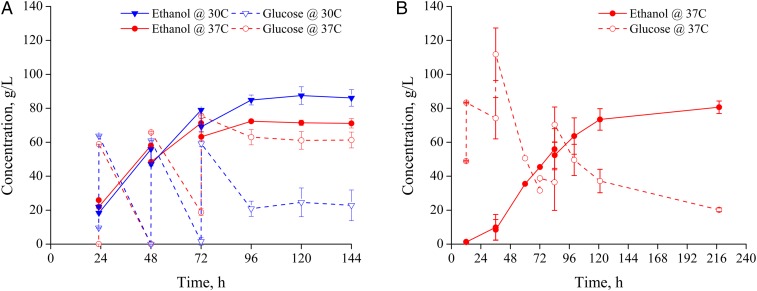
S. cerevisiae D5A was selected based on prior reports of its robustness for SSF compared with other native yeast strain (26). The most salient stress factors during SSF of pretreated lignocellulosic biomass are high-temperature culture, carbon starvation from incomplete sugar hydrolysis, and toxicity from high ethanol concentrations (27–30). Other stresses such as inhibitors released from pretreatment can be reduced through washing of the pretreated solids (20). Maintaining strict anaerobic conditions during ethanol production reduces oxidative stresses, and the immediate consumption of glucose released from cellulose in SSF can alleviate some osmotic stresses (31). While high ethanol concentration is a culture stress factor that cannot be avoided in maximizing SSF yields and titers, the effects of individual and additive stresses due to high temperature and carbon starvation on maximum achievable ethanol titers can be characterized. Carbon starvation can occur in SSF due to saccharification rates being slower than glucose consumption. To simulate the effects of carbon starvation in SSF in a simpler system, we first performed fed-batch glucose fermentations using S. cerevisiae D5A at two temperatures and two glucose feeding strategies to evaluate the impact of culture temperature and carbon availability on ethanol concentrations, as shown in Fig. 2.
Fig. 2.
Ethanol and corresponding glucose concentrations resulting from fed-batch glucose fermentations at (A) 30 °C and 37 °C with feeding strategy to induce carbon starvation, and at (B) 37 °C with feeding strategy to ensure adequate carbon supply. Carbon starvation (A) resulted from feeding glucose every 24 h to allow sugar to be depleted and then replenished, whereas more frequent glucose additions (B) avoided carbon starvation by maintaining glucose concentrations at >30 g⋅L−1. Error bars represent upper and lower range of duplicate experiments.
Generally, cell growth and ethanol production is optimal at a culture temperature of about 30 °C (32), with the maximum temperature tolerance ranging between 37.5 and 39.8 °C (27). However, as cellulase enzymes are most active at higher temperatures of about 50 °C (33), 37 °C is often employed for SSF as a compromise to promote enzyme activity while also improving yeast viability (27, 33). Thus, with sugar cultures, we can observe temperature effects and ethanol toxicity on glucose consumption. In Fig. 2A, ethanol and glucose concentrations are tracked during glucose fermentations at 30 °C and 37 °C that employ an interval feeding strategy (outlined in Methods) allowing glucose to be nearly completely depleted, thus initiating carbon starvation, before feeding of additional glucose. Fig. 2B shows ethanol and glucose concentrations for a 37 °C culture while glucose was fed at shorter intervals to maintain adequate carbon supply throughout the culture.
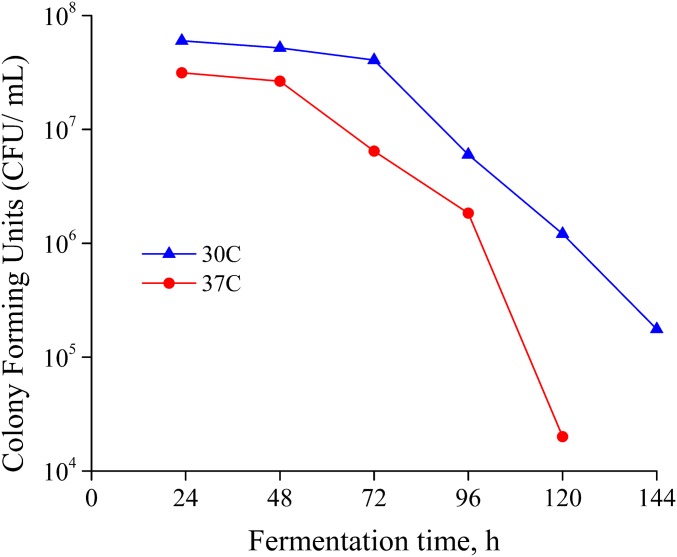
As shown in Fig. 2A, the highest ethanol concentrations in this fed-batch experiment dropped from 87 g⋅L−1 at 30 °C to 73 g⋅L−1 at the typical for SSF temperature of 37 °C, demonstrating reduced tolerance to ethanol at higher temperatures during carbon-limited conditions. Carbon starvation was achieved at 24 and 48 h for both temperatures, but by 72 h, the 37 °C culture failed to reach carbon starvation compared with the 30 °C culture. As a result, the final glucose concentrations remaining after 37 °C culture were about 40 g⋅L−1 higher, indicating a decline in glucose consumption resulting in glucose buildup as increased ethanol concentrations led to increased cell toxicity. Tracking of specific colony-forming units (colony-forming units per milliliter) over time (Fig. S1) supported this hypothesis in that colony-forming units were consistently severalfold lower for 37 °C fed-batch glucose fermentations compared with corresponding 30 °C fermentations after 72 h. High culture temperatures and high ethanol concentrations both have negative effects on yeast cell viability, and their combination results in greater stress than either one alone. More specifically, both stresses increase membrane permeability, which adversely affects nutrient uptake, maintenance of the potassium balance, and the regulation of intracellular pH (33).
Fig. S1.
The effects of fermentation temperature on the D5A yeast cell viability as measured by colony-forming units for pure glucose fermentations.
Although raising the temperature from 30 to 37 °C reduced maximum ethanol concentrations to below 80 g⋅L−1 in carbon-limited conditions; however, when the available glucose concentration was maintained above 30 g⋅L−1, as shown in Fig. 2B, the maximum ethanol titer at 37 °C was restored to over 80 g⋅L−1. This outcome indicated the detrimental impact of low sugar availability on ethanol production that is exacerbated at higher culture temperatures and the importance of maintaining cell viability in late culture. Although fed-batch pure glucose fermentations do not entirely mimic the continual gradual release of glucose during SSF of lignocellulosic biomass, these results highlighted that (i) ethanol toxicity in a carbon-limited situation played a larger role in reducing cell viability, and (ii) maintaining an adequate carbon supply can improve cell viability at higher culture temperatures and potentially improve ethanol tolerance. Thus, successfully achieving higher ethanol yields in SSF would require balancing ethanol concentrations with glucose consumption to maintain cell viability and prevent carbon starvation.
Factors Limiting Ethanol Yields in High-Solids SSF.
It was previously shown that integrating CELF pretreatment with SSF can overcome the barrier to achieving high ethanol yields from biomass at titers greater than the 50 g⋅L−1 threshold needed for effective downstream ethanol recovery from water (21). However, further cost savings can be achieved if even higher ethanol titers can be realized at higher solids loadings in order for SSF strategy to be beneficial over SHF. While the high moisture content of filtered solids produced by CELF pretreatment of corn stover previously limited SSF solids loadings to a maximum of 15.5 wt %, we hydraulically pressed the filtered solids in this study to increase the maximum potential solids loadings up to 23–26 wt %, corresponding to a maximum potential ethanol titer of up to 100 g⋅L−1. We also determined that, from the fed-batch glucose fermentation results and other prior studies (34), maximum ethanol tolerance was expected to be limited to 73–87 g⋅L−1 for S. cerevisiae D5A.
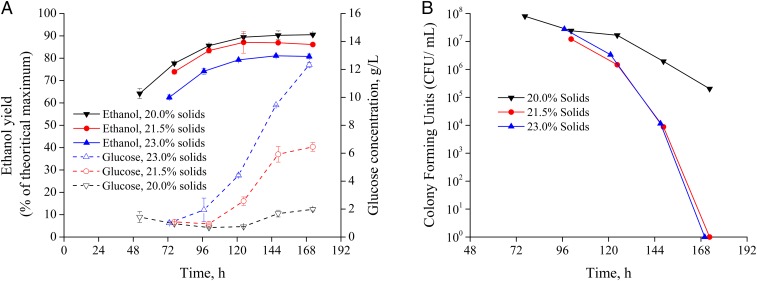
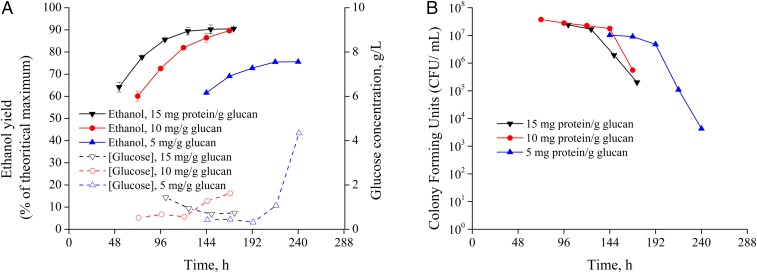
As outlined in Methods, we subjected pressed CELF-pretreated corn stover to SSF and tracked ethanol yield, glucose concentration, and cell viability. As shown in Fig. 3A, we achieved maximum ethanol yields of 90.5%, 86.1%, and 80.8% that corresponded to ethanol titers of 79.2, 81.3, and 85.6 g⋅L−1 at solids loadings of 20 wt %, 21.5 wt %, and 23 wt %, respectively. At 20 wt % solids loading, accumulation of glucose was at a low 2 g⋅L−1 and cell viability was highest after 168 h (7 d) of SSF culture using only 15 mg protein⋅mg glucan−1 enzyme loadings. However, while ethanol titers surpassed 80 g⋅L−1 at solids loadings of 21.5 and 23 wt %, ethanol yields dropped to 86.1% and 80.8%, and greater glucose accumulation occurred after 72 h to over 6 and 12 g⋅L−1, respectively, followed by complete cell death. As shown in Fig. 3B, the drastic drop in cell viability as measured by specific colony-forming units (colony-forming units per milliliter) over time, along with the decreased glucose consumption, supported the hypothesis that ethanol toxicity was the limiting factor to cell viability and ethanol yields at this enzyme loading. While day 6 colony-forming units at a 20 wt % solids loading to SSF dropped by about one order of magnitude, colony-forming units at solids loadings of 21.5 wt % and 23 wt % dropped by about three orders of magnitude. The decreased viability can be related with loss of membrane integrity as ethanol titers exceeded 80 g⋅L−1 due to hyperpolarization of phospholipid cell membranes and resulting greater fluidity and permeability (35). These results are in agreement with the fed-batch glucose fermentations, in that the highest ethanol yields were achieved at 20 wt % solids, where the glucose release was balanced with glucose consumption and ethanol toxicity to maintain greater cell viability.
Fig. 3.
The effects of solids loadings on (A) ethanol yields, glucose concentrations (in grams per liter), and (B) colony-forming units over time in the batch SSF of CELF-pretreated corn stover at a cellulase loading of 15 mg protein⋅g glucan−1. Error bars represent upper and lower range of duplicate experiments.
Understanding factors that limit ethanol yields at commercially desirable ethanol concentrations from SSF is important to identify opportunities for process improvement. The results reported here show that the highly digestible glucan-rich solids produced by CELF pretreatment can be configured at high-solids loadings to overcome the limitation of incomplete saccharification. As shown in Fig. 3A, the extent of saccharification at the completion of each fermentation was nearly identical at all solids loadings, with the total yields of glucose plus cellobiose plus ethanol being 92.7%, 92.1%, and 92.2% for solids loadings of 20.0 wt %, 21.5 wt %, and 23.0 wt %, respectively. Thus, saccharification clearly continued to release glucose and cellobiose even after ethanol yields plateaued due to cell death for solids loadings of 21.5 wt % and 23.0 wt %. Conversely, in a study by Zhang et al. (18), incomplete saccharification was evident at higher solids loadings of steam-exploded corn stover, with ethanol yields being 76.5%, 68%, and 64.8% at solids loadings of 15 wt %, 20 wt %, and 25 wt %, respectively, with no glucose accumulation. On the other hand, our results show that ethanol toxicity played a larger part in limiting ethanol production, with a titer of about 86 g⋅L−1 proving to be the upper limit. Coupling this outcome with the need to maximize ethanol yields translates into a recommended upper limit of 20 wt % solids loading for the following enzyme dosage studies and for potential commercial applications with this strain.
Impact of Enzyme Loadings on Ethanol Production During High-Solids SSF.
As production of cellulolytic enzymes represents a significant cost for cellulosic ethanol production, it is vital to drive down enzyme loadings and enzyme formulation costs while still realizing high yields for a commercial operation to be economically feasible. Unfortunately, many studies report low ethanol yields at economically attractive enzyme loadings due to incomplete or slow saccharification (17, 18, 36). For instance, Zhang et al. (18) showed that lowering the enzyme dosage from 30 to 15 and further to 7.5 FPU⋅g dry matter (DM)−1 for SSF of steam-exploded corn stover reduced yields from 82.8 to 75.9%, and then 52.1%, respectively. Similarly, Zhao et al. (36) reported that dropping enzyme loadings from 20 to 15 and then 10 FPU⋅g DM−1 for SSF of alkaline-pretreated corn stover reduced yields from 0.35 to 0.325 and then 0.258 g ethanol⋅g substrate−1. To study the impact of reduced enzyme loadings on ethanol yield in our system, SSF was performed on 20 wt % solids loading of CELF-pretreated corn stover at enzyme loadings of 5, 10, and 15 mg protein⋅g glucan−1 with ethanol yields, glucose concentration, and cell viability reported in Fig. 4. As shown in Fig. 4A, a maximum 90% ethanol yield of theoretical was achieved at both enzyme loadings of 15 and 10 mg protein⋅g glucan−1 with minimal accumulation of glucose. However, at 5 mg protein⋅g glucan−1 loadings, ethanol yields dropped to 75.6% with a significant increase in glucose accumulation upon termination and an order of magnitude greater cell die-off. Thus, we find that 10 mg protein⋅g glucan−1 at solids loadings of 20 wt % to be the threshold before significant ethanol yield losses would occur. For comparison, it was shown previously (21) that 15.5 wt % loading of unpressed CELF-pretreated corn stover can achieve over 90% ethanol yield corresponding to a titer of 56.4 g⋅L−1 at 5 mg protein⋅g glucan−1 enzyme loading. The need for higher enzyme loadings to achieve similar yields at 20 wt % pressed solids than at 15.5 wt % filtered solids are likely caused by a combination of ethanol toxicity and carbon starvation, as evidenced by the decline in yeast cell viability in Fig. 4B. As glucose concentrations shot up after ethanol yields plateaued and cell viability dropped, it is apparent that, although the enzymes were still active, the rate of saccharification may have been too slow to maintain cell viability.
Fig. 4.
The impact of different cellulase enzyme loadings of 15, 10, and 5 mg protein⋅g glucan−1 on (A) ethanol yields, glucose concentrations (in grams per liter), and (B) specific colony-forming units (colony-forming units per milliliter) over time in the SSF of CELF-pretreated corn stover at 20 wt % solids loadings. Error bars represent upper and lower range of duplicate experiments.
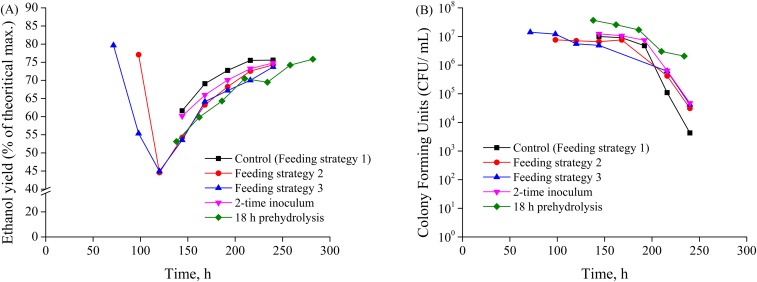
Several strategies to further improve ethanol yields and cell viability were investigated at the enzyme loading of 5 mg protein⋅g glucan−1, including doubling the initial inoculum size from OD of 0.5–1.0, applying a fed-batch feeding strategy to increase initial effective enzyme loadings, and performing an 18-h prehydrolysis step at 50 °C to increase initial sugar availability. While prehydrolysis and fed-batch SSF strategies were intended to increase initial sugar concentrations to prevent a carbon starvation event, doubling the inoculum size was an attempt to enhance initial yeast cell viability. Unfortunately, none of these process strategies resulted in dramatic yield improvements or improved long-term cell viability, as shown in Fig. S2. In summary, while minimizing enzyme loading is an important goal to reducing processing costs, the lower limit to enzyme loadings is governed by late-stage cell viability and glucose consumption. Consequently, improving strain tolerance to fermentation stresses is needed to further improve ethanol yields.
Fig. S2.
The effects of different fermentation strategies on (A) ethanol yields (percentage of theoretical maximum) and (B) cell viability in terms of specific colony-forming units (colony-forming units per milliliter) for SSF of pressed CELF-pretreated solids. The final solids loading was 20% and cellulase loading was 5 mg protein⋅g glucan−1. Feeding strategies 1, 2, and 3 refer to the strategies outlined in Table S1. The 2× inoculum strategy refers to the doubling of the initial inoculum during inoculation. Finally, the 18-h prehydrolysis strategy refers to the added step of prehydrolysis by the addition of enzyme at 50 °C for 18 h before yeast cell inoculation.
Total Mass Balance to Confirm Component Yields.
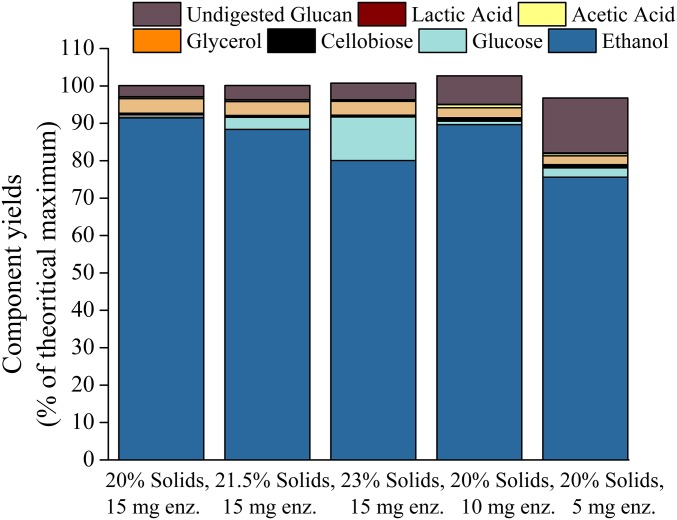
Ethanol yields can be misrepresented by up to 36% when solids loadings are increased to 10–40 wt % if they are calculated using initial liquid volume and final ethanol concentrations in solution (37). Consequently, to minimize this error, ethanol yields reported in this study account for changing liquid volumes, solute concentrations, and liquid density as the fermentations progressed. To confirm this, the fate of the total glucan mass added to each flask was compared with yields of the following glucan-derived fermentation products and by-products: ethanol, glycerol, acetic acid, lactic acid, and undigested glucan in the solid residues after SSF. Fig. 5 shows a summative mass of all components after culture to demonstrate mass closure well within the error range of ±5% and the sum of the component yields based on Eq. S1 equate to ∼100%. As expected, the major fermentation by-product was glycerol (2.4–3.9%), with minimal losses to acetic acid (0.4–0.8%) and lactic acid (0.1–0.2%). It is important to note that losses to by-products were less than in previous studies at lower solids loadings of higher moisture content pretreated corn stover solids, in which the maximum glycerol, acetic acid, and lactic acid yields were 6%, 2.3%, and 1%, respectively (21). However, previous trends of increasing glycerol yields with increasing enzyme loadings were also found in this study, with values of 2.4%, 2.7%, and 3.9% at 5, 10, and 15 mg protein⋅g glucan−1, respectively. Also as shown, increasing glucose component mass after culture termination at higher solids loadings is consistent with yeast die-off from ethanol toxicity as enzymes still remain active at 15 mg protein⋅g glucan−1 enzyme loading in the presence of ethanol.
Fig. 5.
Summative yields of all measured components ethanol, glucose, cellobiose, glucose derived by-products, and undigested glucan from SSF of CELF-pretreated corn stover solids over a range of solids in wt % and enzyme loadings in milligrams protein⋅grams glucan−1.
Conclusions
Here, we demonstrated how CELF pretreatment can overcome limitations to high ethanol yields and titers from biomass. First, using S. cerevisiae D5A, we simulated carbon-limited conditions in glucose fermentations and showed that cell viability at high ethanol concentrations was largely influenced by carbon availability, especially for fermentations at 37 °C culture. We then demonstrated that the highest ethanol titer that can be achieved from this strain was 86 g⋅L−1, representing the upper bound to its ethanol tolerance. Using hydraulically pressed CELF-pretreated corn stover in an SSF configuration, we then achieved ethanol titers of 85.6 g⋅L−1 at 23 wt % solids loading for an enzyme loading of only 15 mg protein⋅g glucan−1, matching the highest ethanol titer from glucose fermentation. Additionally, nearly complete saccharification was achieved, as demonstrated by mass closure. Our findings attribute cell viability as the primary factor limiting high ethanol yields in this process, highlighting the need for strain improvement to address fermentation stresses such as ethanol toxicity as process limitations are overcome. At a reduced enzyme loading of 10 mg protein⋅g glucan−1, we were able to maintain an ethanol yield of 90% at 20 wt % solids corresponding to 80 g⋅L−1 ethanol titer. Further increasing solids loadings or reducing enzyme loadings reduced ethanol yields due to ethanol toxicity and carbon starvation, respectively, that different culture strategies could not overcome. Thus, CELF pretreatment represents a promising approach to improving cellulosic ethanol production by overcoming process limitations, thereby demonstrating an SSF process that was limited by a strain’s metabolic capabilities and tolerance to ethanol.
Materials and Methods
Below is a concise summary of the experimental methods. Detailed descriptions of the materials preparation, pretreatment procedure, culture conditions, sampling procedure, and calculations are found in SI Materials and Methods.
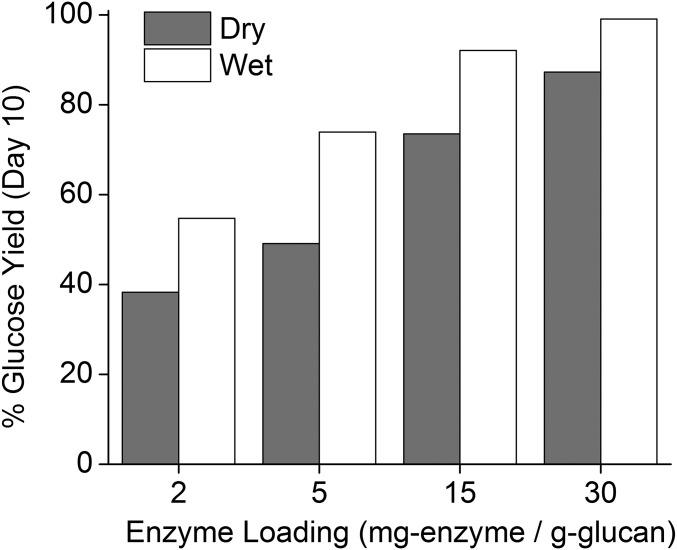
Corn stover was provided by the National Renewable Energy Laboratory (NREL) with composition reported previously (21). DuPont Industrial Biosciences generously provided the cellulolytic enzyme mixture Accellerase 1500 used in this study. The BCA protein concentration and enzyme activity was previously reported as 82 mg⋅mL−1 and 50 filter paper units (FPU)⋅mL−1, respectively (38). S. cerevisiae D5A strain used here was kindly supplied by NREL from which a frozen culture stock was prepared as previously described (20). CELF pretreatment reaction conditions used in this study were previously optimized (20) and conducted at 150 °C for 25 min using 7.5 wt % solids loading, 0.5 wt % (aq.) sulfuric acid loading, and 1:1 (vol) THF:water cosolvent mixture. Reactions were performed in a 1-L Hastelloy Parr autoclave reactor and heated by fluidized sand bath. The pretreated solids were vacuum filtrated, water washed, and then pressed to a moisture content of ∼70 wt % with a hydraulic press (Westward; model 3ZC62G) to final composition of 69.7 ± 1.0% glucan, 4.7 ± 0.2% xylan, and 11.9 ± 0.3% Klason-lignin, and 13.7% remaining components, as measured by NREL protocol (39). Reducing moisture of pretreated solids by air drying must be avoided as it negatively impacts saccharification performance between 2 and 30 mg enzyme⋅g glucan−1 as shown in Fig. S3. Glucose and SSF cultures were performed in duplicate in 125-mL flasks and prepared based on modified NREL protocol (40). Carbon starvation studies were performed in fed-batch configuration with 50 g⋅L−1 initial glucose concentration. To induce carbon starvation, 500 g⋅L−1 glucose solution was fed in 5-mL aliquots at 24-, 48-, and 72-h time points that were predetermined to allow glucose levels to nearly completely deplete before each subsequent feeding. In the studies that prevent carbon starvation, additional glucose feeding was performed at 12, 36, and 84 h to maintain glucose levels sufficiently above 20 g⋅L−1. All flask cultures were performed in a shaker-incubator set at 130 rpm. Table S1 outlines three substrate feeding strategies applied for fed-batch SSF: (i) all at once, (ii) in two equal amounts, or (iii) in three equal amounts to vary initial glucan and enzyme loadings. Colony-forming units were determined with agar plates that were incubated at 37 °C for 48 h. Calculation of product yields are as outlined in previous study (21), and the density of the culture liquid at a specific weight percent of ethanol was determined from published data (41). At the end of each high-solids SSF run, the dry mass and the composition of the remaining solids in each flask were measured to close mass balances and confirm ethanol yields.
Fig. S3.
Comparison of glucose yield by enzymatic hydrolysis of CELF-pretreated corn stover between wet (75% moisture) and dry (air dried at 105 °C to <5% moisture) solids at different loadings (milligram enzyme⋅grams glucan−1) of Accellerase 1500 after 10-d culture. Initial solids loadings into flasks were 15 wt %. Pretreatment conditions are as described in Methods.
Table S1.
Summary of feeding strategies for fed-batch SSF of pressed CELF-pretreated corn stover
| Feeding strategy | Interval | Total elapse culture time, h | Effective glucan loading, g | Effective enzyme loading, mg protein⋅g glucan−1 |
| All at once | 1 | 0–240 | 7 | 5 |
| Two batches | 1 | 0–48 | 3.5 | 10 |
| 2 | 48–240 | 7 | 5 | |
| Three batches | 1 | 0–24 | 2.33 | 15 |
| 2 | 24–72 | 4.66 | 7.5 | |
| 3 | 72–240 | 7 | 5 |
CELF-pretreated corn stover solids were added to flasks all at once, in two batches, or in three batches, resulting in different effective glucan and enzyme loadings during the first 3 d. Glucan and enzyme loadings were the same at the end of all fermentations at 7 g of glucan and 5 mg protein·g glucan−1, respectively.
SI Materials and Methods
Materials Preparation.
Air-dried Kramer corn stover kindly provided by the National Renewable Energy Laboratory (NREL) was milled to pass through a 1-mm particle size interior sieve using a laboratory mill (model 4; Arthur H. Thomas Company). As reported previously, the composition of the raw corn stover was 34.2 ± 0.3% glucan, 23.7 ± 0.2% xylan, 3.8 ± 0.1% arabinan, 17.9 ± 0.9% Klason-lignin, and 20.2% of components that included acetate, extractives, organic acids, inorganics, and ash (20). DuPont Industrial Biosciences generously provided the cellulolytic enzyme mixture Accellerase 1500. The BCA protein concentration and activity were about 82 mg⋅mL−1 and 50 filter paper units (FPU)⋅mL−1, respectively, as reported by Kumar et al. (38). The non–xylose-fermenting S. cerevisiae D5A yeast strain used for SSF was kindly supplied by NREL from which a frozen culture stock was prepared as previously described (20).
Production of Pressed CELF-Pretreated Corn Stover.
CELF pretreatment parameters such as acid concentration, THF cosolvent concentration, reaction temperature, and duration were selected based on conditions previously determined to maximize total sugar yields (20). Accordingly, the reaction solutions contained 0.5 wt % sulfuric acid (Ricca Chemical Company) in water and cosolvent THF (histological grade, from Fisher Scientific) at a 1:1 volume ratio. Before each pretreatment, corn stover (at 7.5 wt % solids loading) was added to the cosolvent mixture and soaked overnight at 4 °C. The contents were then transferred to a 1-L Hastelloy Parr autoclave reactor (236HC Series; Parr Instruments Company) equipped with a double-stacked pitch blade impeller rotating at 200 rpm and heated to 150 °C for 25 min. All reactions were maintained within ±1 °C of the target temperature by heating with a 4-kW fluidized sand bath (model SBL-2D; Techne), as previously described (20), with the reactor temperature directly measured by an in-line K-type thermocouple (Omega Engineering). Immediately after, the solids were separated from the liquor by vacuum filtration through glass fiber filter paper (from Fisher Scientific) and washed with deionized (DI) water until the filtrate reached a neutral pH. The separated and washed solids were then pressed to a moisture content of 68–72% with a hydraulic press (Westward; model 3ZC62G). The composition of the pretreated corn stover solids as determined according to the established NREL procedure (version 8-03-2012) (39) was 69.7 ± 1.0% glucan, 4.7 ± 0.2% xylan, and 11.9 ± 0.3% Klason-lignin, and 13.7% remaining components. Reducing moisture of pretreated solids by air-drying must be avoided as it negatively affects saccharification performance between 2 and 30 mg enzyme⋅g glucan−1 as shown in Fig. S3.
Pure Glucose Fermentations, High-Solids SSF, and Fed-Batch SSF.
Seed inoculum was prepared by thawing and transferring the stock into a 250-mL baffled flask with 50 mL of sterilized yeast–peptone–dextrose (YPD) medium for propagation in a shaker incubator (New Brunswick Scientific Classic C25) set at 130 rpm and 37 °C. The inoculum was then centrifuged and resuspended in sterile DI water twice for washing and prepared for inoculation at an optical density (OD) of 0.5–1. Glucose fermentations and SSF were performed in duplicate in 125-mL flasks with a total working mass of 50 g that included 50 mM sodium citrate buffer (pH 4.9), 10 g⋅L−1 yeast extract (Becton Dickinson), 20 g⋅L−1 peptone (Becton Dickinson), 40 mg⋅L−1 tetracycline (Sigma-Aldrich) as an antibiotic agent, and yeast inoculum at an initial OD of 0.5 or 1.0 as noted.
Procedures for SSF flask preparations followed NREL standard protocols (40) other than slight modifications to account for intentional changes to the fermentation strategy as outlined. Fed-batch glucose fermentation flasks were first loaded with micrometer-filtered reverse osmosis water (MilliQ), while high-solids and fed-batch SSF flasks were initially loaded with pressed solids from CELF pretreatment of corn stover to target final solids loadings of 20.0, 21.5, or 23.0 wt %. The flasks were then autoclaved and cooled in a laminar flow hood (Baker and Baker Ruskin) for aseptic addition of presterilized MilliQ water to replenish any water losses and bring the final working mass of each flask to 50 g, followed by addition of yeast extract, citrate buffer, tetracycline, and D5A inoculum. For fed-batch glucose fermentations, 50 g⋅L−1 glucose was initially added aseptically to the flasks before inoculation. To induce carbon starvation, 500 g⋅L−1 glucose solution was fed in 5-mL aliquots at 24-, 48-, and 72-h time points that were predetermined to allow glucose levels to nearly completely deplete before each subsequent feeding. In the studies that prevent carbon starvation, additional glucose feeding was performed at 12, 36, and 84 h to maintain glucose levels sufficiently above 20 g⋅L−1. For high-solids and fed-batch SSF of CELF solids, Accellerase 1500 cellulase was added at loadings of either 5, 10, or 15 mg protein⋅g glucan−1. All SSF flasks were placed in a shaker-incubator set at 130 rpm and 37 °C. Fed-batch glucose flasks were performed at both 30 and 37 °C.
Table S1 outlines three substrate feeding strategies applied for fed-batch SSF in which pressed and autoclaved solids from CELF pretreatment of corn stover were added to flasks (i) all at once, (ii) in two equal amounts, or (iii) in three equal amounts to provide different effective glucan and enzyme loadings during the first 3 d. Enzymes were added all at once with the initial batch. The final cellulase loading for all flasks was 5 mg protein⋅total g glucan−1. For prehydrolyzed SSF runs, the flasks were first inoculated with just enzymes for prehydrolysis in an incubator set at 150 rpm and 50 °C and prehydrolyzed for 18 h. After allowing the flasks to cool, yeast inoculum was added, and flasks were placed in an incubator set at the aforementioned culture conditions.
Sampling and Analytical Procedures.
Initial fermentation samples of about 400 µL were withdrawn at 24 h or as soon as liquid was apparent in SSF flasks and every 24 h thereafter to track concentrations of product and colony-forming units. For the latter, 100 µL of serial diluted samples were spread on YPD agar plates that were incubated at 37 °C for 48 h followed by colony counts. In tracking products and by-products formation, samples were centrifuged at 21,130 × g for 10 min so the supernatant could be withdrawn for analysis of sugars, ethanol, lactic acid, and acetic acid. All chemical analyses were based on Laboratory Analytical Procedures documented by NREL, in which liquid samples along with appropriate calibration standards were analyzed by HPLC (Waters Alliance 2695 system equipped with a Bio-Rad Aminex HPX-87H column and Waters 2414 RI detector) with a 5 mM sulfuric acid eluent flow rate of 0.6 mL⋅min−1. Chromatograms were integrated by an Empower 2 software package (Waters Company).
Ethanol Yield Calculations at High-Solids Operations.
As the insoluble solid mass dissolved during SSF by hydrolysis and subsequent fermentation to ethanol, the aqueous liquid volume increased and the specific gravity dropped. Consequently, ethanol yields were calculated [S1] according to the following equations, as described previously:
| [S1] |
| [S2] |
in which [C] is the ethanol concentration in grams per liter, MG is the mass of glucan in grams, and VL is the volume of the liquid phase in liters after fermentation, Mw is the mass of liquid initially loaded, MDS is the mass of dissolved solids (including the reacted products of the dissolved solids), ρ is the density of the solution as estimated from the weight percent ethanol in solution, and the 1.111 and 0.51 factors account for the stoichiometry for conversion of glucan to glucose and glucose to ethanol, respectively. The mass of dissolved substrates, MDS, was estimated by multiplying the final volume of liquid times the sum of the concentrations of ethanol, glucose, cellobiose, glycerol, acetic acid, lactic acid, xylitol, and xylose. The density of the culture liquid at a specific weight percent of ethanol was determined from published data (41).
Post-SSF Solids Harvesting and Compositional Analysis for Mass Balance Closures.
At the end of each high-solids SSF run, the dry mass and the composition of the remaining solids in each flask were measured to close mass balances and confirm ethanol yields. The solids were separated from the slurries by centrifugation at 501 × g for 10 min, washed three times with DI water, and dried in an oven for 48 h at 105 °C. After determination of the final dry weight, the solids were ground with a mortar and pestle and mixed well to provide uniform samples for triplicate compositional analysis according to the established NREL procedure (version 8-03-2012) (39).
Acknowledgments
We acknowledge the Center for Environmental Research and Technology (CE-CERT) of the Bourns College of Engineering for providing the facilities and the Ford Motor Company for funding the Chair in Environmental Engineering that facilitates projects such as this one. We also acknowledge support from the Office of Biological and Environmental Research in the Department of Energy Office of Science through the BioEnergy Science Center (BESC) at Oak Ridge National Laboratory (Contract DE-PS02-06ER64304). The award of a fellowship to the lead author by the National Science Foundation (Grant 2013142496) made her participation in this project possible.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704652114/-/DCSupplemental.
References
- 1.Lynd LR, Cushman JH, Nichols RJ, Wyman CE. Fuel ethanol from cellulosic biomass. Science. 1991;251:1318–1323. doi: 10.1126/science.251.4999.1318. [DOI] [PubMed] [Google Scholar]
- 2.Wyman CE. ACS National Meeting. American Chemical Society; Washington, DC: 1990. Biological production of chemicals from renewable feedstocks. [Google Scholar]
- 3.Lynd LR, Wyman CE, Gerngross TU. Biocommodity engineering. Biotechnol Prog. 1999;15:777–793. doi: 10.1021/bp990109e. [DOI] [PubMed] [Google Scholar]
- 4.Wyman CE. What is (and is not) vital to advancing cellulosic ethanol. Trends Biotechnol. 2007;25:153–157. doi: 10.1016/j.tibtech.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Inoue H, Yano S, Endo T, Sakaki T, Sawayama S. Combining hot-compressed water and ball milling pretreatments to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Biotechnol Biofuels. 2008;1:2. doi: 10.1186/1754-6834-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu W, Zhu JY, Gleisner R, Pan XJ. On energy consumption for size-reduction and yields from subsequent enzymatic saccharification of pretreated lodgepole pine. Bioresour Technol. 2010;101:2782–2792. doi: 10.1016/j.biortech.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 7.Kumar R, Wyman CE. Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals. Wiley; Chichester, UK: 2013. Physical and chemical features of pretreated biomass that influence macro-/micro-accessibility and biological processing; pp. 281–310. [Google Scholar]
- 8.Yang B, Wyman CE. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod Biorefin. 2008;2:26–40. [Google Scholar]
- 9.Lloyd TA, Wyman CE. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour Technol. 2005;96:1967–1977. doi: 10.1016/j.biortech.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 10.Wyman CE, et al. Hydrolysis of cellulose and hemicellulose. In: Dumitriu S, editor. Polysaccharides: Structural Diversity and Functional Versatility. 2nd Ed. Marcel Dekker; New York: 2005. pp. 995–1033. [Google Scholar]
- 11.Lu Y, Warner R, Sedlak M, Ho N, Mosier NS. Comparison of glucose/xylose cofermentation of poplar hydrolysates processed by different pretreatment technologies. Biotechnol Prog. 2009;25:349–356. doi: 10.1002/btpr.158. [DOI] [PubMed] [Google Scholar]
- 12.Öhgren K, Bura R, Lesnicki G, Saddler J, Zacchi G. A comparison between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using steam-pretreated corn stover. Process Biochem. 2007;42:834–839. [Google Scholar]
- 13.Wingren A, Galbe M, Zacchi G. Techno-economic evaluation of producing ethanol from softwood: Comparison of SSF and SHF and identification of bottlenecks. Biotechnol Prog. 2003;19:1109–1117. doi: 10.1021/bp0340180. [DOI] [PubMed] [Google Scholar]
- 14.Cannella D, Jørgensen H. Do new cellulolytic enzyme preparations affect the industrial strategies for high solids lignocellulosic ethanol production? Biotechnol Bioeng. 2014;111:59–68. doi: 10.1002/bit.25098. [DOI] [PubMed] [Google Scholar]
- 15.Klein-Marcuschamer D, Oleskowicz-Popiel P, Simmons BA, Blanch HW. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol Bioeng. 2012;109:1083–1087. doi: 10.1002/bit.24370. [DOI] [PubMed] [Google Scholar]
- 16.Humbird D, et al. 2011. Process design and economics for biochemical conversion of lignocellulosic biomass to ethanol. (National Renewable Energy Laboratory, Golden, CO) National Renewable Energy Laboratory Technical Report NREL TP-5100-47764.
- 17.Agrawal R, et al. Pilot scale pretreatment of wheat straw and comparative evaluation of commercial enzyme preparations for biomass saccharification and fermentation. Biochem Eng J. 2015;102:54–61. [Google Scholar]
- 18.Zhang J, et al. Simultaneous saccharification and ethanol fermentation at high corn stover solids loading in a helical stirring bioreactor. Biotechnol Bioeng. 2010;105:718–728. doi: 10.1002/bit.22593. [DOI] [PubMed] [Google Scholar]
- 19.Mohagheghi A, Tucker M, Grohmann K, Wyman C. High solids simultaneous saccharification and fermentation of pretreated wheat straw to ethanol. Appl Biochem Biotechnol. 1992;33:67–81. [Google Scholar]
- 20.Nguyen TY, Cai CM, Kumar R, Wyman CE. Co-solvent pretreatment reduces costly enzyme requirements for high sugar and ethanol yields from lignocellulosic biomass. ChemSusChem. 2015;8:1716–1725. doi: 10.1002/cssc.201403045. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TY, Cai CM, Osman O, Kumar R, Wyman CE. CELF pretreatment of corn stover boosts ethanol titers and yields from high solids SSF with low enzyme loadings. Green Chem. 2016;18:1581–1589. [Google Scholar]
- 22.Luo X, Zhu JY. Effects of drying-induced fiber hornification on enzymatic saccharification of lignocelluloses. Enzyme Microb Technol. 2011;48:92–99. doi: 10.1016/j.enzmictec.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 23.Sathitsuksanoh N, Zhu Z, Wi S, Zhang YH. Cellulose solvent-based biomass pretreatment breaks highly ordered hydrogen bonds in cellulose fibers of switchgrass. Biotechnol Bioeng. 2011;108:521–529. doi: 10.1002/bit.22964. [DOI] [PubMed] [Google Scholar]
- 24.Esteghlalian AR, Bilodeau M, Mansfield SD, Saddler JN. Do enzymatic hydrolyzability and Simons’ stain reflect the changes in the accessibility of lignocellulosic substrates to cellulase enzymes? Biotechnol Prog. 2001;17:1049–1054. doi: 10.1021/bp0101177. [DOI] [PubMed] [Google Scholar]
- 25.Wang QQ, et al. Evaluations of cellulose accessibilities of lignocelluloses by solute exclusion and protein adsorption techniques. Biotechnol Bioeng. 2012;109:381–389. doi: 10.1002/bit.23330. [DOI] [PubMed] [Google Scholar]
- 26.Spindler DD, Wyman CE, Grohmann K, Mohagheghi A. Simultaneous saccharification and fermentation of pretreated wheat straw to ethanol with selected yeast strains and beta-glucosidase supplementation. Appl Biochem Biotechnol. 1989;20-21:529–540. [Google Scholar]
- 27.Walsh RM, Martin PA. Growth of Saccharomyces cerevisiae and Saccharomyces uvarium in a temperature gradient incubator. J Inst Brew. 1977;83:169–172. [Google Scholar]
- 28.Sa-Correia I, Van Uden N. Ethanol-induced death of Saccharomyces cerevisiae at low and intermediate growth temperatures. Biotechnol Bioeng. 1986;28:301–303. [Google Scholar]
- 29.Cheng C, Kao KC. Microbiology. How to survive being hot and inebriated. Science. 2014;346:35–36. doi: 10.1126/science.1260127. [DOI] [PubMed] [Google Scholar]
- 30.D’Amore T, Panchal CJ, Russell I, Stewart GG. A study of ethanol tolerance in yeast. Crit Rev Biotechnol. 1990;9:287–304. doi: 10.3109/07388558909036740. [DOI] [PubMed] [Google Scholar]
- 31.Pratt PL, Bryce JH, Stewart GG. The effects of osmotic pressure and ethanol on yeast viability and morphology. J Inst Brew. 2003;109:218–228. [Google Scholar]
- 32.Bergman LW. Growth and maintenance of yeast. Methods Mol Biol. 2001;177:9–14. doi: 10.1385/1-59259-210-4:009. [DOI] [PubMed] [Google Scholar]
- 33.Banat I, Nigam P, Singh D, Marchant R, McHale A. Review: Ethanol production at elevated temperatures and alcohol concentrations: Part I–Yeasts in general. J Microbiol Biotechnol. 1998;14:809–821. [Google Scholar]
- 34.Lam FH, Ghaderi A, Fink GR, Stephanopoulos G. Biofuels. Engineering alcohol tolerance in yeast. Science. 2014;346:71–75. doi: 10.1126/science.1257859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thammasittirong SN-R, Thirasaktana T, Thammasittirong A, Srisodsuk M. Improvement of ethanol production by ethanol-tolerant Saccharomyces cerevisiae UVNR56. Springerplus. 2013;2:583. doi: 10.1186/2193-1801-2-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Xia L. Simultaneous saccharification and fermentation of alkaline-pretreated corn stover to ethanol using a recombinant yeast strain. Fuel Process Technol. 2009;90:1193–1197. [Google Scholar]
- 37.Kristensen JB, Felby C, Jørgensen H. Determining yields in high solids enzymatic hydrolysis of biomass. Appl Biochem Biotechnol. 2009;156:127–132. doi: 10.1007/s12010-008-8375-0. [DOI] [PubMed] [Google Scholar]
- 38.Kumar R, et al. Carbohydrate derived-pseudo-lignin can retard cellulose biological conversion. Biotechnol Bioeng. 2013;110:737–753. doi: 10.1002/bit.24744. [DOI] [PubMed] [Google Scholar]
- 39.Sluiter JB, et al. Determination of Structural Carbohydrates and Lignin in Biomass. National Renewable Energy Laboratory; Boulder, CO: 2012. [Google Scholar]
- 40.Dowe N, McMillan J. SSF Experimental Protocols—Lignocellulosic Biomass Hydrolysis and Fermentation. National Renewable Energy Laboratory; Golden, CO: 2008. [Google Scholar]
- 41.Osborne NS, McKelvy EC, Bearce HW. Density and Thermal Expansion of Ethyl Alcohol and of Its Mixtures with Water. UNT Digital Library; Washington, DC: 1913. [Google Scholar]