Significance
Diabetes mellitus type 1 is an autoimmune disease that results in irreversible destruction of insulin-producing beta cells. Substantial advances have been made in beta cell replacement therapies during the last decades. However, lack of eligible donor organs and the need for chronic immunosuppression to prevent rejection critically limit widespread application of these strategies. In this manuscript, we present an experimental study using a bioartificial pancreas device for the transplantation of xenogeneic islet without affecting the immune system in nonhuman primates. We could demonstrate stable graft function and adequate glucose-regulated insulin secretion without the need for immunosuppressive medication. This strategy opens up new avenues for more widespread and safe application of various cell-based therapies.
Keywords: diabetes, porcine islets, beta-cell replacement, immune barrier
Abstract
Transplantation of pancreatic islets for treating type 1 diabetes is restricted to patients with critical metabolic lability resulting from the need for immunosuppression and the shortage of donor organs. To overcome these barriers, we developed a strategy to macroencapsulate islets from different sources that allow their survival and function without immunosuppression. Here we report successful and safe transplantation of porcine islets with a bioartificial pancreas device in diabetic primates without any immune suppression. This strategy should lead to pioneering clinical trials with xenotransplantation for treatment of diabetes and, thereby, represents a previously unidentified approach to efficient cell replacement for a broad spectrum of endocrine disorders and other organ dysfunctions.
Type 1 diabetes (T1D) is an autoimmune disease with increasing incidence during the last decades (1). Despite major advances in the treatment of T1D, it is still associated with substantial morbidities and a significant reduction in life expectancy (2). The transplantation of isolated pancreatic islets is an established therapy for a subset of patients with T1D that can effectively restore normoglycemia, prevent hypoglycemia, and stabilize the long-term complications of T1D (3, 4). However, several problems prohibit this therapy from becoming more widespread. First, there is a lack of suitable human donor organs. Second, human islet transplantation currently requires lifelong immunosuppression, and third, the long-term effects of the graft are unsatisfactory because of immunological and inflammatory reactions, which are also associated with a suboptimal microenvironment of the islet transplant (5).
To overcome these limitations, strategies for physical barriers that protect transplanted cells from immune attacks have been investigated (5–7). If successful, this would create a customized microenvironment to facilitate survival and function of grafts and allow a safe use of alternative cell sources such as xenogeneic and stem cell-derived insulin-producing cells (5, 6, 8, 9).
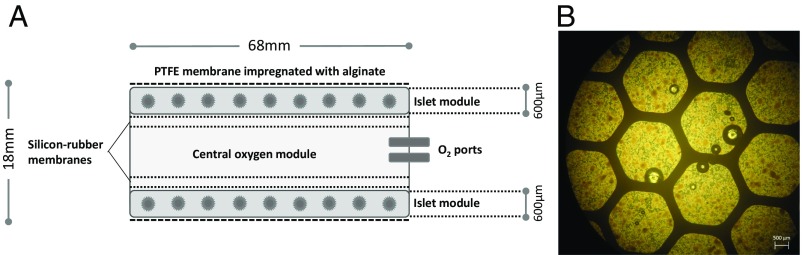
We have previously reported a strategy for macroencapsulation of islets that provides sufficient immune isolation and allows endogenous regulation of insulin secretion (Fig. 1). This BetaO2 bioartificial pancreas device (10) consists of two islet compartments and an oxygen reservoir that can be refilled through port connections that maintain physiological oxygen pressure without direct contact with the bloodstream. The outside of the chamber is covered with porous membranes of hydrophilized polytetrafluoroethylene that are impregnated with high mannuronic acid alginate to prevent immunologic communication between the recipient and the graft tissue (Fig. 1). In previous studies, this concept was successfully tested in models of rat and pig xeno- and allotransplantation (10–13). These initial studies established islet macroencapsulation as a feasible approach to transplantation. We have also performed a pioneer application in a patient with T1D by transplanting encapsulated allogeneic islets isolated from a human donor and followed the patient over a period of 10 mo without immunosuppression. Viable transplanted islets were successfully retrieved after 10 mo, and we found no evidence of an immune response against the transplanted islets (14). The availability of human islets is, however, very limited. Therefore, we progressed to an alternative cell source of porcine islets, which is an intriguing option for various replacement therapies in view of the consistent lack of sufficient human organs for the increasing number of patients on the waiting lists. In particular, given the recent prospects of devices for safe encapsulation as presented here, xenotransplantation offers a potentially unlimited source of material for islet grafts (15). Although stem cell–derived therapies may offer great long-term promise for diabetes therapy, xenotransplants may represent an option with potentially shorter horizons to the clinic (16).
Fig. 1.
Schematic view of the chamber system for islet macroencapsulation and encapsulated porcine islets. (A) The system is built from two islet compartments containing the islets immobilized in alginate and an oxygen module in center, connected to access ports for exogenous oxygen refilling. These ports allow direct injection of oxygen-enriched gas mixture (95% oxygen at 1.4 ATM; 1,011 Torr) into the central cavity. Oxygen is diffusing via the gas permeable membranes into two external chambers and via additional two gas permeable membranes into the cell chambers, where it is dissolved and consumed by the islets. According to mathematical models, refueling of oxygen every 24 h ensures minimal pO2 within the islet module at above critical value of 60 Torr at all islets, (29). The plastic housing of the chamber is covered by porous membranes of hydrophylized polytetrafluoroethylene impregnated with alginate. Drawing is not to scale. (B) Microscopic image (brightfield) of porcine islets immobilized in alginate ready for integration into the chamber system.
Results
Nonhuman Primate Model.
The combination of surgical subtotal pancreatectomy plus streptozotocin resulted in reproducibly C-peptide-deficient recipients. Because of the insulin and glucagon deficiency, the glycemic profile was highly unstable, and complex diabetes care as practice in completely insulin-deficient patients with diabetes was necessary to achieve acceptable control of blood glucose (BG). As the result of an extensive training program, to which every recipient animal was exposed before entering the study, glucose monitoring, exogenous insulin treatment, and the daily oxygen refueling of the device were performed without complications. The animals showed adequate recovery from the interventions, food and water intake was quickly normalized, and body weight development was recorded as equivalent to that of healthy controls (Table 1).
Table 1.
Summary of demographic and metabolic data (n = 3)
| Animal characteristics | Before transplantation | 6 mo after transplantation |
| Age, y | 6.4 ± 1.2 | 6.9 ± 1.2 |
| Weight, kg | 8.5 ± 1.4 | 9.0 ± 1.4 |
| Daily insulin requirement, IU/d | 18.9 ± 1.6 | 10.8 ± 2.4 |
| Fasting BG, mmol/L | 9.9 ± 1.7 | 7.8 ± 11.3 |
| Stimulated C-peptide, ng/mL | — | 16.7 ± 3.0 |
| Fructosamine, µmol/L | 260 ± 31 | 206 ± 8 |
Data are shown as mean ± SD.
Metabolic Follow-Up.
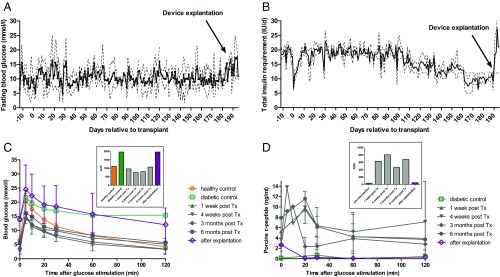
Graft function was evaluated by frequent BG measurements and determination of systemic basal and stimulated C-peptide [i.v. glucose tolerance tests (ivGTT)]. On transplantation, the animals showed a steadily improving glycemic control (Fig. 2 A and B), whereas insulin demand could be decreased by approximately half of pretransplantation needs (pretransplantation: 18.9 ± 1.6 IU/d; day 151–180 posttransplantation: 10.8 ± 2.4 IU/d; Table 1). This marked reduction in insulin use was not at the expense of glycemic control, as indicated by the assessment of serum fructosamine levels, which are reflecting glucose levels during the previous 2–3 wk. At baseline, animals showed mean fructosamine levels of 194 ± 12 µmol/L. The normal range for this species is indicated as 160–230 µmol/L (17). All animals showed an increase in fructosamine levels after diabetes induction (260 ± 31 µmol/L) and a steady decrease after transplantation reaching normal ranges at 12 wk (213 ± 12 µmol/L) and 24 wk (206 ± 8 µmol/L) after transplantation. For the determination of direct graft function, porcine C-peptide was measured during ivGTTs. We observed BG kinetics comparable to healthy control animals and adequate C-peptide secretion on glucose challenge (Fig. 2 C and D). A summary of metabolic characteristics is provided in Table 1.
Fig. 2.
Efficacy study of macroencapsulated porcine islets transplanted into diabetic nonhuman primates (n = 3). (A) The levels of fasting BG showed persistent stable glycemic control over time, despite a stepwise reduction in daily exogenous insulin dose (B). On explantation, the insulin demand immediately increased to prevent hyperglycemia. Values are shown as mean (solid line) ± SD (dashed line) for all three animals. Total insulin was composed of long-acting insulin (glargine; Lantus, Sanofi-Aventis) and short-acting insulin (glulisine; Apidra, Sanofi-Aventis). All animals were challenged by ivGTT before intervention and 1 wk, 4 wk, 3 mo, and 6 mo after transplantation, as well as after explantation of the islet graft. (C) During glucose challenge, there was adequate BG lowering comparable to healthy control, accompanied by porcine-specific C-peptide secretion (D). (Insets) AUC for BG (mmol × min × L−1) and porcine C-peptide (ng × min × mL−1) during the glucose challenge for each time.
Biocompatibility and Immune Assessment.
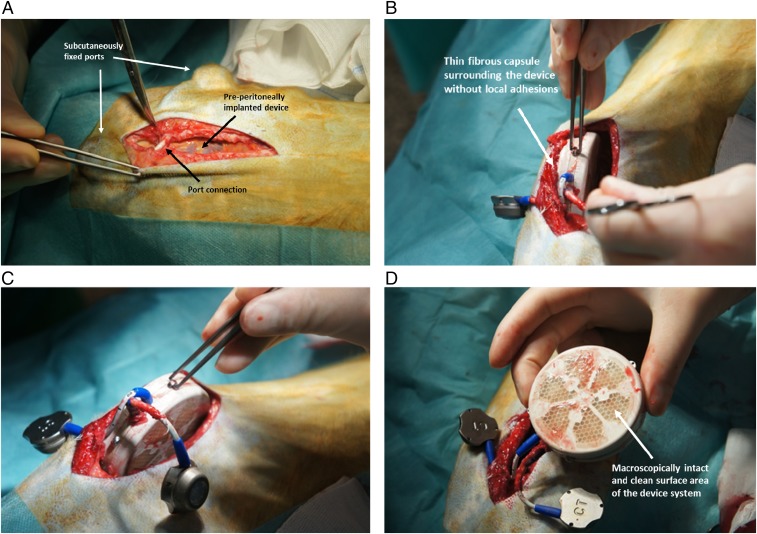
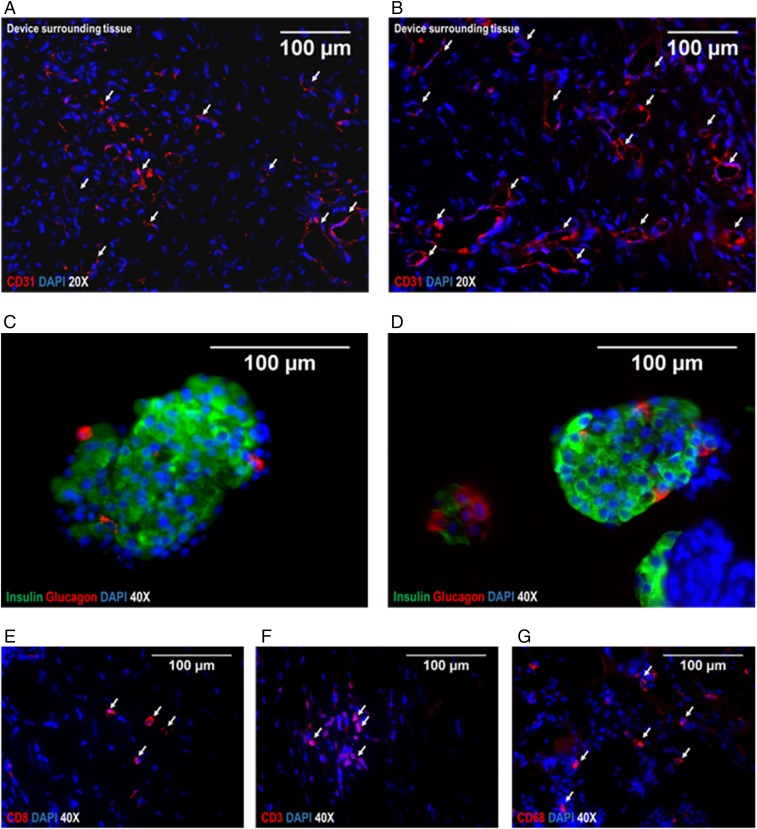
On the retrieval at 6 mo, the device was macroscopically intact, the membrane surface was clean and without any adhesions, and the implantation site appeared as a thin fibrous capsule (Fig. 3) that was strongly vascularized, as shown by immunohistochemical analysis for CD31 (Fig. 4 A and B). The extracted alginate-immobilized islet slabs were analyzed immunohistochemically and revealed a well-preserved architecture with regular distribution of cell types, as typical for adult porcine islets (18). The explanted islet grafts showed no morphological difference in comparison with the islets implanted initially (Fig. 4 C and D). The surrounding tissue showed no signs of inflammation, and immunohistochemical staining for immune markers such as CD8, CD3, and CD68 identified only rare, apparently sporadic positive cells (Fig. 4 E, F, and G).
Fig. 3.
Implantable device containing porcine islets. (A) Intraoperative situs with the device, embedded in a bluntly dissected pocket between the parietal peritoneum and the fascia of the abdominal muscles and s.c. fixed port connections for oxygen refueling. (B and C) Smooth explantation of device and port system without relevant fibrous adhesions. (D) Retrieved bioartificial pancreas device with intact and clean membrane surfaces.
Fig. 4.
Immunohistochemical analysis of islets after explantation and device surrounding tissue. (A and B) Representative images of tissue surrounding the device on the peritoneal site, stained for CD31 to visualize strong vascularization as crucial for exchange of glucose/hormones. (Red) CD31, (blue) DAPI. (C and D) Representative images of porcine islets immobilized in alginate analyzed after explantation of the device at 6 mo. (Green) insulin, (red) Glucagon, (blue) DAPI. The architecture and cell composition of explanted islet grafts were not different to the structure at the time of implantation. Although beta cells are often found as single cells or grouped together to form the core of islets at younger age, the retired breeder animals as used in this study typically show a rather compact structure with beta cells scattered throughout the islet and alpha cells predominantly in the periphery. For analysis of local immune reactions at the transplantation site, the surrounding tissue was stained for CD8+ T-cells (E), CD3+ activated cytotoxic T-cells (F), and CD68+ macrophages (G); (red) respective CD-molecules, (blue) DAPI. Arrows indicate the relevant structures in the respective images (exemplary).
Discussion
This encapsulation strategy was applied by us to a preclinical model of porcine islet xenografts in diabetic nonhuman primates (NHP). We demonstrated a comprehensive safety profile of the xenograft in the BetaO2 bioartificial pancreas device, with no detectable transmission of porcine cytomegalovirus or porcine endogenous retroviruses to the NHP hosts (19) and persistent graft function with regulated insulin secretion without any immunosuppressive therapy. In addition to the indirect clinical benefits such as glycemic stability and prevention of hypoglycemia that have been shown in other approaches (9), the detection of endogenous porcine-specific C-peptide provided a direct proof of graft function. The failure to achieve complete independence to insulin in our system is certainly associated in part with the well-described limitations of the NHP model (20, 21), but is also a result of the intrinsic limitations of any encapsulation system in which nutrient and hormonal passage over the physical barriers are solely dependent on diffusion (22). The lack of insulin independence also prevents checking the safety of the device with regard to delayed insulin response and systemic insulin effect on glucose challenge and consecutive hypoglycemia. However, this potential challenge of any diffusion device has been addressed in previous studies with the BetaO2 device (13). Also, in a single-patient study carried out with encapsulated human islets (14), no episodes of hypoglycemia have been detected even though the patient was temporarily substituted with basal insulin only. Overall, the challenge of insulin secretory kinetics using encapsulation devices is a crucial one, and because of the critical limitations of animal models with respect to the extrapolation of results to the clinical situation, it seems likely a satisfactory answer can only be generated in clinical pilot trials. In summary, this concept of islet macroencapsulation has previously been successfully proven in different transplant models in small and large animals, and now in a xenogeneic NHP model with regard to biocompatibility, safety, efficacy, and sufficient immunoprotection. Ongoing refinements of the device with a particular focus on increasing the transplantable islet mass and optimized, simplified approaches for oxygen supply are targeted on a larger cohort of subjects eligible for this beta cell replacement approach. Furthermore, functional potency of porcine islets can be optimized by pretreatment strategies such as the application of GHRH agonists (23), which we have shown to be highly beneficial for improving graft survival and function (11, 24–26). The coupling of bioactive components to the device membrane surface (e.g., anti-inflammatory, revascularization enhancing substances) might further improve this concept. In conclusion, the described strategy is a major step for a future curative approach to diabetes, supporting further in-depth studies to optimize cell sources and application devices. Importantly, it also presents a previously untried and more efficient strategy of cell replacement for a broad spectrum of endocrine disorders and other organ dysfunctions.
Materials and Methods
All animal studies were approved and in accordance with the strict guidelines established by the University of Dresden Institutional Animal Care and Use Committee and the respective authorities.
Donor Animals and Organ Retrieval.
Porcine islets were isolated from Goettingen minipigs (Ellegard). Before organ retrieval, the animals were housed under standard conditions (19–23 °C; 40–70% relative humidity; 12:12 h day:night cycle) and fed twice daily with special minipig pelleted food (Ellegard). Water was provided ad libitum. For this study, female retired breeder minipigs with an average age of 3–4 y and average pregnancies of three to four were used ensuring for reproducible islet yields and quality (27). The procedure of pancreas organ retrieval has been described in detail previously (27). In brief, after premedication, the anesthesia was performed as total i.v. anesthesia. After median laparotomy, the peritoneal layer covering the pancreas was incised and the organ was mobilized from its adjacent tissue, leaving the major pancreas supplying vasculature intact. Organ perfusion was performed systemically through a perfusion catheter inserted in the suprarenal aorta, using cold Custodiol-HTK solution (Köhler Chemie GmbH).
Porcine Islet Isolation and Encapsulation.
Islet isolation was performed by a modified Ricordi method using collagenase NB8 (4 U/g tissue) and neutral protease (0.6 U/g tissue) purchased from SERVA and DNase (100 mg; Roche Diagnostics) for digestion. Purification was performed by discontinuous gradient centrifugation. Isolated islets were cultured in CMRL medium supplemented with 10% heat-inactivated FBS (Gibco), and l-glutathione 32.5 mM (Sigma-Aldrich). Islet isolation outcome was determined by assessing the number of islet particles and their volume converted into islet equivalents, using standard dithizone staining. The encapsulation procedure was performed 24 h after isolation, according to our established good manufacturing practice (GMP)-like protocols.
Bioartificial Pancreas Device.
The detailed construction of the chamber system used for islet macroencapsulation was previously described (10–13). Briefly, the device is 68 mm in diameter and 18 mm in thickness (Fig. 1), composed of a 600-µm-thick immobilized islets module attached to an oxygen module by a gas-permeable silicon-rubber membrane. The oxygen module is connected to two polyurethane access ports implanted s.c. A gas mixture containing 95% O2 at 1.4 atm and 5% CO2 is refilled daily. The outside of the chamber consists of hydrophilic 0.4 µm porous polytetrafluoroethylene membranes and is impregnated with high mannuronic acid alginate to prevent immunologic communication between the recipient and the graft tissue.
Diabetes Induction in Recipient Animals.
Rhesus macaques (Macaca mulatta) of ∼10 kg body weight were used for these studies that were performed entirely at the German Primate Center, Göttingen, Germany. Before entering the study, the animals were extensively trained for BG monitoring and the necessary oxygen refueling of the device. For surgical diabetes induction, the animals were fasted overnight with free access to water. After premedication, the anesthesia was performed as total i.v. anesthesia. The abdominal region was cleaned and shaved before disinfection and covered with surgical drapes. After median laparotomy, the pancreas was carefully dissected from the surrounding tissue and liberated. Particular precaution was exercised in the duodenal part of the pancreas, preserving the duodenal vascular arcade and thereby preventing ischemic complications. Therefore, a very small part of pancreatic tissue was left in situ. Furthermore, this strategy of subtotal pancreatectomy allowed for preserving the anatomical drainage of the bile duct. The abdomen and the skin were sutured with absorbable material. Postoperatively, insulin therapy was started by multiple daily injection of insulin (long-acting insulin glargine: Lantus, Sanofi-Aventis; short-acting insulin glulisine: Apidra, Sanofi-Aventis) according to clinical practice. Insulin glargine was given twice daily for ensuring persistent basal insulin levels at a total dose of ∼1 IU/kg body weight (BW). Dose was adjusted to reach fasting BG levels between 4 and 10 mmol/L. Insulin glulisine was administered before feeding and for correcting hyperglycemia according to BG readings (2–4 IU/regular meal; 1 IU for lowering BG by 1 mmol/L; target BG level of 8 mmol/L). One week after pancreatectomy, the animals received a single dose of streptozotocin of 80 mg/kg BW to induce complete insulin deficiency (28), which was proven by negative monkey-specific C-peptide measurement during ivGTT.
Transplantation of Encapsulated Animals.
Diabetic rhesus macaques were then transplanted using ∼20,000 islets/kg BW, encapsulated within the BetaO2 bioartificial pancreas device, and followed for 6 mo (n = 3). After premedication and establishment of anesthesia, a median relaparotomy was performed and the device containing the islet graft was implanted within a bluntly dissected pocket between the parietal peritoneum and the fascia of the abdominal muscles. The port connections for refueling of oxygen were placed and fixed s.c. in the lateral thoracic region (Fig. 3). The entire study was performed without immunosuppression.
Metabolic Monitoring.
For metabolic assessment, BG levels were closely monitored and exogenous insulin treatment adjusted accordingly. Basal and short-acting insulin doses were reduced subtly, ensuring continuous acceptable glycemic control and avoiding induction of hypoglycemia. Concomitantly, serum samples were taken before intervention, at the time of transplantation, 1, 4, 12, and 24 wk after transplantation for determination of fructosamine as marker for glycemic control (cobas 8000, fructosamine immunoassay; Roche Diagnostics). ivGTTs were conducted at regular intervals before intervention, after 1 and 4 wk, 3 mo, and 6 mo after transplantation, as well as after explantation of the islet graft. Samples were taken at 5, 10, 20, 40, 60, and 120 min after glucose challenge and measured for glucose and porcine-specific C-peptide (10-1256-01 porcine C-peptide ELISA immunoassay; Mercodia). The ELISA was performed according to manufacturer’s instructions.
Immunohistochemical Analysis.
After retrieval of the device, islet grafts and the surrounding tissue were fixed overnight in 4% paraformaldehyde and embedded in Tissue-Tek O.C.T (Sakura Finetek). Immunohistochemistry was performed on 6-μm sections using primary antibodies Guinea pig polyclonal anti-insulin antibody (1:100, ab7842; abcam), mouse monoclonal anti-glucagon antibody (1:2,000, G2654; Sigma), rabbit anti-CD3 (1:100, C7930; Sigma), mouse monoclonal anti CD4 antibody (4B12; 1:100, MA5-12259; Thermo Fisher Scientific), rabbit monoclonal anti-CD8(SP16) antibody (1:100, SAB5500074; Sigma), and mouse monoclonal anti CD68 Antibody (KP1; 1:100, MA5-13324; Thermo Fisher Scientific). After blocking unspecific antibody binding sites with background sniper (Biocare medical) for 11 min at room temperature, sections were incubated with primary antibodies overnight at 4 °C. After washing in PBS with 0.5% Tween, secondary antibodies goat anti-guinea pig Alexa Flour 488 (1:1,000, A11073; Life Technologies), goat anti-rabbit Alexa Flour 568 (1:1,000, A11011; Life Technologies), and goat anti-mouse Alexa Flour 568 (1:1,000, A11031; Life Technologies) were applied for 1 h at room temperature. DAPI (10236276001; Roche) was applied for cell nucleus-specific staining. Immunofluorescence microscopy was performed on Zeiss Axiovert200M with AxioCamMRc5.
Acknowledgments
We thank the German Primate Center for highly professional support of the nonhuman primate studies. This study was supported by the Deutsche Forschungsgemeinschaft (DFG) Grant BR1179 (to B.L.) and Grants CRC/TRR 127 and 205 (to B.L. and S.R.B.), the German Ministry for Education and Research to the German Centre for Diabetes Research (DZD e.V.), and the DFG-Center for Regenerative Therapies Dresden, Germany.
Footnotes
The authors declare no conflict of interest.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Livingstone SJ, et al. Scottish Diabetes Research Network epidemiology group Scottish Renal Registry Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313:37–44. doi: 10.1001/jama.2014.16425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choudhary P, et al. Evidence-informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care. 2015;38:1016–1029. doi: 10.2337/dc15-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson DM, et al. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation. 2011;91:373–378. doi: 10.1097/TP.0b013e31820437f3. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett ST, et al. Report from IPITA-TTS opinion leaders meeting on the future of β-cell replacement. Transplantation. 2016;100(Suppl 2):S1–S44. doi: 10.1097/TP.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludwig B, Ludwig S. Transplantable bioartificial pancreas devices: Current status and future prospects. Langenbecks Arch Surg. 2015;400:531–540. doi: 10.1007/s00423-015-1314-y. [DOI] [PubMed] [Google Scholar]
- 7.Markmann JF, et al. Executive summary of IPITA-TTS opinion leaders report on the future of β-cell replacement. Transplantation. 2016;100:e25–e31. doi: 10.1097/TP.0000000000001054. [DOI] [PubMed] [Google Scholar]
- 8.Desai T, Shea LD. Advances in islet encapsulation technologies. Nat Rev Drug Discov. 2017;16:338–350. doi: 10.1038/nrd.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto S, Abalovich A, Wechsler C, Wynyard S, Elliott RB. Clinical benefit of islet xenotransplantation for the treatment of type 1 diabetes. EBioMedicine. 2016;12:255–262. doi: 10.1016/j.ebiom.2016.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkai U, et al. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 2013;22:1463–1476. doi: 10.3727/096368912X657341. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig B, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci USA. 2012;109:5022–5027. doi: 10.1073/pnas.1201868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludwig B, et al. A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res. 2010;42:918–922. doi: 10.1055/s-0030-1267916. [DOI] [PubMed] [Google Scholar]
- 13.Neufeld T, et al. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS One. 2013;8:e70150. doi: 10.1371/journal.pone.0070150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig B, et al. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci USA. 2013;110:19054–19058. doi: 10.1073/pnas.1317561110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkel JM. Xenotransplantation makes a comeback. Nat Biotechnol. 2016;34:3–4. doi: 10.1038/nbt0116-3. [DOI] [PubMed] [Google Scholar]
- 16. (January 8, 2016) Xenotransplantation 2.0. Nat Biotechnol, 10.1038/nbt.3466.
- 17.Williams-Fritze MJ, Smith PC, Zelterman D, Scholz JA. Fructosamine reference ranges in rhesus macaques (Macaca mulatta) J Am Assoc Lab Anim Sci. 2011;50:462–465. [PMC free article] [PubMed] [Google Scholar]
- 18.Cohrs CM, et al. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology. 2017;158:1373–1385. doi: 10.1210/en.2016-1184. [DOI] [PubMed] [Google Scholar]
- 19.Morozov VA, et al. Islet cell transplantation from Göttingen minipigs to cynomolgus monkeys: Analysis of virus safety. Xenotransplantation. 2016;23:320–327. doi: 10.1111/xen.12252. [DOI] [PubMed] [Google Scholar]
- 20.Graham ML, Schuurman HJ. Validity of animal models of type 1 diabetes, and strategies to enhance their utility in translational research. Eur J Pharmacol. 2015;759:221–230. doi: 10.1016/j.ejphar.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 21.Graham ML, Bellin MD, Papas KK, Hering BJ, Schuurman HJ. Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: A comparison with allotransplantation. Xenotransplantation. 2011;18:328–342. doi: 10.1111/j.1399-3089.2011.00676.x. [DOI] [PubMed] [Google Scholar]
- 22.Colton CK. Oxygen supply to encapsulated therapeutic cells. Adv Drug Deliv Rev. 2014;67–68:93–110. doi: 10.1016/j.addr.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Cai R, et al. Synthesis of new potent agonistic analogs of growth hormone-releasing hormone (GHRH) and evaluation of their endocrine and cardiac activities. Peptides. 2014;52:104–112. doi: 10.1016/j.peptides.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig B, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107:12623–12628. doi: 10.1073/pnas.1005098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubert U, et al. Transplantation of pancreatic islets to adrenal gland is promoted by agonists of growth-hormone-releasing hormone. Proc Natl Acad Sci USA. 2013;110:2288–2293. doi: 10.1073/pnas.1221505110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Beneficial effects of growth hormone-releasing hormone agonists on rat INS-1 cells and on streptozotocin-induced NOD/SCID mice. Proc Natl Acad Sci USA. 2015;112:13651–13656. doi: 10.1073/pnas.1518540112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steffen A, et al. Production of high-quality islets from goettingen minipigs: Choice of organ preservation solution, donor pool, and optimal cold ischemia time. Xenotransplantation. 2017;24:12284. doi: 10.1111/xen.12284. [DOI] [PubMed] [Google Scholar]
- 28.Heinke S, et al. Diabetes induction by total pancreatectomy in minipigs with simultaneous splenectomy: A feasible approach for advanced diabetes research. Xenotransplantation. 2016;23:405–413. doi: 10.1111/xen.12255. [DOI] [PubMed] [Google Scholar]
- 29.Barkai U, Rotem A, de Vos P. Survival of encapsulated islets: More than a membrane story. World J Transplant. 2016;6:69–90. doi: 10.5500/wjt.v6.i1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]