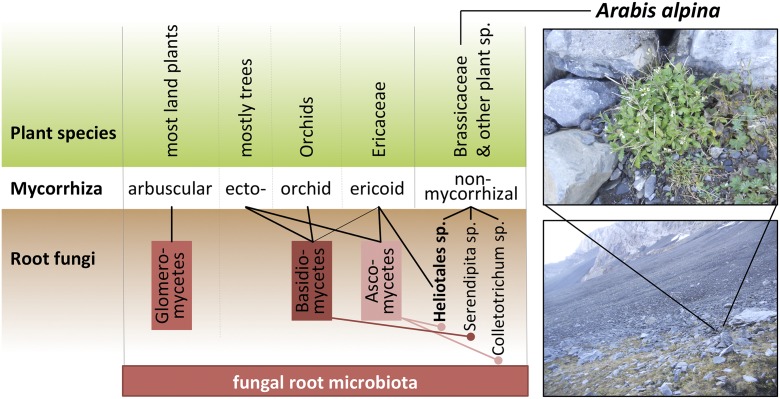
Plants accommodate a specific microbiota on and in their roots that, similar to the microbial communities in human or animal guts, supports the host in nutrient acquisition (1). Beneficial associations with fungi are widespread in the plant kingdom and probably best known are so-called mycorrhizal symbioses (Fig. 1), which are formed between soil fungi and ∼90% of land plants (2). In these partnerships, fungi provide limiting nutrients such as phosphorus (P) in return for photosynthetically fixed carbon from the plant host. Up to 80% of plant P can be derived from the symbionts, underpinning the importance of these associations for plant nutrition. However, ∼10% of all plants do not form mycorrhizal associations, and this prompts the question how nonmycorrhizal plants like the Brassicaceae manage to scavenge sufficient amounts of soil nutrients, especially when growing in nutrient poor environments?
Fig. 1.
Associations between plants and the fungal root microbiota. Different types of mycorrhizal associations include Glomeromycete fungi forming arbuscular mycorrhiza with most vascular plants. Ascomycete and Basidiomycete members form ecto-mycorrhizal symbiosis (mostly with trees), orchid mycorrhiza with orchids, and ericoid mycorrhizas with plants of the Ericaceae family. Moreover, recent studies demonstrate that the Mucoromycotina, a basal fungal lineage close to the Glomeromycetes, forms symbiotic associations with a wide range of plants (not shown) (16, 17). Emerging studies indicate that various nonmycorrhizal plants have the ability to form a functional symbiosis with Ascomycetous and Basidiomycetous soil fungi. Arabis alpina (Top Right) is a nonmycorrhizal Brassicaceae that is native to harsh arctic–alpine environments (site: Lenk i.S., Switzerland) and typically exposed to water limitations, fluctuating temperatures, and extremely low nutrient availability (18). In recent years, Arabis became a model to study adaptation to extreme environments, flowering control, or perennialism, and therefore also for root microbiome research (19).
A Functional Plant–Fungal Association in a Nonmycorrhizal Plant
In PNAS, Almario et al. (3) identify a novel functional association between a fungus belonging to the Helotiales order and the nonmycorrhizal plant Arabis alpina (hereafter: Arabis). First evidence pointing to the potential importance of this group of Ascomycete fungi came from cultivation-independent root microbiota profiling. The comparison of different growth conditions revealed that this taxon is a recurrent and enriched member of the root microbiota of Arabis with a particularly high abundance under low-P conditions. Subsequently, Almario et al. performed a functional analysis with a Helotiales strain, which was isolated from the Arabis root microbiota, and they revealed colonization of the root interior without causing disease symptoms, transport of P to the host, promotion of shoot biomass, and enhanced shoot P content in native low-P soil—all of these functions being hallmarks of mycorrhizal symbiosis. What makes these findings special is the fact that the fungus provided substantial amounts of P to the plant [e.g., Arabis plants inoculated with the Helotiales fungus contained up to 60% more P, a number comparable to what has been found for plants forming associations with arbuscular mycorrhizal or ectomycorrhizal fungi (2)]. Furthermore, it is intriguing that members of the Helotiales order commonly form ericoid mycorrhizal associations (4). Moreover, genome analysis revealed an enlarged set of carbohydrate-active enzymes (involved in plant cell wall degradation), which was consistent with other plant beneficial fungi including a fungus forming ericoid mycorrhizal symbiosis. Taken together, Almario et al. (3) discover a beneficial Helotiales root fungus that forms a functional association with its nonmycorrhizal host Arabis.
Continuum of Root–Fungal Symbioses
Based on the presented functional evidence (e.g., symptomless root colonization, P transfer to the host, fitness benefit under low-P conditions), the identified Arabis–Helotiales association resembles a mycorrhizal symbiosis. Mycorrhiza originates from the Greek terms for fungus and root and refers to a symbiotic association between a fungus and plant roots. Although the Arabis–Helotiales association would fit such a liberal definition, there are a few distinctions compared with mycorrhizal symbioses. Below, we highlight particularities and communalities in a continuum of functionally similar interactions between root fungi and plants existing in nature.
On one end of the continuum, the mycorrhizal symbiosis with the biotrophic Glomeromycetes is monophyletic and has evolved ∼450 million years ago (Mya) to a highly intimate host–fungus association with tree-like intracellular organs for nutrient exchange called arbuscules (5). In contrast, the wide range of ectomycorrhizal symbioses has a multitude of evolutionary origins with primitive associations dating back to 140–180 Mya. Here, the hyphae from Basidiomycetes and Ascomycetes differentiate to characteristic nutrient exchange structures composed of a series of hyphal layers surrounding the plant roots into a mantle (6). The continuum of mycorrhizal symbioses also includes ericoid and orchid mycorrhizal fungi, thought to have evolved more recently, and they form characteristic structures for nutrient exchange with intracellular hyphal coils and hyphal complexes called pelotons, respectively (2). In contrast to these well-defined mycorrhizal symbioses, the Helotiales strain F229 provides P to its host but without apparent cellular structures inside plant roots, and also the abundance of the fungus inside the roots appears, at least at first sight, rather low (3). Thus, this association shares characteristics with root endophyte interactions (7).
Fungal root endophytes, primarily termed in reference to the habitat where they were found, present other important players in the continuum of root–fungal interactions. There is accumulating evidence that nonmycorrhizal plants have the ability to form beneficial associations with endophytic fungi. For instance, the Ascomycete Colletotrichum tofieldiae forms subtle cellular structures (some epidermal and cortical cells become packed with swollen hyphal cells) and transfers P to its nonmycorrhizal host Arabidopsis thaliana (8). C. tofieldiae presents a native fungal root endophyte of Arabidopsis and only promoted growth under low-P conditions. This is in contrast to the Helotiales fungus (transfer of P occurs under low- and high-P conditions) or the Basidiomycete Serendipita indica [formerly Piriformospora indica (9)], which promotes plant growth under both low- and high-P conditions. Although, S. indica transfers P to nonmycorrhizal Arabidopsis thaliana under laboratory conditions, the ecological context of this interaction remains doubtful, as this fungus has not been isolated from this species. In summary, nutrient transfer from the fungus to the host appears to be a common trait in the continuum of symbioses between root fungi and their hosts, including some nonmycorrhizal plants.
Emerging Questions
A key question now is, how widespread and how relevant are these plant–Helotiales associations in nature? Helotiales present a large group of root-associated fungi (10) and comprise well-known fungal partners in ericoid symbiosis (2, 4). Further work is needed to clarify the host specificity and host range of the Helotiales fungus from Arabis and to test whether, for instance, it has the ability to form an mycorrhizal symbiosis with plants of the Ericacea family. There is also evidence for recurrent associations with plants in the Brassicaceae, both with perennial Arabis (3) and the annual Microthlaspi genus (11). Future work needs to investigate whether this association confers fitness benefits to the host under natural conditions (e.g., by manipulating the fungus in the field). Such studies will also clarify the evolutionary stability of this novel plant root–fungus interaction. Finally, the physiological mechanisms involving P uptake by the fungus, hyphal P transport, and P delivery to the plant need further attention.
In the ancient arbuscular or ectomycorrhizal symbioses, there is a highly evolved molecular dialogue—including signaling molecules, plant genes required for accommodation, and fungal effectors—
In PNAS, Almario et al. identify a novel functional association between a fungus belonging to the Helotiales order and the nonmycorrhizal plant Arabis alpina.
between the fungus and the host (6, 12). Consistently, it will be interesting to clarify whether there is a genetic basis and to identify the molecular cross talk in plant and Helotiales interactions. While the Brassicaceae have lost the gene complement needed for arbuscular mycorrhizal symbiosis (13), there is emerging evidence that the plant immune system controls at least partly host colonization by beneficial microbial communities (14). The recognition of generic microbial patterns triggers immune responses that affect the “community size” of the root microbiota. For instance, indolic secondary metabolites, being generic defense compounds, were required for restricting endophytic colonization by C. tofieldiae (8).
Microbiomic Toolkit
The advent of high-throughput sequencing methodologies has made it possible to rapidly characterize the continuum of microbes from different kingdoms colonizing plant roots. The microbiomic toolkit offers additional approaches to how novel microbe–microbe or plant–microbe partnerships in the continuum of microbial symbioses at plant roots can be uncovered.
The combined microbiota profiling of bacteria and fungi of plant root samples permits the exploration of co-occurrence patterns of root microbiota members. Network analysis can disentangle intertaxa associations and possibly reveal niche spaces shared by community members or, perhaps, direct symbiotic relationships. Numerous root microbiota members have traits to solubilize or mineralize P, and co-occurrence analyses could permit the identification of candidate bacterial partners complementing the P transfer activities of mycorrhizal fungi. The combined investigation of root bacteria and fungi presents a logical next step for a more holistic understanding of rhizo-microbial interactions.
A caveat of marker genes studies is that they do not provide information about the functions in a community. While cultivation approaches, as exemplified with Helotiales sp. F229, reveal functional abilities of individual microbiota members, such approaches preclude community-level insights and are often limited by the inability to culture a large fraction of microbes. The drop in sequencing costs and the advances in metagenomics offer new opportunities, such as single-cell genomics or metagenome-based reconstructions of individual genomes, to investigate the metabolic potential encoded in a microbiota. The latter approach has recently succeeded in the reconstruction of more than 7,500 genomes from diverse environments including samples from the gut, freshwater, or soil (15). The advantage of such reconstructed genomes is that both the taxonomy and the functional information of an organism are linked and can be used to infer the metabolic potential of an interconnected community. While DNA-based approaches reveal the metabolic potential, a major frontier is still to investigate the functional relationship, for example, using transcriptomic or cultivation and microbiota manipulation approaches.
Conclusions
The merit of the work by Almario et al. (3) includes that their discovery broadens the functional continuum of root–fungal symbioses. Aside from classical mycorrhizal plants, also the nonmycorrhizal Arabis relies on fungal allies for nutrient acquisition and, perhaps, the association with the Helotiales fungus permits adaptation to the nutrient-limiting conditions. Taken together, this study underpins the importance of the plant root microbiota for host nutrition and adaptation to environment.
Acknowledgments
M.G.A.v.d.H. (Grant 166079) and K.S. (Grant 31003A_165891) are supported by the Swiss National Science Foundation and the Swiss State Secretariat for Education, Research and Innovation (Grant C15.0111).
Footnotes
The authors declare no conflict of interest.
See companion article on page E9403.
References
- 1.Hacquard S, et al. Microbiota and host nutrition across plant and animal kingdoms. Cell Host Microbe. 2015;17:603–616. doi: 10.1016/j.chom.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 2.van der Heijden MGA, Martin FM, Selosse MA, Sanders IR. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015;205:1406–1423. doi: 10.1111/nph.13288. [DOI] [PubMed] [Google Scholar]
- 3.Almario J, et al. Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proc Natl Acad Sci USA. 2017;114:E9403–E9412. doi: 10.1073/pnas.1710455114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker JF, et al. Diverse Helotiales associated with the roots of three species of Arctic Ericaceae provide no evidence for host specificity. New Phytol. 2011;191:515–527. doi: 10.1111/j.1469-8137.2011.03703.x. [DOI] [PubMed] [Google Scholar]
- 5.Brundrett MC. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002;154:275–304. doi: 10.1046/j.1469-8137.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- 6.Martin F, Kohler A, Murat C, Veneault-Fourrey C, Hibbett DS. Unearthing the roots of ectomycorrhizal symbioses. Nat Rev Microbiol. 2016;14:760–773. doi: 10.1038/nrmicro.2016.149. [DOI] [PubMed] [Google Scholar]
- 7.Weiß M, Waller F, Zuccaro A, Selosse M-A. Sebacinales—one thousand and one interactions with land plants. New Phytol. 2016;211:20–40. doi: 10.1111/nph.13977. [DOI] [PubMed] [Google Scholar]
- 8.Hiruma K, et al. Root endophyte Colletotrichum tofieldiae confers plant fitness benefits that are phosphate status dependent. Cell. 2016;165:464–474. doi: 10.1016/j.cell.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav V, et al. A phosphate transporter from the root endophytic fungus Piriformospora indica plays a role in phosphate transport to the host plant. J Biol Chem. 2010;285:26532–26544. doi: 10.1074/jbc.M110.111021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Wang Z, Johnston PR, Takamatsu S, Spatafora JW, Hibbett DS. Toward a phylogenetic classification of the leotiomycetes based on rDNA data. Mycologia. 2006;98:1065–1075. doi: 10.3852/mycologia.98.6.1065. [DOI] [PubMed] [Google Scholar]
- 11.Glynou K, et al. The local environment determines the assembly of root endophytic fungi at a continental scale. Environ Microbiol. 2016;18:2418–2434. doi: 10.1111/1462-2920.13112. [DOI] [PubMed] [Google Scholar]
- 12.Parniske M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat Rev Microbiol. 2008;6:763–775. doi: 10.1038/nrmicro1987. [DOI] [PubMed] [Google Scholar]
- 13.Delaux P-M, et al. Comparative phylogenomics uncovers the impact of symbiotic associations on host genome evolution. PLoS Genet. 2014;10:e1004487. doi: 10.1371/journal.pgen.1004487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hacquard S, Spaepen S, Garrido-Oter R, Schulze-Lefert P. Interplay between innate immunity and the plant microbiota. Annu Rev Phytopathol. 2017;55:565–589. doi: 10.1146/annurev-phyto-080516-035623. [DOI] [PubMed] [Google Scholar]
- 15.Parks DH, et al. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat Microbiol. September 11, 2017 doi: 10.1038/s41564-017-0012-7. [DOI] [PubMed] [Google Scholar]
- 16.Field KJ, et al. First evidence of mutualism between ancient plant lineages (Haplomitriopsida liverworts) and Mucoromycotina fungi and its response to simulated Palaeozoic changes in atmospheric CO2. New Phytol. 2015;205:743–756. doi: 10.1111/nph.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orchard S, et al. Fine endophytes (Glomus tenue) are related to Mucoromycotina, not Glomeromycota. New Phytol. 2017;213:481–486. doi: 10.1111/nph.14268. [DOI] [PubMed] [Google Scholar]
- 18.Koch MA, et al. Three times out of Asia Minor: The phylogeography of Arabis alpina L. (Brassicaceae) Mol Ecol. 2006;15:825–839. doi: 10.1111/j.1365-294X.2005.02848.x. [DOI] [PubMed] [Google Scholar]
- 19.Dombrowski N, et al. Root microbiota dynamics of perennial Arabis alpina are dependent on soil residence time but independent of flowering time. ISME J. 2017;11:43–55. doi: 10.1038/ismej.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]