Abstract
Purpose
More than two decades ago, an international working group established the International Neuroblastoma Response Criteria (INRC) to assess treatment response in children with neuroblastoma. However, this system requires modification to incorporate modern imaging techniques and new methods for quantifying bone marrow disease that were not previously widely available. The National Cancer Institute sponsored a clinical trials planning meeting in 2012 to update and refine response criteria for patients with neuroblastoma.
Methods
Multidisciplinary investigators from 13 countries reviewed data from published trials performed through cooperative groups, consortia, and single institutions. Data from both prospective and retrospective trials were used to refine the INRC. Monthly international conference calls were held from 2011 to 2015, and consensus was reached through review by working group leadership and the National Cancer Institute Clinical Trials Planning Meeting leadership council.
Results
Overall response in the revised INRC will integrate tumor response in the primary tumor, soft tissue and bone metastases, and bone marrow. Primary and metastatic soft tissue sites will be assessed using Response Evaluation Criteria in Solid Tumors (RECIST) and iodine-123 (123I) –metaiodobenzylguanidine (MIBG) scans or [18F]fluorodeoxyglucose–positron emission tomography scans if the tumor is MIBG nonavid. 123I-MIBG scans, or [18F]fluorodeoxyglucose–positron emission tomography scans for MIBG-nonavid disease, replace technetium-99m diphosphonate bone scintigraphy for osteomedullary metastasis assessment. Bone marrow will be assessed by histology or immunohistochemistry and cytology or immunocytology. Bone marrow with ≤ 5% tumor involvement will be classified as minimal disease. Urinary catecholamine levels will not be included in response assessment. Overall response will be defined as complete response, partial response, minor response, stable disease, or progressive disease.
Conclusion
These revised criteria will provide a uniform assessment of disease response, improve the interpretability of clinical trial results, and facilitate collaborative trial designs.
INTRODUCTION
Neuroblastoma, a cancer of the sympathetic nervous system, is responsible for 12% of deaths associated with cancer in children younger than 15 years of age. It is a heterogeneous disease, with nearly 50% of patients having a high-risk phenotype characterized by widespread disease dissemination and poor long-term survival. In contrast, patients diagnosed with low- or intermediate-risk neuroblastoma have excellent long-term survival.1
Collaborative clinical trials have led to improved outcomes for patients with high-risk neuroblastoma and decreased therapy-related toxicity in patients with non–high-risk disease.2-11 Unfortunately, a lack of consensus regarding the definition of clinically relevant disease response has hampered the development of more effective therapy for high-risk neuroblastoma and impaired our ability to define the optimal management for the majority of patients with low- and intermediate-risk neuroblastoma. Development of more effective therapeutic approaches for all children with neuroblastoma must be a primary goal in prospective clinical trials, in which standardized methods to interpret response are used to efficiently advance therapy for neuroblastoma.
The International Neuroblastoma Response Criteria (INRC) consensus was last updated in 199312 and has significant limitations in accurately defining response at metastatic bone and bone marrow sites, the most common sites of relapse.13 Since 1993, iodine-123 (123I) metaiodobenzylguanidine (MIBG) imaging has become widely available and provides more sensitive and specific imaging of neuroblastoma in soft tissue and bone sites.14 For MIBG-nonavid neuroblastomas, [18F]fluorodeoxyglucose (FDG) –positron emission tomography (PET) is also a useful imaging technique.15-18 Limited guidance for using nuclear medicine modalities for response was included in the INRC published in 1993. In addition, further experience quantifying bone marrow disease using morphology and evolving molecular modalities to accurately quantify minimal marrow disease provide the basis for better assessment of bone marrow response.19 Incorporation of these technical advances and cumulative clinical trial data into an international consensus on response is needed to identify optimally effective treatment strategies and facilitate the development of international collaborative clinical trials.
In 2005, an International Neuroblastoma Risk Group (INRG) task force evaluated the prognostic impact of biologic and clinical data and established criteria for an internationally accepted risk group classification system.20,21 The INRG task force also released consensus statements on molecular and radiographic techniques and assessment of minimal residual disease,22-25 setting the stage for the current revision in neuroblastoma response criteria. Single-institution retrospective analyses have confirmed that MIBG imaging rather than anatomic imaging is more likely to detect recurrent disease.14 The New Approaches to Neuroblastoma Clinical Trials Consortium,26-30 Children’s Oncology Group (COG),31-33 International Society of Pediatric Oncology European Neuroblastoma (SIOPEN),34 and German Pediatric Oncology and Hematology (GPOH)35,36 have also piloted novel response criteria incorporating MIBG scoring in anatomic sectors for bone metastases and developed definitions of bone marrow response using morphology. As a consequence of this previous work, neuroblastoma investigators are now poised to develop uniform response criteria using state-of-the-art imaging and molecular methods.
A National Cancer Institute–appointed executive planning committee, representing neuroblastoma leadership from COG and its international counterparts from SIOPEN, GPOH, and the Japan Children’s Cancer Group, selected a panel of 52 international investigators from 13 countries with oncology, pathology, radiology, nuclear medicine, surgery, biology, and statistical expertise (Appendix Table A1, online only) to develop and implement a revised consensus response criteria for neuroblastoma.
METHODS
Methodology for determining response and definitions of response in pediatric neuroblastoma were reviewed using the previously published INRC and results from neuroblastoma clinical trials published from 2005 to 2015 (Appendix Table A2, online only).2-11,14,23,26-28,31-34,37-39 A database established by the INRG task force21,40 was used to identify prevalence and characteristics of metastatic sites of disease at diagnosis in patients with neuroblastoma.
Response assessment will include anatomic imaging for primary and metastatic soft tissue disease, nuclear medicine imaging using 123I-MIBG or FDG-PET for assessment of soft tissue and bone disease and bilateral bone marrow aspirates and trephine biopsies for assessment of marrow disease. Tissue biopsies may be used as an adjunct to verify the presence of viable neuroblastoma or ganglioneuroblastoma that is evaluable for response. Urine catecholamine levels will not be used to evaluate response because of a lack of standardization in specimen collection and analysis and the influence of diet on results.41,42
Primary and Metastatic Soft Tissue Disease
Soft tissue disease should be evaluated using either computed tomography (CT) or magnetic resonance imaging (MRI) scans to determine if a lesion is considered measurable.22 Measurement of irregularly shaped primary tumors in children with neuroblastoma presents significant challenges for assessment of response. The current INRC includes assessment of tumor volumes using three-dimensional reconstructions from CT and MRI scans.12 Although the availability of three-dimensional reconstructions from anatomic imaging has increased in recent years, the trend in the broader field of oncology has been away from the use of multidimensional measurements and toward assessment of response using change in the single longest dimension. The RECIST guidance, published in 2000 and revised in 2009, relies on measurement of nonnodal target lesions based on the longest single diameter,43-46 whereas discrete lymph nodes are assessed using the short axis as a single dimension.
A multi-institution, retrospective analysis of 229 patients with high-risk neuroblastoma was conducted to identify the preferred method of primary tumor response assessment for use in a revision of the INRC.47 No statistically significant difference in outcome was observed when comparing the use of three-dimensional volumetric measurement versus RECIST single longest dimension measurement. Given the complexity of three-dimensional measurement followed by calculation of resultant volume, primary tumor sites in children should be defined as measurable in accordance with RECIST criteria, using the single longest dimension in any orthogonal plane. The RECIST criteria will also be used for defining measurable soft tissue metastatic lesions and response as defined by changes in longest dimension for non–lymph node tumor lesions and changes in the short-axis diameter of malignant lymph nodes.
123I-MIBG in conjunction with anatomic imaging will define measurable lesions and will be used to assess primary and metastatic soft tissue tumor response in the majority of patients. Neuroblastoma is a tumor derived from the sympathetic nervous system, and neuroblastoma cells typically express the norepinephrine transporter, which mediates active intracellular uptake of radiolabeled MIBG in approximately 90% of patients,18 regardless of stage of disease, risk group, or age at presentation. MIBG is a derivative of guanethidine and a norepinephrine analog, which is highly sensitive and specific for imaging both primary tumor and metastatic neuroblastoma when labeled with radioisotopes of iodine.22,23 MIBG uptake resolves when a tumor is necrotic or involutes and often when maturation occurs (only 20% of ganglioneuromas concentrate MIBG).48 In patients whose tumors do not concentrate MIBG, FDG-PET is an alternative modality for tumor detection, although FDG is less specific than MIBG because of uptake of FDG in inflammatory lesions, as well as normal and cytokine-stimulated bone marrow.15-17,49 Because FDG is less specific for neuroblastoma, a tissue biopsy of at least one of the lesions may be required to confirm that FDG-avid, MIBG-nonavid lesions are histologically confirmed to be neuroblastoma and/or ganglioneuroblastoma. Adding the use of 123I-MIBG or FDG to the RECIST response by CT or MRI will provide a more specific and sensitive definition of response for soft tissue lesions in neuroblastoma.
Both 123I-MIBG and FDG-PET scans must be interpreted carefully in light of physiologic sites of uptake. MIBG will normally concentrate in salivary glands, myocardium, liver, intestines, and brown fat and is excreted via the urinary tract. FDG is concentrated in the brain, myocardium, liver, and brown fat and is excreted via the urinary tract. Questions about uptake in tumor versus physiologic uptake or uptake in soft tissue versus bone are commonly resolved with three-dimensional imaging with combined 123I-MIBG single-photon emission computed tomography (SPECT; or MIBG-SPECT/CT) or FDG-PET/CT.15,24,49 Three-dimensional imaging may also identify lesions not seen with planar imaging. If a three-dimensional imaging modality is available and used at baseline, this same modality must be used for all disease response evaluations to ensure appropriate comparisons. In some cases, however, patients may require a biopsy in addition to nuclear imaging to confirm the presence of viable tumor.
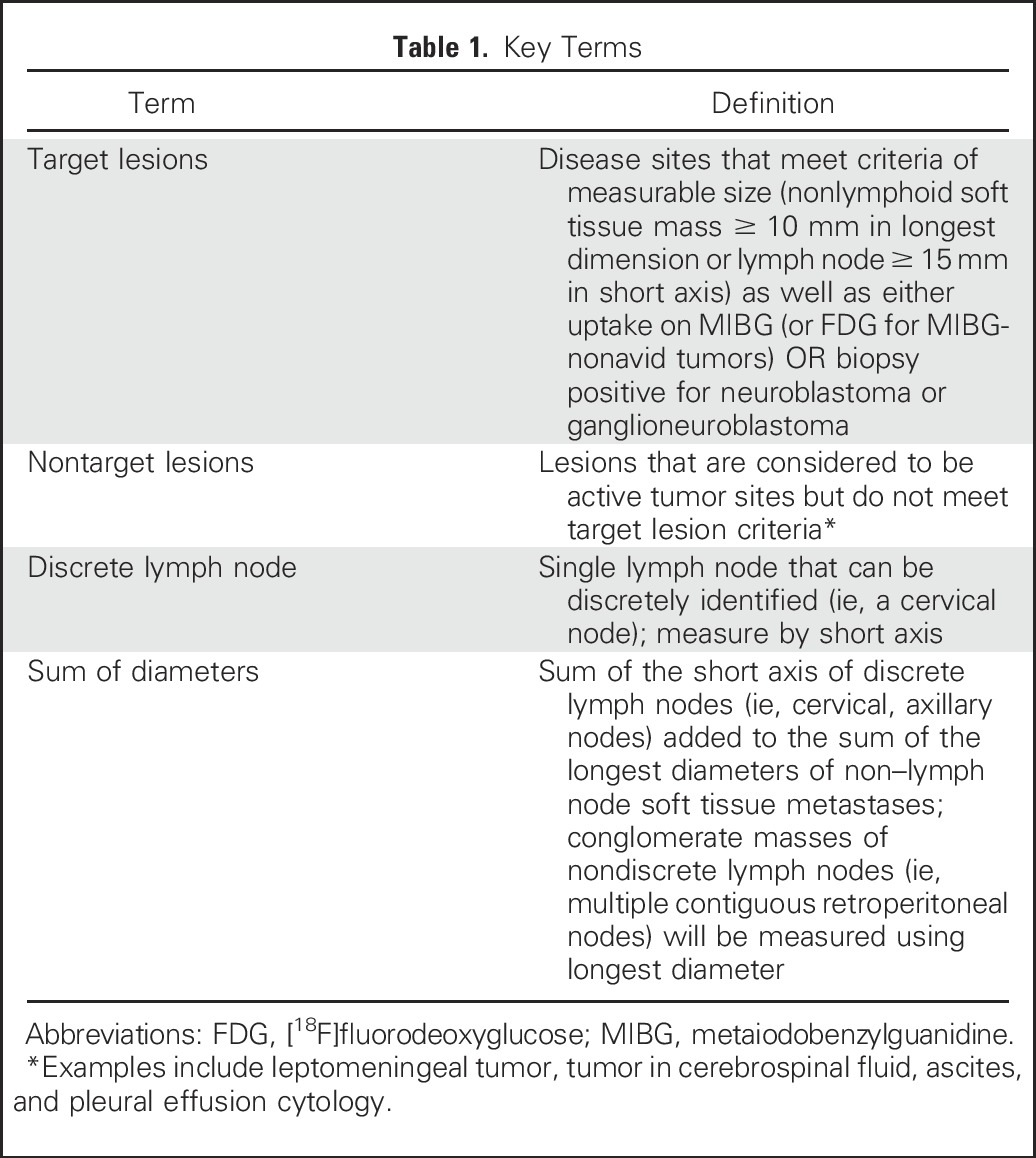
MIBG uptake (or FDG for tumors that are not MIBG avid) will be used to determine which metastatic soft tissue lesions considered measurable by RECIST will be deemed target lesions for response assessment (Table 1). Nontarget soft tissue lesions will include leptomeningeal tumor, tumor in cerebrospinal fluid, ascites, or pleural effusion, and lesions smaller than 10 mm that are considered likely to be active tumor based on clinical correlation (eg, hepatic and pulmonary nodules). Small (< 10 mm) soft tissue lesions and lymph nodes that measure shorter than 15 mm on short axis will be considered nontarget lesions if they are biopsied and proven to consist of viable tumor. Non–lymph node soft tissue lesions at least 10 mm in diameter and lymph nodes larger than 15 mm on short axis that are not MIBG or FDG avid and do not contain viable tumor (if biopsied) will not be considered either target or nontarget lesions.
Table 1.
Key Terms
For certain subgroups of patients with localized tumors with favorable histology and genomics, differentiation of the tumor can occur during therapy and can be associated with an apparent increase in the size of the tumor, as well as persistent MIBG uptake.48 In the absence of new tumor sites, serial evaluation of histology may be helpful to accurately define response.
Metastatic Bone Disease
Because of its higher sensitivity and specificity, 123I-MIBG uptake will replace technetium-99m (99mTc) bone scintigraphy for evaluation of response at osteomedullary lesions.22,23,50 For patients whose tumors do not concentrate MIBG, FDG-PET or PET/CT scan will be used for tumor detection in bone. Anatomic imaging will not be used to evaluate osteomedullary lesions, because these lesions may not shrink in size using CT/MRI even in the absence of residual viable tumor. In addition, osseous lesions without a soft tissue mass are considered nonmeasurable by RECIST. The measurable extramedullary soft tissue components of bone lesions will be assessed using the same criteria used for other soft tissue sites.
Metastatic Bone Marrow Disease
Assessment of bone marrow involvement is achieved via evaluation of bilateral aspirates and bilateral trephine biopsies, a total of four sampled sites. The 1993 INRC on bone marrow response are based on the number of sites positive for tumor but do not incorporate modern techniques to better quantitate disease burden within the bone marrow. The revised guidelines require assessment of bone marrow aspirates and trephines for neuroblastoma cells using morphologic criteria in conjunction with appropriate antibodies to confirm the identity of neuroblastoma cells by immunocytology (if available) and/or immunohistochemistry. Only bone marrow samples of suitable quality should be investigated, as detailed by Burchill et al.19 Although more advanced techniques, including automatic immunofluorescence plus fluorescent in situ hybridization51 and reverse transcription quantitative polymerase chain reaction (RTqPCR),4,52-57 are available for assessment of bone marrow status, further prospective validation across clinical trials using standardized reporting is required before these can be incorporated into a revised INRC.
RESULTS
Data collected by the INRG task force was used to evaluate characteristics of metastatic disease at diagnosis in neuroblastoma.21,40 This database consists of clinical data from 17,938 patients diagnosed with neuroblastoma from 1974 to 2015. Specifics of metastatic disease sites were not identified in 11,430 patient cases. Of the remaining 6,508 patients, 3,496 (54%) had documented metastatic disease at diagnosis. Bone marrow (n = 1,940; 56%) and bone (n = 1,625; 47%) are the most common sites of metastatic disease, highlighting the importance of including these sites as components of the proposed revised response criteria. Metastatic disease involving soft tissue sites includes lymph nodes (n = 846; 24%), liver (n = 727; 21%), and, less commonly, skin (n = 155; 4%), lung (n = 101; 3%), and CNS (n = 38; 1%).
On the basis of review of the scientific literature and consensus among the experts of this panel, the following revised INRC are proposed. Response will be based on all components of disease, taking into consideration soft tissue, bone, and bone marrow disease sites.
Primary Tumor
Response of primary tumor using both RECIST criteria and MIBG (or FDG if tumor is MIBG nonavid) uptake will be used (Table 2). In patients with bilateral adrenal lesions, response will be based on the sum of the longest dimensions of both sites unless biopsy proves one to be ganglioneuroma. In patients with multifocal nonadrenal disease, the largest tumor will be considered the primary tumor, and additional lesions will be assessed as metastatic sites unless biopsy proven to be ganglioneuroma.
Table 2.
Primary (soft tissue) Tumor Response*
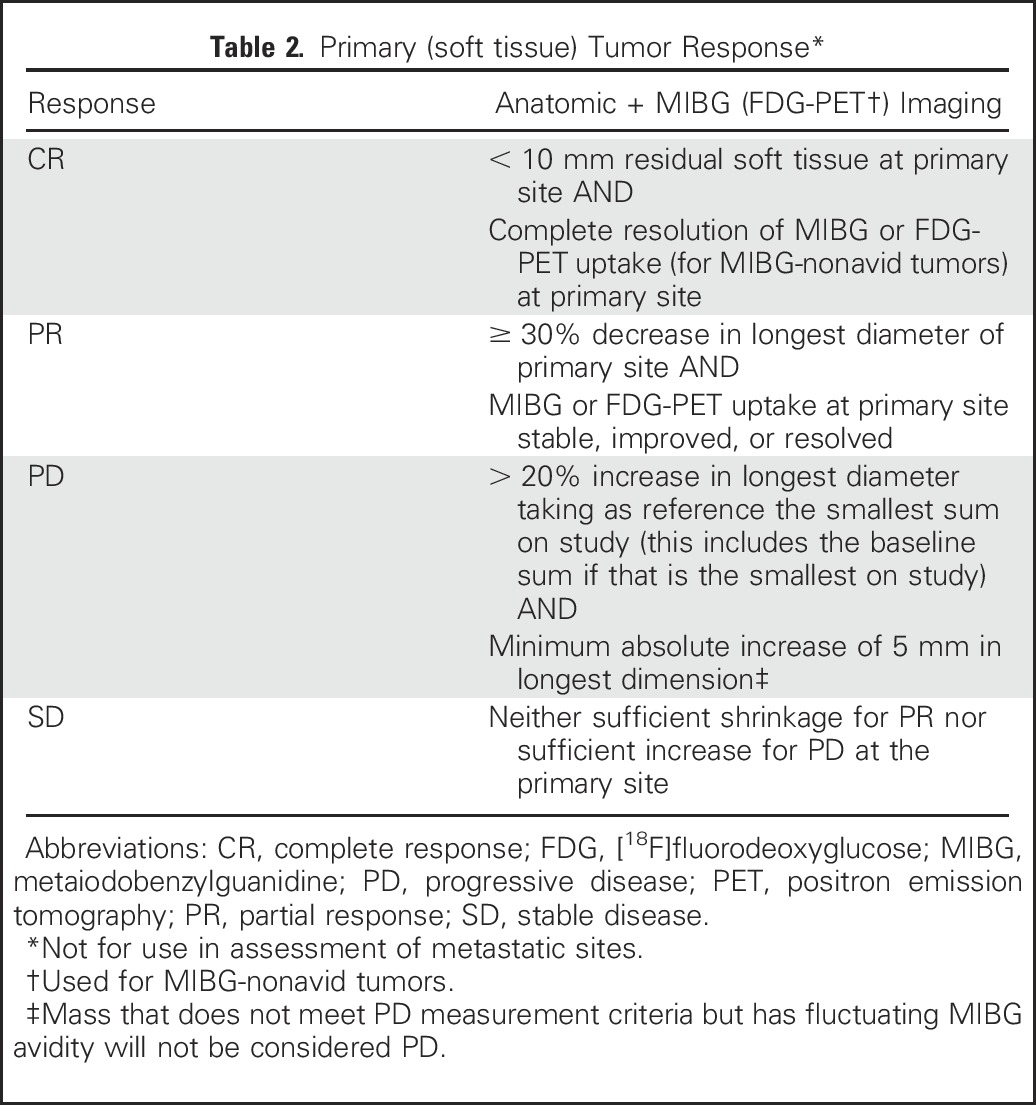
In some patients, it may be difficult to distinguish postoperative changes in soft tissues in the primary tumor bed from true residual neuroblastoma using anatomic imaging alone. This is particularly true when residual soft tissue masses are small (< 1 cm at longest diameter). For this reason, patients with MIBG-nonavid lesions measuring less than 1 cm in diameter would be considered to have achieved complete response (CR) in the primary site if the tumor was initially MIBG avid. For patients with MIBG-nonavid tumors at the time of diagnosis, small residual tumors must not demonstrate increased metabolic activity by FDG-PET imaging and, if biopsied, must not demonstrate neuroblastoma or ganglioneuroblastoma.
Metastatic Soft Tissue and Bone Disease
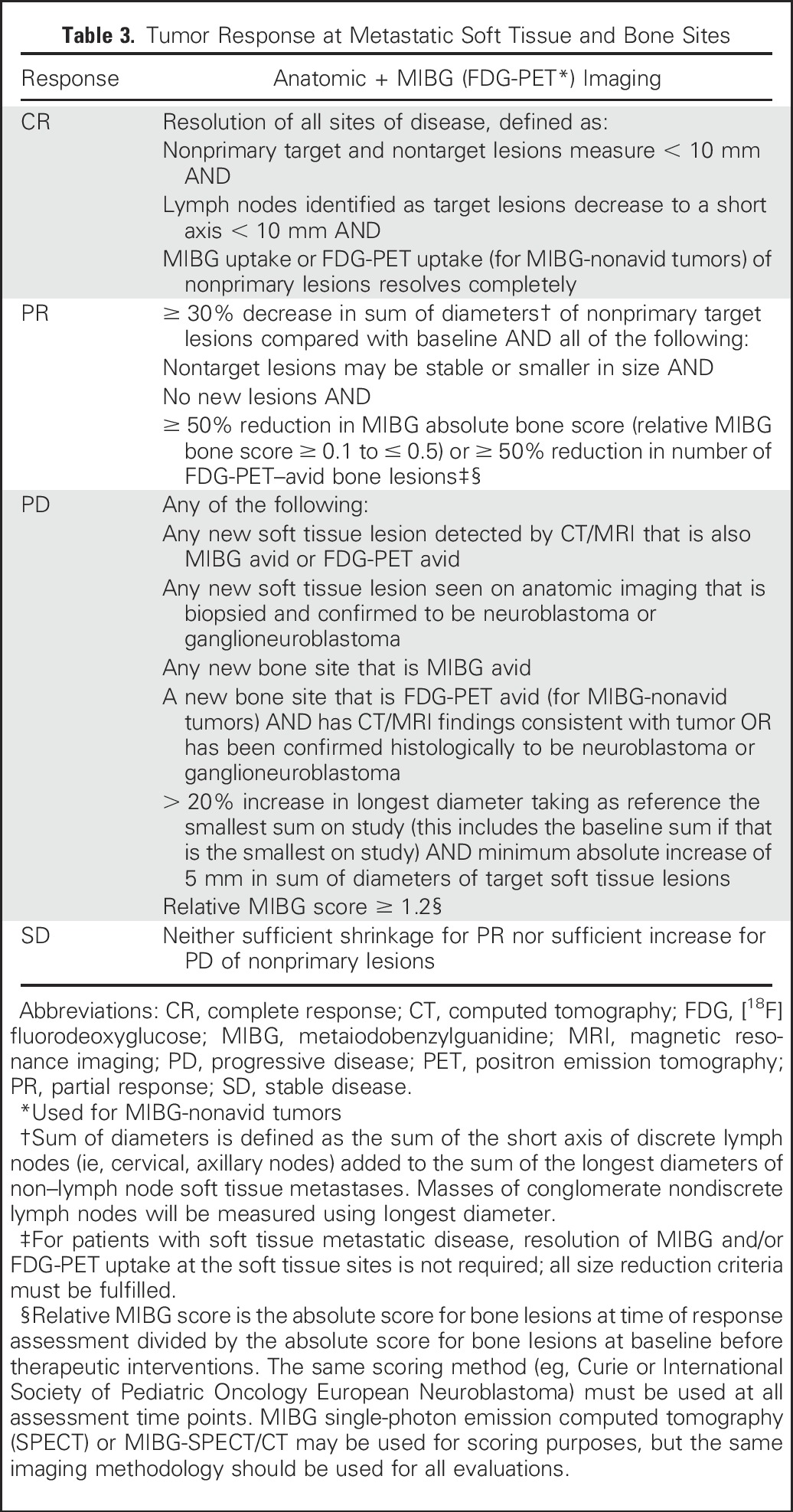
A combination of anatomic imaging and radionuclide scans will be used to assess response in soft tissue (including lymph node and non–lymph node) and bone metastases (Table 3). MIBG semiquantitative scoring systems have been previously used for response assessment,37,50,58-62 with international consensus developed for use of these scoring systems in disease response.23 Although differences exist in the approach to absolute scoring in the various systems, comparisons of the relative scores as defined by the SIOPEN scoring system62 and the Curie scoring system61 (used in COG) have yielded consistent designations of response and have validated MIBG relative scoring as prognostic for overall response and patient outcome in patients with newly diagnosed neuroblastoma.36 The consensus recommendation is to use the MIBG relative score on bone sectors (the absolute score of bone lesions at time of response assessment divided by the absolute score of bone lesions at baseline before therapeutic interventions) for response assessment. The same scoring method (eg, Curie, SIOPEN) should be used at each time point of response assessment. MIBG-SPECT or MIBG-SPECT/CT may be used for scoring purposes, but the same imaging methodology should be used for all evaluations.
Table 3.
Tumor Response at Metastatic Soft Tissue and Bone Sites
Bone Marrow Metastases
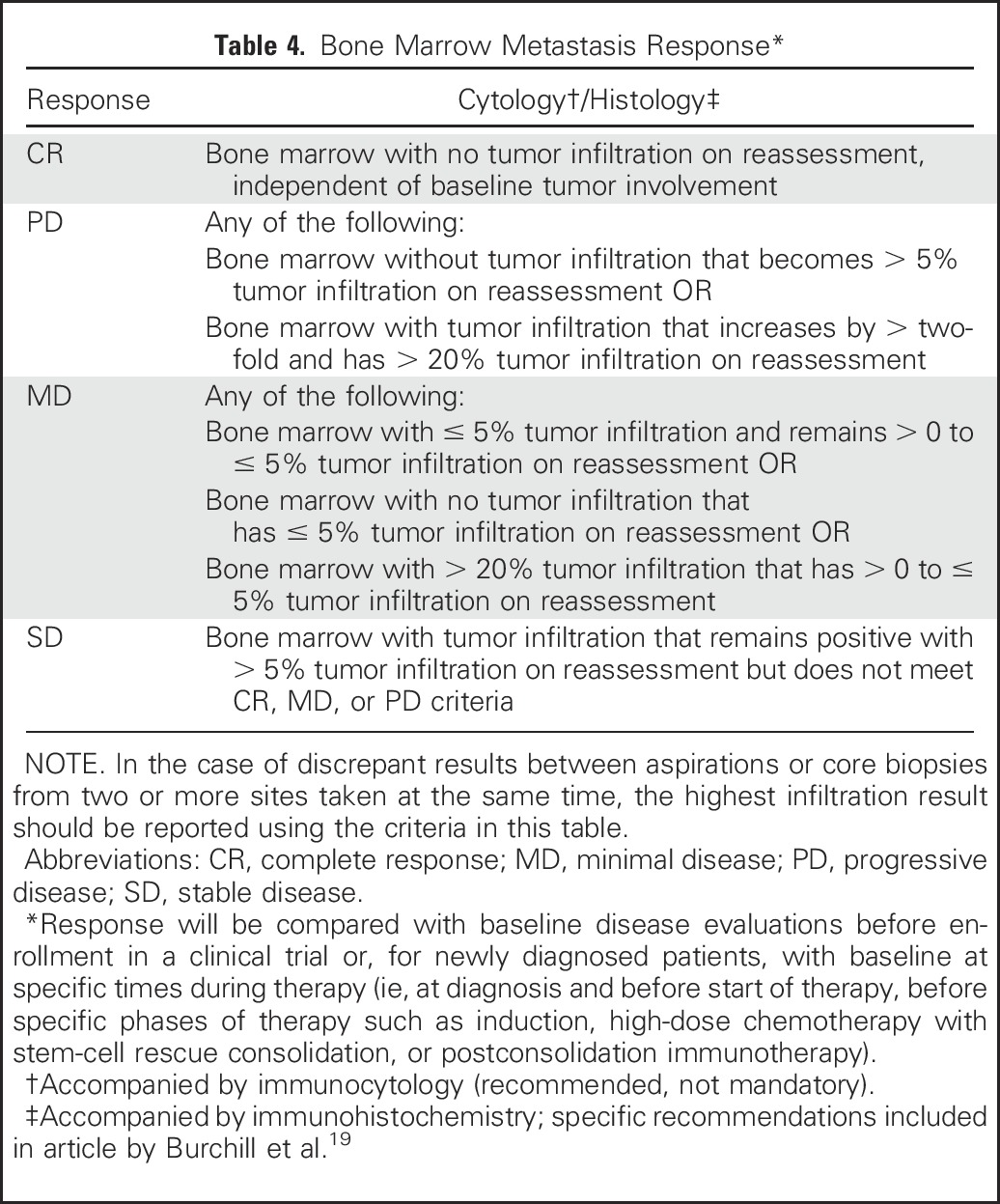
Exact quantification of bone marrow involvement at all sites should be reported; the percentage of tumor infiltration of bone marrow space assessed by histologic evaluation of trephine or biopsy (with immunohistochemical staining encouraged) or counting of the number of tumor cells in aspirates by cytology or immunocytology (recommended if available) divided by the number of hematopoietic or mononuclear cells evaluated to obtain a percentage of involvement (methodology described by Burchill et al19). The bone marrow sample with the highest percentage of tumor infiltration is used in the response algorithm. Neuroblastoma infiltration in the marrow can be heterogeneously distributed throughout the skeleton.63,64 Because the clinical impact of this heterogeneity has not yet been fully evaluated, detection of more than 0% to ≤ 5% tumor infiltration in bone marrow will represent a new category of minimal disease (Table 4).
Table 4.
Bone Marrow Metastasis Response*
Overall Response
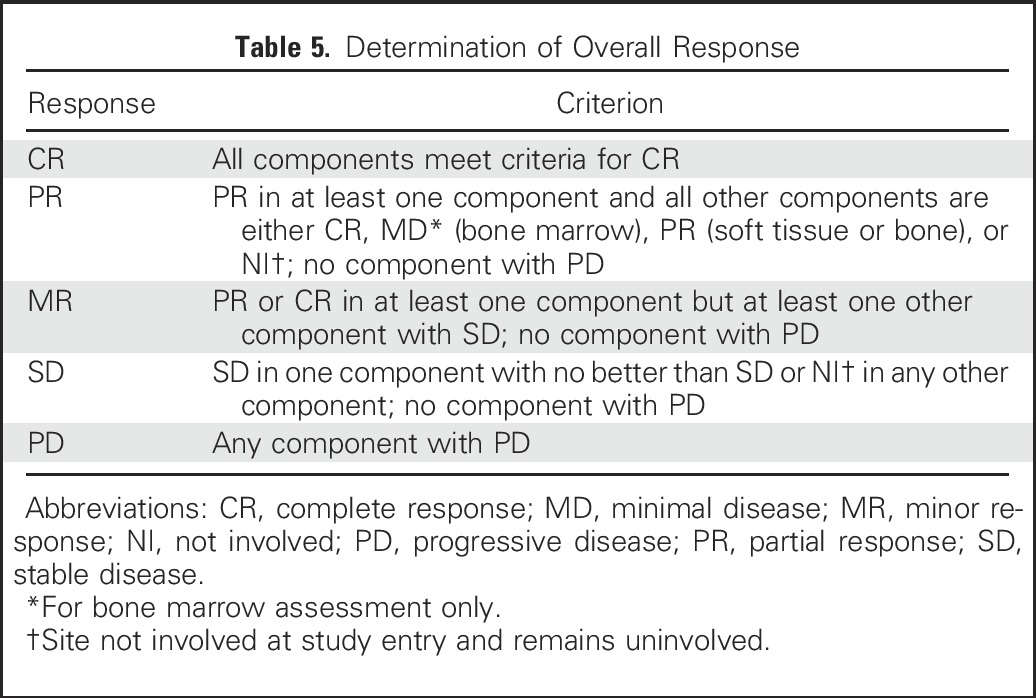
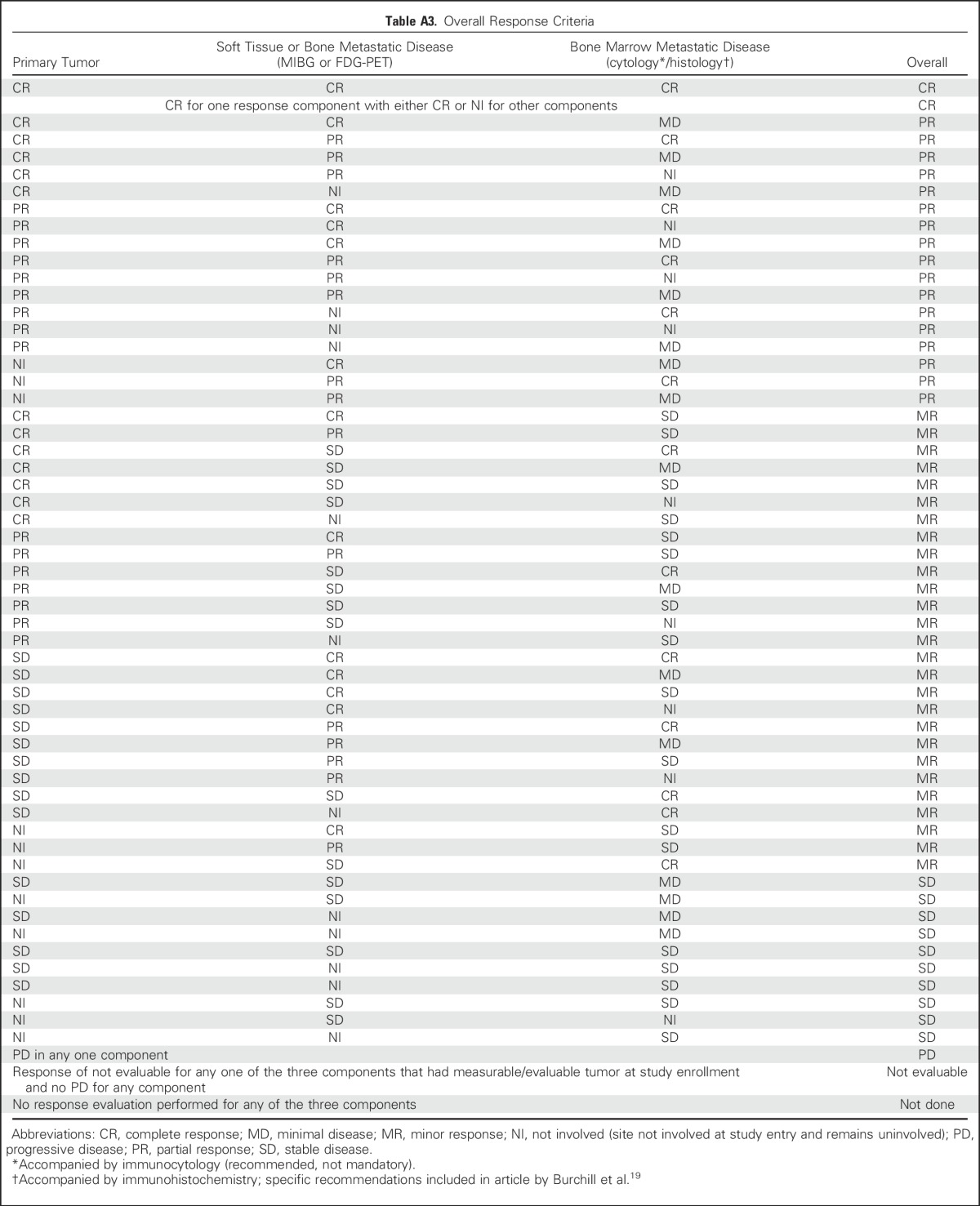
Overall response will be defined by combining response of the individual components (ie, soft tissue, bone, and bone marrow disease). All components must be evaluated and of sufficient quality to fully assess overall response (Table 5; Appendix Table A3, online only). An overall CR requires that all involved components have a CR. An overall partial response includes a partial response of all soft tissue and bone sites or noninvolvement in one of these components but allows residual minimal disease in the bone marrow. The prior category of mixed response has been eliminated, and a new category, minor response, has been included. Minor response requires a partial response or CR in at least one component, stable disease for at least one component, and no evidence of progressive disease in any component. Progressive disease in any one component defines overall progressive disease.
Table 5.
Determination of Overall Response
DISCUSSION
The INRC revisions described in this consensus statement represent an evolution of neuroblastoma response criteria, with the incorporation of functional imaging, the widespread application of advanced techniques for analysis of marrow involvement, and the recognition that clinically significant minimal bone marrow disease can be assessed by discontinuous sampling. The goal of the current INRC revision is to eliminate modalities for tumor assessment that are less sensitive and/or specific for neuroblastoma (eg, bone scintigraphy and catecholamine levels) and replace these with nuclear imaging modalities (123I-MIBG and FDG-PET imaging) that increase the likelihood of detection of viable neuroblastoma and/or ganglioneuroblastoma in soft tissue and bone metastatic sites and to include the use of histopathologic techniques that quantitate the extent of bone marrow involvement. These newer modalities are being widely used; however, until now, they have not been uniformly incorporated into neuroblastoma disease assessment. As a result, there have been significant challenges in interpretation of clinical trial results across institutions and consortia, highlighting the need for revisions to the INRC. In addition, the prior INRC were developed with a focus on newly diagnosed neuroblastoma and were not easily applicable to phase I or II clinical trials for patients with recurrent or refractory disease, where bone and bone marrow are frequently the only sites of tumor involvement.
Functional imaging is the cornerstone of these revised criteria for neuroblastoma response. Bone scintigraphy with 99mTc biphosphonates, although sensitive for detecting osseous metastatic sites at diagnosis, lacks specificity when assessing disease response to therapy, because of tracer uptake in remodeling bone.65 123I-MIBG imaging provides superior sensitivity compared with bone scintigraphy for detecting viable neuroblastoma and will be used for assessment of response of bony metastatic disease. Postoperative changes in resected soft tissue sites and therapy-induced tumor differentiation require the use of functional imaging to complement the use of RECIST guidelines, which are based on anatomic imaging for response assessment of soft tissue sites. In the minority of patients with MIBG-nonavid disease, FDG-PET provides an alternative modality to assess disease status at primary or metastatic soft tissue sites and bony sites. Thus, in contrast to the majority of adult trials that use only RECIST criteria to define response, the revised INRC will fully incorporate the sensitive and specific nuclear medicine modalities available to better define neuroblastoma response.
Bone marrow represents the most common site of metastatic disease at diagnosis and is one of the most common sites of relapse in patients with neuroblastoma. The INRC 1993 version12 incorporated the number of involved bone marrow sites (assessing bilateral bone marrow aspirates and biopsies) into response assessment. However, the level of disease at each site was not assessed, and no distinction was made between patients who had a single neuroblastoma cell clump in the bone marrow and those who had near-complete replacement of the bone marrow compartment with tumor. Consequently, assessment of bone marrow response lacked precision, which could lead to over- or underestimation of response. Bone marrow response criteria piloted in the NANT consortium27,29-31 and subsequently adopted in COG early-phase clinical trials have used the maximum percentage of tumor found at any of the four sampled bone marrow sites in response assessment and defined intermittently positive detection of low levels of tumor infiltration in the marrow as stable disease rather than progression. Multiple published early-phase clinical trials have confirmed the feasibility of incorporating these criteria to evaluate bone marrow response assessment in neuroblastoma.27,29-31,33 The revised INRC now include quantitative assessment of bone marrow involvement, and the bone marrow response criteria reflect the current uncertainty regarding the clinical impact of detecting intermittent minimal disease. The optimal methodology for quantification of tumor in bone marrow is still under evaluation. The article by Burchill et al19 provides details about the methodologies incorporated in the revised INRC to standardize the definition of bone marrow response. The precise amount of tumor cell infiltration in bone marrow aspirates and trephines or biopsies must be collected in future clinical trials, allowing for better assessment of the relationship between marrow response with overall outcome and providing data for evidence-based refinement of future revisions of the INRC.
Because minimal residual marrow disease is frequently observed post-therapy, these revised response criteria will include a new categorization of patients with minimal marrow disease. This will provide the opportunity to more uniformly study the prognostic importance of minimal disease and the effect of new agents in the setting of minimal marrow disease. Furthermore, this new classification provides the opportunity to prospectively study the clinical use of newer techniques, such as RTqPCR,4,52-57 which provides an objective, rapid throughput method to precisely quantitate neuroblastoma load in the bone marrow. Although RTqPCR for neuroblastoma mRNAs has been shown to be of prognostic and predictive value in several clinical trials,4,54 it has not yet been widely adopted in clinical practice and has not been incorporated into these revised INRC. Additional studies are required to define how best to incorporate such highly sensitive detection methods into future refinements of disease response criteria.
The revised INRC will apply to both newly diagnosed and recurrent or refractory neuroblastoma. Because these criteria can be used to assess disease response in patients with only bone and/or bone marrow metastases, they will enable the evaluation of disease response in patients without measurable soft tissue disease who are eligible for early-phase trials.
We anticipate that the INRC will continue to evolve as newer technologies are incorporated into clinical practice. In addition to novel techniques for marrow detection, detection of circulating tumor cells and use of novel functional imaging techniques, such as diffusion-weighted imaging MRI and PET-MRI, and new radiotracers, such as gallium-68–labeled somatostatin analogs,66,67 Iodine-124–MIBG,68 and [18F]fluorodopa,69 may further provide improved sensitivity for metastatic tumor detection. As these modalities become more widely available, their incorporation into future INRC revisions will require prospective study in the context of clinical trials for the validation of their utility and clinical applicability.
Great strides have been made in using clinical and biologic characteristics to more precisely assign therapy for children with neuroblastoma.21 These international consensus response criteria are an additional step in providing a common international language to assess clinical trial outcomes and enhance the opportunity for international collaborative clinical trials.
ACKNOWLEDGMENT
Presented at Advances in Neuroblastoma Research, Toronto, Canada, June 18-21, 2012, and Advances in Neuroblastoma Research, Cairns, Australia, June 19-23, 2016.
Appendix
Table A1.
NCI Clinical Trial Planning Investigators
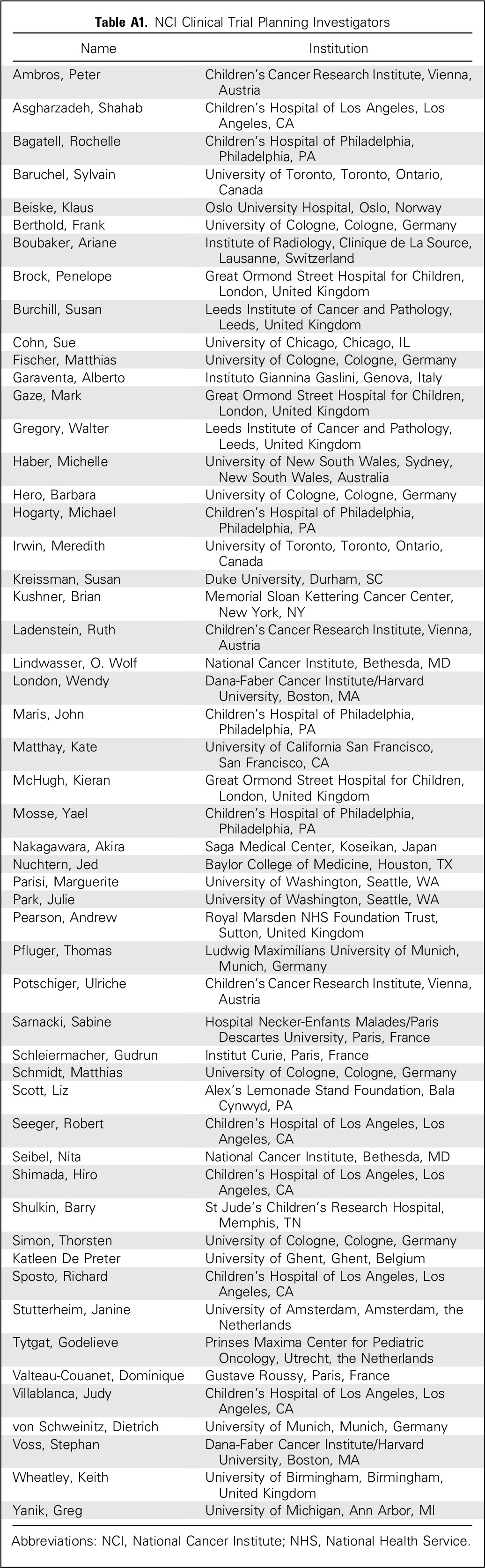
Table A2.
Clinical Trials Included in INRG Database
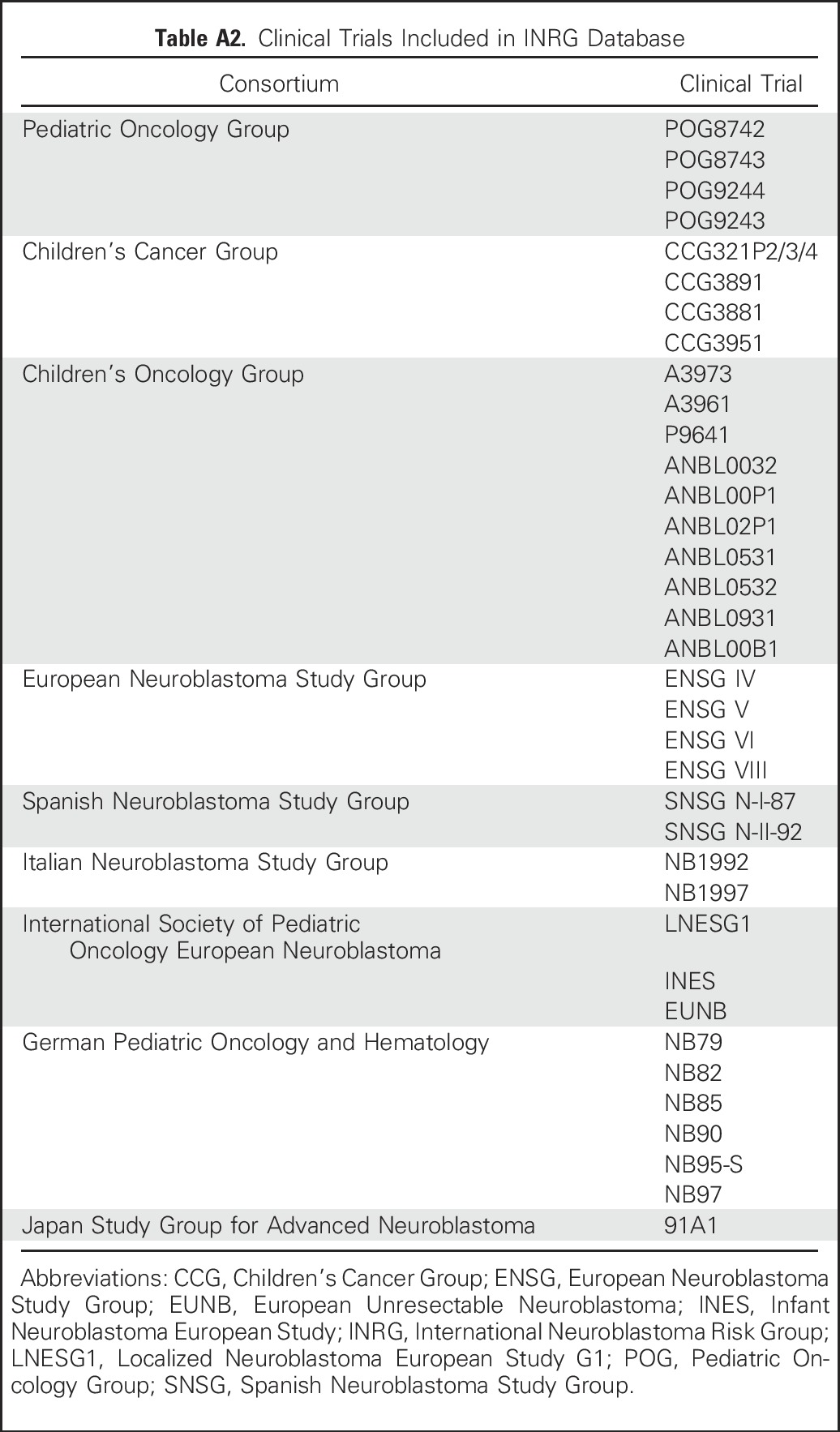
Table A3.
Overall Response Criteria
Footnotes
Supported by the National Cancer Institute Pediatric and Adolescent Solid Tumor Steering Committee, Alex’s Lemonade Stand Foundation, the Ben Towne Foundation, the EVAN Foundation, Cancer Research UK Institut of Cancer Research (ICR) Core Award No. C347/A15403, and the National Institute for Health Research Research Methods Programme/ICR Biomedical Research Centre.
AUTHOR CONTRIBUTIONS
Conception and design: Julie R. Park, Rochelle Bagatell, Susan L. Cohn, Andrew D. Pearson, Judith G. Villablanca, Frank Berthold, Susan Burchill, Ariane Boubaker, Kieran McHugh, Jed G. Nuchtern, Nita L. Seibel, O. Wolf Lindwasser, John M. Maris, Penelope Brock, Gudrun Schleiermacher, Ruth Ladenstein, Katherine K. Matthay, Dominque Valteau-Couanet
Financial support: Julie R. Park
Administrative support: O. Wolf Lindwasser
Collection and assembly of data: Julie R. Park, Rochelle Bagatell, Susan L. Cohn, Andrew D. Pearson, Judith G. Villablanca, Frank Berthold, Susan Burchill, Kieran McHugh, Jed G. Nuchtern, Wendy B. London, John M. Maris, Ruth Ladenstein, Katherine K. Matthay, Dominque Valteau-Couanet
Data analysis and interpretation: Julie R. Park, Rochelle Bagatell, Susan L. Cohn, Andrew D. Pearson, Judith G. Villablanca, Frank Berthold, Susan Burchill, Ariane Boubaker, Kieran McHugh, Jed G. Nuchtern, Wendy B. London, Nita L. Seibel, John M. Maris, Penelope Brock, Ruth Ladenstein, Katherine K. Matthay, Dominque Valteau-Couanet
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Revisions to the International Neuroblastoma Response Criteria: A Consensus Statement From the National Cancer Institute Clinical Trials Planning Meeting
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Julie R. Park
Travel, Accommodations, Expenses: Roche
Rochelle Bagatell
No relationship to disclose
Susan L. Cohn
Stock or Other Ownership: United Therapeutics (I), Varian Medical Systems (I), Hansen Medical, United Therapeutics, Universal Health Services, Varian Medical Systems, Vermillion, Fresenius Medical Care (I), Resmed (I), Universal Health Services (I), AstraZeneca (I), Merck (I), Dr. Reddy’s Laboratories, Novartis, Henry Schein, TEVA Pharmaceuticals Industries, AstraZeneca, Merck, Stryker (I), Cigna (I), Cigna, Ensign Group, Express Scripts, Perrigo, Smith & Nephew, Stryker, Valent Pharmaceuticals, Joint Chiropractic Company (I)
Research Funding: United Therapeutics (Inst), Merck (Inst)
Andrew D. Pearson
Travel, Accommodations, Expenses: Boehringer Ingelheim
Judith G. Villablanca
No relationship to disclose
Frank Berthold
Consulting or Advisory Role: United Therapeutics
Research Funding: United Therapeutics
Travel, Accommodations, Expenses: United Therapeutics
Susan Burchill
No relationship to disclose
Ariane Boubaker
No relationship to disclose
Kieran McHugh
No relationship to disclose
Jed G. Nuchtern
Stock or Other Ownership: Lexicon, Johnson & Johnson, CVS, Insulet, Dexcom, Tandem Diabetes Care
Wendy B. London
No relationship to disclose
Nita L. Seibel
No relationship to disclose
O. Wolf Lindwasser
No relationship to disclose
John M. Maris
No relationship to disclose
Penelope Brock
Honoraria: Fennec Pharma
Consulting or Advisory Role: Fennec Pharma
Travel, Accommodations, Expenses: Fennec Pharma
Gudrun Schleiermacher
No relationship to disclose
Ruth Ladenstein
Honoraria: Apeiron Biologics, Boehringer Ingelheim
Consulting or Advisory Role: Apeiron Biologics, Boehringer Ingelheim
Research Funding: Apeiron Biologics (Inst)
Patents, Royalties, Other Intellectual Property: Apeiron Biologics (Inst)
Expert Testimony: Apeiron Biologics
Travel, Accommodations, Expenses: Apeiron Biologics
Katherine K. Matthay
No relationship to disclose
Dominque Valteau-Couanet
No relationship to disclose
REFERENCES
- 1.Irwin MS, Park JR. Neuroblastoma: Paradigm for precision medicine. Pediatr Clin North Am. 2015;62:225–256. doi: 10.1016/j.pcl.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 2.Pearson AD, Pinkerton CR, Lewis IJ, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: A randomised trial. Lancet Oncol. 2008;9:247–256. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]
- 3.Ladenstein R, Valteau-Couanet D, Brock P, et al. Randomized trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: The European HR-NBL1/SIOPEN study. J Clin Oncol. 2010;28:3516–3524. doi: 10.1200/JCO.2009.27.3524. [DOI] [PubMed] [Google Scholar]
- 4.Kreissman SG, Seeger RC, Matthay KK, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): A randomised phase 3 trial. Lancet Oncol. 2013;14:999–1008. doi: 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon T, Hero B, Faldum A, et al. Long term outcome of high-risk neuroblastoma patients after immunotherapy with antibody ch14.18 or oral metronomic chemotherapy. BMC Cancer. 2011;11:21. doi: 10.1186/1471-2407-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berthold F, Boos J, Burdach S, et al. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: A randomised controlled trial. Lancet Oncol. 2005;6:649–658. doi: 10.1016/S1470-2045(05)70291-6. [DOI] [PubMed] [Google Scholar]
- 7.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma. N Engl J Med. 2010;363:1313–1323. doi: 10.1056/NEJMoa1001527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strother DR, London WB, Schmidt ML, et al. Outcome after surgery alone or with restricted use of chemotherapy for patients with low-risk neuroblastoma: Results of Children’s Oncology Group study P9641. J Clin Oncol. 2012;30:1842–1848. doi: 10.1200/JCO.2011.37.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Bernardi B, Gerrard M, Boni L, et al. Excellent outcome with reduced treatment for infants with disseminated neuroblastoma without MYCN gene amplification. J Clin Oncol. 2009;27:1034–1040. doi: 10.1200/JCO.2008.17.5877. [DOI] [PubMed] [Google Scholar]
- 10.De Bernardi B, Mosseri V, Rubie H, et al. Treatment of localised resectable neuroblastoma: Results of the LNESG1 study by the SIOP Europe Neuroblastoma Group. Br J Cancer. 2008;99:1027–1033. doi: 10.1038/sj.bjc.6604640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simon T, Hero B, Benz-Bohm G, et al. Review of image defined risk factors in localized neuroblastoma patients: Results of the GPOH NB97 trial. Pediatr Blood Cancer. 2008;50:965–969. doi: 10.1002/pbc.21343. [DOI] [PubMed] [Google Scholar]
- 12.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 13.Matthay KK, Atkinson JB, Stram DO, et al. Patterns of relapse after autologous purged bone marrow transplantation for neuroblastoma: A Children’s Cancer Group pilot study. J Clin Oncol. 1993;11:2226–2233. doi: 10.1200/JCO.1993.11.11.2226. [DOI] [PubMed] [Google Scholar]
- 14.Kushner BH, Kramer K, Modak S, et al. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol. 2009;27:1041–1046. doi: 10.1200/JCO.2008.17.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taggart DR, Han MM, Quach A, et al. Comparison of iodine-123 metaiodobenzylguanidine (MIBG) scan and [18F]fluorodeoxyglucose positron emission tomography to evaluate response after iodine-131 MIBG therapy for relapsed neuroblastoma. J Clin Oncol. 2009;27:5343–5349. doi: 10.1200/JCO.2008.20.5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papathanasiou ND, Gaze MN, Sullivan K, et al. 18F-FDG PET/CT and 123I-metaiodobenzylguanidine imaging in high-risk neuroblastoma: Diagnostic comparison and survival analysis. J Nucl Med. 2011;52:519–525. doi: 10.2967/jnumed.110.083303. [DOI] [PubMed] [Google Scholar]
- 17.Melzer HI, Coppenrath E, Schmid I, et al. 123I-MIBG scintigraphy/SPECT versus 18F-FDG PET in paediatric neuroblastoma. Eur J Nucl Med Mol Imaging. 2011;38:1648–1658. doi: 10.1007/s00259-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 18.Shulkin BL, Shapiro B. Current concepts on the diagnostic use of MIBG in children. J Nucl Med. 1998;39:679–688. [PubMed] [Google Scholar]
- 19.Burchill SA, Beiske K, Shimada H, et al. Recommendations for the standardization of bone marrow disease assessment and reporting in children with neuroblastoma: On behalf of the International Neuroblastoma Response Criteria Bone Marrow Working Group Cancer [epub ahead of print on December 16, 2016] [DOI] [PubMed] [Google Scholar]
- 20.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: An INRG Task Force report. J Clin Oncol. 2009;27:298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG Task Force report. J Clin Oncol. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brisse HJ, McCarville MB, Granata C, et al. Guidelines for imaging and staging of neuroblastic tumors: Consensus report from the International Neuroblastoma Risk Group project. Radiology. 2011;261:243–257. doi: 10.1148/radiol.11101352. [DOI] [PubMed] [Google Scholar]
- 23.Matthay KK, Shulkin B, Ladenstein R, et al. Criteria for evaluation of disease extent by (123)I-metaiodobenzylguanidine scans in neuroblastoma: A report for the International Neuroblastoma Risk Group (INRG) Task Force. Br J Cancer. 2010;102:1319–1326. doi: 10.1038/sj.bjc.6605621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beiske K, Burchill SA, Cheung IY, et al. Consensus criteria for sensitive detection of minimal neuroblastoma cells in bone marrow, blood and stem cell preparations by immunocytology and QRT-PCR: Recommendations by the International Neuroblastoma Risk Group Task Force. Br J Cancer. 2009;100:1627–1637. doi: 10.1038/sj.bjc.6605029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambros PF, Ambros IM, Brodeur GM, et al. International consensus for neuroblastoma molecular diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br J Cancer. 2009;100:1471–1482. doi: 10.1038/sj.bjc.6605014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthay KK, Quach A, Huberty J, et al. Iodine-131–metaiodobenzylguanidine double infusion with autologous stem-cell rescue for neuroblastoma: A new approaches to neuroblastoma therapy phase I study. J Clin Oncol. 2009;27:1020–1025. doi: 10.1200/JCO.2007.15.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurer BJ, Kang MH, Villablanca JG, et al. Phase I trial of fenretinide delivered orally in a novel organized lipid complex in patients with relapsed/refractory neuroblastoma: A report from the New Approaches to Neuroblastoma Therapy (NANT) consortium. Pediatr Blood Cancer. 2013;60:1801–1808. doi: 10.1002/pbc.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yanik GA, Villablanca JG, Maris JM, et al. 131I-metaiodobenzylguanidine with intensive chemotherapy and autologous stem cell transplantation for high-risk neuroblastoma: A new approaches to neuroblastoma therapy (NANT) phase II study. Biol Blood Marrow Transplant. 2015;21:673–681. doi: 10.1016/j.bbmt.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 29.Villablanca JG, Volchenboum SL, Cho H, et al. A phase I new approaches to neuroblastoma therapy study of buthionine sulfoximine and melphalan with autologous stem cells for recurrent/refractory high-risk neuroblastoma. Pediatr Blood Cancer. 2016;63:1349–1356. doi: 10.1002/pbc.25994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuBois SG, Marachelian A, Fox E, et al. Phase I study of the aurora A kinase inhibitor alisertib in combination with irinotecan and temozolomide for patients with relapsed or refractory neuroblastoma: A NANT (New Approaches to Neuroblastoma Therapy) trial. J Clin Oncol. 2016;34:1368–1375. doi: 10.1200/JCO.2015.65.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villablanca JG, London WB, Naranjo A, et al. Phase II study of oral capsular 4-hydroxyphenylretinamide (4-HPR/fenretinide) in pediatric patients with refractory or recurrent neuroblastoma: A report from the Children’s Oncology Group. Clin Cancer Res. 2011;17:6858–6866. doi: 10.1158/1078-0432.CCR-11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bagatell R, London WB, Wagner LM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: A Children’s Oncology Group study. J Clin Oncol. 2011;29:208–213. doi: 10.1200/JCO.2010.31.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shusterman S, London WB, Gillies SD, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: A Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Giannatale A, Dias-Gastellier N, Devos A, et al. Phase II study of temozolomide in combination with topotecan (TOTEM) in relapsed or refractory neuroblastoma: A European Innovative Therapies for Children With Cancer-SIOP-European neuroblastoma study. Eur J Cancer. 2014;50:170–177. doi: 10.1016/j.ejca.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Simon T, Berthold F, Borkhardt A, et al. Treatment and outcomes of patients with relapsed, high-risk neuroblastoma: Results of German trials. Pediatr Blood Cancer. 2011;56:578–583. doi: 10.1002/pbc.22693. [DOI] [PubMed] [Google Scholar]
- 36.Decarolis B, Schneider C, Hero B, et al. Iodine-123 metaiodobenzylguanidine scintigraphy scoring allows prediction of outcome in patients with stage 4 neuroblastoma: Results of the Cologne interscore comparison study. J Clin Oncol. 2013;31:944–951. doi: 10.1200/JCO.2012.45.8794. [DOI] [PubMed] [Google Scholar]
- 37.Yanik GA, Parisi MT, Shulkin BL, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: A report from the Children’s Oncology Group. J Nucl Med. 2013;54:541–548. doi: 10.2967/jnumed.112.112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.London WB, Castel V, Monclair T, et al. Clinical and biologic features predictive of survival after relapse of neuroblastoma: A report from the International Neuroblastoma Risk Group project. J Clin Oncol. 2011;29:3286–3292. doi: 10.1200/JCO.2010.34.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DuBois SG, Allen S, Bent M, et al. Phase I/II study of (131)I-MIBG with vincristine and 5 days of irinotecan for advanced neuroblastoma. Br J Cancer. 2015;112:644–649. doi: 10.1038/bjc.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol. 2015;33:3008–3017. doi: 10.1200/JCO.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sundell IB, Hallmans G, Nilsson TK, et al. Plasma glucose and insulin, urinary catecholamine and cortisol responses to test breakfasts with high or low fibre content: The importance of the previous diet. Ann Nutr Metab. 1989;33:333–340. doi: 10.1159/000177555. [DOI] [PubMed] [Google Scholar]
- 42.Weetman RM, Rider PS, Oei TO, et al. Effect of diet on urinary excretion of VMA, HVA, metanephrine, and total free catecholamine in normal preschool children. J Pediatr. 1976;88:46–50. doi: 10.1016/s0022-3476(76)80725-1. [DOI] [PubMed] [Google Scholar]
- 43.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 44.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Schwartz LH, Bogaerts J, Ford R, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–267. doi: 10.1016/j.ejca.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: A review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 47.Bagatell R, McHugh K, Naranjo A, et al. Assessment of primary site response in children with high-risk neuroblastoma: An international multicenter study. J Clin Oncol. 2016;34:740–746. doi: 10.1200/JCO.2015.63.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marachelian A, Shimada H, Sano H, et al. The significance of serial histopathology in a residual mass for outcome of intermediate risk stage 3 neuroblastoma. Pediatr Blood Cancer. 2012;58:675–681. doi: 10.1002/pbc.23250. [DOI] [PubMed] [Google Scholar]
- 49.Sharp SE, Shulkin BL, Gelfand MJ, et al. 123I-MIBG scintigraphy and 18F-FDG PET in neuroblastoma. J Nucl Med. 2009;50:1237–1243. doi: 10.2967/jnumed.108.060467. [DOI] [PubMed] [Google Scholar]
- 50.Suc A, Lumbroso J, Rubie H, et al. Metastatic neuroblastoma in children older than one year: Prognostic significance of the initial metaiodobenzylguanidine scan and proposal for a scoring system. Cancer. 1996;77:805–811. doi: 10.1002/(sici)1097-0142(19960215)77:4<805::aid-cncr29>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 51.Méhes G, Luegmayr A, Kornmüller R, et al. Detection of disseminated tumor cells in neuroblastoma: 3 log improvement in sensitivity by automatic immunofluorescence plus FISH (AIPF) analysis compared with classical bone marrow cytology. Am J Pathol. 2003;163:393–399. doi: 10.1016/S0002-9440(10)63669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Wezel EM, Stutterheim J, Vree F, et al. Minimal residual disease detection in autologous stem cell grafts from patients with high risk neuroblastoma. Pediatr Blood Cancer. 2015;62:1368–1373. doi: 10.1002/pbc.25507. [DOI] [PubMed] [Google Scholar]
- 53.Cheung IY, Lo Piccolo MS, Kushner BH, et al. Early molecular response of marrow disease to biologic therapy is highly prognostic in neuroblastoma. J Clin Oncol. 2003;21:3853–3858. doi: 10.1200/JCO.2003.11.077. [DOI] [PubMed] [Google Scholar]
- 54.Viprey VF, Gregory WM, Corrias MV, et al. Neuroblastoma mRNAs predict outcome in children with stage 4 neuroblastoma: A European HR-NBL1/SIOPEN study. J Clin Oncol. 2014;32:1074–1083. doi: 10.1200/JCO.2013.53.3604. [DOI] [PubMed] [Google Scholar]
- 55.Cai JY, Pan C, Tang YJ, et al. Minimal residual disease is a prognostic marker for neuroblastoma with bone marrow infiltration. Am J Clin Oncol. 2012;35:275–278. doi: 10.1097/COC.0b013e318210f51b. [DOI] [PubMed] [Google Scholar]
- 56.Stutterheim J, Ichou FA, den Ouden E, et al. Methylated RASSF1a is the first specific DNA marker for minimal residual disease testing in neuroblastoma. Clin Cancer Res. 2012;18:808–814. doi: 10.1158/1078-0432.CCR-11-0849. [DOI] [PubMed] [Google Scholar]
- 57.Choi YB, Bae GE, Lee NH, et al. Clinical significance of persistent tumor in bone marrow during treatment of high-risk neuroblastoma. J Korean Med Sci. 2015;30:1062–1067. doi: 10.3346/jkms.2015.30.8.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt M, Simon T, Hero B, et al. The prognostic impact of functional imaging with (123)I-mIBG in patients with stage 4 neuroblastoma >1 year of age on a high-risk treatment protocol: Results of the German neuroblastoma trial NB97. Eur J Cancer. 2008;44:1552–1558. doi: 10.1016/j.ejca.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 59.Boubaker A, Bischof Delaloye A. MIBG scintigraphy for the diagnosis and follow-up of children with neuroblastoma. Q J Nucl Med Mol Imaging. 2008;52:388–402. [PubMed] [Google Scholar]
- 60.Kushner BH, Yeh SD, Kramer K, et al. Impact of metaiodobenzylguanidine scintigraphy on assessing response of high-risk neuroblastoma to dose-intensive induction chemotherapy. J Clin Oncol. 2003;21:1082–1086. doi: 10.1200/JCO.2003.07.142. [DOI] [PubMed] [Google Scholar]
- 61.Matthay KK, Edeline V, Lumbroso J, et al. Correlation of early metastatic response by 123I-metaiodobenzylguanidine scintigraphy with overall response and event-free survival in stage IV neuroblastoma. J Clin Oncol. 2003;21:2486–2491. doi: 10.1200/JCO.2003.09.122. [DOI] [PubMed] [Google Scholar]
- 62.Ady N, Zucker JM, Asselain B, et al. A new 123I-MIBG whole body scan scoring method: Application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31A:256–261. doi: 10.1016/0959-8049(94)00509-4. [DOI] [PubMed] [Google Scholar]
- 63.Burchill SA. Micrometastases in neuroblastoma: Are they clinically important? J Clin Pathol. 2004;57:14–20. doi: 10.1136/jcp.57.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheung NK, Heller G, Kushner BH, et al. Detection of metastatic neuroblastoma in bone marrow: When is routine marrow histology insensitive? J Clin Oncol. 1997;15:2807–2817. doi: 10.1200/JCO.1997.15.8.2807. [DOI] [PubMed] [Google Scholar]
- 65.Sisson JC, Shulkin BL. Nuclear medicine imaging of pheochromocytoma and neuroblastoma. Q J Nucl Med. 1999;43:217–223. [PubMed] [Google Scholar]
- 66.Gains JE, Bomanji JB, Fersht NL, et al. 177Lu-DOTATATE molecular radiotherapy for childhood neuroblastoma. J Nucl Med. 2011;52:1041–1047. doi: 10.2967/jnumed.110.085100. [DOI] [PubMed] [Google Scholar]
- 67.Kiratli PO, Tuncel M, Bar-Sever Z. Nuclear medicine in pediatric and adolescent tumors. Semin Nucl Med. 2016;46:308–323. doi: 10.1053/j.semnuclmed.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 68.Cistaro A, Quartuccio N, Caobelli F, et al. 124I-MIBG: A new promising positron-emitting radiopharmaceutical for the evaluation of neuroblastoma. Nucl Med Rev Cent East Eur. 2015;18:102–106. doi: 10.5603/NMR.2015.0024. [DOI] [PubMed] [Google Scholar]
- 69.Piccardo A, Lopci E, Conte M, et al. Comparison of 18F-dopa PET/CT and 123I-MIBG scintigraphy in stage 3 and 4 neuroblastoma: A pilot study. Eur J Nucl Med Mol Imaging. 2012;39:57–71. doi: 10.1007/s00259-011-1938-2. [DOI] [PubMed] [Google Scholar]


