Abstract
Kuding tea is implicated in alleviating metabolic disorders in traditional Chinese medicine. However, the role of Ilex latifolia Thunb (kuding tea), one of the large leaf kuding tea species, in the prevention of the development of obesity remains to be determined. We show here that 7-week-old male mice treated with an Ilex latifolia Thunb supplement for 14 weeks were resistant to HFD-induced body weight gain and hepatic steatosis, accompanied by improved insulin sensitivity. Ilex latifolia Thunb supplementation dramatically reduced the systemic and tissue inflammation levels of mice via reducing pro-inflammatory cytokine levels, increasing anti-inflammatory cytokine levels in the circulation and inhibiting p38 MAPK and p65 NF-κB signaling in adipose tissue. Together, these results indicate that Ilex latifolia Thunb protects mice from the development of obesity and is a potential compound pool for the development of novel anti-obesity drugs.
Introduction
Green tea and kuding tea are two of the most popular beverages in China1,2. Green tea has been well studied for its health benefits in previous reports, but the biological functions of kuding tea are not as well studied3–5. The kuding teas most commonly found in the markets of China are typically classified into two groups: one group, which includes at least two species, Ilex latifolia Thunb and Ilex kudingcha C.J. Tseng (Ilex kaushue), has been well characterized as “large-leaved Kudingcha”; the other group, consisting of the species Ligustrum robustum, has been classified as “small-leaved Kudingcha”6. Kuding tea has been widely used in Chinese medicine for more than 2,000 years as a beverage to quench thirst, remove phlegm, refresh the mind and improve eyesight6,7. Previous studies have investigated pharmaceutical functions of Ilex kudingcha C.J. Tseng, including anti-oxidant, anti-hypertensive, anti-obesity, anti-diabetic, hepatoprotective and anti-cancer roles8–10. However, experimental data on the effects of Ilex latifolia Thunb on health are lacking.
Here, we found that aqueous extract of Ilex latifolia Thunb inhibited lipogenesis via suppressing the expression of lipogenic genes. Aqueous extract of Ilex latifolia Thunb prevented body weight gain and hepatic steatosis, enhanced insulin sensitivity and reduced chronic inflammation in mice. The alleviation of body weight gain by Ilex latifolia Thunb suggested that herbal supplements of Ilex latifolia Thunb could be used in the intervention of development of obesity.
Results
Aqueous extract of Ilex latifolia Thunb decreases adipocyte lipid accumulation
Ilex kudingcha C.J. Tseng (kuding tea) has been implicated in the prevention of obesity and diabetes in traditional Chinese medicine1,11. To investigate the function of Ilex latifolia Thunb on obesity development, we first examined the effects of the aqueous extract of Ilex latifolia Thunb on the prevention of adipocyte differentiation of OP9 mouse stromal cells. Rosiglitazone was used as an inducer to promote the adipocyte differentiation of OP9 cells, and then an aqueous extract of Ilex latifolia Thunb was added into the medium from day 1 to day 5. Compared with the control, Ilex latifolia Thunb significantly decreased lipid accumulation, indicating an inhibitory effect of Ilex latifolia Thunb on lipid accumulation (Fig. 1a,b). Moreover, the aqueous extract of Ilex latifolia Thunb displayed significantly stronger inhibitory effects on OP9 cell lipogenesis than the aqueous extract of Ilex kudingcha C.J. Tseng (Fig. S1).
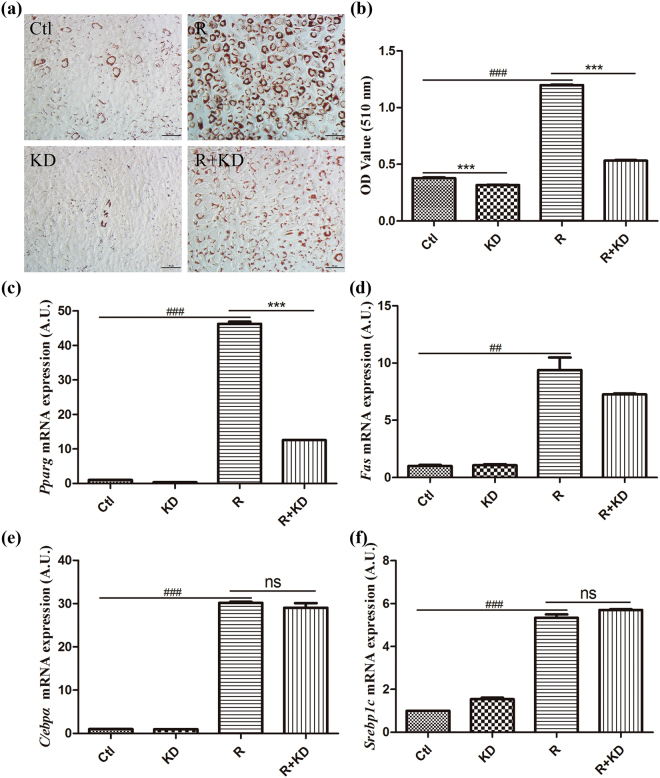
Figure 1.
Adipose differentiation in OP9 mouse stromal cells. (a) The cells were stained with oil red O at day 5. (b) The absorbance was measured at 510 nm. (c–f) qPCR was used to determine mRNA levels in OP9 mouse stromal cells. The aqueous extract of Ilex latifolia Thunb was added to the medium at 4 μg/mL. PBS was used as the vehicle control. Pparg: Ppar γ; Ctl: control; R: rosiglitazone; KD: aqueous extract of Ilex latifolia Thunb. β-actin was used as an internal control. Data are presented as the mean ± SEM. Significant differences between Ctl and R are indicated as ##P < 0.01, ###P < 0.001; significant difference versus R + KD or KD are indicated as ***P < 0.001.
Previous studies have shown that Ppar γ, C/ebpα, Srebp1c and Fas are critical genes in the regulation of adipogenesis and accumulation of fatty acids12–14. To verify whether their expressions were altered by Ilex latifolia Thunb, we first examined the mRNA levels of Ppar γ, C/ebpα, C/ebpβ, Adiponectin, Srebp1c and Fas in cells by a quantitative PCR approach. We found that the mRNA levels of Ppar γ were decreased in Ilex latifolia Thunb-treated cells compared to control cells, indicating that Ilex latifolia Thunb inhibits lipid accumulation via Ppar γ signaling (Fig. 1c). However, no change in C/ebpα, C/ebpβ and Srebp1c mRNA levels was observed in Ilex latifolia Thunb-treated cells compared to control cells, suggesting that C/ebpα or Srebp1c signaling is not involved in the inhibition of lipogenesis by Ilex latifolia Thunb (Figs 1d–f and S2b). The treatment of aqueous extract of Ilex latifolia Thunb increased Adiponectin mRNA levels in OP9 cells (Fig. S2a). Taken together, these results suggest that Ilex latifolia Thunb inhibits lipid accumulation due to suppression of Ppar γ transcription.
Ilex latifolia Thunb alleviates HFD-induced body weight gain
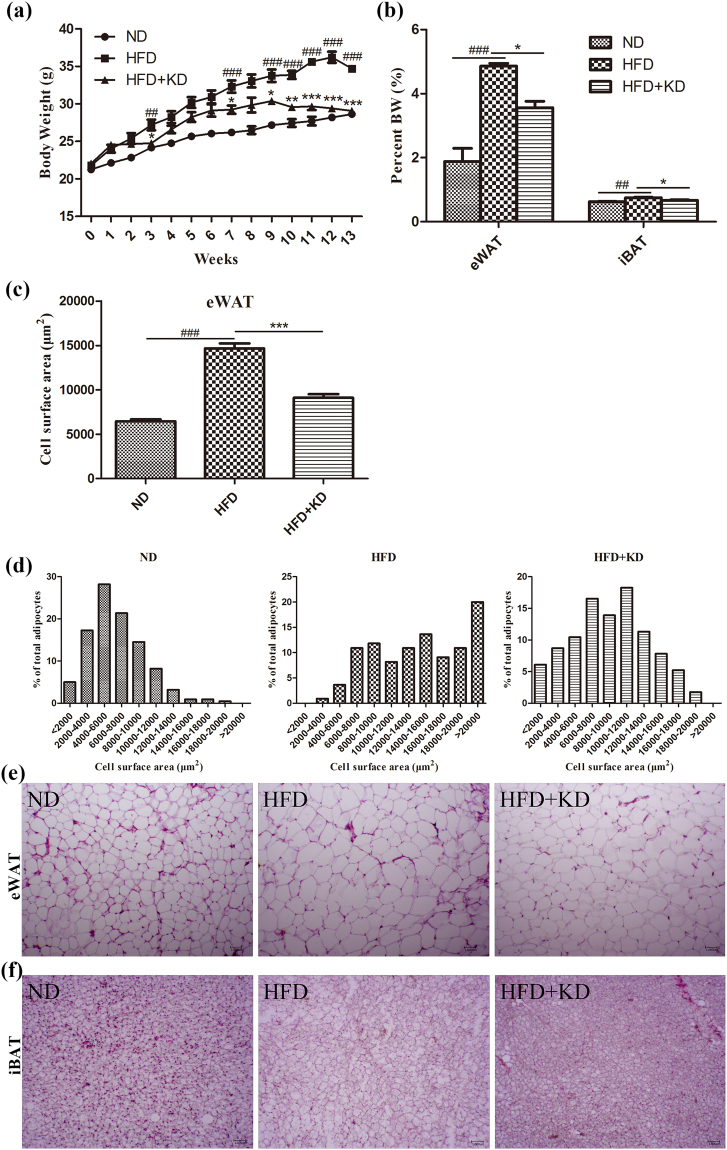
As described above, an aqueous extract of Ilex latifolia Thunb inhibited fatty acid accumulation in cells, raising the question of whether Ilex latifolia Thunb can prevent the development of obesity in animal models. To address this question, mice were fed a high-fat diet (HFD) either with or without aqueous extract of Ilex latifolia Thunb supplement. After mice were fed a HFD for 14 weeks, we found that mice treated with different dosage of aqueous extract of Ilex latifolia Thunb displayed similarly significantly reduced body weight gain compared to mice without treatment with Ilex latifolia Thunb (Figs 2a and S3). So middle-dose aqueous extract of Ilex latifolia Thunb was used to perform further experiments in this research. Mice treated with Ilex latifolia Thunb had significantly lower indices of epididymal white adipose tissue (eWAT) and intrascapular brown adipose tissue (iBAT) versus body weight than mice without Ilex latifolia Thunb, indicating a protective role of Ilex latifolia Thunb in the development of obesity (Fig. 2b). In the HFD group, mice fed Ilex latifolia Thunb had a smaller white and brown adipocyte size than controls, suggesting the inhibition of fatty acid accumulation (Fig. 2c–f). These observations demonstrate that aqueous extract of Ilex latifolia Thunb protected mice from fatty acid accumulation and reduced the body weight gain of the mice in the HFD group.
Figure 2.
Body weight. Mice were fed ND(normal diet), HFD, or HFD + KD (0.33%) for 14 weeks. (a) Body weight. (b) The indices of eWAT and iBAT versus body weight (BW). (c) Cell surface area (CSA) of eWAT adipocytes. (d) Adipocyte size distribution of eWAT of all experimental groups. (e and f) Hematoxylin and eosin (H&E) staining of eWAT and iBAT adipocytes, magnification 100 × . n = 8 in each group. Significant differences between ND and HFD are indicated as ###P < 0.001; significant differences between HFD and HFD + KD are indicated as *P < 0.05, **P < 0.01, ***P < 0.001.
Ilex latifolia Thunb enhances energy expenditure
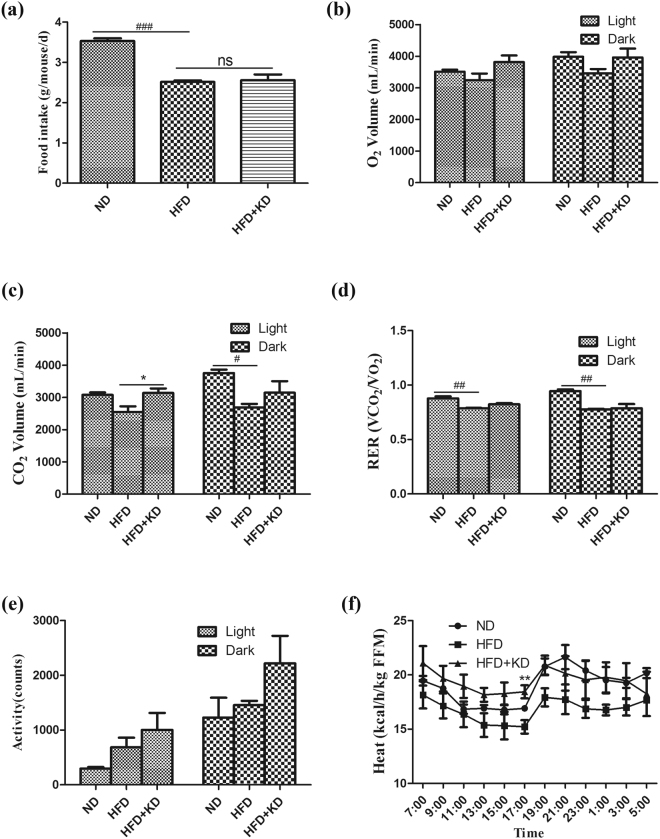
To investigate the energy balance, we monitored food intake amounts and found no change in food consumption in mice treated with Ilex latifolia Thunb compared to mice without Ilex latifolia Thunb when fed a HFD, indicating that neither food intake nor the appetites of the mice were modulated by Ilex latifolia Thunb (Fig. 3a). After the mice were HFD-fed for 12 weeks, mice treated with Ilex latifolia Thunb showed more CO2 release than did controls (Fig. 3b–d). And heat productions were increased in mice treated with Ilex latifolia Thunb compared to mice without Ilex latifolia Thunb on a HFD (Fig 3e, f), supporting denser iBAT in Fig. 2f. These data suggest that Ilex latifolia Thunb attenuated HFD-induced obesity due to enhanced energy expenditure.
Figure 3.
Energy balance. (a) Food intake (n = 8). (b–f) Metabolic chambers analysis of O2, CO2, RER, spontaneous physical activity and heat (n = 4). FFM: fat free mass. Significant differences between ND and HFD are indicated as ###P < 0.001; significant differences between HFD and HFD + KD are indicated as *P < 0.05, **P < 0.01.
Ilex latifolia Thunb protects against hepatic steatosis
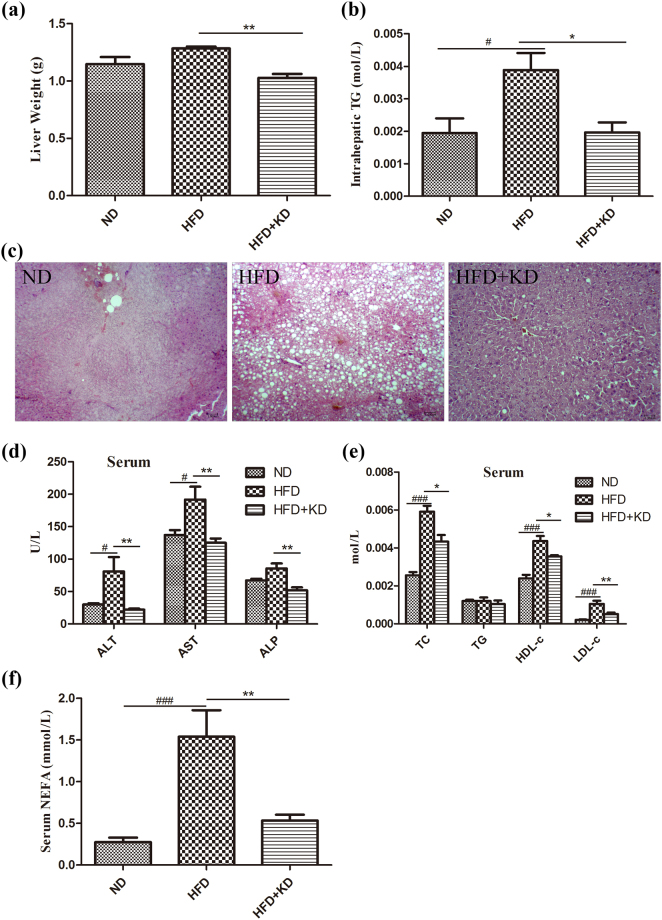
After being placed on a HFD for 14 weeks, mice treated with Ilex latifolia Thunb showed significantly lower liver weight than mice fed a HFD without Ilex latifolia Thunb (Fig. 4a). The analysis of intrahepatic triglyceride (TG) content revealed elevated TG levels in the livers of mice fed a HFD, which was prevented by Ilex latifolia Thunb treatment (Fig. 4b). Notably, we found that mice on a HFD showed macrovesicular steatosis, but mice treated with Ilex latifolia Thunb displayed markedly fewer fat droplets in the liver than the controls on a HFD, as revealed by hematoxylin and eosin (H&E) staining, indicating an inhibitory role of Ilex latifolia Thunb in hepatic steatosis (Fig. 4c). Ilex latifolia Thunb decreased the elevated alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) levels in mice fed a HFD compared to controls, suggesting a protective role of Ilex latifolia Thunb in hepatic injury (Fig. 4d). Moreover, mice treated with Ilex latifolia Thunb had significantly lower serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c) and low-density lipoprotein cholesterol (LDL-c) levels than mice fed a HFD (Fig. 4e). However, the serum TG level was similar between mice fed a HFD plus Ilex latifolia Thunb and controls fed a HFD. In contrast, the HFD group showed a significantly higher level of serum non-esterified fatty acids (NEFA) compared with the normal diet (ND) group, and Ilex latifolia Thunb reduced the level of NEFA in circulation (Fig. 4f). Thus, these results demonstrate that an aqueous extract of Ilex latifolia Thunb prevents HFD-induced hepatic steatosis.
Figure 4.
Liver function and serum lipid levels. (a) Weight of the liver. (b) Intrahepatic concentrations of TG. (c) H&E staining (×100) of liver sections. (d) The quantitative results of ALT, AST and ALP contents in serum. (e) Serum TC, TG, HDL-c and LDL-c levels were measured. (f) NEFA level in serum was measured. n = 8 for each group. Significant differences between ND and HFD are indicated as ###P < 0.001; significant differences between HFD and HFD + KD are indicated as *P < 0.05, **P < 0.01.
Ilex latifolia Thunb improves insulin sensitivity
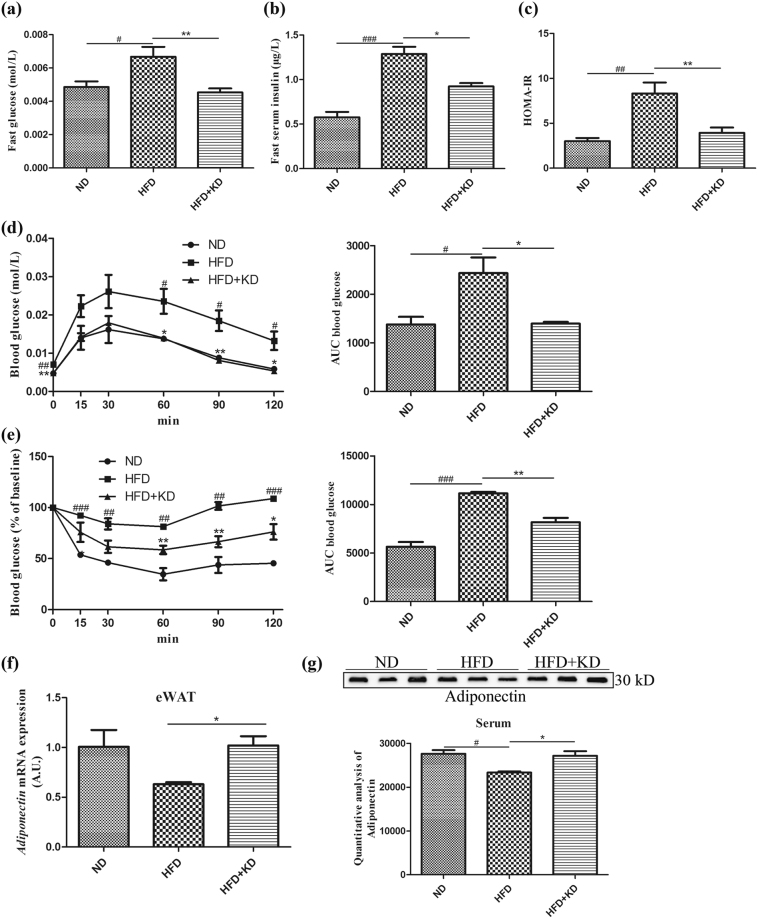
The beneficial effects of Ilex latifolia Thunb on the development of obesity prompted us to investigate whether insulin sensitivity and glucose homeostasis of mice were affected by Ilex latifolia Thunb supplementation. As shown in Fig. 5a and b, fasting glucose and blood insulin levels were similarly elevated in mice fed a HFD compared to mice fed a ND, indicating the status of obesity-related hyperglycemia and hyperinsulinemia. In contrast, mice fed a HFD with Ilex latifolia Thunb exhibited normal blood insulin and glucose concentrations, suggesting the improvement of obesity-related insulin sensitivity and glucose homeostasis. Indeed, the HOMA-IR was significantly decreased in mice fed a HFD with Ilex latifolia Thunb compared to controls fed a HFD (Fig. 5c). To verify this improvement, we performed glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) on mice after they were fed on a HFD for 12 weeks (Fig. 5d and e). The mice fed a HFD showed a decreased response to glucose load, indicating the status of HFD-induced glucose intolerance. In contrast, mice fed a HFD with Ilex latifolia Thunb showed normal glucose tolerance levels compared to controls fed a ND, suggesting improved glucose homeostasis. Mice on a ND were more insulin sensitive than mice fed a HFD, indicating the development of obesity-related insulin resistance. The insulin sensitivity was similar between mice fed a HFD with Ilex latifolia Thunb and controls fed a ND, indicating that Ilex latifolia Thunb enhanced insulin sensitivity. Consistent with reports that adiponectin expression is associated with insulin sensitivity15, the mRNA level of Adiponectin was dramatically elevated in eWAT in mice fed a HFD supplemented with Ilex latifolia Thunb compared to that in HFD-control mice (Fig. 5f). The serum level of Adiponectin was also significantly elevated in mice treated with Ilex latifolia Thunb (Fig. 5g). Furthermore, the phosphorylation levels of Akt were increased in both the livers and the eWAT of Ilex latifolia Thunb-treated mice, as well as IRS1 protein level in the eWAT (Fig. S4). These results described above suggest that aqueous extract of Ilex latifolia Thunb protects mice from obesity-related insulin resistance.
Figure 5.
Insulin sensitivity. (a) Fasting blood glucose (n = 8). (b) Fasting insulin levels on ND, HFD or HFD + KD. (c): HOMA-IR. (d) Intraperitoneal GTT from mice fed each diet (left) and area under curve (AUC). (e) ITT for mice fed ND, HFD or HDF + KD (left) and area under curve (AUC). (f) Real-time qPCR results of gene expression levels of Adiponectin in eWAT. (g) Immunoblotting analysis of Adiponectin signaling in serum. Full-length blots are presented in Supplementary Figure S7. For b-g, n = 5 in each group. Significant differences between ND and HFD are indicated as ###P < 0.001; significant differences between HFD and HFD + KD are indicated as *P < 0.05, **P < 0.01.
Ilex latifolia Thunb decreases the expression of lipogenic genes in liver and adipose tissue
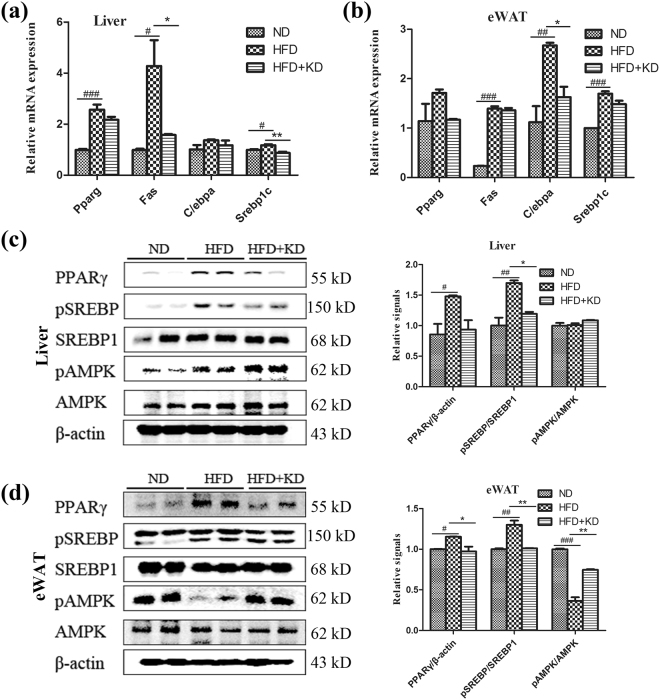
To probe the underlying molecular mechanism for the protective effects of Ilex latifolia Thunb on the development of obesity, we investigated the expression of lipogenic genes in both liver and adipose tissues. Quantitative PCR analysis showed that the mRNA levels of Ppar γ, Fas and Srebp1c in the liver were reduced in Ilex latifolia Thunb-treated mice compared with those in control mice, indicating an inhibitory effect of Ilex latifolia Thunb in liver lipogenesis (Fig. 6a). Indeed, immunoblotting results showed that PPARγ protein level and phosphorylation level of SREBP-1c were significantly enhanced in the liver of mice fed a HFD, compared to mice fed a ND, supporting the development of hepatic steatosis in Fig. 4c (Fig. 6c). Mice fed a HFD with Ilex latifolia Thunb exhibited lower levels of PPARγ protein and SREBP-1c phosphorylation in liver than control mice fed a HFD, indicating that Ilex latifolia Thunb inhibited the lipogenic signaling in liver. Consistent with the liver data, the mRNA levels of C/ebpα were decreased in the eWAT of mice fed a HFD with Ilex latifolia Thunb, suggesting that Ilex latifolia Thunb inhibited lipogenesis in adipose tissue (Fig. 6b). Indeed, significantly reduced PPARγ protein and phosphorylated-SREBP-1c levels in eWAT were observed in mice fed a HFD with Ilex latifolia Thunb (Fig. 6d). Meanwhile, the mRNA expression of lipolytic genes Atgl, Hsl, Plin1 and Adrb3 were elevated in the eWAT of HFD mice, suggesting a positive feedback regulation in lipolytic states (Fig. S5)16,17. Moreover, under the HFD condition, mice showed significantly higher phosphorylated-AMPK level in liver tissue than did controls. Ilex latifolia Thunb also increased the level of phosphorylated-AMPK in liver tissue, supporting the increased activity in Figure 3e 18. Notably, a significantly reduced phosphorylated-AMPK level was observed in the eWAT of mice fed a HFD compared to controls. However, Ilex latifolia Thunb supplement restored the phosphorylated-AMPK in eWAT to control levels, suggesting enhanced energy expenditure in those mice (Fig. 3f). Thus, these data suggest that Ilex latifolia Thunb protects mice from obesity by reducing lipogenesis.
Figure 6.
The expression of lipogenic genes in liver and adipose tissues. (a) mRNA expression levels of Pparg(Ppar γ ), Fas, C/ebpα and Srebp1c in liver. (b) mRNA expression levels of Pparg, Fas, C/ebpα and Srebp1c in eWAT. (c) PPARγ, p-SREBP1c, SREBP-1c, p-AMPK and AMPK levels in liver. (d) PPARγ, p-SREBP1c, SREBP-1c, p-AMPK and AMPK levels in eWAT. Full-length blots are presented in Supplementary Figure S7. n = 5 for each group. Significant differences between ND and HFD are indicated as ###P < 0.001; significant differences between HFD and HFD + KD are indicated as *P < 0.05, **P < 0.01.
Ilex latifolia Thunb decreases inflammation
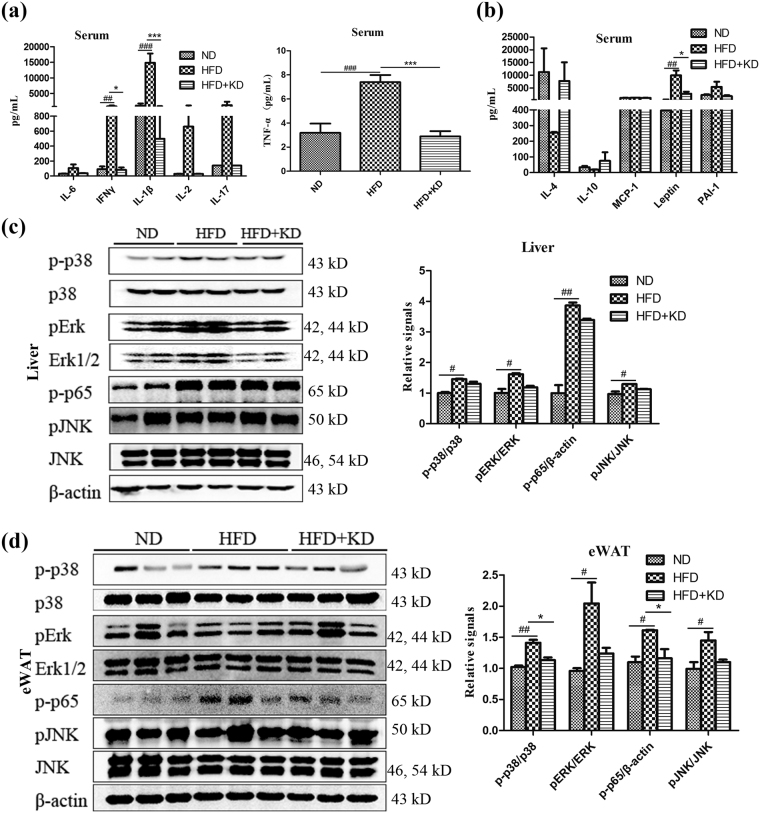
Obesity is often associated with an inflammatory state in metabolic tissues and is defined as low-grade and chronic inflammation in response to excess nutrients and energy19. The inflammation in metabolic tissues including adipose and liver leads to a state of insulin insensitivity and metabolic dysfunction. Since aqueous extract of Ilex latifolia Thunb improved insulin sensitivity and ameliorated metabolic disorders, we next examined whether Ilex latifolia Thunb could prevent low-grade inflammation in mice under HFD conditions. Mice fed a HFD showed higher serum pro-inflammatory cytokine levels of IL-6, TNF-alpha, IFN-gamma, IL-1β, IL-2 and IL-17 and lower serum anti-inflammatory cytokine levels of IL-4 and IL-10 than those of mice fed a ND, suggesting an inflammatory status. Remarkably, under the HFD condition, pro-inflammatory cytokine levels of IL-6, TNF-alpha, IFN-gamma, IL-1β, IL-2 and IL-17 and anti-inflammatory cytokine concentrations of IL-4 and IL-10 were restored to control levels in mice treated with Ilex latifolia Thunb, indicating that Ilex latifolia Thunb reduces pro-inflammatory markers in the circulation (Fig. 7a, b). Consistently, phosphorylated p38 MAPK and p-p65 (p65 constitutes a subunit of NF-κB) levels in eWAT were significantly increased in mice fed a HFD compared to controls fed a ND, and they were dramatically decreased by Ilex latifolia Thunb, whereas the activation of pErk1/2 and pJNK in both liver and eWAT were similar between Ilex latifolia Thunb-treated mice and controls, indicating Ilex latifolia Thunb decreased activity of pro-inflammatory signaling pathways such as p38 MAPK and p65 NF-κB in eWAT (Fig. 7c and d). Moreover, the aqueous extract of Ilex latifolia Thunb decreased mRNA levels of Il6 and Mcp1 in the eWAT in HFD + KD mice compared to HFD fed mice (Fig. S6). In addition to inflammatory cytokines, the levels of the adipokine leptin were also elevated in mice fed a HFD compared to controls, and Ilex latifolia Thunb supplement reversed the high levels of leptin to normal concentrations, suggesting a regulatory role of Ilex latifolia Thunb in adipokine production (Fig. 7b). Together, these observations reveal that Ilex latifolia Thunb reduces HFD-induced inflammation via the modulation of cytokine levels and via p38 MAPK and p65 NF-κB signaling.
Figure 7.
Inflammatory cytokine levels and cell signaling. (a and b) Cytokine levels in serum. (c) Immunoblotting analysis of p38 MAPK, Erk1/2, NF-κB p-p65 and JNK signaling in liver. (d) Immunoblotting analysis of p38 MAPK, Erk1/2, NF-κB p-p65 and JNK signaling in eWAT. Full-length blots are presented in Supplementary Figures S8 and S9. For a and b, n = 8 in each group, for c and d, n = 5 per group. Significant differences between ND and HFD are indicated as ##P < 0.01; significant differences between HFD and HFD + KD are indicated as *P < 0.05, ***P < 0.001.
Discussion
In this study, we provide the evidence that an aqueous extract of Ilex latifolia Thunb prevents HFD-induced body weight gain. The aqueous extract of Ilex latifolia Thunb decreases gain in body weight as well as the indices of eWAT and iBAT versus body weight, and it improves liver function, obesity-related insulin resistance and chronic inflammation in HFD-fed mice. Lipid levels in liver and blood glucose of mice were significantly reduced, and the expression of lipogenic genes was dramatically altered by Ilex latifolia Thunb treatment. These results suggest that an aqueous extract of Ilex latifolia Thunb may play a protective role in obesity, T2D, non-alcoholic fatty liver disease (NAFLD) and chronic inflammation.
Previous studies have shown that the ethanolic extract but not the aqueous extract of Ilex kudingcha C.J. Tseng inhibited adipocyte differentiation and the development of obesity, suggesting that liposoluble components of Ilex kudingcha C.J. Tseng, including lupeol and ursolic acid, may act as the anti-adipogenic and anti-metabolic syndrome active compound candidates1. In contrast, the aqueous extract of Ilex kudingcha C.J. Tseng was found to possess an anti-diabetic activity, and chlorogenic acids, dicaffeoylquinic acids, flavonoids, triterpenoid saponins and other natural products were speculated as being active compound candidates7,11. Therefore, the truly active compounds from Ilex kudingcha C.J. Tseng are still unknown. Here, the anti-obesity active components present in Ilex latifolia Thunb were water soluble, and their role in lipogenesis is totally different from the aqueous and ethanolic extracts of Ilex kudingcha C.J. Tseng, suggesting different active components in the aqueous extracts of Ilex latifolia Thunb and Ilex kudingcha C.J. Tseng. However, further experiments are required for the identification of the active components of Ilex latifolia Thunb that have an anti-obesity role.
Kuding tea is a traditional beverage in China. Like the popular green tea, kuding tea is used in health care formulas to ameliorate metabolic disorders such as obesity. The large-leaved kuding tea has exhibited many biological functions on health6. Kuding tea Ilex kudingcha C.J. Tseng is able to lower the levels of serum TC, LDL-c, and HDL-c in mice, suppress rat liver mitochondrial peroxidation and prevent the progression of atherosclerosis20,21. Similarly, in the current study, body weight and serum TC, LDL-c, HDL-c, ALT, AST, ALP and glucose levels were significantly increased in mice fed a HFD for 14 weeks. The aqueous extract of Ilex latifolia Thunb treatment reversed body weight gain, serum levels of TC, LDL-c, HDL-c, ALT, AST, and ALP, and glucose tolerance and insulin sensitivity in mice, suggesting that Ilex latifolia Thunb prevents the development of metabolic syndrome. However, more detailed analysis is required to dissect the molecular mechanism of the protective role of Ilex latifolia Thunb in dyslipidemia.
Although reduction of food intake may cause body weight loss and reduce the levels of blood glucose and lipids, we did not observe a difference in food intake between mice fed a HFD and mice fed a HFD with the aqueous extract of Ilex latifolia Thunb, suggesting no change in appetite. However, CO2 release and heat production were increased in mice treated with Ilex latifolia Thunb compared to controls. Therefore, the protective roles of the aqueous extract of Ilex latifolia Thunb against body weight gain may be associated with enhanced energy expenditure but independent of food intake or appetite. Consistently, the activation of AMPK, an energy expenditure marker, was significantly increased in adipose tissue by Ilex latifolia Thunb supplement. Additionally, adipocyte differentiation plays a key role in the pathogenesis of obesity22. Weight gain is a consequence of the increase in adipocyte volume (hypertrophy) and numbers (hyperplasia) caused by excess calories stored as TG, while weight loss was usually caused by a reduction of the adipocyte mass and number by suppression of energy intake or overburn of excess calories23,24. Here, the anti-adipogenesis and anti-obesity effects of Ilex latifolia Thunb were further examined in OP9 mouse stromal cells. The Ilex latifolia Thunb treatment inhibited the adipocyte differentiation and accumulation of fatty acids in OP9 mouse stromal cells, suggesting an inhibitory effect of Ilex latifolia Thunb in lipogenesis. Indeed, mice treated with Ilex latifolia Thunb have smaller adipocytes and fewer fat droplets in the liver than controls fed a HFD. However, markers for adipogenesis Cebpα and Cebpβ gene expression is not changed in OP9 cells and tissue from mice treated with the extract of Ilex latifolia Thunb, indicating complicated effects of the extract of Ilex latifolia Thunb on lipogenesis. Further experiments are required to determine how Ilex latifolia Thunb inhibits lipogenesis and increases energy expenditure.
Low-grade inflammation is closely related with HFD-induced obesity. A HFD induces the expression of several pro-inflammatory cytokines (TNF-α, NF-κB, IL-6, IL-1β and MCP-1) as well as the activation of JNK (c-Jun N-terminal kinase) in white adipose tissue and liver25. In our study, similar inflammatory responses were observed in HFD-fed mice compared to ND controls. As a pure natural health herb, Ilex latifolia Thunb suppressed HFD-induced chronic inflammation, as evidenced by a modulated cytokine profile compared with HFD models, although a more detailed analysis is required to elucidate the molecular mechanism. These findings suggest that the aqueous extract of Ilex latifolia Thunb may be used as a potential dietary strategy for preventing metabolic disorders such as obesity, diabetes, non-alcoholic steatohepatitis and atherosclerosis. The potential of using naturally occurring dietary supplements to regulate body weight and lipid metabolism is attractive. Because this traditional beverage is safe and inexpensive, it should be considered as a dietary therapy for metabolic syndrome. This type of therapy is particularly important because weight loss and existing treatments for NAFLD have poor long-term success rates. Further investigations are required to identify the active compounds in Ilex latifolia Thunb, their roles in metabolic diseases, and the molecular mechanisms by which this supplement protects against obesity.
Materials and Methods
Cell culture and differentiation
The leaves of Ilex latifolia Thunb were from Hainan. Rosiglitazone (R) was purchased from Sigma Ltd. (USA). Ilex latifolia Thunb with a material:liquid ratio of 1:10 were boiled in distilled water for 3 h. The extracts were concentrated on a rotary evaporator under reduced pressure. OP9 mouse stromal cells were obtained from the ATCC (USA) and maintained in MEM-α containing L-Glutamine, with 20% FBS (Secure fetal bovine serum; Gibco, Australia), 100 U/mL penicillin, and 100 μg/mL streptomycin26. For adipocyte differentiation, 1 μM rosiglitazone was added into the culture for differentiation at 37 °C in 5% CO2. Aqueous extract of Ilex latifolia Thunb was added into the medium at 4 μg/mL for 5 days. Three wells of OP9 cells were used for each experiment. After 5 days of treatment, the cells were washed twice with PBS, fixed with 4% paraformaldehyde at room temperature for 30 minutes and then stained with oil red O (Sigma, USA), and the absorbance was measured at 510 nm.
Quantitative real-time PCR
Total RNA was extracted using TRIzol (Ambion, USA). cDNA was synthesized by a reverse transcription reagent kit (RT Reagent Kit with gDNA Eraser RR047A, TaKaRa, Japan). Gene expression levels were analyzed by quantitative real-time PCR using the BIO-RAD CFX Connect Real-Time System (CA, USA). The primers of Pparγ (NM_001127330.2): 5′-TCGCTGATGCACTGCCTATG-3, 5′-GAGAGGTCCACAGAGCTGATT-3′; Fas (NM_007988.3): 5′-GGAGGTGGTGATAGCCGGTAT-3′, 5′-TGGGTAATCCATAGAGCCCAG-3′; C/ebpα (NM_001287514.1): 5′-CAAGAACAGCAACGAGTACCG-3′, 5′-GTCACTGGTCAACTCCAGCAC-3′; C/ebpβ (NM_001287738.1): 5′-CGACTTCAGCGCCTACATTGA-3′, 5′-CTAGCGACAGACCCCACAC-3′; Srebp1c (NM_001313979.1): 5′-GAGCGAGCGTTGAACTGTAT-3′, 5′-ATGCTGGAGCTGACAGAGAA-3′; Adiponectin (NM_009605.5): 5′-TGTTCCTCTTAATCCTGCCCA-3′, 5′-CCAACCTGCACAAGTTCCCTT-3′; Il6 (NM_001314054.1): 5′-CCAAGAGGTGAGTGCTTCCC-3′, 5′-CTGTTGTTCAGACTCTCTCCCT-3′; Mcp1 (NM_011333.3): 5′-TCACCTGCTGCTACTCATTC-3′, 5′-TACAGAAGTGCTTGAGGTGG-3′; Atgl (NM_001163689.1): 5′-CAACGCCACTCACATCTACGG-3′, 5′-GGACACCTCAATAATGTTGGCAC-3′; Hsl (NM_001039507.2): 5′-TCCCTCAGTATCTAGGCCAGA-3′, 5′-GGCTCATTTGGGAGACTTTGTTT-3′; Plin1 (NM_001113471.1): 5′-GGGACCTGTGAGTGCTTCC-3′, 5′-GTATTGAAGAGCCGGGATCTTTT-3′; Adrb3 (NM_013462.3): 5′-GGCCCTCTCTAGTTCCCAG-3′, 5′-TAGCCATCAAACCTGTTGAGC-3′; β-actin (NM_007393.5): 5′-GGCTGTATTCCCCTCCATCG-3′, 5′-CCAGTTGGTAACAATGCCATGT-3′ were used to perform qPCR.
HFD feed and Metabolic assays
The animal protocols used in this study were approved by the Animal Ethics Committee of Jiangnan University, China. All experiments were performed in accordance with China regulations for the administration of affairs concerning experimental animals 2017. Male C57BL/6 mice (6 weeks old) were purchased from the Xi Nuo Sai BioScience, Inc. (Suzhou, China) and were randomly separated into three groups: normal diet (ND; chow diet, 10% of calories derived from fat); high-fat diet (HFD; 45% of calories derived from fat, Research Diets, Beijing, China; D12451); and HFD + KD (0.33% aqueous extract of Ilex latifolia Thunb was added to the HFD) after acclimatization for 1 week. The diet study was continued for 14 weeks. Hematoxylin and eosin (H&E) staining of epididymal white adipose tissue (eWAT), intrascapular brown adipose tissue (iBAT) and liver were performed as in previous reports27,28. The eWAT adipocyte cell surface area (CSA) from at least 150 cells/section was measured using the software Adobe Photoshop CS529. Intrahepatic triglyceride (TG) content was measured by colorimetric methods (Triglyceride Quantification Kit, Bio Vision, USA)30. Respiratory exchange ratio (RER, the volume ratio of CO2 exhaled versus O2 consumed), heat (kcal/h/kg FFM) and spontaneous locomotor activity (X Ambulatory counts) were measured using metabolic chambers (Columbus Instruments, Columbus, OH). After 14 weeks of HFD feeding, the mice were sacrificed, and serum was collected. Serum TG, TC, HDL-c, LDL-c, ALT, AST and ALP levels were measured using Roche Modular P800 Automatic Analyzer (Roche, Basel, Switzerland). Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs) were performed at the end of this experiment as in previous reports31. For the GTT, mice were fasted overnight for 12 hours, and glucose (2 g/kg body weight) was injected intraperitoneally. For the ITT, insulin (0.75 U/kg body weight) was injected intraperitoneally. The blood samples were collected for the determination of glucose values with an Accu-chek glucometer (Roche Diagnostics, Basel, Switzerland). Insulin levels in serum were measured using an ELISA kit (Mouse Insulin ELISA, Mercodia, Sweden) according to the standard procedure. The intra- and inter-assay coefficient of variation for the insulin ELISA kit are < 4% and < 6%, respectively. Mouse cytokines in serum were measured using Milliplex Map kits (EMD Millipore Corporation, USA) by Luminex (USA) and a TNF-α ELISA kit (BioLegend, USA). The intra- and inter-assay coefficient of variation for the Luminex assays are < 5% and < 10%. Levels of non-esterified fatty acids (NEFA) in serum were determined by an enzymatic kit (Nanjing Jiancheng Bioengineering Institute, China).
Cell signaling
The tissues were lysed with RIPA buffer containing protease inhibitors (Sangon Biotech, Shanghai). Immunoblotting was performed as in previous reports28,32. Tissue lysates were immunoblotted for SREBP-1c (Santa Cruz Biotechnology), p-AMPK (Ser485, Santa Cruz Biotechnology), β-actin (Santa Cruz Biotechnology), PPARγ (Cell Signaling), p-SREBP1c (Ser372, Cell Signaling), AMPK (Cell Signaling), p-p38 (Thr180/Tyr182, Cell Signaling), p38 MAPK (Cell Signaling), p-Erk1/2 (Thr202/Tyr204, Cell Signaling), Erk1/2 (Cell Signaling), p-Akt (Ser 473, Cell Signaling), Akt (Cell Signaling), NF-κB p-p65 (Ser 536, Cell Signaling), p-JNK (Thr183/Tyr185, Cell Signaling), JNK (Cell Signaling), IRS1 (Cell Signaling) and Adiponectin (Abcam)31,32.
Statistical analysis
Each experiment was repeated at least three times. All data are presented as the mean ± SEM. Data were analyzed by one-way ANOVA or two-way ANOVA. *P < 0.05; **P < 0.01; ***P < 0.001.
Electronic supplementary material
Acknowledgements
This research was supported by the National Natural Science Foundation of China Grant No. 31471321 (Z.H.), the Young-Thousand-talents Plan (Z.H.) and Jiangsu Province Recruitment Plan for High-level, Innovative and Entrepreneurial Talents (Z.H.).
Author Contributions
Hailan Wu and Zhao He designed the research; Hailan Wu performed the research; Hailan Wu, Yueyuan Yu, Jin Zang, Yikuan Wu, Yue-lei Chen and Zhao He analyzed the data; Yue-lei Chen provided the cell line; Hailan Wu and Zhao He wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Hailan Wu and Yue-Lei Chen contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15292-x.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fan S, et al. Extract of Kuding tea prevents high-fat diet-induced metabolic disorders in C57BL/6 mice via liver X receptor (LXR) beta antagonism. PloS one. 2012;7:e51007. doi: 10.1371/journal.pone.0051007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. The Journal of nutritional biochemistry. 2007;18:179–183. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu C, Zhu W, Shen CL, Gao W. Green tea polyphenols reduce body weight in rats by modulating obesity-related genes. PloS one. 2012;7:e38332. doi: 10.1371/journal.pone.0038332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang X, Yin L, Li T, Chen Z. Green tea extracts reduce adipogenesis by decreasing expression of transcription factors C/EBPalpha and PPARgamma. International journal of clinical and experimental medicine. 2014;7:4906–4914. [PMC free article] [PubMed] [Google Scholar]
- 5.Santamarina AB, et al. Decaffeinated green tea extract rich in epigallocatechin-3-gallate prevents fatty liver disease by increased activities of mitochondrial respiratory chain complexes in diet-induced obesity mice. The Journal of nutritional biochemistry. 2015;26:1348–1356. doi: 10.1016/j.jnutbio.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Li L, et al. The large-leaved Kudingcha (Ilex latifolia Thunb and Ilex kudingcha C.J. Tseng): a traditional Chinese tea with plentiful secondary metabolites and potential biological activities. Journal of natural medicines. 2013;67:425–437. doi: 10.1007/s11418-013-0758-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li L, et al. Quantitative Analysis of Five Kudinosides in the Large-leaved Kudingcha and Related Species from the GenusIlexby UPLC-ELSD. Phytochemical Analysis. 2012;23:677–683. doi: 10.1002/pca.2372. [DOI] [PubMed] [Google Scholar]
- 8.Zhao X, Wang Q, Qian Y, Song JL. Ilex kudingcha C.J. Tseng (Kudingcha) has in vitro anticancer activities in MCF-7 human breast adenocarcinoma cells and exerts anti-metastatic effects in vivo. Oncology letters. 2013;5:1744–1748. doi: 10.3892/ol.2013.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu K, et al. In vitro and in vivo anti-cancer activities of Kuding tea (Ilex kudingcha C.J. Tseng) against oral cancer. Experimental and therapeutic medicine. 2014;7:709–715. doi: 10.3892/etm.2013.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao X, Wang Q, Qian Y, Song JL. Ilex kudingcha C.J. Tseng (Kudingcha) prevents HCl/ethanolinduced gastric injury in SpragueDawley rats. Molecular medicine reports. 2013;7:1613–1616. doi: 10.3892/mmr.2013.1402. [DOI] [PubMed] [Google Scholar]
- 11.Song C, Xie C, Zhou Z, Yu S, Fang N. Antidiabetic Effect of an Active Components Group from Ilex kudingcha and Its Chemical Composition. Evidence-based complementary and alternative medicine: eCAM. 2012;2012:423690. doi: 10.1155/2012/423690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Ojeda, F. J., Ruperez, A. I., Gomez-Llorente, C., Gil, A. & Aguilera, C. M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. International journal of molecular sciences17, 10.3390/ijms17071040 (2016). [DOI] [PMC free article] [PubMed]
- 13.Li P, et al. Adipocyte NCoR Knockout Decreases PPARγ Phosphorylation and Enhances PPARγ Activity and Insulin Sensitivity. Cell. 2011;147:815–826. doi: 10.1016/j.cell.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker AK, et al. A Conserved SREBP-1/Phosphatidylcholine Feedback Circuit Regulates Lipogenesis in Metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khatib, S. A., Rossi, E. L., Bowers, L. W. & Hursting, S. D. Reducing the burden of obesity-associated cancers with anti-inflammatory long-chain omega-3 polyunsaturated fatty acids. Prostaglandins & other lipid mediators, 10.1016/j.prostaglandins.2016.07.011 (2016). [DOI] [PMC free article] [PubMed]
- 16.Ding, L. et al. Reduced lipolysis response to adipose afferent reflex involved in impaired activation of adrenoceptor-cAMP-PKA-hormone sensitive lipase pathway in obesity. Scientific reports6, 10.1038/srep34374 (2016). [DOI] [PMC free article] [PubMed]
- 17.Geng B, et al. Increase or decrease hydrogen sulfide exert opposite lipolysis, but reduce global insulin resistance in high fatty diet induced obese mice. PloS one. 2013;8:e73892. doi: 10.1371/journal.pone.0073892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature cell biology. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 20.Song C, et al. The Hypolipidemic Effect of Total Saponins from Kuding Tea in High-Fat Diet-Induced Hyperlipidemic Mice and Its Composition Characterized by UPLC-QTOF-MS/MS. Journal of food science. 2016;81:H1313–1319. doi: 10.1111/1750-3841.13299. [DOI] [PubMed] [Google Scholar]
- 21.Thuong PT, et al. Antioxidant activity and principles of Vietnam bitter tea Ilex kudingcha. Food Chemistry. 2009;113:139–145. doi: 10.1016/j.foodchem.2008.07.041. [DOI] [Google Scholar]
- 22.Peterson SJ, et al. Oxidized HDL is a potent inducer of adipogenesis and causes activation of the Ang-II and 20-HETE systems in human obese females. Prostaglandins & other lipid mediators. 2016;123:68–77. doi: 10.1016/j.prostaglandins.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Rosen ED, Spiegelman Bruce M. What We Talk About When We Talk About Fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang QA, Scherer PE, Gupta RK. Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. Journal of lipid research. 2014;55:605–624. doi: 10.1194/jlr.R046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Current opinion in pharmacology. 2016;29:77–89. doi: 10.1016/j.coph.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolins NE, et al. OP9 mouse stromal cells rapidly differentiate into adipocytes: characterization of a useful new model of adipogenesis. Journal of lipid research. 2006;47:450–460. doi: 10.1194/jlr.D500037-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Kim SY, et al. Inhibitory effect of anthocyanin-rich black soybean testa (Glycine max (L.) Merr.) on the inflammation-induced adipogenesis in a DIO mouse model. Journal of Functional Foods. 2015;14:623–633. doi: 10.1016/j.jff.2015.02.030. [DOI] [Google Scholar]
- 28.He Z, et al. Nonreceptor tyrosine phosphatase Shp2 promotes adipogenesis through inhibition of p38 MAP kinase. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E79–88. doi: 10.1073/pnas.1213000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendez-Gimenez L, et al. Sleeve Gastrectomy Reduces Hepatic Steatosis by Improving the Coordinated Regulation of Aquaglyceroporins in Adipose Tissue and Liver in Obese Rats. Obesity surgery. 2015;25:1723–1734. doi: 10.1007/s11695-015-1612-z. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez A, et al. Leptin administration restores the altered adipose and hepatic expression of aquaglyceroporins improving the non-alcoholic fatty liver of ob/ob mice. Scientific reports. 2015;5:12067. doi: 10.1038/srep12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He, Z. et al. Shp2 Controls Female Body Weight and Energy Balance by Integrating Leptin and Estrogen Signals. Molecular and Cellular Biology32(10), 1867–1878, doi:10.1128/MCB.06712-11 (2012). [DOI] [PMC free article] [PubMed]
- 32.Wu S, et al. Omega-3 free fatty acids inhibit tamoxifen-induced cell apoptosis. Biochemical and biophysical research communications. 2015;459:294–299. doi: 10.1016/j.bbrc.2015.02.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.