Abstract
Setting: Tuberculosis (TB) is the leading cause of death among people living with the human immunodeficiency virus (PLHIV) in Papua New Guinea. Despite a policy for isoniazid preventive therapy (IPT) among PLHIV, implementation has been slow.
Objective: We prospectively evaluated a standardized guided screening process, including TB diagnostic support, to increase IPT initiation in adult PLHIV on antiretro-viral treatment.
Design: The guided process included a paper-based IPT screening tool that prompted review of patient history and TB symptoms and sputum analysis by smear microscopy and Xpert® MTB/RIF. Chest X-ray was performed at the provider's discretion. We quantified the yield of this guided process on IPT initiation and detection of TB and rifampicin resistance, and examined the contributions of each diagnostic modality.
Results: Among 532 patients, TB was ruled out and IPT initiated in 450 (84%). TB was diagnosed and treatment was started in 82 (15%) patients. Xpert detected rifampicin resistance in one of 21 patients (5%, 95%CI 0.24–21.3) with a positive Xpert result. All TB cases were diagnosed by chest X-ray and/or Xpert. No cases were diagnosed by sputum smear alone.
Conclusion: A standardized guided process, including TB diagnostic support, successfully enabled IPT initiation and identified a large burden of undetected TB.
Keywords: latent tuberculous infection, Xpert MTB/RIF, smear microscopy, chest X-ray, tuberculosis screening
Abstract
Contexte : La tuberculose (TB) est la première cause de décès parmi les personnes vivant avec le VIH (PVVIH) en Papouasie Nouvelle Guinée. En dépit d'une politique en faveur du traitement préventif par isoniazide (TPI) parmi les PVVIH, la mise en oeuvre a été lente.
Objectif : Nous avons prospectivement évalué un processus de dépistage standardisé guidé, incluant le soutien au diagnostic de la TB, pour augmenter la mise en route du TPI chez les adultes PVVIH sous traitement antirétroviral.
Schéma : Le processus guidé a inclus un outil de dépistage sur support papier du TPI qui a suscité la revue des antécédents des patients, les symptômes de TB et l'analyse des crachats par microscopie de frottis et Xpert® MTB/RIF. Une radiographie pulmonaire a été réalisée à la discrétion du prestataire de soins. Nous avons quantifié le rendement de ce processus guidé relatif à la mise en route du TPI et à la détection de la TB et de la résistance à la rifampicine et examiné les contributions de chaque modalité de diagnostic.
Résultats : Parmi 532 patients, la TB a été éliminée et le TPI a été initié chez 450 (84%) patients. Une TB a été diagnostiquée et le traitement a été mis en œuvre chez 82 (15%) patients. L'Xpert a détecté la résistance à la rifampicine chez un des 21 patients (5% ; IC95% 0,24–21,3) avec un résultat d'Xpert positif. Tous les cas de TB ont été diagnostiqués par radiographie pulmonaire et/ou Xpert. Aucun cas n'a été diagnostiqué par frottis de crachats seul.
Conclusion : Un processus guidé standardisé, incluant la soutien au diagnostic de la TB, a permis la mise en route du TPI et identifié une large charge de TB non détectée.
Abstract
Marco de referencia: La tuberculosis (TB) es la principal causa de muerte en las personas infectadas por el virus de la inmunodeficiencia humana (VIH) en Papua Nueva Guinea. Pese a las políticas vigentes en materia de tratamiento preventivo con isoniazida (TPI) en las personas afectadas por el VIH, su aplicación ha sido lenta.
Objetivo: Se evaluó de manera prospectiva un procedimiento normalizado dirigido de detección sistemática, que comprendía el apoyo al diagnóstico de la TB, con el objeto de aumentar la tasa de iniciación del TPI en los adultos aquejados de infección por el VIH que recibían tratamiento antirretrovírico.
Método: El procedimiento dirigido comportaba un instrumento impreso de detección para el TPI, que incitaba la evaluación de los antecedentes del paciente, los síntomas de TB y un análisis del esputo mediante la baciloscopia y la prueba Xpert® MTB/RIF. La radiografía de tórax se practicó según el criterio del profesional de salud. Se cuantificó el efecto de este método dirigido sobre la iniciación del TPI, la detección de la TB y la resistencia a rifampicina y se examinaron además las contribuciones de cada modalidad diagnóstica.
Resultados: De los 532 pacientes, en 450 se descartó el diagnóstico de TB y se inició el TPI (84%). Se diagnosticó la TB y se inició el tratamiento a 82 personas (15%). Mediante la prueba Xpert se detectó la resistencia a rifampicina en uno de los 21 pacientes con resultado positivo a esta prueba (5%; intervalo de confianza del 95% de 0,24 a 21,3). Todos los casos de TB se diagnosticaron mediante la radiografía de tórax, la prueba Xpert o ambas. La baciloscopia por sí sola no permitió el diagnostico de ningún caso.
Conclusión: Un procedimiento normalizado dirigido, que comportaba apoyo al diagnóstico de la TB, hizo posible la iniciación del TPI y favoreció el reconocimiento de una gran carga de morbilidad por TB no detectada.
Tuberculosis (TB) remains the most common cause of death worldwide for individuals positive for the human immunodeficiency virus (HIV);1 prevention and timely diagnosis of TB in people living with HIV (PLHIV) is therefore of utmost importance. The World Health Organization (WHO) recommends systematic screening for TB in PLHIV in low-resource settings, which includes an assessment of TB symptoms at each clinical encounter.2 In 2011, following the endorsement of the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA), the WHO recommended the use of Xpert in individuals presumed to have HIV-associated TB.3 Studies from high-burden HIV settings have assessed the use of Xpert as a screening tool for TB in PLHIV during routine clinical evaluations and concluded that Xpert successfully detected otherwise unrecognized TB.4,5
Isoniazid preventive therapy (IPT) is an effective strategy for preventing progression from latent tuberculous infection to active TB disease, and has been shown to reduce severe illness and mortality in HIV-infected individuals.6–9 Despite IPT's advantages, a number of resource-limited countries have documented suboptimal IPT coverage in PLHIV, citing reasons such as stock-outs, perceived fears of poor adherence and proliferation of isoniazid (INH) resistance, a lack of local or national commitment, and perceived difficulty of ruling out active TB.10–12 While these country reports provide some insight into the barriers and the limited reach of IPT implementation, global coverage of IPT, although thought to be low, is not entirely understood due to incomplete reporting by countries.13
In accordance with the WHO recommendations,2 the Papua New Guinea (PNG) Department of Health guidelines indicate IPT for all PLHIV in the absence of TB symptoms or suggestive history. While data on the provision of IPT for PLHIV are not currently collected on the national level in PNG,13 a review of the National HIV Program in 2013 suggested that IPT implementation was minimal. The review also described poor coverage of TB symptom screening and that less than half of the estimated incident cases of TB among PLHIV began anti-tuberculosis treatment. These findings represent missed opportunities for active case finding and prevention of TB in PLHIV.14
We implemented and prospectively evaluated a standardized guided screening process, inclusive of diagnostic support, among PLHIV in PNG primarily to facilitate IPT uptake and, as a secondary objective, to describe symptoms and diagnostic modalities leading to a diagnosis of TB.
MATERIALS AND METHODS
Program setting
Implementation of the guided screening process took place in the Eastern Highlands province of PNG, which reported an HIV prevalence of 0.91% in 2013.15 Eastern Highlands is predominantly rural, with 95% of the population engaged in agricultural activity.16 Every clinic providing HIV care in the province was invited to participate in implementation through a quarterly meeting of antiretroviral therapy (ART) providers. The pilot implementation was conducted by Goroka General Hospital and Eastern Highlands Provincial Health Services in partnership with the Clinton Health Access Initiative (CHAI, Boston, MA, USA), and with the support of the National Department of Health (Port Moresby, PNG).
Programme design
Per national treatment guidelines, all adult PLHIV who were not currently on IPT or TB treatment were eligible for 6 months of IPT once TB was ruled out.17 Because national guidelines rely on symptom screening for ruling out TB, and we intended to address provider uncertainty with regard to ruling out TB, we prospectively evaluated a standardized guided screening process for TB among PLHIV eligible for IPT. The guided process included a paper-based tool that prompted review of patient history (household members with TB in the last year, previous history of TB treatment or IPT, and contraindications for IPT), a simplified symptom screen using three questions with high positive predictive value (cough or fever of any duration in the previous 4 weeks, night sweats in 3 of the past 4 weeks),18 and sputum analyses by acid-fast bacilli (AFB) smear and Xpert, regardless of symptoms (Figure 1). We selected the three-question symptom screen based on evidence that these symptoms can accurately rule out TB in the vast majority of PLHIV.18,19 Chest X-ray (CXR) was conducted selectively when clinical suspicion of TB persisted in the absence of positive smear microscopy or Xpert results. When a patient was unable to produce a sputum sample, sputum induction with nebulized hypertonic saline was recommended. TB was ruled out or diagnosed based on smear, Xpert or CXR findings, at which time patients were referred for initiation of IPT or anti-tuberculosis treatment. Extra-pulmonary TB (EPTB) was primarily clinically diagnosed without bacteriologic confirmation of non-sputum clinical samples. While TB symptom screening was undertaken routinely at each clinic visit, the guided screening process, with diagnostic support regardless of symptoms, occurred once per participant during the program period. This is because the goal of the screening process was to facilitate IPT uptake.
FIGURE 1.
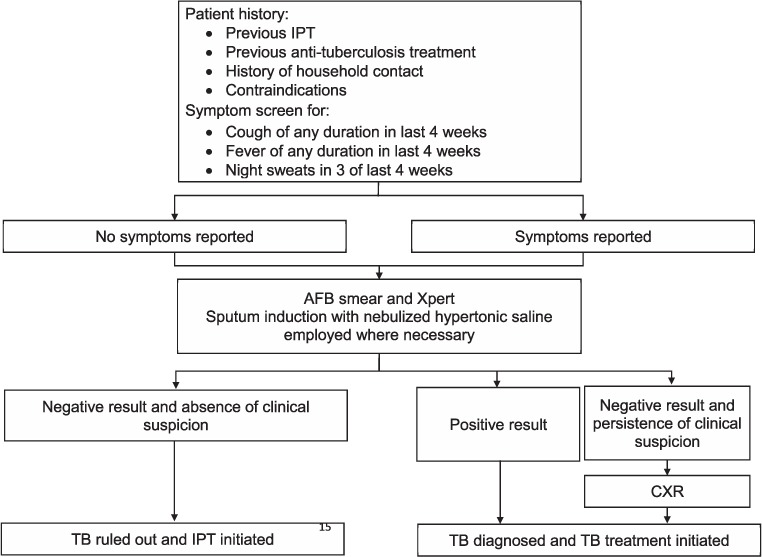
Screening algorithm* to facilitate IPT initiation. * Represents the ideal screening algorithm; however, both Xpert® MTB/RIF and AFB smear were not conducted in all participants (n = 50) and a minority of participants received CXR despite Xpert positivity (n = 4). IPT = isoniazid preventive therapy; AFB = acid-fast bacilli; CXR = chest X-ray; TB = tuberculosis.
The Research Advisory Committee of the PNG National AIDS Council Secretariat (Boroko, PNG), the Provincial AIDS Committee (Boroko), and the Harvard University Faculty of Medicine Committee on Human Subjects (Boston, MA, USA) approved this study and deemed it to be non-human subject research based on the routinely and programmatically collected nature of the data.
Standard of care
All HIV care was provided in accordance with national care and treatment guidelines.17 This included the provision of daily INH (5 mg/kg/day) and vitamin B6 (pyridoxine, 25 mg/day) for 6 months for all PLHIV once active TB was ruled out.
RESULTS
Between January 2013 and March 2016, 532 HIV-positive adult patients seeking routine HIV care at a large provincial hospital clinic (63%), or one of 13 satellite clinics (37%), underwent IPT eligibility screening using the paper-based guided screening process. Of these, 500 (94%) received an Xpert test, 492 (93%) received an AFB smear, and 95 (18%) received CXR. TB disease was diagnosed in 82 patients (15%) and ruled out in 450 (85%). Of those diagnosed with TB, 56 (68%) had pulmonary TB and 26 (32%) had EPTB (Figure 1). No patients were diagnosed by smear alone. Resistance to rifampin was detected by Xpert in 1/21 patients (5%, 95% confidence interval [CI] 0.24–21.3) with a positive Xpert result; a second sample from this person was sent for confirmatory resistance testing. All patients diagnosed with TB began treatment as described in the PNG National TB Program guidelines.20 Of those for whom TB was ruled out, 450 (100%) initiated IPT; however, four patients should have been excluded from receiving IPT due to contraindications (Figure 2).
FIGURE 2.
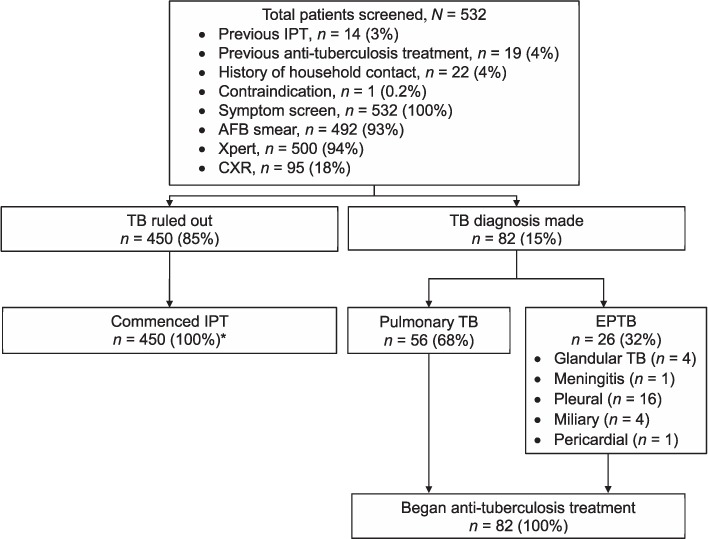
IPT commencement and TB treatment among patients with and without TB. * Four patients should have been excluded based on 1) receiving IPT in the previous 24 months (n = 3), and 2) jaundice (n = 1). IPT = isoniazid preventive therapy; AFB = acid-fast bacilli; CXR = chest X-ray; TB = tuberculosis; EPTB = extra-pulmonary TB.
Twenty-two patients (4%) reported having a household member who was diagnosed with TB or treated for TB in the last year. Of these, four (18%) were subsequently diagnosed with TB, although the diagnosis of TB was not significantly associated with the presence of a household member with TB (P = 0.76). A prior history of IPT was reported in only 14 (3%) patients; the outcome of previous IPT completion was known in 10 (71%). Among these, two (20%) patients reported having completed the full course of IPT.
Clinical and epidemiological characteristics
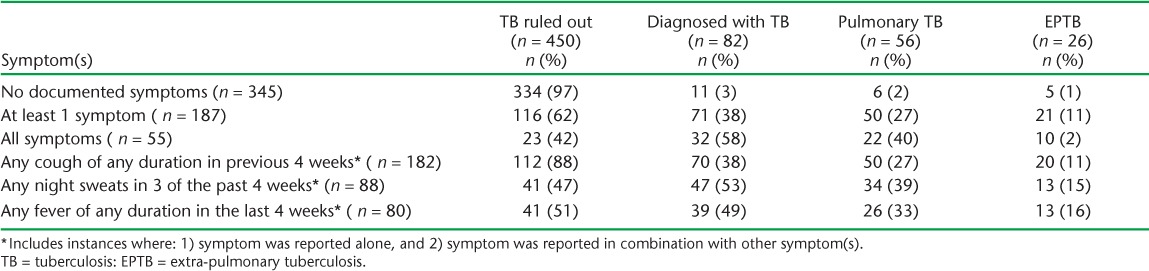
Over one third (35%) of the patients reported at least one of three symptoms (i.e., cough, fever, night sweats). TB was ruled out in the vast majority of patients without documented symptoms (97%) (Table). Among those with at least one reported symptom, 38% were diagnosed with TB. Fever was the least reported symptom across all groups. Reporting of at least one symptom was significantly associated with TB (P < 0.0001); however, when analyzed by individual symptom, fever was not significantly associated with a TB diagnosis (P = 0.07) (data not shown).
TABLE.
Symptom screen for cough, night sweats, and fever (n = 532)
Diagnostic methods in patients with TB
Figure 3 details the percentage of patients with a documented result for each diagnostic test, and positivity by diagnostic test, amongst all patients diagnosed with TB. In patients who were diagnosed with TB, 65 (82%) had an Xpert test result, 62 (78%) had an AFB smear result, and 56 (71%) had a CXR result. Three (4%) patients with TB did not have a documented Xpert, AFB smear, or CXR result. Ten patients were diagnosed by Xpert alone, while 52 (63%) were diagnosed by CXR alone. All 10 patients diagnosed through Xpert alone had pulmonary TB (Figure 4A), while the vast majority of patients with EPTB were diagnosed through CXR alone, representing 20 of the 24 patients with EPTB who had a documented diagnostic test (Figure 4B).
FIGURE 3.
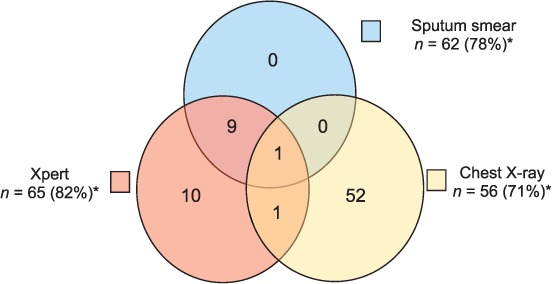
Positive result by diagnostic modality in patients with TB (n = 79).* * Three patients were clinically diagnosed and did not have documentation of a diagnostic test. † n = number of patients who received the specified diagnostic modality (percentage who received the specified diagnostic modality among the total population with TB). TB = tuberculosis.
FIGURE 4.
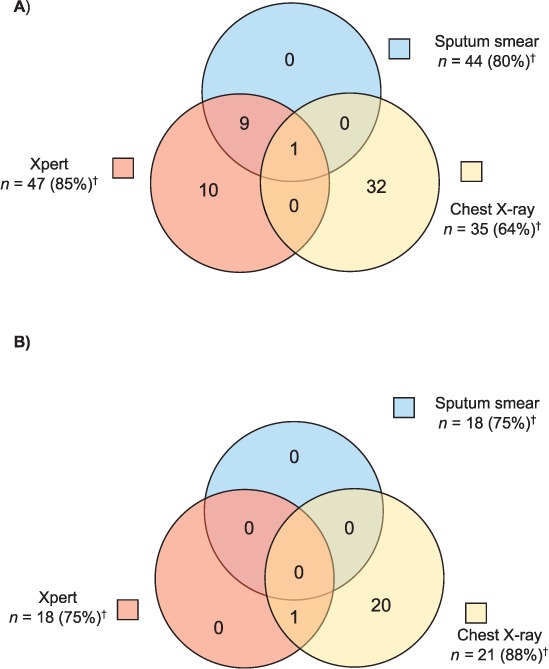
Positive result by diagnostic modality in patients with pulmonary TB and EPTB receiving sputum smear, Xpert® MTB/RIF, and/or chest X-ray (n = 79).* A) Among patients with pulmonary TB (n = 55). B) Among patients with EPTB (n = 24). * Three patients were clinically diagnosed and did not have documentation of a diagnostic test. † n = the number of patients who received the specified diagnostic modality (percentage who received the specified modality among the total in the specified population). TB = tuberculosis; EPTB = extra-pulmonary TB.
Among the 79 patients diagnosed with TB who had a documented diagnostic test, the majority (n = 70, 89%) reported at least one symptom (Figure 5A). This population was largely diagnosed by CXR alone (n = 47, 67%), with the remaining 19 (27%) patients diagnosed by Xpert alone (n = 8, 11%), or a combination of CXR and/or AFB smear and Xpert (n = 11, 16%). In those without symptoms who had at least one documented diagnostic test (n = 9, 11%), 5 (55%) were diagnosed through CXR, suggesting clinical suspicion, and 2 (22%) were diagnosed by Xpert.
FIGURE 5.
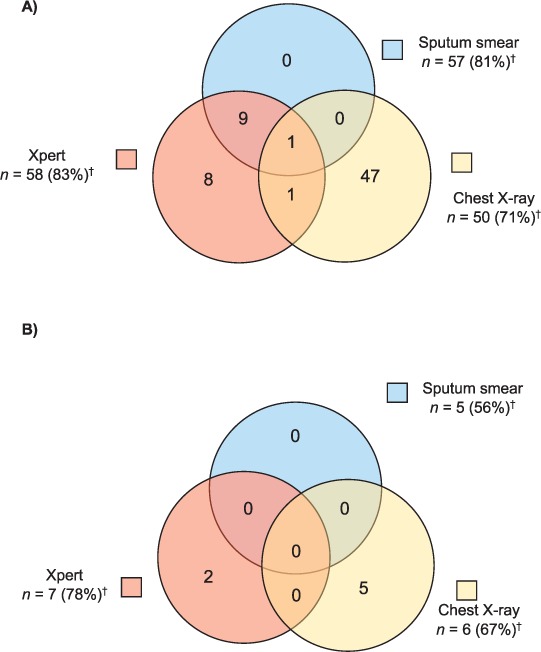
Positive result by diagnostic modality in patients with TB, with and without symptoms, among those receiving sputum smear, Xpert® MTB/RIF, and/or chest X-ray (n = 79).* A) Among patients with symptoms (n = 70). B) Among patients without symptoms (n = 9). * Three patients were clinically diagnosed and did not have documentation of a diagnostic test. † n = the number of patients who received the specified diagnostic modality (percentage who received the specified modality among the total in the specified population). TB = tuberculosis; EPTB = extra-pulmonary TB.
DISCUSSION
We found that a standardized guided screening process with TB diagnostic support facilitated the uptake of IPT for those in whom TB had been ruled out and led to the detection of TB in a substantial percentage (15%) of patients. All patients eligible for IPT or TB treatment were commenced on the respective treatment regimens in the program. We conducted sputum examination in all patients, regardless of symptoms, with the aim of alleviating health provider uncertainty in ruling out active TB. Providers participating in the program expressed that a negative Xpert result provided them with greater confidence in ruling out TB and prescribing IPT. A small number of patients initiated IPT in spite of contraindications, highlighting the need for close attention to factors such as jaundice, hepatitis, heavy alcohol use, and previous IPT when determining IPT eligibility.
Reporting of symptoms was significantly associated with a TB diagnosis. While 9 (11%) TB patients with a documented diagnostic test did not report any symptoms, five of these patients were diagnosed by CXR, indicating clinical suspicion despite the absence of the three TB symptoms. The remaining four patients were either symptom-free or had symptoms that were not documented on the symptom screening form. These findings provide evidence that triaging patients for further diagnostic assessment by Xpert and CXR based on a symptom questionnaire may be a strategy for capturing most PLHIV who have TB and that,18 in patients without TB symptoms or clinical suspicion, IPT can be safely initiated with minimal risk of active TB. However, clinical judgement on behalf of providers is still needed to direct further assessments, such as CXR,22 especially in diagnostically challenging cases such as patients with EPTB. Indeed, CXR was critical for the diagnosis of EPTB in this group of patients: all EPTB cases had a positive CXR, and for all but one patient, CXR was the sole positive test. Guidance on intensified case finding in PLHIV suggests a four-symptom questionnaire, including cough, night sweats, fever, and weight loss.23,24 It is unknown whether inclusion of weight loss as a symptom may have contributed to capturing those who did not report the three symptoms. Finally, while no patients were diagnosed with TB by AFB smear alone, AFB smear remains valuable for monitoring the treatment of those who are AFB smear-positive.
In addition to finding otherwise undetected cases of TB and initiating patients on IPT once disease had been ruled out, providers anecdotally expressed satisfaction with the guided process screening form because of its role in prompting the provider to carry out each portion of the screening process and follow up on the next steps required. The providers expressed surprise at the volume of EPTB cases that were diagnosed, and asserted their belief that they would not have identified the majority of these cases without the screening form. The guided process has become part of local practice as providers continue to use it to guide screening. These findings provide confirmation that the inclusion of a form may be helpful in guiding and reminding providers to carry out and follow up on the steps of an evaluation for IPT initiation. We found unconfirmed rifampin resistance detected by Xpert in one (5%) of 21 patients with a positive Xpert result. Although confirmatory test results for this patient were not available to us, this rate is comparable to that reported in a recent national population-based survey of drug resistance, which found rifampin resistance in over 3% of new TB patients.21 Given the limited access to drug susceptibility testing in PNG, Xpert offers a critical contribution not only to TB diagnosis but also to rapid detection of drug resistance in this setting.
There were operational challenges to executing screening using both smear microscopy and Xpert. First, some sputum samples were not processed by Xpert due to a 6-month cartridge stock-out. During this time, screening without Xpert continued and providers relied on AFB smear for bacteriologic confirmation, demonstrating that the guided process itself, and not a confirmatory Xpert result, gave providers the confidence to rule out TB. Stock-outs are a surmountable barrier to implementation with appropriate national and local supply chain investments and usage of available data to inform forecasting, procurement, and distribution. Second, executing an IPT screening algorithm that calls for sputum analysis, either by microscopy or Xpert, as the primary diagnostic requires the collection of sputum from patients who are unlikely to be able to produce it. Establishing sputum induction so that sputum samples could be obtained from patients was a complex process involving training, equipment and consumable procurement, as well as facility management to create sputum induction areas and infection control procedures, in accordance with the guidelines of the International Center for AIDS Care and Treatment Programs (ICAP) at Columbia University (New NY, USA).25 Sputum induction, although not systematically documented for the purposes of this study, was well received by both patients and providers despite operational challenges. Providers expressed that they valued sputum induction as a means of carrying out IPT screening; however, sputum induction was not available at all clinical sites during the implementation of the program, which may have diminished participation from smaller rural sites. Finally, among the small number of patients in this cohort who had a history of IPT, a sizeable proportion had documented non-completion of prior IPT. While adherence to the IPT initiated during our evaluation was not assessed, in practice IPT adherence monitoring is conducted at each clinical review. Variable completion rates documented in those with prior IPT use may reflect INH supply chain issues, insufficient patient education or adherence counseling, perceived stigma, or side effects, and drug toxicity.26,27 As in other settings,10 the ART providers in PNG cited inconsistent INH availability as a key barrier to IPT implementation prior to this screening algorithm. To eliminate this barrier and ensure initiation, clinicians set aside 6 months of IPT for each patient at the time of initiation, and recorded the date that treatment commenced.
In conclusion, a paper-based guided screening process with diagnostic support—most valuably, CXR and Xpert—was effective for the categorization of screened patients as IPT- or TB treatment-eligible and subsequent initiation of IPT or TB treatment in a large cohort of adult PLHIV in rural PNG. We recommend the addition of a guided tool to facilitate the screening process.
Acknowledgments
The authors would like to acknowledge the Australian Department of Foreign Affairs and Trade (Canberra, ACT, Australia) who generously supported the implementation of this adult human immunodeficiency virus treatment program.
Footnotes
Conflicts of interest: none declared.
References
- 1. Joint United Nations Programme on HIV/AIDS. . Fact Sheet 2016. Geneva, Switzerland: UNAIDS, 2016. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf Accessed July 2017. [Google Scholar]
- 2. World Health Organization. . WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. WHO/HTM/TB2012.1 Geneva, Switzerland: WHO, 2012. [PubMed] [Google Scholar]
- 3. World Health Organization. . Rapid implementation of the Xpert MTB/RIF diagnostic test: technical and operational ‘how-to’ practical considerations. WHO/HTM/TB/2011.2 Geneva, Switzerland: WHO, 2011. [Google Scholar]
- 4. Balcha T T, Sturegard E, Winqvist N, . et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PLOS Med 2014; 9: e85478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawn S D, Kerkhoff A D, Burton R, . et al. Rapid microbiological screening for tuberculosis in HIV-positive patients on the first day of acute hospital admission by systematic testing of urine samples using Xpert MTB/RIF: a prospective cohort in South Africa. BMC Med 2015; 13: 1– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danel C, Moh R, Gabillard D, . et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med 2015; 373: 808– 822. [DOI] [PubMed] [Google Scholar]
- 7. Frigati L J, Kranzer K, Cotton M F, Schaaf H S, Lombard C J, Zar H J.. The impact of isoniazid preventive therapy and antiretroviral therapy on tuberculosis in children infected with HIV in a high tuberculosis incidence setting. Thorax 2011; 66: 496– 501. [DOI] [PubMed] [Google Scholar]
- 8. Grant A D, Charalambous S, Fielding K L, . et al. Effect of routine isoniazid preventive therapy on tuberculosis incidence among HIV-infected men in South Africa: a novel randomized incremental recruitment study. JAMA 2005; 293: 2719– 2725. [DOI] [PubMed] [Google Scholar]
- 9. Akolo C, Adetifa I, Shepperd S, Volmink J.. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev 2010; 1: CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teklay G, Teklu T, Legesse B, Tedla K, Klinkenberg E.. Barriers in the implementation of isoniazid preventive therapy for people living with HIV in Northern Ethiopia: a mixed quantitative and qualitative study. BMC Public Health 2016; 16: 840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moolphate S, Lawpoolsri S, Pungrassami P, Sanguanwongse N, Yamada N, Kaewkungwal J.. Barriers to and motivations for the implementation of a treatment programme for latent tuberculosis infection using isoniazid for people living with HIV, in upper northern Thailand. Glob J Health Sci 2013; 5: 60– 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lester R, Hamilton R, Charalambous S, . et al. Barriers to implementation of isoniazid preventive therapy in HIV clinics: a qualitative study. AIDS 2010; 24 Suppl 5: S45– S48. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization. . Global tuberculosis report, 2016. WHO/HTM/TB/2016.13 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 14. Papua New Guinea National Department of Health. . Interim Global AIDS response progress and universal access reports, Papua New Guinea, 31 March 2014. Port Moresby, PNG: National Department of Health, 2014. http://files.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2014countries/PNG_narrative_report_2014.pdf Accessed July 2017. [Google Scholar]
- 15. Papua New Guinea National AIDS Council. . Papua New Guinea 2013, HIV/AIDS estimations and projections. Port Moresby, PNG: National AIDS Council, 2013. [Google Scholar]
- 16. The National Research Institute. . 2010. Papua New Guinea District and Provincial Profiles. Port Moresby, PNG: The National Research Institute, 2010. [Google Scholar]
- 17. Papua New Guinea National Department of Health. . Guidelines for HIV care and treatment in Papua New Guinea. Port Moresby, PNG: National Department of Health, 2009. http://www.who.int/hiv/pub/guidelines/papua_art.pdf Accessed July 2017. [Google Scholar]
- 18. Cain K P, McCarthy K D, Heilig C M, . et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. New Eng J Med 2010; 362: 707– 716. [DOI] [PubMed] [Google Scholar]
- 19. Menberu M A. Performance of the WHO 2011 TB symptom screening algorithm for pulmonary TB diagnosis among HIV-infected patients in Gondar University Referral Hospital, Ethiopia. Int J Microbiol 2016; 2016: 9058109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Tuberculosis Program. . Papua New Guinea National Tuberculosis Management Protocol. Port Moresby, PNG: National Department of Health, 2011. http://www.adi.org.au/wp-content/uploads/2016/11/National-Tuberculosis-Management-Protocol-PNG-2011.pdf Accessed July 2017. [Google Scholar]
- 21. Aia P, Kal M, Lavu E, . et al. The burden of drug-resistant tuberculosis in Papua New Guinea: results of a large population-based survey. PLOS ONE 2016; 11: e0149806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization. . Chest radiography in tuberculosis detection—summary of current WHO recommendations and guidance on programmatic approaches. WHO/HTM/TB/2016.20 Geneva, Switzerland: WHO, 2016. [Google Scholar]
- 23. World Health Organization. . Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource constrained settings. Geneva, Switzerland: WHO, 2011. [Google Scholar]
- 24. Getahun H, Kittikraisak W, Heilig C M, Corbett E L, Ayles H, Cain K P.. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLOS Med 2011; 8: e1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. International Center for AIDS Care and Treatment Programs. . Sputum induction. Standard operating procedures for health care workers, HIV care and treatment facilities. Version 1. February 2010. New York, NY, USA: ICAP, 2010. http://www.learningace.com/doc/1835783/4e20596a6bc9a331a0135cc0b81ec931/icap-sa-tb-hiv-sputum-induction-sop-final. Accessed 21 November 2016. [Google Scholar]
- 26. van Griensven J, Choun K, Chim B, Thai S, Lorent N, Lynen L.. Implementation of isoniazid preventive therapy in an HIV clinic in Cambodia: high rates of discontinuation when combined with antiretroviral therapy. Trop Med Int Health 2015; 20: 1823– 1831. [DOI] [PubMed] [Google Scholar]
- 27. Munseri P J, Talbot E A, Mtei L, Fordham von Reyn C.. Completion of isoniazid preventive therapy among HIV-infected patients in Tanzania. Int J Tuberc Lung Dis 2008; 12: 1037– 1041. [PubMed] [Google Scholar]


