Abstract
The salivary duct cyst (SDC) is a reactive ductal ectasia most frequently seen in major salivary glands, and likely caused by obstruction. The aim of this study is to define the clinical and histopathologic spectrum of intraoral SDCs. Cases were retrieved from the archives of Harvard School of Dental Medicine/StrataDx, Inc. from January 2012 to August 2014. There were 177 cases of which 103 (58.2%) occurred in females, with a median age of 56 (range 2–95). Approximately half of cases (45.8%) presented in the area of the buccal mucosa, lower lip mucosa, or mandibular vestibule, and 23.2% presented in the floor of mouth. SDCs were lined at least focally by 1–2 layers of cuboidal/columnar epithelium in 85.3% of cases and showed varying degrees of metaplasia (oncocytic, mucous cell, squamous, ciliated, apocrine-like) in 68.4% of cases. Intraluminal mucous stasis was present in 41.8% of SDCs, incipient calcification was present within 4.5% of SDCs, and chronic obstructive sialadenitis was seen in 90.2% of cases. No cysts showed adenomatous ductal proliferations or true papillary structures with fibrovascular cores, although 41.2% exhibited reactive undulation of cyst lining. Thirty-nine ‘papillary oncocytic cystadenoma-like’ SDCs (22.0%) demonstrated complete oncocytic metaplasia and marked undulation. An additional seven such cysts (4.0%) had a ‘Warthin tumor-like’ lymphoplasmacytic infiltrate. Intraoral SDCs occur most commonly in the sixth decade of life in locations distinct from extravasation mucoceles, likely secondary to intraluminal obstruction. SDCs show diverse histopathology and certain phenotypic variants may be mistaken for papillary oncocytic cystadenoma or Warthin tumor.
Keywords: Salivary duct cyst, Sialocyst, Mucous retention cyst, Cystadenoma, Mucocele, Warthin tumor
Introduction
The salivary duct cyst (SDC), also known as sialocyst, is an acquired cystic dilatation of salivary ducts that is thought to arise secondary to ductal obstruction, which may be transient or persistent [1, 2]. SDCs typically occur in the major salivary glands and 80% occur in the parotid, where they present as unilateral, asymptomatic, compressible nodules in adult patients [3].
SDCs may also occur in the minor salivary glands, where some investigators have used the term mucous retention cyst, or even mucocele [4, 5]. The term salivary duct cyst seems preferable over mucous retention cyst for several reasons. Salivary duct cyst is already commonly used to describe these cysts in the major salivary glands [2, 3]. Mucous retention cyst was initially described as a histopathologic subtype of SDC with identifiable intraluminal mucin and, while not incorrect, does not adequately describe the phenotypic variation seen in these cysts [4]. And lastly, avoidance of the terms mucous retention cyst or mucous retention phenomenon emphasizes distinction from the more common mucocele (mucous extravasation phenomenon), which has a different etiology, clinical presentation and histopathology. The conventional mucocele (mucous extravasation phenomenon) is much more common in the oral cavity than SDC and is a distinct entity representing mucous extravasation into surrounding connective tissue as a result of traumatic injury to, and loss of integrity of, an excretory salivary duct [6].
There has been considerable confusion regarding the etiology and nomenclature of SDCs as well as their relationship to mucoceles. Many older studies of ‘mucoceles’ have included both mucoceles and SDCs in their series, with some reporting the data separately and some in aggregate [7–10]. These authors considered SDC to be a histopathologic variant of mucocele that represents a mucous retention phenomenon as opposed to the mucous extravasation phenomenon of conventional mucoceles [11, 12]. Other authors have considered the mucocele as a variant of SDC, generating only further confusion [13]. The aim of this study is to characterize the clinical and histopathologic features of a large series of intraoral SDCs to clarify their etiology and to elaborate on their differential diagnosis.
Materials and Methods
Cases diagnosed as SDC were identified in a 20-month period from January 2012 to August 2014 from the archives of StrataDX, the surgical pathology laboratory affiliated with the Harvard School of Dental Medicine in Boston, MA, USA. All cases fulfilled the diagnostic criteria for SDC, namely the presence of one or more cystically dilated salivary ducts with no evidence of duct rupture or mucous extravasation [4]. Sialoliths within ectatic ducts were excluded. Submitting pathology requisition forms and hematoxylin and eosin (H&E) slides were available in all instances and clinical and histopathologic features were documented.
Results
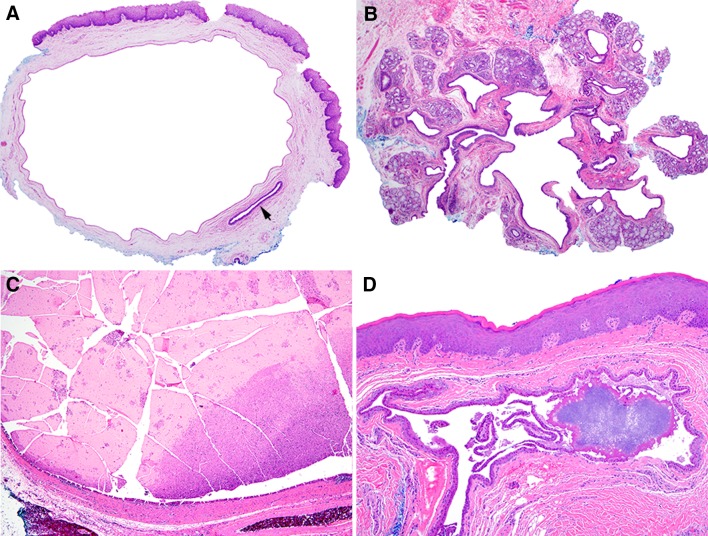
One hundred and seventy-seven cases of SDC were identified. The male:female ratio was 1:1.4. The median age was 56 years (range 2–95) (Table 1). The contiguous area of the buccal mucosa, lower lip mucosa, and mandibular vestibule represented the most common location of SDCs (45.8%); the lower lip mucosa was affected in only 15.8% of cases. The single most common site was the floor of mouth (23.2%). Lesions were clinically described as nodules that were most often yellow/white or blue. In the majority of cases, mucocele was the diagnostic impression at the time of excision (Fig. 1).
Table 1.
Clinical features of intraoral salivary duct cysts
| Gender | |
| Male | 74 (41.8%) |
| Female | 103 (58.2%) |
| Age | |
| Median | 56 years |
| Range | 2–95 years |
| Site | |
| Buccal mucosa/lower lip mucosa/mandibular vestibule | 81 (45.8%) |
| Buccal mucosa | 29 (16.4%) |
| Lower lip | 28 (15.8%) |
| Mandibular vestibule | 24 (13.6%) |
| Floor of mouth | 41 (23.2%) |
| Hard or soft palatal mucosa | 28 (15.8%) |
| Upper lip | 10 (5.6%) |
| Tongue | 10 (5.6%) |
| Othera | 7 (4.0%) |
| Common clinical diagnosesb | |
| Mucocele | 99 (57.9%) |
| Minor salivary gland tumor | 19 (11.1%) |
| Sialadenitis | 15 (8.8%) |
| Traumatic/irritation fibroma | 11 (6.4%) |
| Salivary duct cyst | 11 (6.4%) |
aIncludes lip mucosa, NOS (3), maxillary vestibule (2), retromolar pad (1), lip vestibule, NOS (1)
bOut of 171 cases with accompanying information
Fig. 1.
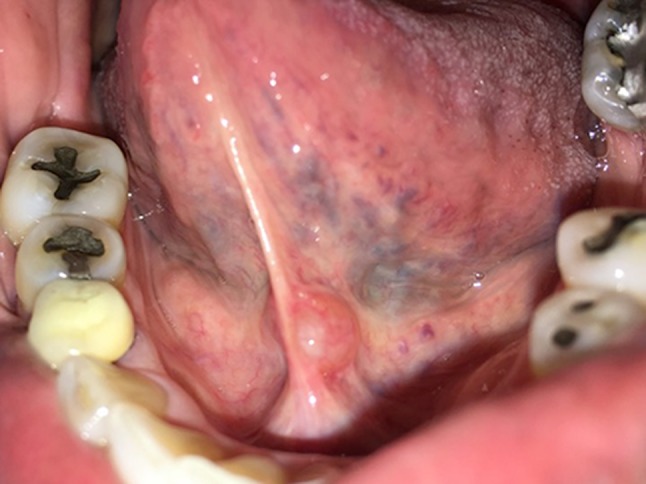
Mucosal-colored, mildly translucent SDC of left sublingual caruncle
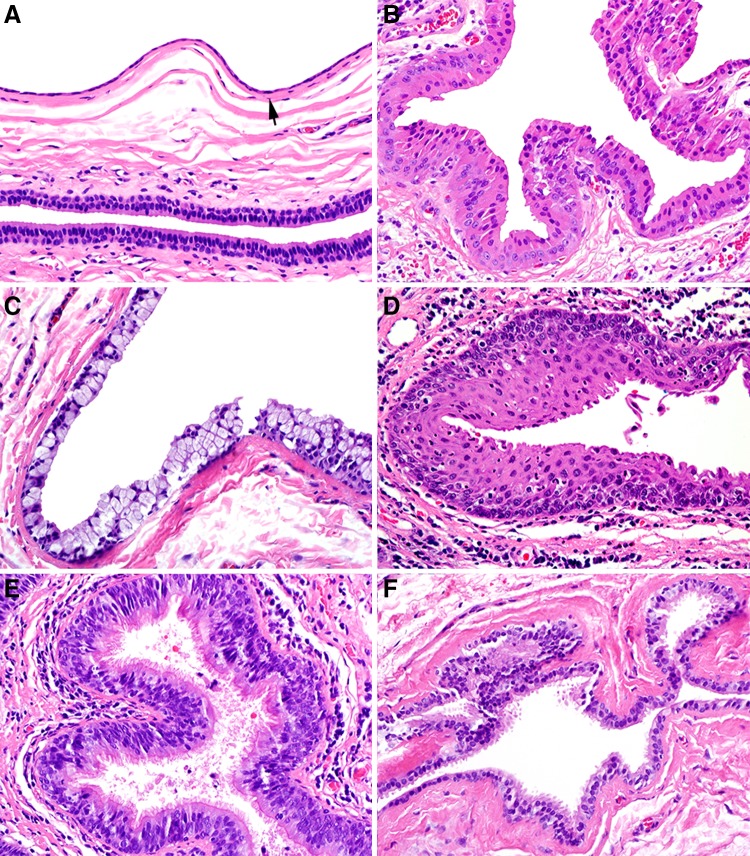
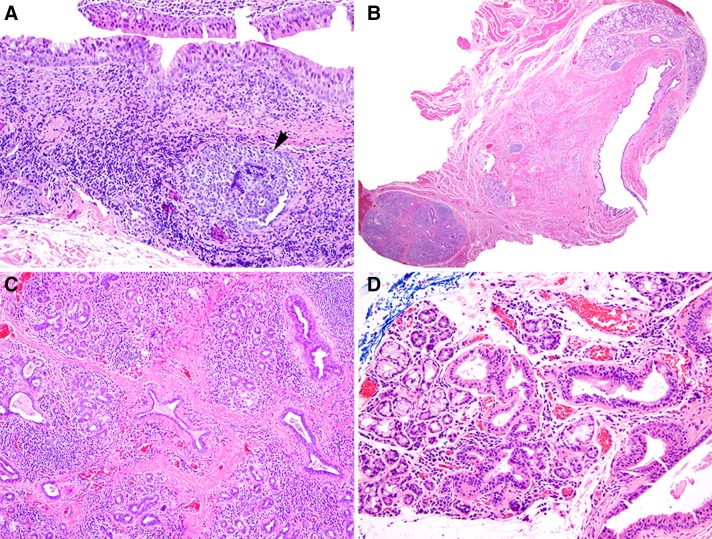
Histopathologic features are summarized in Table 2. A single epithelium-lined cyst was noted in 92.7% of cases and two or more cystic structures in 7.3% of cases (Fig. 2a, b). Intraluminal mucin was present in 41.8% of cysts and this varied from focal mucin accumulation to a well-formed mucous plug (Fig. 2c). Focal, incipient calcification was present within 4.5% of cysts (Fig. 2d). The cysts were lined, at least partially, by 1–2 layers of flattened, cuboidal-columnar epithelium in 85.3% of cases (Fig. 3a) and exhibited metaplasia ranging from focal to complete involvement of the lining in 68.4% of cases. The most common metaplastic changes were oncocytic (50.8%), mucous cell (36.2%) and squamous (23.7%); ciliated cell metaplasia (16.4%) and apocrine-like metaplasia (14.1%) were less commonly noted (Fig. 3b–f). In addition, 40.1% of cysts showed more than one type of metaplasia. Hyalinization of the basement membrane zone beneath the cyst lining was noted in 19.2% of cysts. An acute or chronic inflammatory infiltrate that was generally mild to moderate was present in 71.8% of cysts (Fig. 4a). Chronic obstructive sialadenitis, as defined by ductal dilatation, acinar atrophy, interstitial fibrosis, and interstitial inflammation, was present in 90.2% of cases within associated minor salivary gland lobules (Fig. 4b, c). Many salivary gland lobules exhibited metaplasia of intra- and extra-lobular ductal epithelium, similar to changes seen in the associated SDC (Fig. 4d).
Table 2.
Histopathologic features of intraoral salivary duct cysts
| Cyst architecture | |
| One epithelium-lined cyst | 164 (92.7%) |
| Two or more epithelium-lined cysts | 12 (7.3%) |
| Undulating (papillary-like) lining | 73 (41.2%) |
| True papillaea | 0 (0.0%) |
| Adenomatous plaques | 0 (0.0%) |
| Intraluminal secretion | |
| Mucous | 74 (41.8%) |
| Incipient calcification | 8 (4.5%) |
| Cyst lining | |
| Cuboidal/columnar | 151 (85.3%) |
| Oncocytic metaplasia | 90 (50.8%) |
| Mucous cell metaplasia | 64 (36.2%) |
| Squamous metaplasia | 42 (23.7%) |
| Ciliated cells | 29 (16.4%) |
| Apocrine-like cells | 25 (14.1%) |
| Basement membrane hyalinization | 34 (19.2%) |
| Inflammatory infiltrate | |
| Acute | 0 (0.0%) |
| Chronic | 74 (41.8%) |
| Mixed | 53 (29.9%) |
| Total | 127 (71.8%) |
| Chronic obstructive sialadenitis | 101b (90.2%) |
| Papillary oncocytic cystadenoma-likec | 39 (22.0%) |
| Warthin tumor-liked | 7 (4.0%) |
aAs defined by the presence of fibrovascular cores
bOut of 112 cases with associated minor salivary glands
cAs defined by complete oncocytic metaplasia and undulating architecture
dAs defined by complete oncocytic metaplasia, undulating architecture, and associated lymphoplasmacytic infiltrate
Fig. 2.
a Typical SDC with cystically dilated excretory duct, uninvolved excretory duct (arrow) is noted bottom right (×40). b SDC involving excretory duct as well as multiple smaller intralobular ducts located within distinct minor salivary gland lobules (×40). c SDC with intraluminal mucous plug (×40). d Early calcification occurring within longstanding SDC (×100)
Fig. 3.
a SDC (top, arrow) lined by 1–2 layers of low cuboidal/columnar epithelium and juxtaposed to normal excretory duct epithelium (bottom) consisting of columnar luminal and cuboidal abluminal cells (×400). b Cyst lining exhibiting oncocytic metaplasia (×400). c Cyst lining exhibiting mucous cell metaplasia (×400). d Cyst lining exhibiting squamous metaplasia (×400). e Cyst lining exhibiting ciliated cell metaplasia (×400). f Cyst lining exhibiting apocrine-like metaplasia (×400)
Fig. 4.
a Inflamed SDC exhibiting metaplastic changes and moderate chronic inflammation with germinal center formation (arrow) (×200). b Minor salivary gland lobule proximal to SDC exhibiting severe chronic obstructive sialadenitis (×20). c Chronic obstructive sialadenitis characterized by acinar atrophy, ductal dilatation, interstitial fibrosis, and interstitial chronic inflammation (×100). d Chronic obstructive sialadenitis exhibiting metaplasia of intralobular ductal epithelium (×200)
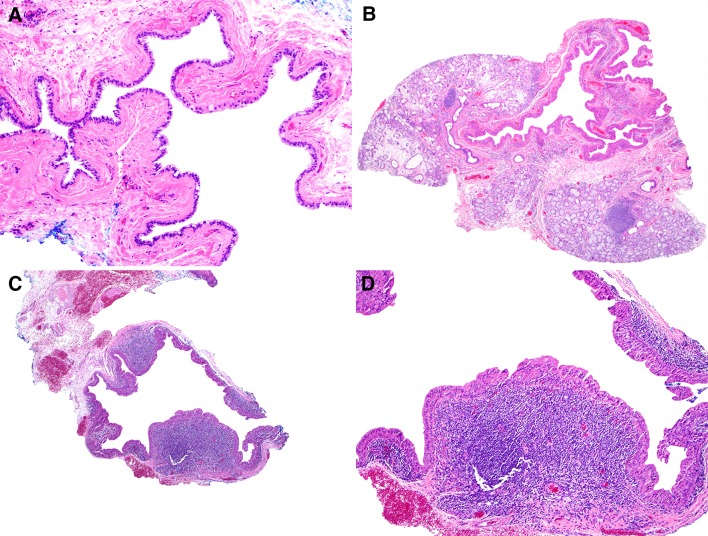
A prominent undulating architecture of the cyst lining was present in 41.2% of cases (Fig. 5a); however, no true papillary structures with fibrovascular cores and no adenomatous plaques, as seen in papillary cystadenoma, were identified. SDCs that were characterized by complete oncocytic metaplasia and undulating architecture of the cyst lining were designated as ‘papillary oncocytic cystadenoma-like’ and comprised 20.3% of cases (Fig. 5b). A further 3.6% of cases had, in addition to the aforementioned features, a prominent lymphoplasmacytic inflammatory infiltrate surrounding the cyst and were designated as ‘Warthin tumor-like’ (WT-like) (Fig. 4e, f).
Fig. 5.
a SDC with prominent undulating lining representing collapse of previously distended cyst; true papillae with fibrovascular cores are not seen. b Collapsed ‘papillary oncocytic cystadenoma-like’ SDC with undulating cyst lining and uniform oncocytic metaplasia, but no papillae. c, d ‘Warthin tumor-like’ SDC with undulating cyst lining, uniform oncocytic metaplasia, and prominent lymphoplasmacytic inflammatory infiltrate subjacent to cyst lining
Discussion
SDCs are not uncommon in the oral cavity but occur much less frequently than mucoceles. Over the same 20-month time period, 2275 mucoceles were accessioned, and SDCs comprised 7.8% of the total of these two entities. SDCs and mucoceles both present clinically as asymptomatic nodules but with two important clinical differences. First, mucoceles show a striking predilection for the lower lip mucosa (59–82%) [6, 14, 15], a frequent site of trauma; SDCs are more evenly distributed between the contiguous area of the buccal mucosa/lower lip mucosa/mandibular vestibule, the floor of mouth, and the hard/soft palatal mucosa (84.8% in aggregate), sites less prone to trauma. Only 15.8% of SDCs occurred on the lower lip mucosa. Second, mucoceles are most common in the first to fourth decades of life whereas SDCs are most common in the sixth decade of life [6, 14, 15].
The histopathology of SDC is diverse, as is typical for reactive conditions. The majority of cysts (85.3%) were lined at least focally by 1–2 layers of cuboidal or columnar epithelium, consistent with normal salivary duct phenotype. In addition, 68.4% of cysts showed at least some evidence of metaplastic change, as may be seen in salivary obstructive disease [16, 17]. This was most commonly oncocytic (50.8%) or mucous cell (36.2%) metaplasia, with squamous (23.7%), ciliated cell (16.4%), and apocrine-like (14.1%) metaplasia noted less often. SDC with oncocytic metaplasia has previously been referred to as oncocytic sialocyst [4]. Apocrine-like cells may represent true apocrine differentiation but more likely only appear apocrine morphologically and instead represent heterophagy of stagnant secretory material [18].
Importantly, 41.8% of SDCs showed evidence of mucous stasis, which suggests that nearly half of SDCs had an identifiable cause of obstruction. Mucous stasis varied from focal intraluminal mucin to a well-formed mucous plug that obstructed the cyst lumen, supporting the hypothesis that SDCs develop secondary to idiopathic stasis in secretion [1, 2, 4]. The fact that approximately half of SDCs had patent lumens and that rare SDCs (4.5%) showed focal intraluminal calcification suggests that this stasis transiently resolves or on occasion provides a nidus for sialolith formation [19]. Over time, sufficient calcification may occur for the clinical presentation to be of a firm mass suspicious for a sialolith. In these cases lesional tissue consists primarily of the excised sialolith, with or without scant fragments of excretory duct epithelium peripherally attached. However, as the most common site for minor salivary gland sialoliths is the upper lip, where only 5.6% of SDCs occur, it is likely that sialolith formation is an uncommon consequence of SDC [20, 21].
Additionally, 41.2% of SDCs had a noticeably undulating cyst lining. The fact that this finding tended to occur in cysts with patent lumens suggests that these undulations simply represent folding over of distended cystic epithelium after resolution of mucous stasis and collapse of SDC, and should not be interpreted as papillary proliferations. This is the same undulating architecture that is also frequently seen in collapsed mucoceles.
Chronic obstructive sialadenitis, with intraparenchymal ductal epithelium frequently exhibiting dilatation and metaplastic change as seen in the adjacent SDC, was seen in 90.2% of cases, further supporting the hypothesis of an obstructive etiology. Occasional SDCs consisted of multiple cystic compartments within multiple adjacent salivary gland lobules. This likely is secondary to dilatation of intralobular ducts proximal to the source of obstruction, and should be distinguished from tangential sectioning of a solitary, tortuous SDC where the cystic cavities are closely approximated.
The differential diagnosis for SDC includes cystadenoma and low-grade mucoepidermoid carcinoma (LG-MEC). Cystadenoma is a well-circumscribed or encapsulated neoplasm consisting of a proliferation of ducts that most commonly occurs in the parotid but may arise in other sites; histopathologic variants include papillary cystadenoma, papillary oncocytic cystadenoma, and papillary mucinous cystadenoma [3]. Thirty-nine ‘papillary oncocytic cystadenoma-like’ SDCs (22.0%) in this series were characterized by complete oncocytic metaplasia of the cyst lining with a prominent undulating architecture reminiscent of the papillary infoldings of a cystadenoma. However, none of these exhibited true papillae with fibrovascular cores, adenomatous plaques, or encapsulation, as often seen in cystadenoma. There have been less than 35 reported cases of papillary cystadenoma arising in minor salivary glands [22–42]. Careful review of published photomicrographs suggests that some of these cases may, in fact, represent SDCs with extensive oncocytic metaplasia and a reactive undulation to the cyst lining [25, 26, 33–35, 38, 39]. One large case series illustrates both SDC and cystadenoma [42].
LG-MEC consists of an unencapsulated proliferation of mucous, intermediate, and epidermoid cells and may enter the differential diagnosis when consisting mostly of cystic structures or a single large cyst [43, 44]. However, the cystic components show tumor plaques with a complex arrangement of mucous, intermediate, and epidermoid cells and the tumor should show at least focal evidence of stromal invasion by solid areas. Moreover, p63 is typically diffusely expressed in MEC while positivity is restricted to the basal/abluminal cells of SDC and cystadenoma, and this may aid in the differential diagnosis [45, 46].
There have been less than 25 reported cases of intraoral Warthin tumor,[47] as reviewed by Iwai et al. [48] WT is a benign, polyclonal tumor of unknown etiology with near-exclusive involvement of the parotid (sometimes bilateral), an association with smoking, and a male predilection [49–51]. An origin from heterotopic salivary gland tissue within lymph nodes is favored based on the predilection of WT for the parotid, the occasional occurrence of WT within periparotid lymph nodes, and the rare finding of nodal lymphomas, tuberculosis, and metastatic deposits within the lymphoid stroma of WT [52–55]. Seven ‘WT-like’ SDCs (4.0%) in this series were characterized by complete oncocytic metaplasia of the cyst lining, an undulating architecture, and a prominent lymphoplasmacytic infiltrate. However, these did not have the intense (primarily lymphocytic) infiltrate of parotid WT, true papillae with fibrovascular cores, and intraluminal eosinophilic proteinaceous material. A careful review of all reported cases with accompanying photomicrographs showed histopathologic features consistent with these seven WT-like SDCs. True ‘intraoral WT’ are difficult to explain and may represent a florid inflammatory response to an SDC [56].
Conclusion
In conclusion, intraoral SDCs are a reactive ductal ectasia that develop secondary to intraluminal obstruction and may reach a size that warrants clinical attention. They are most common in the sixth decade of life and are frequently seen in the area of the buccal mucosa/lower lip mucosa/mandibular vestibule or in the floor of mouth. Unlike mucoceles, they are not caused by trauma. Histopathologically they can exhibit oncocytic, mucous and squamous metaplasia and certain phenotypic variants should not be misdiagnosed as papillary oncocytic cystadenoma or interpreted as evidence of intraoral WT.
Acknowledgments
Funding
No funding was received during the study or in preparation of the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed Consent
Informed consent was not obtained as this study consists of secondary research performed on excess tissue of specimens used for diagnostic purposes. Research was conducted using deidentified samples and private patient information was not accessed at any point during the design of the study, data collection/interpretation, or manuscript preparation.
References
- 1.Work WP Cysts and congenital lesions of the parotid gland. Otolaryngol Clin North Am. 1977;10(2):339–343. [PubMed] [Google Scholar]
- 2.Batsakis JG, Raymond AK. Sialocysts of the parotid glands. Ann Otol Rhinol Laryngol. 1989;98(6):487–489. doi: 10.1177/000348948909800618. [DOI] [PubMed] [Google Scholar]
- 3.Ellis GL, Auclair PL, American Registry of Pathology. Armed Forces Institute of Pathology(U.S.) Tumors of the salivary glands. AFIP atlas of tumor pathology Fourth series. Washington, DC: American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2008. [Google Scholar]
- 4.Eversole LR. Oral sialocysts. Arch Otolaryngol—Head Neck Surg. 1987;113(1):51–56. doi: 10.1001/archotol.1987.01860010055014. [DOI] [PubMed] [Google Scholar]
- 5.Southam JC. Retention mucoceles of the oral mucosa. J Oral Pathol. 1974;3(4):197–202. doi: 10.1111/j.1600-0714.1974.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 6.Chi AC, Lambert PR, 3rd, Richardson MS, Neville BW. Oral mucoceles: a clinicopathologic review of 1,824 cases, including unusual variants. J Oral Maxillofac Surg. 2011;69(4):1086–1093. doi: 10.1016/j.joms.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 7.Cohen L. Mucoceles of the oral cavity. Oral Surg Oral Med Oral Pathol. 1965;19:365–372. doi: 10.1016/0030-4220(65)90048-4. [DOI] [PubMed] [Google Scholar]
- 8.Harrison JD. Salivary mucoceles. Oral Surg Oral Med Oral Pathol. 1975;39(2):268–278. doi: 10.1016/0030-4220(75)90228-5. [DOI] [PubMed] [Google Scholar]
- 9.Yamasoba T, Tayama N, Syoji M, Fukuta M. Clinicostatistical study of lower lip mucoceles. Head Neck. 1990;12(4):316–320. doi: 10.1002/hed.2880120407. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira DT, Consolaro A, Freitas FJ. Histopathological spectrum of 112 cases of mucocele. Braz Dent J. 1993;4(1):29–36. [PubMed] [Google Scholar]
- 11.Granholm C, Olsson Bergland K, Walhjalt H, Magnusson B. Oral mucoceles; extravasation cysts and retention cysts. A study of 298 cases. Swed Dent J. 2009;33(3):125–130. [PubMed] [Google Scholar]
- 12.Thompson LD. Mucocele: retention and extravasation types. Ear Nose Throat J. 2013;92(3):106–108. doi: 10.1177/014556131309200307. [DOI] [PubMed] [Google Scholar]
- 13.Seifert G. Tumour-like lesions of the salivary glands. The new WHO classification. Pathol Res Pract. 1992;188(7):836–846. [PubMed] [Google Scholar]
- 14.Elzay RP, Sweeney WT, Peters PB, Malbon BA. Oral mucoceles: a review of 161 cases. Va Dent J. 1968;45(3):15–19. [PubMed] [Google Scholar]
- 15.Cataldo E, Mosadomi A. Mucoceles of the oral mucous membrane. Arch Otolaryngol. 1970;91(4):360–365. doi: 10.1001/archotol.1970.00770040518011. [DOI] [PubMed] [Google Scholar]
- 16.Testa Riva F, Riva A, Puxeddu P. Ciliated cells in the main excretory duct of the submandibular gland in obstructive sialadenitis: a SEM and TEM study. Ultrastruct Pathol. 1987;11(1):1–10. doi: 10.3109/01913128709023177. [DOI] [PubMed] [Google Scholar]
- 17.Triantafyllou A, Hunt JL, Devaney KO, Ferlito A. A perspective of comparative salivary and breast pathology. Part I: microstructural aspects, adaptations and cellular events. Eur Arch oto-rhino-laryngol. 2014;271(4):647–663. doi: 10.1007/s00405-013-2488-y. [DOI] [PubMed] [Google Scholar]
- 18.Triantafyllou A, Harrison JD, Garrett JR. Microenvironmental adaptations in the parotid of ferret investigated by electron microscopy. Arch Oral Biol. 2007;52(8):768–777. doi: 10.1016/j.archoralbio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Marchal F, Kurt AM, Dulguerov P, Lehmann W. Retrograde theory in sialolithiasis formation. Arch Otolaryngol—Head Neck Surg. 2001;127(1):66–68. doi: 10.1001/archotol.127.1.66. [DOI] [PubMed] [Google Scholar]
- 20.Jensen JL, Howell FV, Rick GM, Correll RW. Minor salivary gland calculi. A clinicopathologic study of forty-seven new cases. Oral Surg Oral Med Oral Pathol. 1979;47(1):44–50. doi: 10.1016/0030-4220(79)90100-2. [DOI] [PubMed] [Google Scholar]
- 21.Anneroth G, Hansen LS. Minor salivary gland calculi. A clinical and histopathological study of 49 cases. Int J Oral Surg. 1983;12(2):80–89. doi: 10.1016/S0300-9785(83)80002-7. [DOI] [PubMed] [Google Scholar]
- 22.Collins EM. Papillary cystadenoma of accessory salivary gland. Am J Surg. 1958;96(6):749–750. doi: 10.1016/0002-9610(58)90992-9. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhry AP, Gorlin RJ, Mitchell DF. Papillary cystadenoma of minor salivary gland origin. Report of a case. Oral Surg Oral Med Oral Pathol. 1960;13:452–454. doi: 10.1016/0030-4220(60)90350-9. [DOI] [PubMed] [Google Scholar]
- 24.Calhoun NR, Cerine FC, Mathews MJ. Papillary cystadenoma of the upper lip. Report of a case. Oral Surg Oral Med Oral Pathol. 1965;20(6):810–813. doi: 10.1016/0030-4220(65)90145-3. [DOI] [PubMed] [Google Scholar]
- 25.Parnes EI. Papillary cystadenoma. Report of a case. Oral Surg Oral Med Oral Pathol. 1966;21(6):782–785. doi: 10.1016/0030-4220(66)90102-2. [DOI] [PubMed] [Google Scholar]
- 26.Wilson DF, MacEntee MI. Papillary cystadenoma of ectopic minor salivary gland origin. Oral Surg Oral Med Oral Pathol. 1974;37(6):915–918. doi: 10.1016/0030-4220(74)90444-7. [DOI] [PubMed] [Google Scholar]
- 27.Kerpel SM, Freedman PD, Lumerman H. The papillary cystadenoma of minor salivary gland origin. Oral Surg Oral Med Oral Pathol. 1978;46(6):820–826. doi: 10.1016/0030-4220(78)90314-6. [DOI] [PubMed] [Google Scholar]
- 28.Kameyama Y, Okada Y, Takehana S, Mizohata M, Nishio S, Enomoto M. Papillary cystadenoma. Int J Oral Surg. 1985;14(6):556–559. doi: 10.1016/S0300-9785(85)80065-X. [DOI] [PubMed] [Google Scholar]
- 29.Martin RW, 3rd, Corio RL, Hood AF, Katz M. Papillary cystadenoma of the lower lip mimicking hidradenoma papilliferum. J Cutan Pathol. 1993;20(6):525–527. doi: 10.1111/j.1600-0560.1993.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 30.Alexis JB, Dembrow V. Papillary cystadenoma of a minor salivary gland. J Oral Maxillofac Surg. 1995;53(1):70–72. doi: 10.1016/0278-2391(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 31.Guccion JG, Redman RS, Calhoun NR, Saini N. Papillary cystadenoma of the palate: a case report and ultrastructural study. J Oral Maxillofac Surg. 1997;55(7):759–764. doi: 10.1016/S0278-2391(97)90594-2. [DOI] [PubMed] [Google Scholar]
- 32.Mahler V, Schell H. Papillary cystadenoma: a rare tumor of the minor salivary glands. Eur J Dermatol. 1999;9(5):387–389. [PubMed] [Google Scholar]
- 33.Tsurumi K, Kamiya H, Yokoi M, Kameyama Y. Papillary oncocytic cystadenoma of palatal minor salivary gland: a case report. J Oral Maxillofac Surg. 2003;61(5):631–633. doi: 10.1053/joms.2003.50120. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzaka K, Kokubu E, Takeda E, Tanaka Y, Shimono M, Inoue T. Papillary cystadenoma arising from the upper lip: a case report. Bull Tokyo Dent Coll. 2003;44(4):213–216. doi: 10.2209/tdcpublication.44.213. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro DA, Costa MR, de Assis GF. Papillary cystadenoma of the minor salivary gland of the lower lip. Dermatol Online J. 2004;10(1):14. [PubMed] [Google Scholar]
- 36.Gallego L, Junquera L, Fresno MF, de Vicente JC. Papillary cystadenoma and cystadenocarcinoma of salivary glands: two unusual entities. Medicina oral, patologia oral y cirugia bucal. 2008;13(7):E460–E463. [PubMed] [Google Scholar]
- 37.Lim CS, Ngu I, Collins AP, McKellar GM. Papillary cystadenoma of a minor salivary gland: report of a case involving cytological analysis and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105(1):e28–e33. doi: 10.1016/j.tripleo.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Halbritter SA, Altermatt HJ, Caversaccio M, Bornstein MM. Apocrine papillary cystadenoma of a minor salivary gland on the lower lip: case presentation. Quintessence Int. 2009;40(2):167–169. [PubMed] [Google Scholar]
- 39.Stathopoulos P, Gagari E. Papillary cystadenoma of the lower lip exhibiting ciliated pseudostratified columnar epithelium: report of a bizarre case and review of the literature. Oral Maxillofac Surg. 2013;17(3):161–164. doi: 10.1007/s10006-012-0357-2. [DOI] [PubMed] [Google Scholar]
- 40.Martins-Filho PR, Reinheimer DM, de Santana Santos T, Mascarenhas-Oliveira AC, Piva MR, Ferreira da Silva LC. Papillary cystadenoma of the upper lip. J Craniofac Surg. 2014;25(5):1932–1933. doi: 10.1097/SCS.0000000000000971. [DOI] [PubMed] [Google Scholar]
- 41.Goto M, Ohnishi Y, Shoju Y, Wato M, Kakudo K. Papillary oncocytic cystadenoma of a palatal minor salivary gland: a case report. Oncol Lett. 2016;11(2):1220–1222. doi: 10.3892/ol.2015.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tjioe KC, de Lima HG, Thompson LD, Lara VS, Damante JH, de Oliveira-Santos C. Papillary cystadenoma of minor salivary glands: report of 11 cases and review of the English literature. Head Neck Pathol. 2015;9(3):354–359. doi: 10.1007/s12105-014-0602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82(7):1217–1224. doi: 10.1002/(SICI)1097-0142(19980401)82:7<1217::AID-CNCR2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 44.Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25(7):835–845. doi: 10.1097/00000478-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Fonseca FP, de Andrade BA, Lopes MA, Pontes HA, Vargas PA, de Almeida OP. P63 expression in papillary cystadenoma and mucoepidermoid carcinoma of minor salivary glands. Oral surgery oral medicine oral pathology oral radiology. 2013;115(1):79–86. doi: 10.1016/j.oooo.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Lanzel EA, Pourian A, Sousa Melo SL, Brogden KA, Hellstein JW. Expression of membrane-bound mucins and p63 in distinguishing mucoepidermoid carcinoma from papillary cystadenoma. Head Neck Pathol. 2016 doi: 10.1007/s12105-016-0735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, et al. Burkitt’s lymphoma. The Lancet. 2012;379(9822):1234–1244. doi: 10.1016/S0140-6736(11)61177-X. [DOI] [PubMed] [Google Scholar]
- 48.Iwai T, Baba J, Murata S, Mitsudo K, Maegawa J, Nagahama K, et al. Warthin tumor arising from the minor salivary gland. J Craniofac Surg. 2012;23(5):e374–e376. doi: 10.1097/SCS.0b013e318254359f. [DOI] [PubMed] [Google Scholar]
- 49.Takezawa K, Jackson C, Gnepp DR, King TC. Molecular characterization of Warthin tumor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(5):569–575. doi: 10.1016/S1079-2104(98)90293-1. [DOI] [PubMed] [Google Scholar]
- 50.Honda K, Kashima K, Daa T, Yokoyama S, Nakayama I. Clonal analysis of the epithelial component of Warthin’s tumor. Hum Pathol. 2000;31(11):1377–1380. doi: 10.1016/S0046-8177(00)80007-6. [DOI] [PubMed] [Google Scholar]
- 51.Patel DK, Morton RP. Demographics of benign parotid tumours: Warthin’s tumour versus other benign salivary tumours. Acta Otolaryngol. 2016;136(1):83–86. doi: 10.3109/00016489.2015.1081276. [DOI] [PubMed] [Google Scholar]
- 52.Teymoortash A, Werner JA. Tissue that has lost its track: Warthin’s tumour. Virchows Arch. 2005;446(6):585–588. doi: 10.1007/s00428-005-1276-5. [DOI] [PubMed] [Google Scholar]
- 53.Seifert G, Bull HG, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Arch. 1980;388(1):13–38. doi: 10.1007/BF00430674. [DOI] [PubMed] [Google Scholar]
- 54.Park CK, Manning JT, Jr, Battifora H, Medeiros LJ. Follicle center lymphoma and Warthin tumor involving the same anatomic site. Report of two cases and review of the literature. Am J Clin Pathol. 2000;113(1):113–119. doi: 10.1309/MJH0-RQGX-U128-VFC6. [DOI] [PubMed] [Google Scholar]
- 55.Hwang JY, Kim SW, Yang SC, Kim CD. Extraparotid Warthin tumor in upper cervical lymph node accompanied by primary cervical tuberculosis. Otolaryngol Head Neck Surg. 2011;144(4):646–647. doi: 10.1177/0194599811398187. [DOI] [PubMed] [Google Scholar]
- 56.Fantasia JE, Miller AS. Papillary cystadenoma lymphomatosum arising in minor salivary glands. Oral Surg Oral Med Oral Pathol. 1981;52(4):411–416. doi: 10.1016/0030-4220(81)90340-6. [DOI] [PubMed] [Google Scholar]